Metabolic Rewiring by Human Placenta-Derived Mesenchymal Stem Cell Therapy Promotes Rejuvenation in Aged Female Rats
Abstract
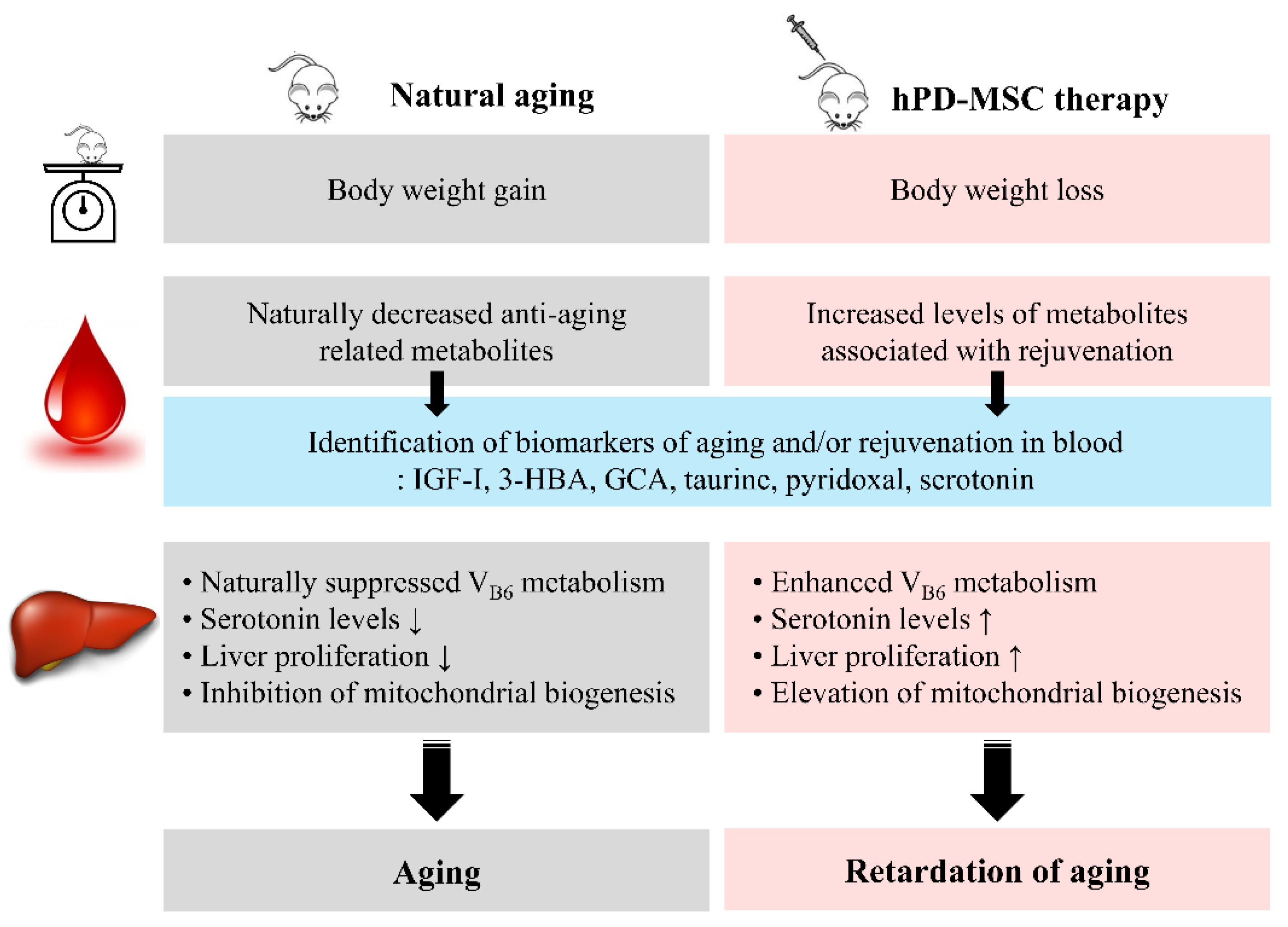
:1. Introduction
2. Results
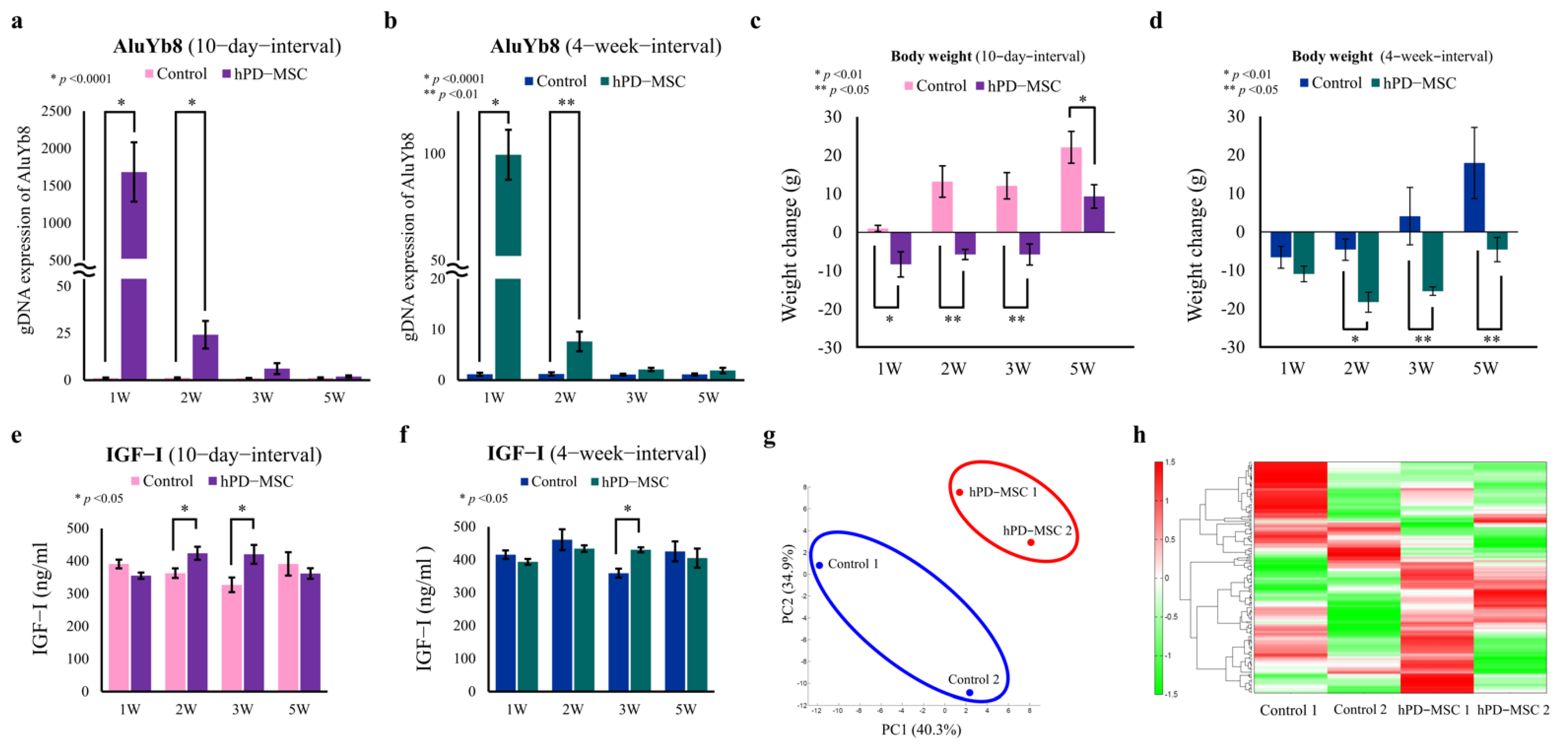
2.1. hPD-MSC Therapy Alters Metabolic Patterns in the Advanced-Age Rat Model
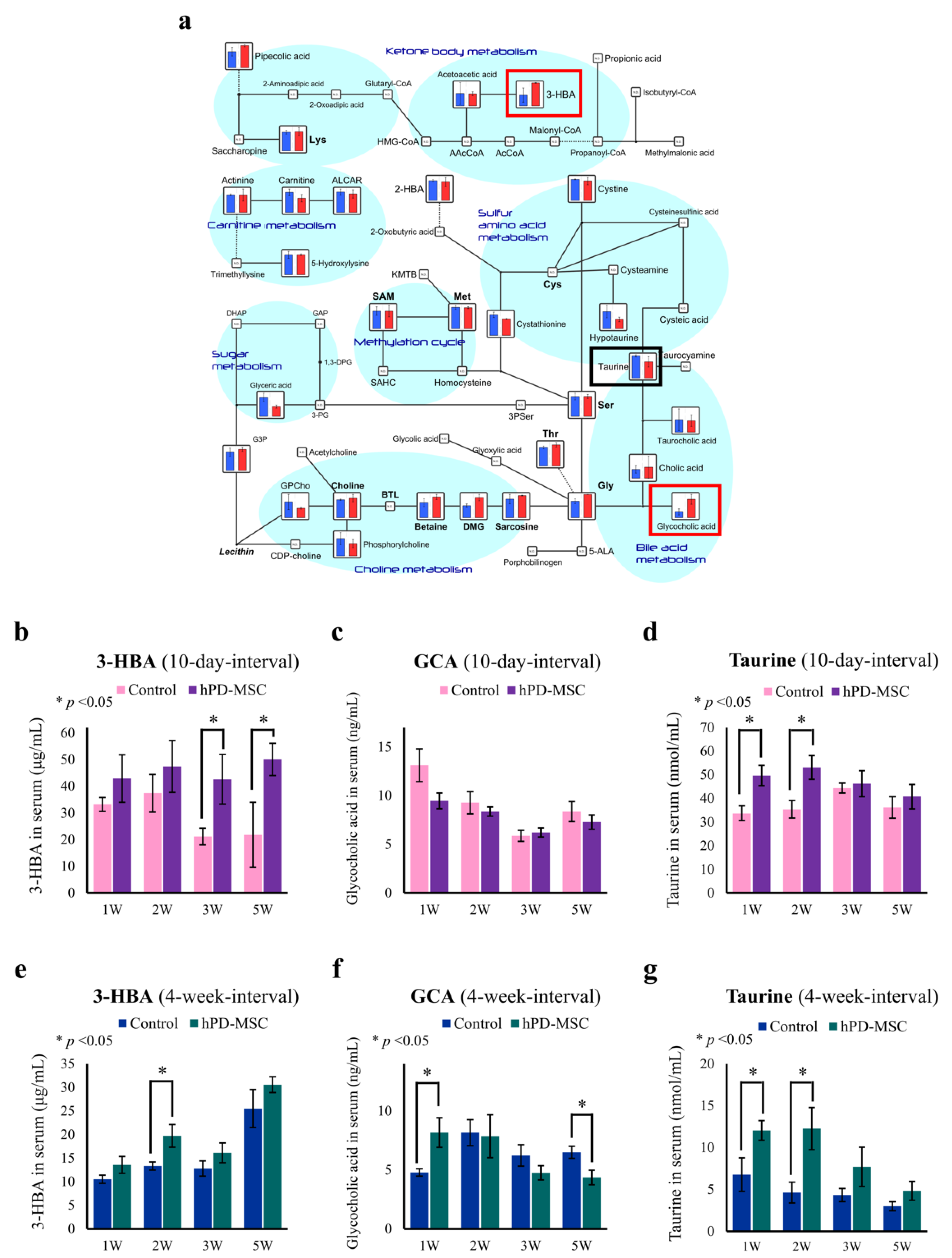
2.2. hPD-MSC Therapy Drives Antiaging-Associated Metabolic Changes
2.3. hPD-MSC Therapy Enhances the Production of Serotonin during Aging via Vitamin B6 Metabolism
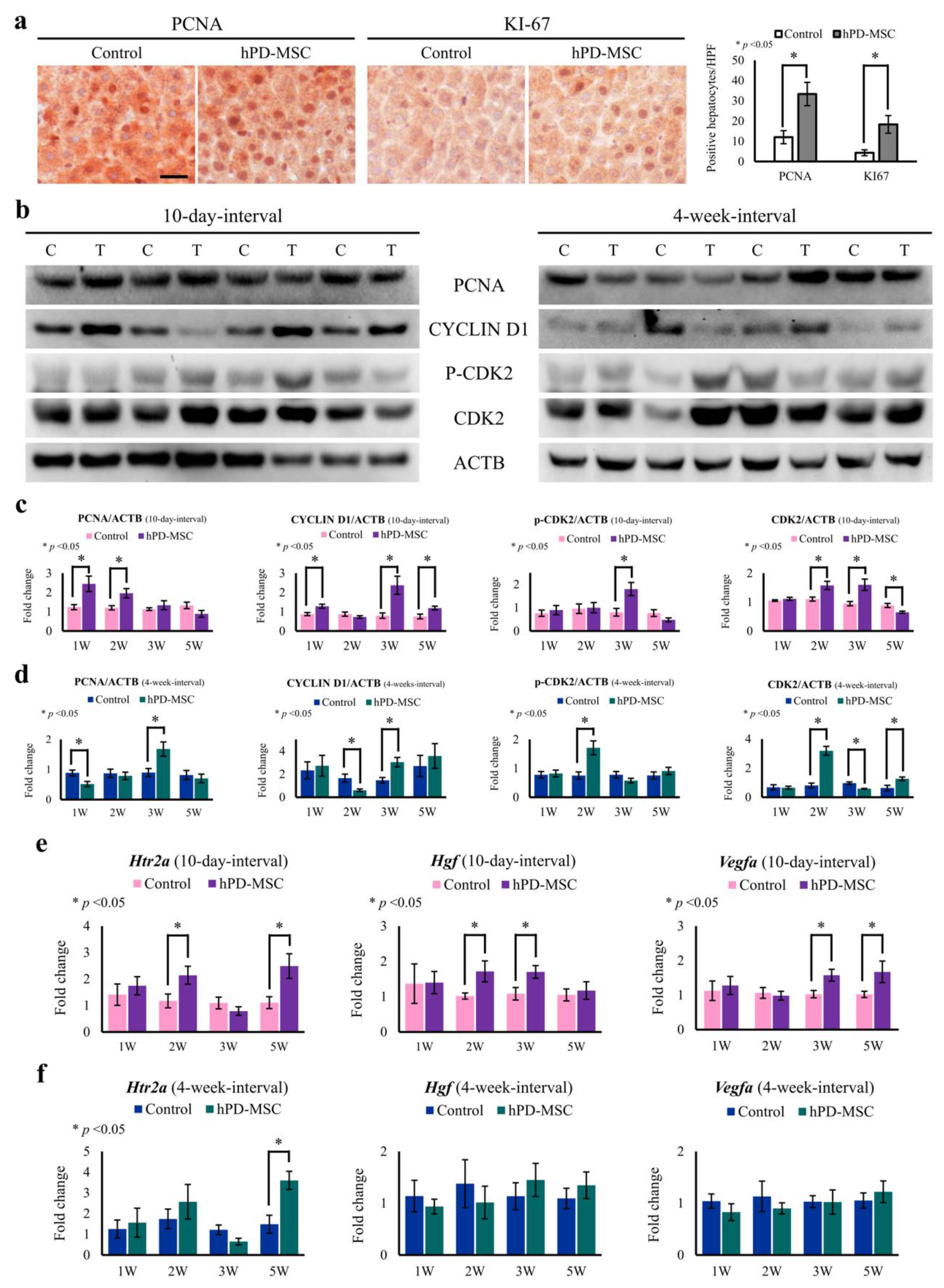
2.4. hPD-MSC Therapy-Derived Serotonin Reverses the Failure of Hepatocyte Proliferation Caused by Aging
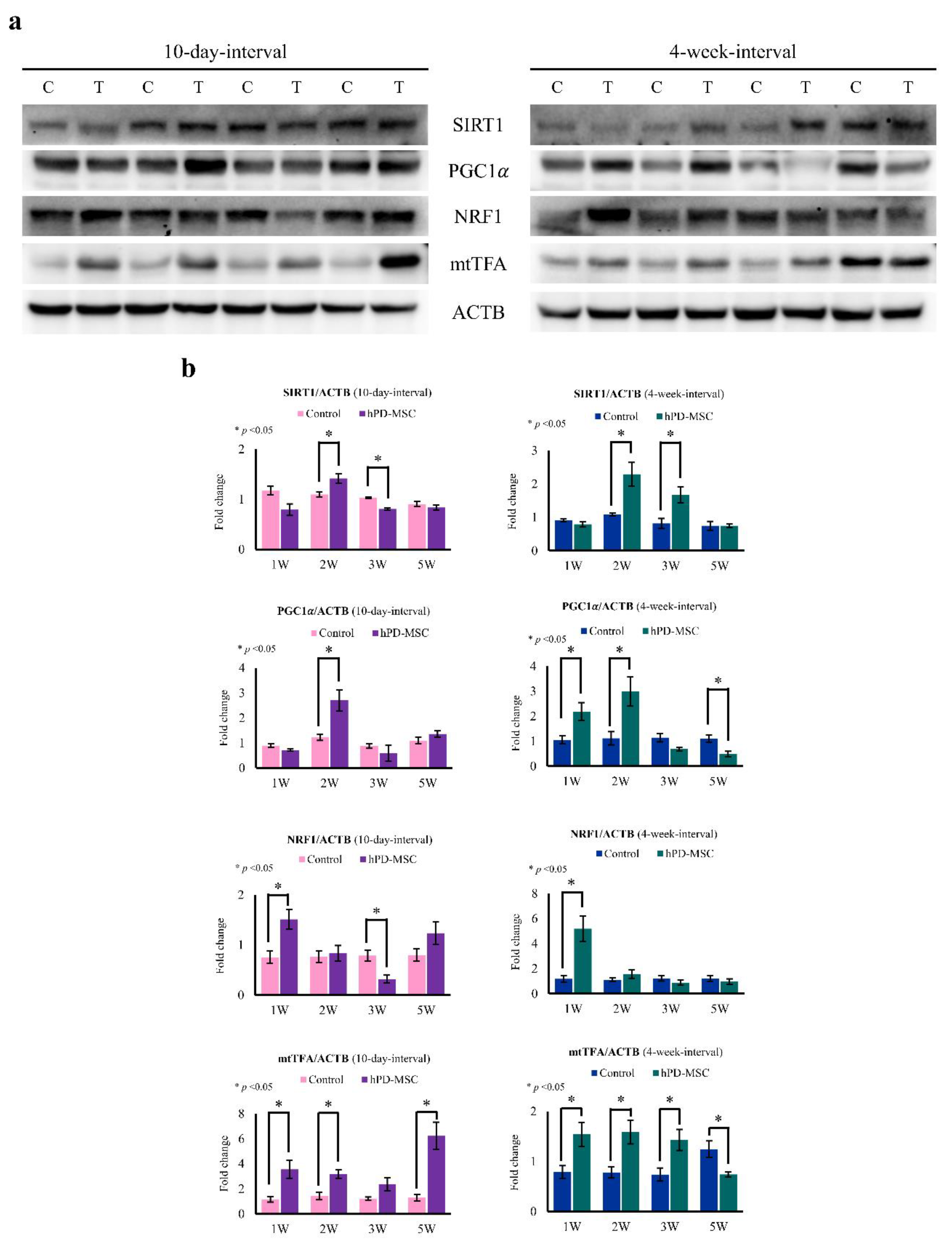
2.5. hPD-MSC Therapy Induces Mitochondrial Biogenesis in the Liver with Aging
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Culture of hPD-MSCs
4.3. Multiple Injection of hPD-MSC Therapy
4.4. RNA Extraction and Quantitative Real-Time RT–PCR (qRT–PCR)
4.5. Genome Extraction
4.6. Human Alu Sequence and qRT–PCR
4.7. Metabolome Profile of Serum by CE-TOF/MS Analysis
4.8. Western Blotting
4.9. Serum and Liver Metabolite Levels
4.10. Immunohistochemistry (IHC)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hPD-MSCs | Human placenta-derived mesenchymal stem cells |
| IGF-I | Insulin-like growth factor-I |
| CE-TOF/MS | Capillary electrophoresis-time-of-flight mass spectrometry |
| MSCs | Mesenchymal stem cells |
| Ovx | Ovariectomized |
| PCA | Principal component analysis |
| 3-HBA | 3-Hydroxybutyric acid |
| GCA | Glycocholic acid |
| PLP | Pyridoxal 5′-phosphate |
| THP1 | Tryptophan hydroxylase 1 |
| DDC | Dopa decarboxylase |
| CDK2 | Cyclin-dependent kinase 2 |
| HTR2A | 5-Hydroxytryptamine receptor 2a |
| HGF | Hepatocyte growth factor |
| VEGFA | Vascular endothelial growth factor a |
| PGC1α | Peroxisome proliferator-activated receptor-γ coactivator 1α |
| mtTFA | Mitochondrial transcription factor A |
| SIRT1 | Sirtuin 1 |
| NRF1 | Nuclear respiration factor 1 |
| GCDCA | Glycochenodeoxycholic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| 5-HT | 5-Hydroxytryptamine |
References
- Davidovic, M.; Sevo, G.; Svorcan, P.; Milosevic, D.P.; Despotovic, N.; Erceg, P. Old age as a privilege of the selfish ones. Aging Dis. 2010, 1, 139–146. [Google Scholar] [PubMed]
- Erikson, E.H.; Erikson, J.M. The Life Cycle Completed; W.W. Norton & Company: New York, NY, USA, 1997. [Google Scholar]
- Villar, F. Successful ageing and development: The contribution of generativity in older age. Ageing Soc. 2012, 32, 1087–1105. [Google Scholar] [CrossRef] [Green Version]
- Burbank, P.M. Psychosocial theories of aging: A critical evaluation. ANS Adv. Nurs. Sci. 1986, 9, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.W.; Martin, P.; Bishop, A.; Cho, J.; da Rosa, G.; Deshpande, N.; Hensley, R.; Macdonald, M.; Margrett, J.; Randall, G.K.; et al. Understanding centenarians’ psychosocial dynamics and their contributions to health and quality of life. Curr. Gerontol. Geriatr. Res. 2010, 2010, 680657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K. Modern biological theories of aging. Aging Dis. 2010, 1, 72–74. [Google Scholar]
- He, S.; Sharpless, N.E. Senescence in health and disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Galluzzi, L.; Freije, J.M.P.; Madeo, F.; Kroemer, G. Metabolic control of longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef] [Green Version]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, D.K.; Hollywood, K.A.; Goodacre, R. Metabolomics for the masses: The future of metabolomics in a personalized world. New Horiz. Transl. Med. 2017, 3, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Sun, H.; Yan, G.; Wang, P.; Wang, X. Metabolomics for biomarker discovery: Moving to the clinic. BioMed Res. Int. 2015, 2015, 354671. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [Green Version]
- Chaleckis, R.; Murakami, I.; Takada, J.; Kondoh, H.; Yanagida, M. Individual variability in human blood metabolites identifies age-related differences. Proc. Natl. Acad. Sci. USA 2016, 113, 4252–4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, C.; Ulloor, J.; Dillon, E.L.; Dahodwala, Q.; Franklin, B.; Storer, T.; Sebastiani, P.; Sheffield-Moore, M.; Urban, R.J.; Bhasin, S.; et al. Identification of serum biomarkers for aging and anabolic response. Immun. Ageing 2011, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.Y.; Hwang, H.; Kim, S.K.; Choi, J.Y.; Lee, S.M.; Bang, H.; Kwon, E.S.; Lee, K.P.; Chung, S.G.; Kwon, K.S. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci. Rep. 2018, 8, 8574. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-mediated mitophagy triggers the visual cycle by enhancing mitochondrial functions in a H2O2-injured rat model. Cells 2021, 10, 1117. [Google Scholar] [CrossRef]
- Na, J.; Song, J.; Kim, H.H.; Seok, J.; Kim, J.Y.; Jun, J.H.; Kim, G.J. Human placenta-derived mesenchymal stem cells trigger repair system in TAA-injured rat model via antioxidant effect. Aging 2020, 13, 61–76. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, J.H.; Jun, J.H.; Park, S.; Jung, J.; Bae, S.H.; Kim, G.J. Enhanced PRL-1 expression in placenta-derived mesenchymal stem cells accelerates hepatic function via mitochondrial dynamics in a cirrhotic rat model. Stem Cell Res. Ther. 2020, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, S.; Chugh, R.M.; Park, H.S.; Hobeika, E.; Ulin, M.; Al-Hendy, A. Mesenchymal stem cells as a bio-organ for treatment of female infertility. Cells 2020, 9, 2253. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, L.; Wang, S.; Sheng, X.; Yan, G.; Xu, L.; Liu, J.; Liu, M.; Zhen, X.; Ding, L.; et al. Transplantation of umbilical cord-derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. Vitr. Cell. Dev. Biol. Anim. 2019, 55, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, E.Y.; Kim, G.J.; Ko, J.J.; Cha, K.Y.; Koong, M.K.; Lee, K.A. Human placenta-derived mesenchymal stem cells stimulate ovarian function via miR-145 and bone morphogenetic protein signaling in aged rats. Stem Cell Res. Ther. 2020, 11, 472. [Google Scholar] [CrossRef]
- Abd-Allah, S.H.; Shalaby, S.M.; Pasha, H.F.; El-Shal, A.S.; Raafat, N.; Shabrawy, S.M.; Awad, H.A.; Amer, M.G.; Gharib, M.A.; El Gendy, E.A.; et al. Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. Cytotherapy 2013, 15, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Yan, G.; Wang, B.; Xu, L.; Gu, Y.; Ru, T.; Cui, X.; Lei, L.; Liu, J.; Sheng, X.; et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci. China Life Sci. 2018, 61, 1554–1565. [Google Scholar] [CrossRef]
- Singh, N.; Mohanty, S.; Seth, T.; Shankar, M.; Bhaskaran, S.; Dharmendra, S. Autologous stem cell transplantation in refractory Asherman’s syndrome: A novel cell-based therapy. J. Hum. Reprod. Sci. 2014, 7, 93–98. [Google Scholar] [CrossRef]
- Seok, J.; Park, H.; Choi, J.H.; Lim, J.Y.; Kim, K.G.; Kim, G.J. Placenta-derived mesenchymal stem cells restore the ovary function in an ovariectomized rat model via an antioxidant effect. Antioxidants 2020, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kim, T.H.; Seok, J.; Jun, J.H.; Park, H.; Kweon, M.; Lim, J.Y.; Kim, G.J. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model via the VEGF pathway. Lab. Invest. 2021, 101, 304–317. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. The laboratory rat: Age and body weight matter. EXCLI J. 2021, 20, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Tas, E.; Muzumdar, R. Humanin and age-related diseases: A new link? Front. Endocrinol. 2014, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daughaday, W.H.; Rotwein, P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr. Rev. 1989, 10, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Copes, N.; Bradshaw, P.C. D-ss-hydroxybutyrate: An anti-aging ketone body. Oncotarget 2015, 6, 3477–3478. [Google Scholar] [CrossRef] [PubMed]
- Barcena, C.; Quiros, P.M.; Durand, S.; Mayoral, P.; Rodriguez, F.; Caravia, X.M.; Marino, G.; Garabaya, C.; Fernandez-Garcia, M.T.; Kroemer, G.; et al. Methionine restriction extends lifespan in progeroid mice and alters lipid and bile acid metabolism. Cell Rep. 2018, 24, 2392–2403. [Google Scholar] [CrossRef] [Green Version]
- Dawson, R.; Eppler, B.; Patterson, T.A.; Shih, D.; Liu, S. The effects of taurine in a rodent model of aging. Adv. Exp. Med. Biol. 1996, 403, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Cellini, B.; Montioli, R.; Oppici, E.; Astegno, A.; Voltattorni, C.B. The chaperone role of the pyridoxal 5’-phosphate and its implications for rare diseases involving B6-dependent enzymes. Clin. Biochem. 2014, 47, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, M. Liver regeneration in aged mice: New insights. Aging 2018, 10, 1801–1824. [Google Scholar] [CrossRef]
- Mde, L.B.S.; Matias, J.E.; Montibeller, G.R.; Siqueira, L.C.; Nunes Eda, S.; Grassi, C.A. Effect of aging on liver regeneration in rats. Acta Cir. Bras. 2006, 21, 197–202. [Google Scholar] [CrossRef]
- Schmucker, D.L.; Sanchez, H. Liver regeneration and aging: A current perspective. Curr. Gerontol. Geriatr. Res. 2011, 2011, 526379. [Google Scholar] [CrossRef] [Green Version]
- Lesurtel, M.; Graf, R.; Aleil, B.; Walther, D.J.; Tian, Y.; Jochum, W.; Gachet, C.; Bader, M.; Clavien, P.A. Platelet-derived serotonin mediates liver regeneration. Science 2006, 312, 104–107. [Google Scholar] [CrossRef]
- Khiati, S.; Baechler, S.A.; Factor, V.M.; Zhang, H.; Huang, S.Y.; Dalla Rosa, I.; Sourbier, C.; Neckers, L.; Thorgeirsson, S.S.; Pommier, Y. Lack of mitochondrial topoisomerase I (TOP1mt) impairs liver regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 11282–11287. [Google Scholar] [CrossRef] [Green Version]
- Han, L.H.; Dong, L.Y.; Yu, H.; Sun, G.Y.; Wu, Y.; Gao, J.; Thasler, W.; An, W. Deceleration of liver regeneration by knockdown of augmenter of liver regeneration gene is associated with impairment of mitochondrial DNA synthesis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G112–G122. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.F.; Hu, H.B.; Xu, H.Y.; Fu, X.F.; Peng, D.X.; Su, W.Y.; He, Y.L. Human umbilical cord mesenchymal stem cell transplantation restores damaged ovaries. J. Cell. Mol. Med. 2015, 19, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Yang, T.; Li, J.; Yang, X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res. Ther. 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W.; et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Invest. 2020, 100, 342–352. [Google Scholar] [CrossRef]
- Xiao, G.Y.; Liu, I.H.; Cheng, C.C.; Chang, C.C.; Lee, Y.H.; Cheng, W.T.; Wu, S.C. Amniotic fluid stem cells prevent follicle atresia and rescue fertility of mice with premature ovarian failure induced by chemotherapy. PLoS ONE 2014, 9, e106538. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Lee, J.Y.; Park, C.H.; Eum, J.H.; Jung, S.K.; Han, A.R.; Seol, D.W.; Lee, J.S.; Shin, H.S.; Im, J.H.; et al. Cryopreserved human oocytes and cord blood cells can produce somatic cell nuclear transfer-derived pluripotent stem cells with a homozygous HLA type. Stem Cell Rep. 2020, 15, 171–184. [Google Scholar] [CrossRef]
- Krampera, M.; Glennie, S.; Dyson, J.; Scott, D.; Laylor, R.; Simpson, E.; Dazzi, F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003, 101, 3722–3729. [Google Scholar] [CrossRef] [PubMed]
- Beyth, S.; Borovsky, Z.; Mevorach, D.; Liebergall, M.; Gazit, Z.; Aslan, H.; Galun, E.; Rachmilewitz, J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005, 105, 2214–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef] [Green Version]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, C.; Garagnani, P.; Franceschi, C. Genetics of human longevity within an eco-evolutionary nature-nurture framework. Circ. Res. 2018, 123, 745–772. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The critical role of metabolic pathways in aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, G.; Tauchmanova, L.; Di Somma, C.; Musella, T.; Rota, F.; Savanelli, M.C.; Colao, A. Somatopause: Dismetabolic and bone effects. J. Endocrinol. Invest. 2005, 28, 36–42. [Google Scholar] [PubMed]
- O’Neill, C.; Kiely, A.P.; Coakley, M.F.; Manning, S.; Long-Smith, C.M. Insulin and IGF-1 signalling: Longevity, protein homoeostasis and Alzheimer’s disease. Biochem. Soc. Trans. 2012, 40, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Bonafe, M.; Franceschi, C.; Paolisso, G. Insulin/IGF-I-signaling pathway: An evolutionarily conserved mechanism of longevity from yeast to humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1064–E1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Collino, S.; Montoliu, I.; Martin, F.P.; Scherer, M.; Mari, D.; Salvioli, S.; Bucci, L.; Ostan, R.; Monti, D.; Biagi, E.; et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS ONE 2013, 8, e56564. [Google Scholar] [CrossRef]
- Montoliu, I.; Scherer, M.; Beguelin, F.; DaSilva, L.; Mari, D.; Salvioli, S.; Martin, F.P.; Capri, M.; Bucci, L.; Ostan, R.; et al. Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging 2014, 6, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Bunning, B.J.; Contrepois, K.; Lee-McMullen, B.; Dhondalay, G.K.R.; Zhang, W.; Tupa, D.; Raeber, O.; Desai, M.; Nadeau, K.C.; Snyder, M.P.; et al. Global metabolic profiling to model biological processes of aging in twins. Aging Cell 2020, 19, e13073. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Kim, Y.J. Anti-aging effect of the ketone metabolite beta-hydroxybutyrate in Drosophila intestinal stem cells. Int. J. Mol. Sci. 2020, 21, 3497. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, H.D.; Gerhard, G.S. Bile acids in neurodegenerative disorders. Front. Aging Neurosci. 2016, 8, 263. [Google Scholar] [CrossRef] [Green Version]
- Heubi, J.E.; Setchell, K.D.; Jha, P.; Buckley, D.; Zhang, W.; Rosenthal, P.; Potter, C.; Horslen, S.; Suskind, D. Treatment of bile acid amidation defects with glycocholic acid. Hepatology 2015, 61, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, S.; Kim, H.W. Effects and mechanisms of taurine as a therapeutic agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Pierno, S.; De Luca, A.; Camerino, C.; Huxtable, R.J.; Camerino, D.C. Chronic administration of taurine to aged rats improves the electrical and contractile properties of skeletal muscle fibers. J. Pharmacol. Exp. Ther. 1998, 286, 1183–1190. [Google Scholar]
- Vanderschuren, H.; Boycheva, S.; Li, K.T.; Szydlowski, N.; Gruissem, W.; Fitzpatrick, T.B. Strategies for vitamin B6 biofortification of plants: A dual role as a micronutrient and a stress protectant. Front. Plant. Sci. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merigliano, C.; Mascolo, E.; Burla, R.; Saggio, I.; Verni, F. The relationship between vitamin B6, diabetes and cancer. Front. Genet. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J.; Field, M.S. Vitamin B-6. Adv. Nutr. 2015, 6, 132–133. [Google Scholar] [CrossRef]
- Ruddell, R.G.; Mann, D.A.; Ramm, G.A. The function of serotonin within the liver. J. Hepatol. 2008, 48, 666–675. [Google Scholar] [CrossRef] [Green Version]
- Cellini, B.; Zelante, T.; Dindo, M.; Bellet, M.M.; Renga, G.; Romani, L.; Costantini, C. Pyridoxal 5’-phosphate-dependent enzymes at the crossroads of host-microbe tryptophan metabolism. Int. J. Mol. Sci. 2020, 21, 5823. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef]
- Kim, I.H.; Kisseleva, T.; Brenner, D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015, 31, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Jimenez, R.J.; Sharma, K.; Luu, H.Y.; Hsu, B.Y.; Ravindranathan, A.; Stohr, B.A.; Willenbring, H. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell 2020, 26, 27–33. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Takeuchi, S.; Watanabe, T.; Yoshida, T.; Nojiri, S.; Ogawa, M.; Terai, S. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as “conducting cells” for improvement of liver fibrosis and regeneration. Inflamm. Regen. 2019, 39, 18. [Google Scholar] [CrossRef] [Green Version]
- Payton, A.; Gibbons, L.; Davidson, Y.; Ollier, W.; Rabbitt, P.; Worthington, J.; Pickles, A.; Pendleton, N.; Horan, M. Influence of serotonin transporter gene polymorphisms on cognitive decline and cognitive abilities in a nondemented elderly population. Mol. Psychiatry 2005, 10, 1133–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meltzer, C.C.; Price, J.C.; Mathis, C.A.; Butters, M.A.; Ziolko, S.K.; Moses-Kolko, E.; Mazumdar, S.; Mulsant, B.H.; Houck, P.R.; Lopresti, B.J.; et al. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology 2004, 29, 2258–2265. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, C.C.; Smith, G.; DeKosky, S.T.; Pollock, B.G.; Mathis, C.A.; Moore, R.Y.; Kupfer, D.J.; Reynolds, C.F. Serotonin in aging, late-life depression, and Alzheimer’s disease: The emerging role of functional imaging. Neuropsychopharmacology 1998, 18, 407–430. [Google Scholar] [CrossRef] [Green Version]
- Grattagliano, I.; Russmann, S.; Diogo, C.; Bonfrate, L.; Oliveira, P.J.; Wang, D.Q.; Portincasa, P. Mitochondria in chronic liver disease. Curr. Drug Targets 2011, 12, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jung, J.; Na, K.H.; Moon, J.S.; Lee, H.J.; Kim, J.H.; Kim, G.I.; Kwon, S.W.; Hwang, S.G.; Kim, G.J. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl(4)-injured liver: Potential application to the treatment of hepatic diseases. J. Cell. Biochem. 2010, 111, 1453–1463. [Google Scholar] [CrossRef]
- Kim, T.H.; Choi, J.H.; Jun, Y.; Lim, S.M.; Park, S.; Paek, J.Y.; Lee, S.H.; Hwang, J.Y.; Kim, G.J. 3D-cultured human placenta-derived mesenchymal stem cell spheroids enhance ovary function by inducing folliculogenesis. Sci. Rep. 2018, 8, 15313. [Google Scholar] [CrossRef]
- Triant, D.A.; Whitehead, A. Simultaneous extraction of high-quality RNA and DNA from small tissue samples. J. Hered. 2009, 100, 246–250. [Google Scholar] [CrossRef]
- Walker, J.A.; Kilroy, G.E.; Xing, J.; Shewale, J.; Sinha, S.K.; Batzer, M.A. Human DNA quantitation using Alu element-based polymerase chain reaction. Anal. Biochem. 2003, 315, 122–128. [Google Scholar] [CrossRef]
- Lee, M.S.; Han, H.J.; Han, S.Y.; Kim, I.Y.; Chae, S.; Lee, C.S.; Kim, S.E.; Yoon, S.G.; Park, J.W.; Kim, J.H.; et al. Loss of the E3 ubiquitin ligase MKRN1 represses diet-induced metabolic syndrome through AMPK activation. Nat. Commun. 2018, 9, 3404. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Lee, K.-A. Metabolic Rewiring by Human Placenta-Derived Mesenchymal Stem Cell Therapy Promotes Rejuvenation in Aged Female Rats. Int. J. Mol. Sci. 2022, 23, 566. https://doi.org/10.3390/ijms23010566
Kim K-H, Lee K-A. Metabolic Rewiring by Human Placenta-Derived Mesenchymal Stem Cell Therapy Promotes Rejuvenation in Aged Female Rats. International Journal of Molecular Sciences. 2022; 23(1):566. https://doi.org/10.3390/ijms23010566
Chicago/Turabian StyleKim, Kyeoung-Hwa, and Kyung-Ah Lee. 2022. "Metabolic Rewiring by Human Placenta-Derived Mesenchymal Stem Cell Therapy Promotes Rejuvenation in Aged Female Rats" International Journal of Molecular Sciences 23, no. 1: 566. https://doi.org/10.3390/ijms23010566
APA StyleKim, K.-H., & Lee, K.-A. (2022). Metabolic Rewiring by Human Placenta-Derived Mesenchymal Stem Cell Therapy Promotes Rejuvenation in Aged Female Rats. International Journal of Molecular Sciences, 23(1), 566. https://doi.org/10.3390/ijms23010566






