IL-17 Pathway Members as Potential Biomarkers of Effective Systemic Treatment and Cardiovascular Disease in Patients with Moderate-to-Severe Psoriasis
Abstract
1. Introduction
2. Results
2.1. Study Cohort
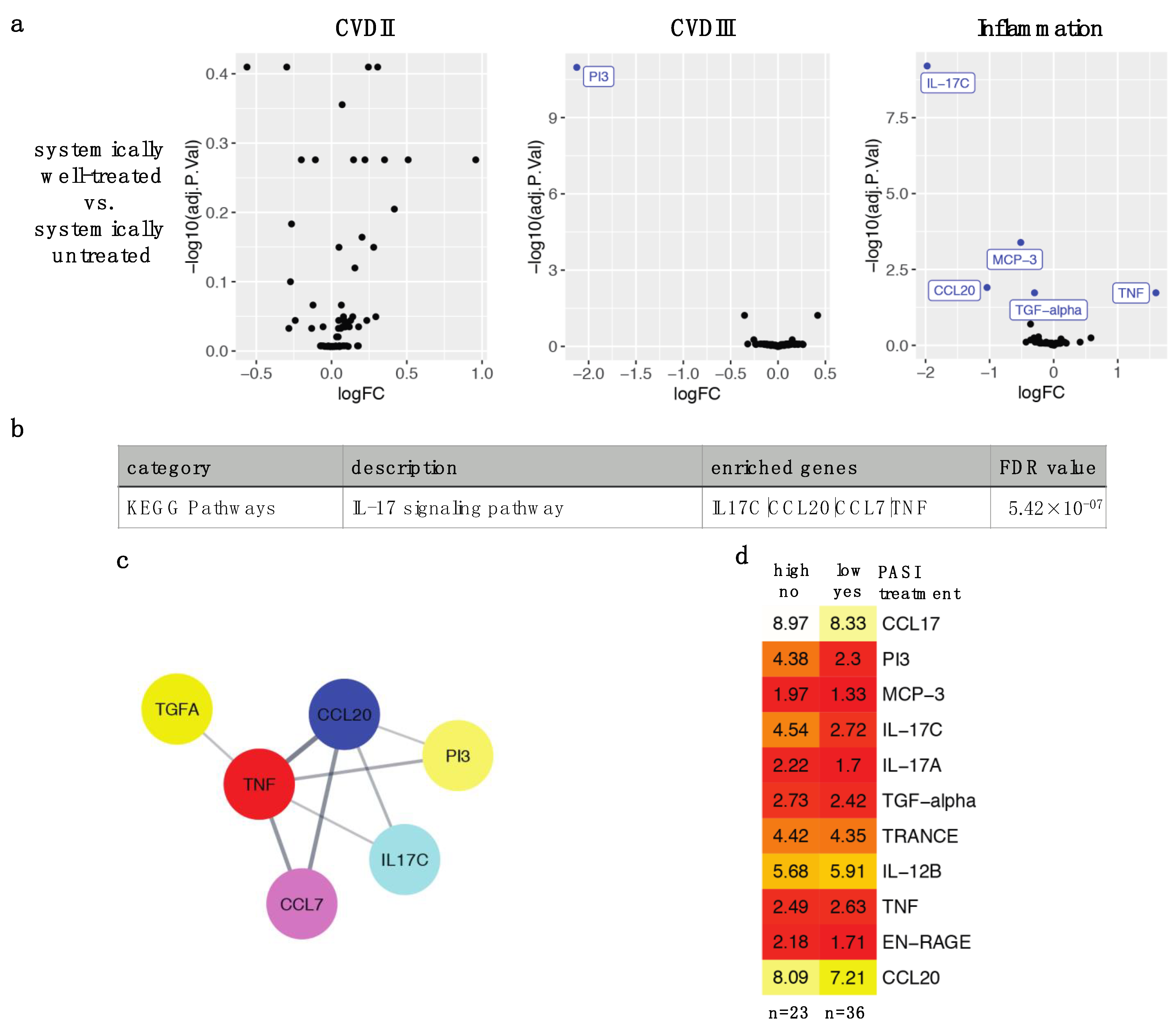
2.2. Effective Systemic Anti-Psoriatic Treatment Is Associated with Decreased Levels of Circulating Proteins in the IL-17 Signaling Pathway
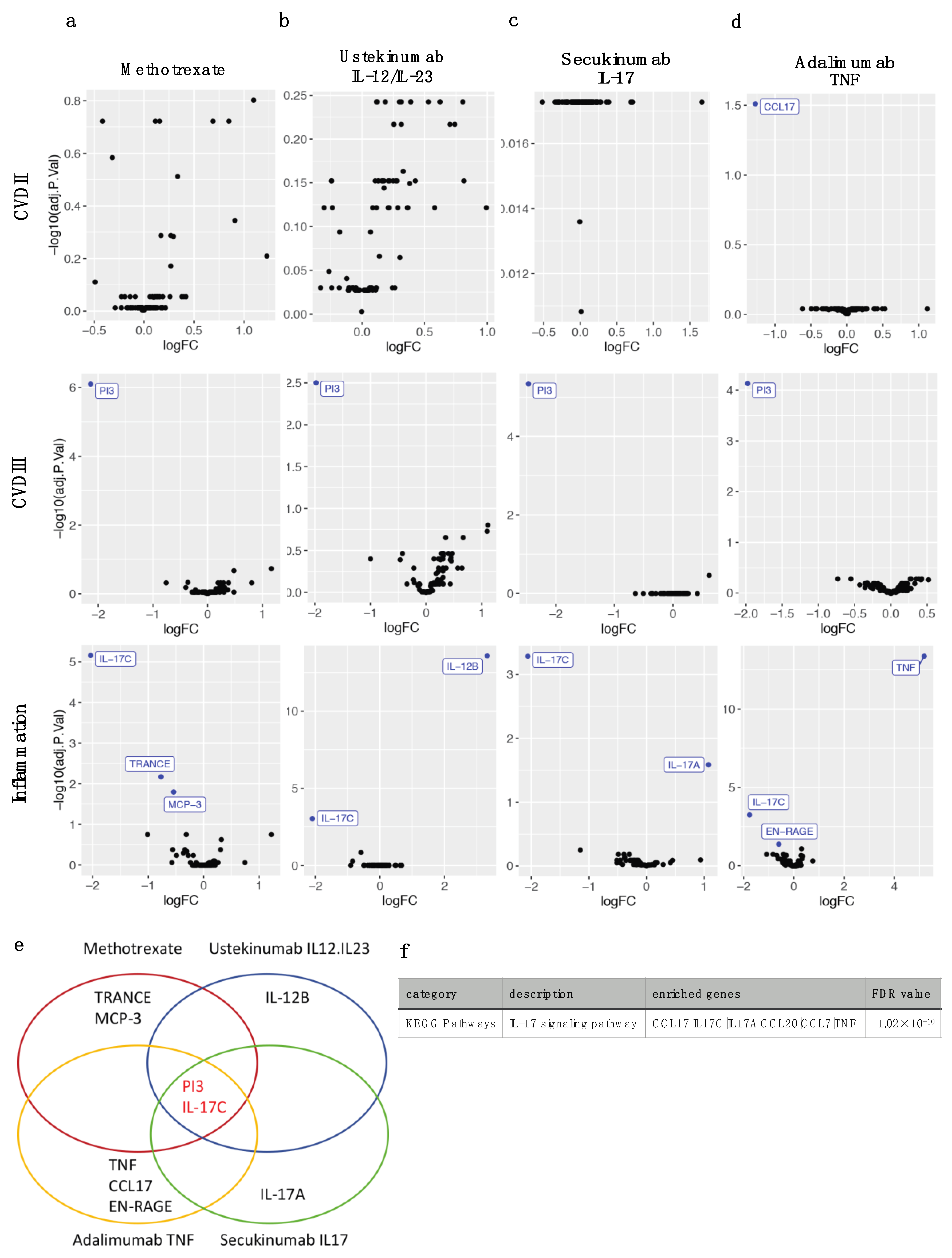
2.3. Four Different Types of Systemic Anti-Psoriatic Treatment Share Association with Lower Levels of PI3 and IL-17C
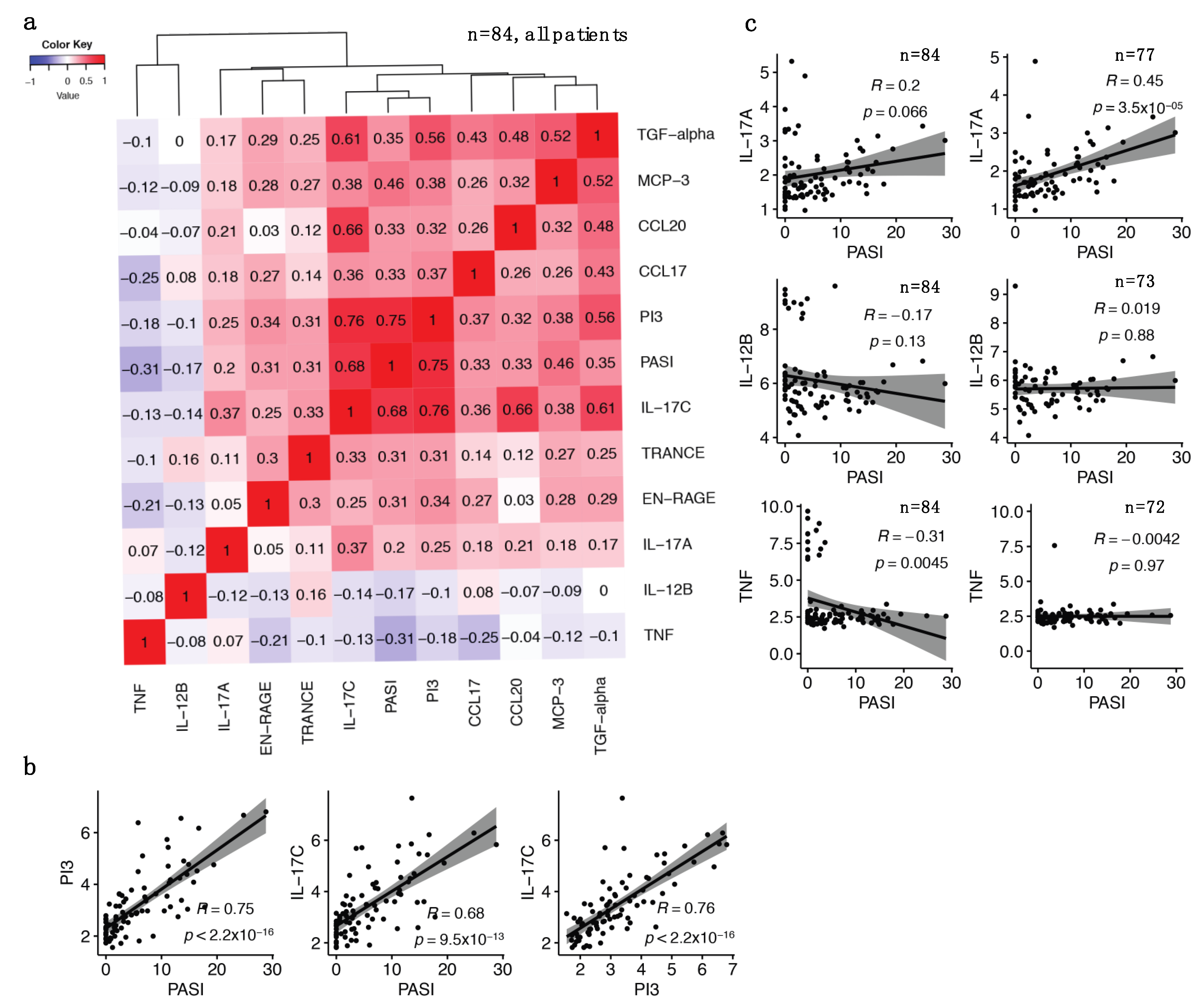
2.4. The Identified IL-17 Pathway Proteins Strongly Correlate with Each Other and with PASI
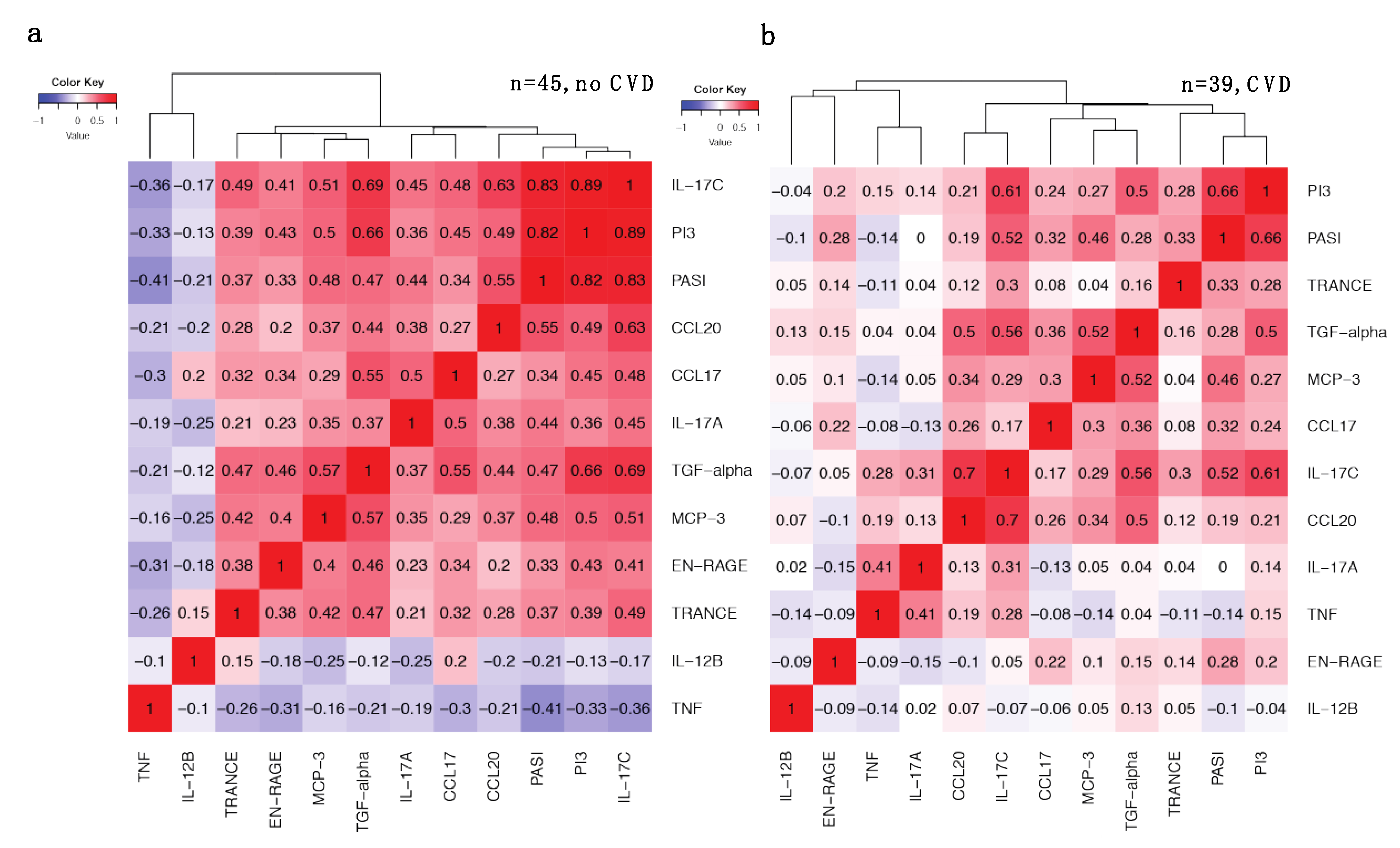
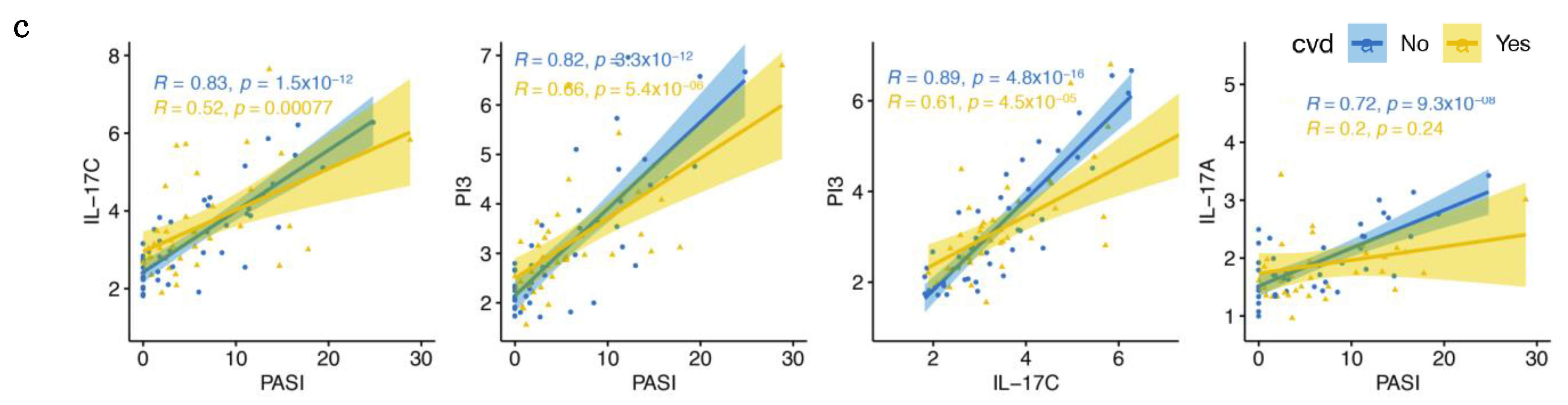
2.5. Increased Correlations between IL-17 Pathway Proteins and PASI in Patients without CVD Are Weakened with CVD
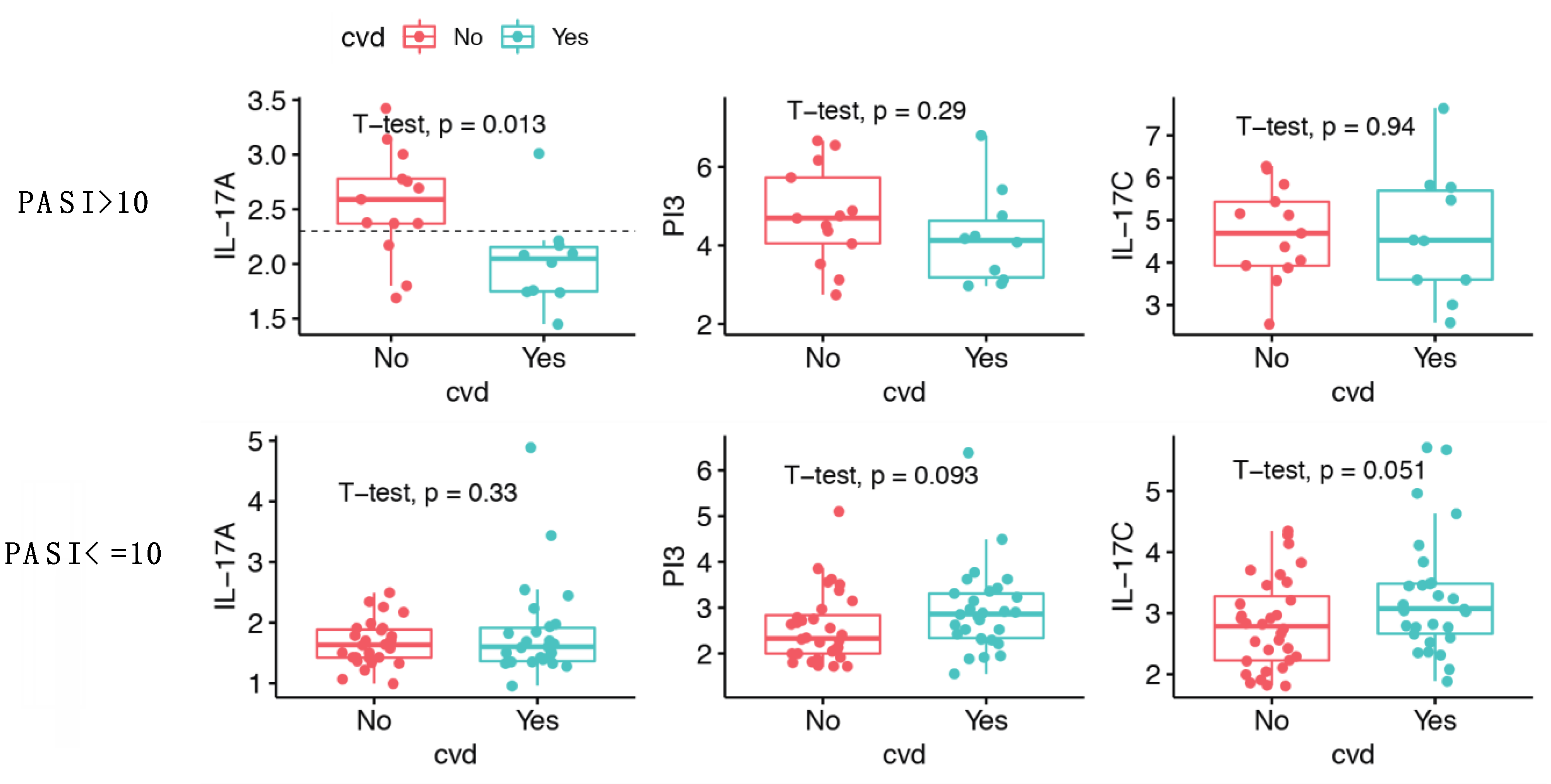
2.6. IL-17A Levels Are Lower in Patients with Moderate-to-Severe Psoriasis and CVD Compared to Patients without CVD
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. Sample Collection and Plasma Protein Quantification
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brembilla, N.C.; Senra, L.; Boehncke, W.H. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front. Immunol. 2018, 9, 1682. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.E.; Yan, B.Y.; Chan, T.C.; Krueger, J.G. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J. Immunol. 2018, 201, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- von Stebut, E.; Boehncke, W.H.; Ghoreschi, K.; Gori, T.; Kaya, Z.; Thaci, D.; Schaffler, A. IL-17A in Psoriasis and Beyond: Cardiovascular and Metabolic Implications. Front. Immunol. 2019, 10, 3096. [Google Scholar] [CrossRef]
- Kim, J.; Krueger, J.G. Highly Effective New Treatments for Psoriasis Target the IL-23/Type 17 T Cell Autoimmune Axis. Annu. Rev. Med. 2017, 68, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Operacz, M.; Sadowska-Przytocka, A. The possibilities and principles of methotrexate treatment of psoriasis-the updated knowledge. Adv. Dermatol. Allergol./Postȩpy Dermatol. Alergol. 2014, 31, 392–400. [Google Scholar] [CrossRef]
- Haustein, U.F.; Rytter, M. Methotrexate in psoriasis: 26 years’ experience with low-dose long-term treatment. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 382–388. [Google Scholar] [CrossRef]
- Yan, K.; Xu, W.; Huang, Y.; Zhang, Z.; Huang, Q.; Xin, K.Z.; Ma, Y.; Han, L. Methotrexate restores the function of peripheral blood regulatory T cells in psoriasis vulgaris via the CD73/AMPK/mTOR pathway. Br. J. Dermatol. 2018, 179, 896–905. [Google Scholar] [CrossRef]
- Harper, E.G.; Guo, C.; Rizzo, H.; Lillis, J.V.; Kurtz, S.E.; Skorcheva, I.; Purdy, D.; Fitch, E.; Iordanov, M.; Blauvelt, A. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: Implications for psoriasis pathogenesis. J. Investig. Dermatol. 2009, 129, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiao, Q.; Liu, M.; He, T.; Shi, J.; Bai, X.; Zhang, Y.; Li, Y.; Cai, W.; Han, S.; et al. IL-17 Promotes Scar Formation by Inducing Macrophage Infiltration. Am. J. Pathol. 2018, 188, 1693–1702. [Google Scholar] [CrossRef]
- Nies, J.F.; Panzer, U. IL-17C/IL-17RE: Emergence of a Unique Axis in TH17 Biology. Front. Immunol. 2020, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Fritz, Y.; Dawes, S.M.; Diaconu, D.; Al-Attar, P.M.; Guzman, A.M.; Chen, C.S.; Fu, W.; Gudjonsson, J.E.; McCormick, T.S.; et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J. Immunol. 2013, 190, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Wiedow, O.; Schröder, J.M.; Gregory, H.; Young, J.A.; Christophers, E. Elafin: An elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J. Biol. Chem. 1990, 265, 14791–14795. [Google Scholar] [CrossRef]
- Alam, S.R.; Newby, D.E.; Henriksen, P.A. Role of the endogenous elastase inhibitor, elafin, in cardiovascular injury: From epithelium to endothelium. Biochem. Pharm. 2012, 83, 695–704. [Google Scholar] [CrossRef]
- Elgharib, I.; Khashaba, S.A.; Elsaid, H.H.; Sharaf, M.M. Serum elafin as a potential inflammatory marker in psoriasis. Int. J. Dermatol. 2019, 58, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Lowes, M.A.; Fuentes-Duculan, J.; Zaba, L.C.; Cardinale, I.; Nograles, K.E.; Khatcherian, A.; Novitskaya, I.; Carucci, J.A.; Bergman, R.; et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J. Immunol. 2008, 181, 7420–7427. [Google Scholar] [CrossRef] [PubMed]
- Leijten, E.; Tao, W.; Pouw, J.; van Kempen, T.; Olde Nordkamp, M.; Balak, D.; Tekstra, J.; Munoz-Elias, E.; DePrimo, S.; Drylewicz, J.; et al. Broad proteomic screen reveals shared serum proteomic signature in patients with psoriatic arthritis and psoriasis without arthritis. Rheumatology 2021, 60, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Renert-Yuval, Y.; Suprun, M.; Esaki, H.; Oliva, M.; Huynh, T.N.; Ungar, B.; Kunjravia, N.; Friedland, R.; Peng, X.; et al. An IL-17-dominant immune profile is shared across the major orphan forms of ichthyosis. J. Allergy Clin. Immunol. 2017, 139, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Deng, J.; Xu, K.; Zhu, T.; Han, L.; Yan, Y.; Yao, D.; Deng, H.; Wang, D.; Sun, Y.; et al. In-depth serum proteomics reveals biomarkers of psoriasis severity and response to traditional Chinese medicine. Theranostics 2019, 9, 2475–2488. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Harskamp, C.T.; Armstrong, A.W. Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. J. Am. Heart Assoc. 2013, 2, e000062. [Google Scholar] [CrossRef] [PubMed]
- Garshick, M.S.; Ward, N.L.; Krueger, J.G.; Berger, J.S. Cardiovascular Risk in Patients With Psoriasis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1670–1680. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B. Risk of myocardial infarction in patients with psoriasis. JAMA 2006, 296, 1735–1741. [Google Scholar] [CrossRef]
- Samarasekera, E.J.; Neilson, J.M.; Warren, R.B.; Parnham, J.; Smith, C.H. Incidence of cardiovascular disease in individuals with psoriasis: A systematic review and meta-analysis. J. Investig. Dermatol. 2013, 133, 2340–2346. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Gislason, G.H.; Charlot, M.; Jorgensen, C.H.; Lindhardsen, J.; Olesen, J.B.; Abildstrom, S.Z.; Skov, L.; Torp-Pedersen, C.; Hansen, P.R. Psoriasis is associated with clinically significant cardiovascular risk: A Danish nationwide cohort study. J. Intern. Med. 2011, 270, 147–157. [Google Scholar] [CrossRef]
- Sajja, A.P.; Joshi, A.A.; Teague, H.L.; Dey, A.K.; Mehta, N.N. Potential Immunological Links Between Psoriasis and Cardiovascular Disease. Front. Immunol. 2018, 9, 1234. [Google Scholar] [CrossRef]
- Flammer, A.J.; Ruschitzka, F. Psoriasis and atherosclerosis: Two plaques, one syndrome? Eur. Heart J. 2012, 33, 1989–1991. [Google Scholar] [CrossRef][Green Version]
- Harrington, C.L.; Dey, A.K.; Yunus, R.; Joshi, A.A.; Mehta, N.N. Psoriasis as a human model of disease to study inflammatory atherogenesis. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H867–H873. [Google Scholar] [CrossRef]
- Spah, F. Inflammation in atherosclerosis and psoriasis: Common pathogenic mechanisms and the potential for an integrated treatment approach. Br. J. Dermatol. 2008, 159 (Suppl. S2), 10–17. [Google Scholar] [CrossRef]
- Micha, R.; Imamura, F.; Wyler von Ballmoos, M.; Solomon, D.H.; Hernan, M.A.; Ridker, P.M.; Mozaffarian, D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am. J. Cardiol. 2011, 108, 1362–1370. [Google Scholar] [CrossRef]
- Elnabawi, Y.A.; Dey, A.K.; Goyal, A.; Groenendyk, J.W.; Chung, J.H.; Belur, A.D.; Rodante, J.; Harrington, C.L.; Teague, H.L.; Baumer, Y.; et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: Results from a prospective observational study. Cardiovasc. Res. 2019, 115, 721–728. [Google Scholar] [CrossRef]
- Mehta, N.N.; Shin, D.B.; Joshi, A.A.; Dey, A.K.; Armstrong, A.W.; Duffin, K.C.; Fuxench, Z.C.; Harrington, C.L.; Hubbard, R.A.; Kalb, R.E.; et al. Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers: A Randomized Placebo-Controlled Trial. Circ. Cardiovasc. Imaging 2018, 11, e007394. [Google Scholar] [CrossRef] [PubMed]
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 480–489. [Google Scholar] [CrossRef]
- Abuabara, K.; Lee, H.; Kimball, A.B. The effect of systemic psoriasis therapies on the incidence of myocardial infarction: A cohort study. Br. J. Dermatol. 2011, 165, 1066–1073. [Google Scholar] [CrossRef]
- Bissonnette, R.; Harel, F.; Krueger, J.G.; Guertin, M.C.; Chabot-Blanchet, M.; Gonzalez, J.; Maari, C.; Delorme, I.; Lynde, C.W.; Tardif, J.C. TNF-alpha Antagonist and Vascular Inflammation in Patients with Psoriasis Vulgaris: A Randomized Placebo-Controlled Study. J. Investig. Dermatol. 2017, 137, 1638–1645. [Google Scholar] [CrossRef]
- Ruiz de Morales, J.M.G.; Puig, L.; Dauden, E.; Canete, J.D.; Pablos, J.L.; Martin, A.O.; Juanatey, C.G.; Adan, A.; Montalban, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Taleb, S.; Tedgui, A.; Mallat, Z. IL-17 and Th17 cells in atherosclerosis: Subtle and contextual roles. Arter. Thromb. Vasc. Biol. 2015, 35, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Reich, J.L.K.; Falk, T.M.; Blodorn-Schlicht, N.; Mrowietz, U.; von Kiedrowski, R.; Pfeiffer, C.; Niesmann, J.; Frambach, Y.; Warren, R.B. Clinical response of psoriasis to subcutaneous methotrexate correlates with inhibition of cutaneous T helper 1 and 17 inflammatory pathways. Br. J. Dermatol. 2019, 181, 859–862. [Google Scholar] [CrossRef]
- Johansen, C.; Vinter, H.; Soegaard-Madsen, L.; Olsen, L.R.; Steiniche, T.; Iversen, L.; Kragballe, K. Preferential inhibition of the mRNA expression of p38 mitogen-activated protein kinase regulated cytokines in psoriatic skin by anti-TNFalpha therapy. Br. J. Dermatol. 2010, 163, 1194–1204. [Google Scholar] [CrossRef]
- Krueger, J.G.; Wharton, K.A., Jr.; Schlitt, T.; Suprun, M.; Torene, R.I.; Jiang, X.; Wang, C.Q.; Fuentes-Duculan, J.; Hartmann, N.; Peters, T.; et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J. Allergy Clin. Immunol. 2019, 144, 750–763. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Shin, D.B.; Alavi, A.; Torigian, D.A.; Werner, T.; Papadopoulos, M.; Takeshita, J.; Noe, M.H.; Dey, A.K.; Playford, M.P.; et al. A Phase IV, Randomized, Double-Blind, Placebo-Controlled Crossover Study of the Effects of Ustekinumab on Vascular Inflammation in Psoriasis (the VIP-U Trial). J. Investig. Dermatol. 2020, 140, 85–93.e2. [Google Scholar] [CrossRef]
- Ohta, K.; Nakajima, T.; Cheah, A.Y.; Zaidi, S.H.; Kaviani, N.; Dawood, F.; You, X.M.; Liu, P.; Husain, M.; Rabinovitch, M. Elafin-overexpressing mice have improved cardiac function after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H286–H292. [Google Scholar] [CrossRef]
- Li, K.; Zhang, F.; Wei, L.; Han, Z.; Liu, X.; Pan, Y.; Guo, C.; Han, W. Recombinant Human Elafin Ameliorates Chronic Hyperoxia-Induced Lung Injury by Inhibiting Nuclear Factor-Kappa B Signaling in Neonatal Mice. J. Interferon Cytokine Res. 2020, 40, 320–330. [Google Scholar] [CrossRef]
- Motta, J.P.; Bermudez-Humaran, L.G.; Deraison, C.; Martin, L.; Rolland, C.; Rousset, P.; Boue, J.; Dietrich, G.; Chapman, K.; Kharrat, P.; et al. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci. Transl. Med. 2012, 4, 158ra144. [Google Scholar] [CrossRef]
- O’Blenes, S.B.; Zaidi, S.H.; Cheah, A.Y.; McIntyre, B.; Kaneda, Y.; Rabinovitch, M. Gene transfer of the serine elastase inhibitor elafin protects against vein graft degeneration. Circulation 2000, 102 (Suppl. S3), III289–III295. [Google Scholar] [CrossRef]
- Small, D.M.; Zani, M.L.; Quinn, D.J.; Dallet-Choisy, S.; Glasgow, A.M.; O’Kane, C.; McAuley, D.F.; McNally, P.; Weldon, S.; Moreau, T.; et al. A functional variant of elafin with improved anti-inflammatory activity for pulmonary inflammation. Mol. Ther. 2015, 23, 24–31. [Google Scholar] [CrossRef]
- Zaidi, S.H.; Hui, C.C.; Cheah, A.Y.; You, X.M.; Husain, M.; Rabinovitch, M. Targeted overexpression of elafin protects mice against cardiac dysfunction and mortality following viral myocarditis. J. Clin. Investig. 1999, 103, 1211–1219. [Google Scholar] [CrossRef]
- Lauffer, F.; Jargosch, M.; Baghin, V.; Krause, L.; Kempf, W.; Absmaier-Kijak, M.; Morelli, M.; Madonna, S.; Marsais, F.; Lepescheux, L.; et al. IL-17C amplifies epithelial inflammation in human psoriasis and atopic eczema. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 800–809. [Google Scholar] [CrossRef]
- Vandeghinste, N.; Klattig, J.; Jagerschmidt, C.; Lavazais, S.; Marsais, F.; Haas, J.D.; Auberval, M.; Lauffer, F.; Moran, T.; Ongenaert, M.; et al. Neutralization of IL-17C Reduces Skin Inflammation in Mouse Models of Psoriasis and Atopic Dermatitis. J. Investig. Dermatol. 2018, 138, 1555–1563. [Google Scholar] [CrossRef]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef]
- Belaaouaj, A.; McCarthy, R.; Baumann, M.; Gao, Z.; Ley, T.J.; Abraham, S.N.; Shapiro, S.D. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 1998, 4, 615–618. [Google Scholar] [CrossRef]
- Korkmaz, B.; Moreau, T.; Gauthier, F. Neutrophil elastase, proteinase 3 and cathepsin G: Physicochemical properties, activity and physiopathological functions. Biochimie 2008, 90, 227–242. [Google Scholar] [CrossRef]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharm. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef]
- Henriksen, P.A.; Sallenave, J.M. Human neutrophil elastase: Mediator and therapeutic target in atherosclerosis. Int. J. Biochem. Cell Biol. 2008, 40, 1095–1100. [Google Scholar] [CrossRef]
- Kui, R.; Gal, B.; Gaal, M.; Kiss, M.; Kemeny, L.; Gyulai, R. Presence of antidrug antibodies correlates inversely with the plasma tumor necrosis factor (TNF)-alpha level and the efficacy of TNF-inhibitor therapy in psoriasis. J. Dermatol. 2016, 43, 1018–1023. [Google Scholar] [CrossRef]
- Ogura, H.; Murakami, M.; Okuyama, Y.; Tsuruoka, M.; Kitabayashi, C.; Kanamoto, M.; Nishihara, M.; Iwakura, Y.; Hirano, T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 2008, 29, 628–636. [Google Scholar] [CrossRef]
- Simon, T.; Taleb, S.; Danchin, N.; Laurans, L.; Rousseau, B.; Cattan, S.; Montely, J.M.; Dubourg, O.; Tedgui, A.; Kotti, S.; et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J. 2013, 34, 570–577. [Google Scholar] [CrossRef]
- Gonzalez-Cantero, A.; Ortega-Quijano, D.; Alvarez-Diaz, N.; Ballester, M.A.; Jimenez-Gomez, N.; Jaen, P.; Gonzalez-Cantero, J.; Gonzalez-Calvin, J.L.; Barderas, M.G.; Shin, D.B.; et al. Impact of Biological Agents on Imaging and Biomarkers of Cardiovascular Disease in Patients with Psoriasis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. J. Investig. Dermatol. 2021, 141, 2402–2411. [Google Scholar] [CrossRef]
- Kaiser, H.; Kvist-Hansen, A.; Becker, C.; Wang, X.; McCauley, B.D.; Krakauer, M.; Gortz, P.M.; Henningsen, K.M.A.; Zachariae, C.; Skov, L.; et al. Multiscale Biology of Cardiovascular Risk in Psoriasis: Protocol for a Case-Control Study. JMIR Res. Protoc. 2021, 10, e28669. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y. Current severe psoriasis and the rule of tens. Br. J. Dermatol. 2005, 152, 861–867. [Google Scholar] [CrossRef]
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of treatment goals for moderate to severe psoriasis: A European consensus. Arch. Dermatol. Res. 2011, 303, 1–10. [Google Scholar] [CrossRef]
- Mahil, S.K.; Wilson, N.; Dand, N.; Reynolds, N.J.; Griffiths, C.E.M.; Emsley, R.; Marsden, A.; Evans, I.; Warren, R.B.; Stocken, D.; et al. Psoriasis treat to target: Defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br. J. Dermatol. 2020, 182, 1158–1166. [Google Scholar] [CrossRef]
- Puig, L. PASI90 response: The new standard in therapeutic efficacy for psoriasis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 645–648. [Google Scholar] [CrossRef]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Bjorkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Rennel Dickens, E.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014, 9, e95192. [Google Scholar]
- Lundberg, M.; Eriksson, A.; Tran, B.; Assarsson, E.; Fredriksson, S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011, 39, e102. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Croft, D.; O’Kelly, G.; Wu, G.; Haw, R.; Gillespie, M.; Matthews, L.; Caudy, M.; Garapati, P.; Gopinath, G.; Jassal, B.; et al. Reactome: A database of reactions, pathways and biological processes. Nucleic Acids Res. 2011, 39, D691–D697. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
| Entire Population (n = 84) | Systemically Well-Treated (n = 36) | Systemically Untreated (n = 23) | p-Value | |
|---|---|---|---|---|
| Age, years | 59.0 ± 10.9 | 59.9 ± 8.9 | 58.7 ± 13.0 | 0.685 |
| Sex, male, n (%) | 61 (72.6) | 24 (66.7) | 20 (87.0) | 0.081 |
| PASI | 3.6 (1.2–11.0) | 0.7 (0.0–1.8) | 13.6 (11.5–16.1) | <0.001 |
| BMI (kg/m2) | 30.0 ± 5.6 | 30.4 ± 5.6 | 30.4 ± 5.6 | 0.981 |
| Atherothrombotic CVD, n (%) | 39 (46.4) | 14 (38.9) | 10 (43.5) | 0.726 |
| Medically treated diabetes, n (%) | 21 (25.0) | 12 (33.3) | 6 (26.1) | 0.555 |
| Statin therapy, n (%) | 41 (48.8) | 19 (52.8) | 12 (52.2) | 0.964 |
| PsA verified by rheumatologist, n (%) | 21 (25.0) | 12 (33.3) | 3 (13.0) | 0.081 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Kaiser, H.; Kvist-Hansen, A.; McCauley, B.D.; Skov, L.; Hansen, P.R.; Becker, C. IL-17 Pathway Members as Potential Biomarkers of Effective Systemic Treatment and Cardiovascular Disease in Patients with Moderate-to-Severe Psoriasis. Int. J. Mol. Sci. 2022, 23, 555. https://doi.org/10.3390/ijms23010555
Wang X, Kaiser H, Kvist-Hansen A, McCauley BD, Skov L, Hansen PR, Becker C. IL-17 Pathway Members as Potential Biomarkers of Effective Systemic Treatment and Cardiovascular Disease in Patients with Moderate-to-Severe Psoriasis. International Journal of Molecular Sciences. 2022; 23(1):555. https://doi.org/10.3390/ijms23010555
Chicago/Turabian StyleWang, Xing, Hannah Kaiser, Amanda Kvist-Hansen, Benjamin D. McCauley, Lone Skov, Peter Riis Hansen, and Christine Becker. 2022. "IL-17 Pathway Members as Potential Biomarkers of Effective Systemic Treatment and Cardiovascular Disease in Patients with Moderate-to-Severe Psoriasis" International Journal of Molecular Sciences 23, no. 1: 555. https://doi.org/10.3390/ijms23010555
APA StyleWang, X., Kaiser, H., Kvist-Hansen, A., McCauley, B. D., Skov, L., Hansen, P. R., & Becker, C. (2022). IL-17 Pathway Members as Potential Biomarkers of Effective Systemic Treatment and Cardiovascular Disease in Patients with Moderate-to-Severe Psoriasis. International Journal of Molecular Sciences, 23(1), 555. https://doi.org/10.3390/ijms23010555






