Intermittent Hypoxic Therapy Inhibits Allogenic Bone-Graft Resorption by Inhibition of Osteoclastogenesis in a Mouse Model
Abstract
:1. Introduction
2. Results
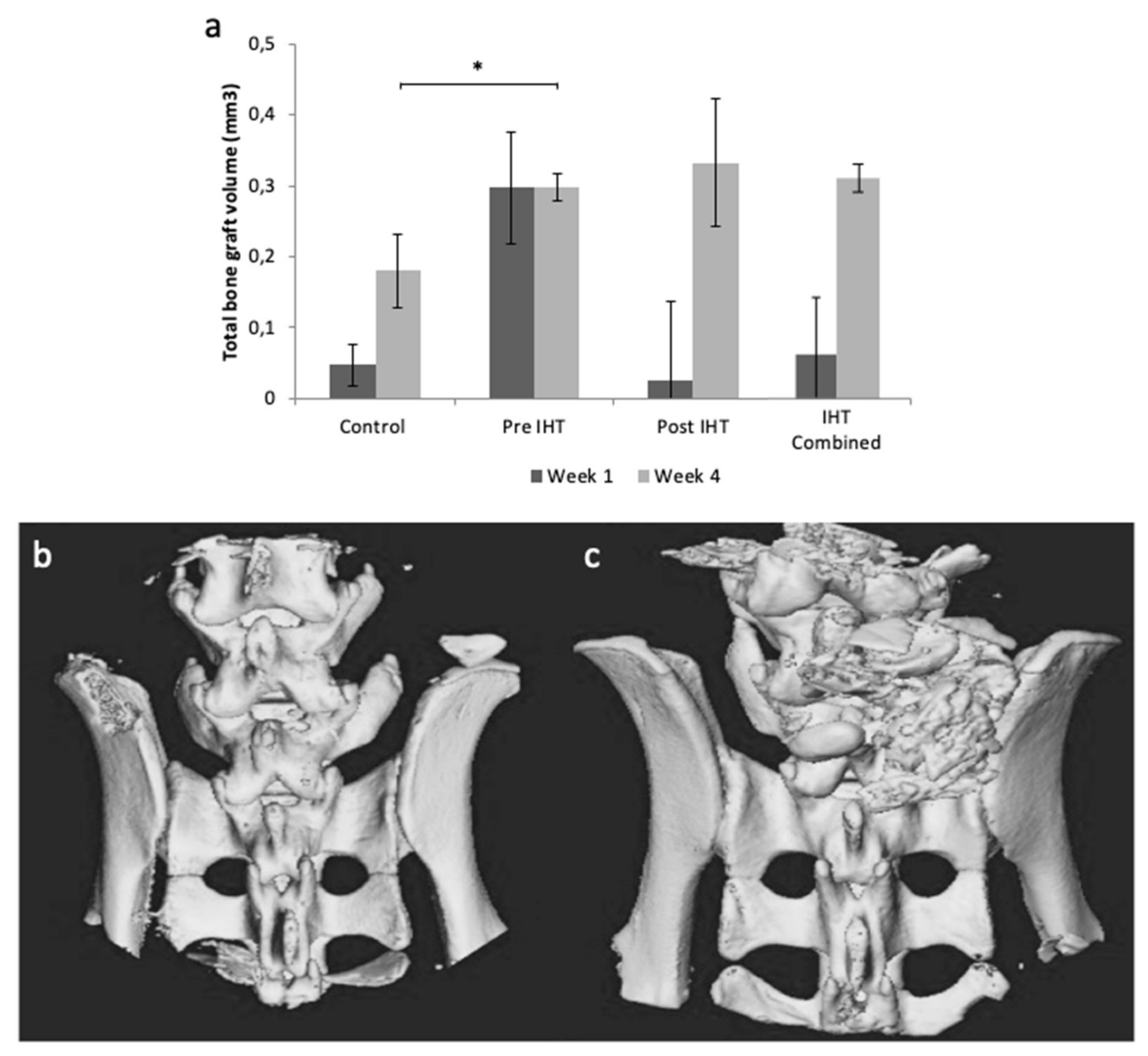
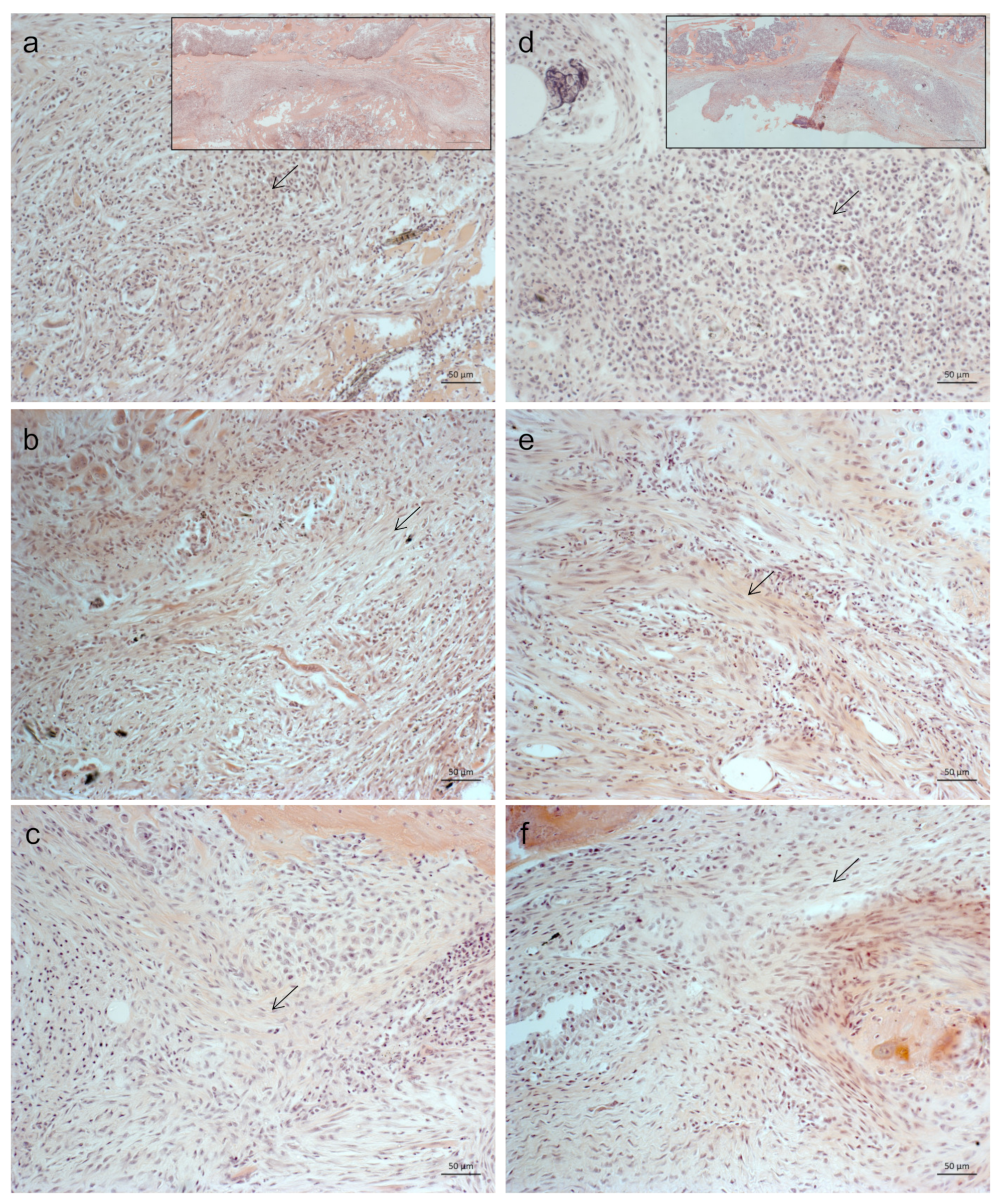
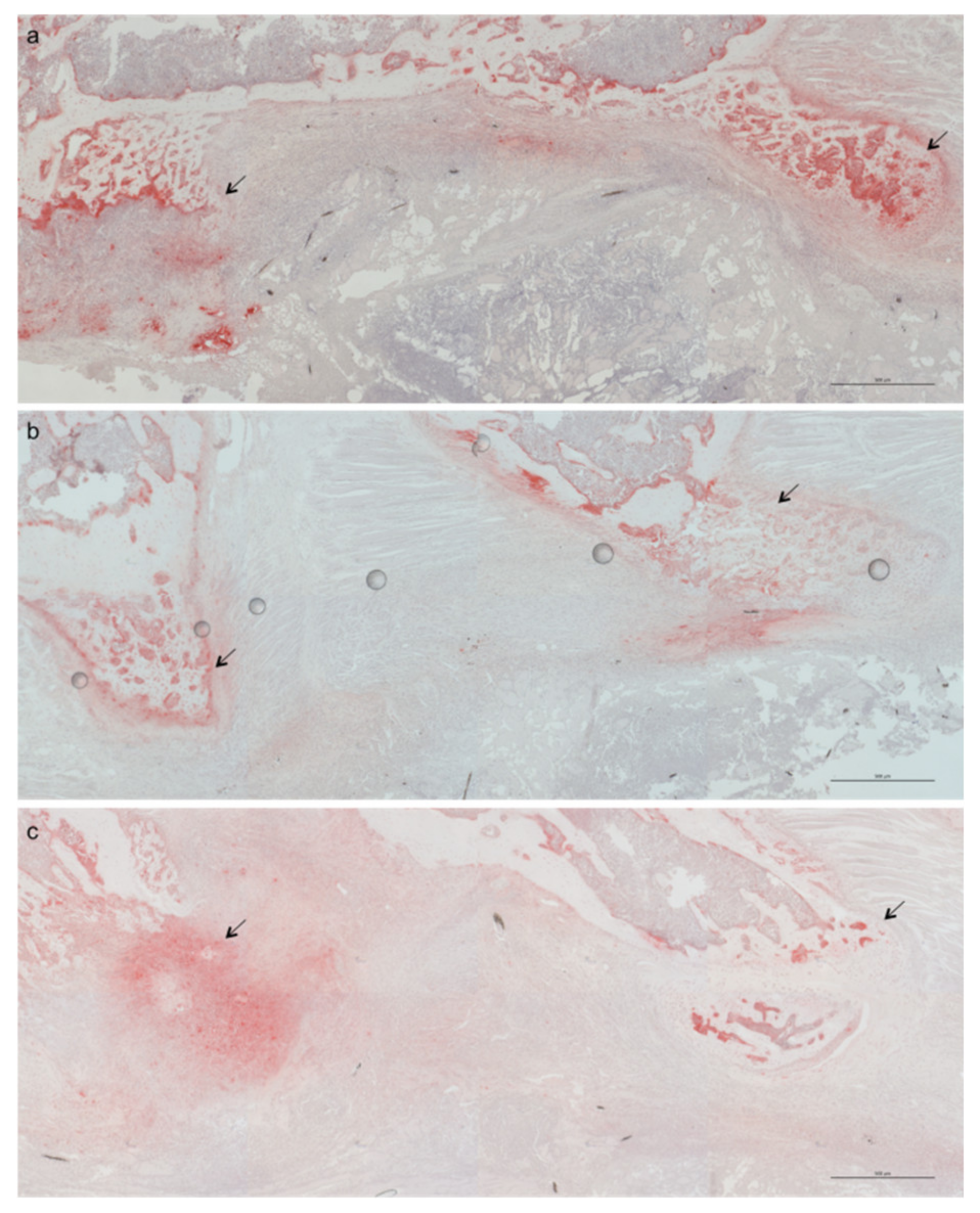
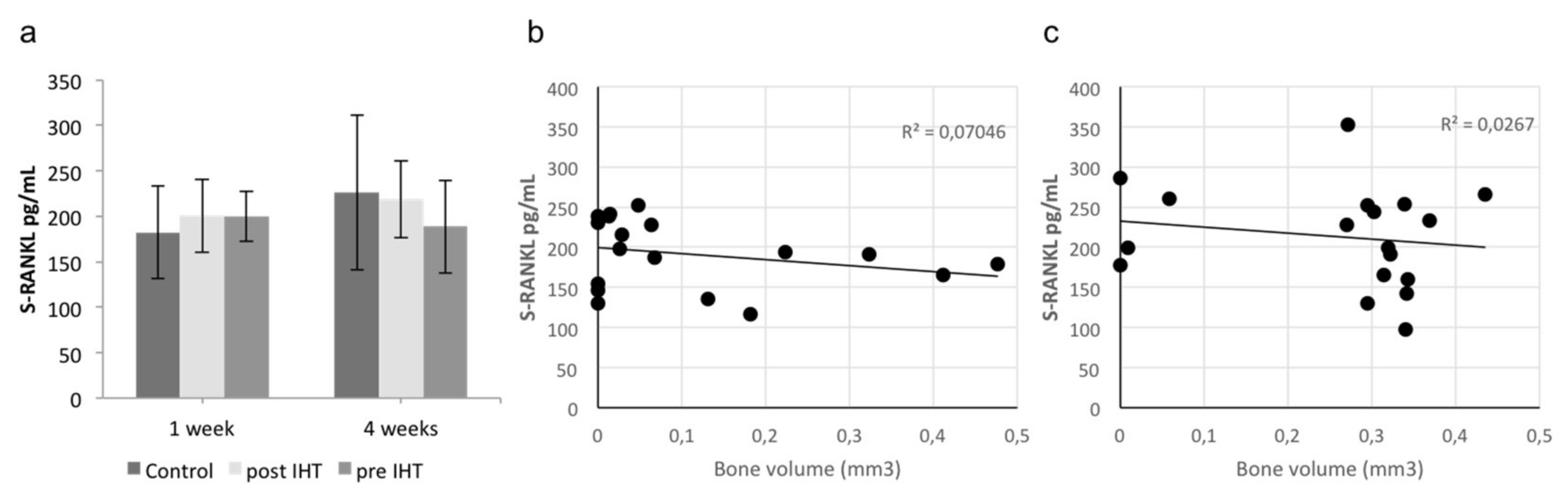
2.1. Less Bone Resorption and Reduced Osteoclast Activity
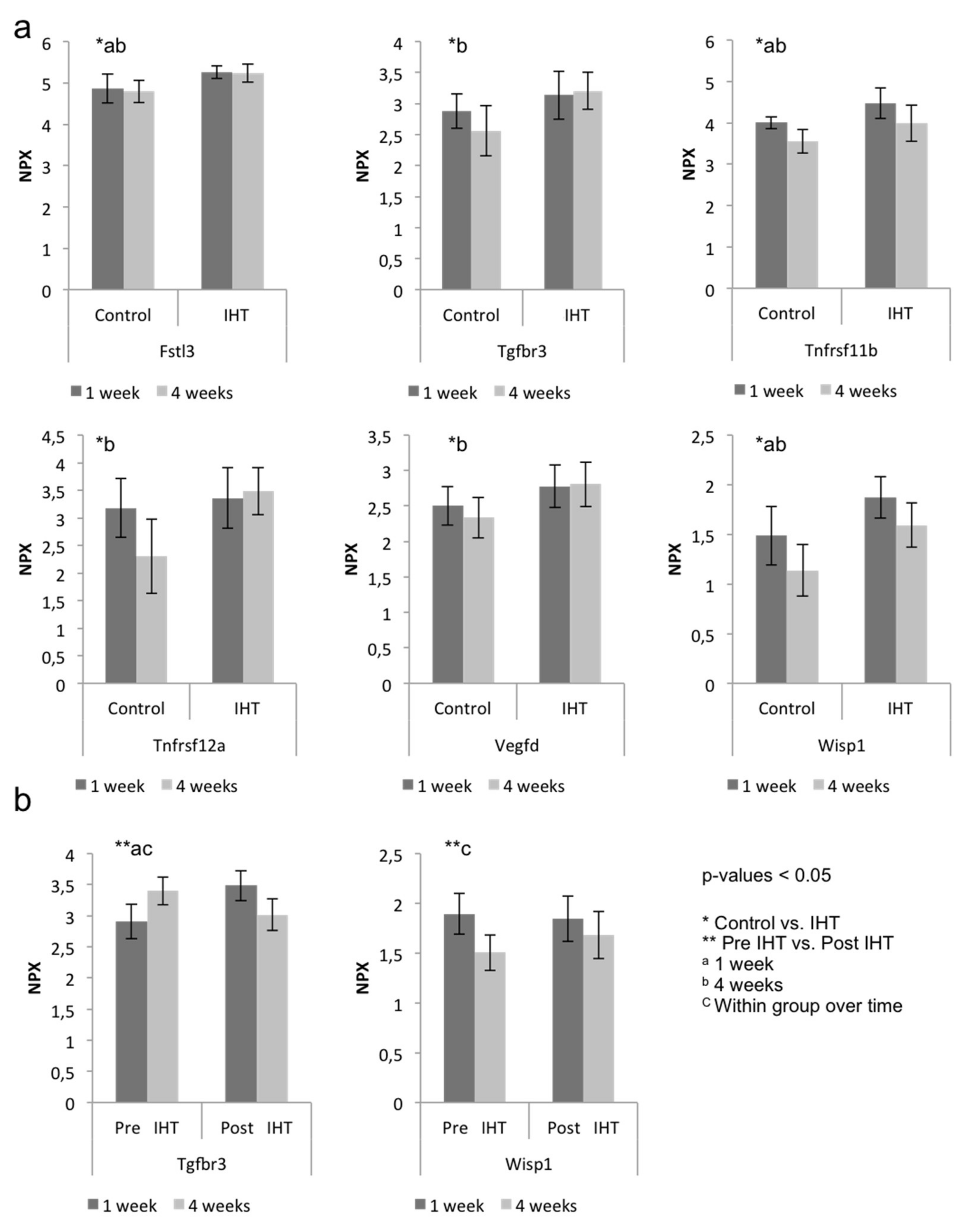
2.2. A Diverse Exploratory Blood Signature of IHT
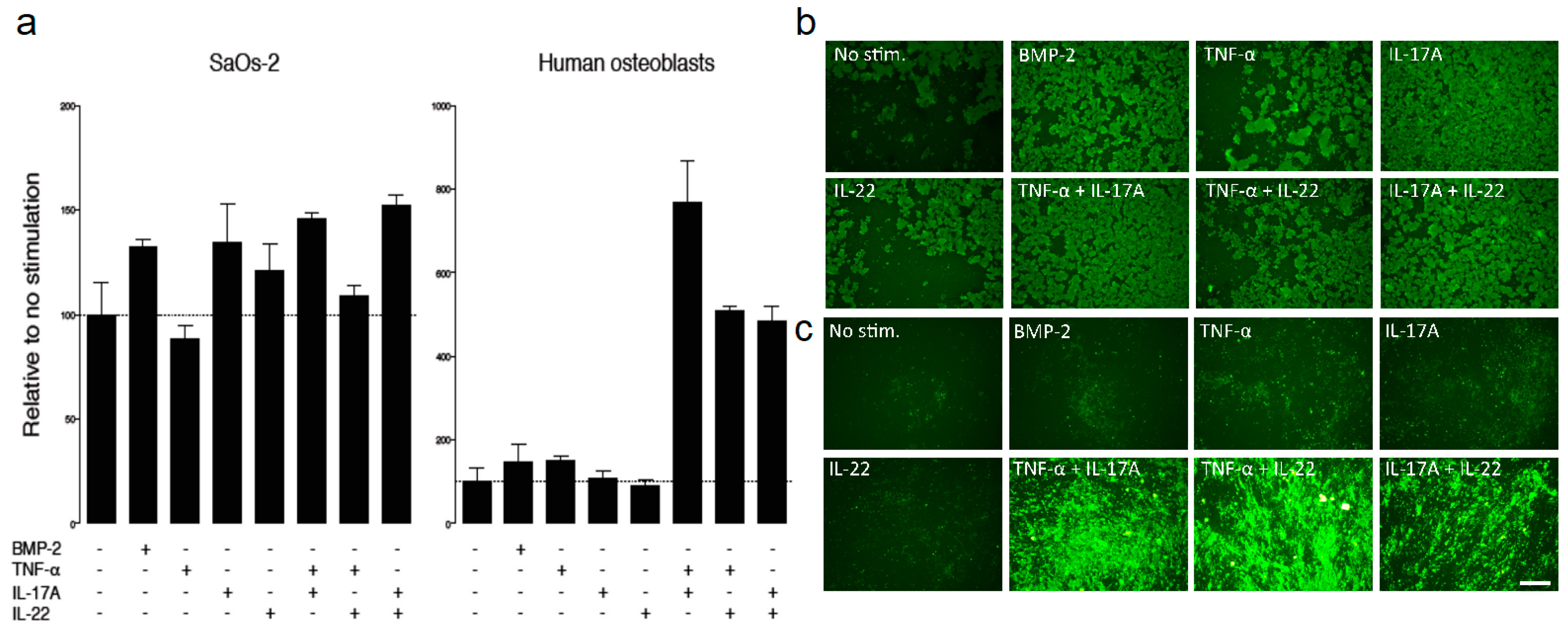
2.3. IHT Promotes an Osteoimmunological Response
3. Discussion
4. Materials and Methods
4.1. Study Procedures
4.2. Intermittent Hypoxic Therapy (IHT)
4.3. Tissue Preparation
4.4. Microcomputed Tomography (μCT)
4.5. Histology
4.6. Osteoblast Mineralization
4.7. Proteomics
4.8. ELISA
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michiels, C. Physiological and pathological responses to hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Dunwoodie, S.L. The Role of Hypoxia in Development of the Mammalian Embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.C.; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef]
- Kim, J.-W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrmann, D.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef]
- Cahill, E.; Rowan, S.C.; Sands, M.; Banahan, M.; Ryan, D.; Howell, K.; McLoughlin, P. The pathophysiological basis of chronic hypoxic pulmonary hypertension in the mouse: Vasoconstrictor and structural mechanisms contribute equally. Exp. Physiol. 2012, 97, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stray-Gundersen, J.; Chapman, R.; Levine, B.D. “Living high-training low” altitude training improves sea level performance in male and female elite runners. J. Appl. Physiol. 2001, 91, 1113–1120. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Osta, B.; Lavocat, F.; Eljaafari, A.; Miossec, P. Effects of Interleukin-17A on Osteogenic Differentiation of Isolated Human Mesenchymal Stem Cells. Front. Immunol. 2014, 5, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifas, L. T-cell cytokine induction of BMP-2 regulates human mesenchymal stromal cell differentiation and mineralization. J. Cell. Biochem. 2006, 98, 706–714. [Google Scholar] [CrossRef]
- Croes, M.; Öner, F.C.; van Neerven, D.; Sabir, E.; Kruyt, M.C.; Blokhuis, T.J.; Dhert, W.J.; Alblas, J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone 2016, 84, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Baraliakos, X.; Deodhar, A.; Baeten, D.; Sieper, J.; Emery, P.; Readie, A.; Martin, R.; Mpofu, S.; Richards, H.B. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann. Rheum. Dis. 2017, 76, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Growth Factors Bone Morphogenetic Proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Eriksson, A.; Tran, B.; Assarsson, E.; Fredriksson, S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011, 39, e102. [Google Scholar] [CrossRef]
- Xiong, D.-H.; Liu, X.-G.; Guo, Y.-F.; Tan, L.-J.; Wang, L.; Sha, B.-Y.; Tang, Z.-H.; Pan, F.; Yang, T.-L.; Chen, X.-D.; et al. Genome-wide Association and Follow-Up Replication Studies Identified ADAMTS18 and TGFBR3 as Bone Mass Candidate Genes in Different Ethnic Groups. Am. J. Hum. Genet. 2009, 84, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.R.; Jacobs, B.H.; Brown, C.B.; Barnett, J.V.; Goudy, S.L. Type III transforming growth factor beta receptor regulates vascular and osteoblast development during palatogenesis. Dev. Dyn. 2015, 244, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.; Perera, P.; Gordon, R.; Jeong, Y.; Blazek, A.; Kim, D.-G.; Tee, B.; Sun, Z.; Eubank, T.; Zhao, Y.; et al. Follistatin-like 3 is a mediator of exercise-driven bone formation and strengthening. Bone 2015, 78, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Maeda, A.; Ono, M.; Holmbeck, K.; Li, L.; Kilts, T.M.; Kram, V.; Noonan, M.; Yoshioka, Y.; McNerny, E.; Tantillo, M.A.; et al. WNT1-induced Secreted Protein-1 (WISP1), a Novel Regulator of Bone Turnover and Wnt Signaling. J. Biol. Chem. 2015, 290, 14004–14018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlandini, M.; Spreafico, A.; Bardelli, M.; Rocchigiani, M.; Salameh, A.; Nucciotti, S.; Capperucci, C.; Frediani, B.; Oliviero, S. Vascular Endothelial Growth Factor-D Activates VEGFR-3 Expressed in Osteoblasts Inducing Their Differentiation. J. Biol. Chem. 2006, 281, 17961–17967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, D.; Uchiyama, H.; Akbarali, Y.; Urashima, M.; Yamamoto, K.; Libermann, T.; Anderson, K.C. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood 1996, 87, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.-Y.; Lee, J.-S.; Kim, K.-W. Bone Graft Volumetric Changes and Clinical Outcomes After Instrumented Lumbar or Lumbosacral Fusion. Spine 2009, 34, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Zindrick, M.; Schwaegler, P.; Vrbos, L.; Collatz, M.A.; Behal, R.; Cram, R. A Comparison of Single-Level Fusions with and Without Hardware. Spine 1991, 16, S455–S458. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, A.B.; Andresen, A.D.K.; Jacobsen, M.K.; Andersen, M.Ø.; Carreon, L.Y. Does Systemic Administration of Parathyroid Hormone After Noninstrumented Spinal Fusion Surgery Improve Fusion Rates and Fusion Mass in Elderly Patients Compared to Placebo in Patients with Degenerative Lumbar Spondylolisthesis? Spine 2019, 44, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.; Baas, J.; Bechtold, J.E.; Elmengaard, B.; Søballe, K. Soaking Morselized Allograft in Bisphosphonate Can Impair Implant Fixation. Clin. Orthop. Relat. Res. 2007, 463, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Park-Snyder, S.H.; Miyazono, K.; Twardzik, D.; Mundy, G.R.; Bonewald, L.F. Characterization and autoregulation of latent transforming growth factor β (TGFβ) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGFβ-binding protein. J. Biol. Chem. 1994, 269, 6815–6821. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-β1–induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Blakytny, R.; Ludlow, A.; Martin, G.E.; Ireland, G.; Lund, L.R.; Ferguson, M.W.; Brunner, G. Latent TGF-? 1 activation by platelets. J. Cell. Physiol. 2004, 199, 67–76. [Google Scholar] [CrossRef]
- López-Casillas, F.; Payne, H.M.; Andres, J.L.; Massague, J. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: Mapping of ligand binding and GAG attachment sites. J. Cell Biol. 1994, 124, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Casillas, F.; Cheifetz, S.; Doody, J.; Andres, J.L.; Lane, W.S.; Massague, J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-β receptor system. Cell 1991, 67, 785–795. [Google Scholar] [CrossRef]
- Huse, M.; Muir, T.W.; Xu, L.; Chen, Y.-G.; Kuriyan, J.; Massagué, J. The TGFβ Receptor Activation Process: An Inhibitor- to Substrate-Binding Switch. Mol. Cell 2001, 8, 671–682. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Worthington, J.J.; Fenton, T.; Czajkowska, B.I.; Klementowicz, J.E.; Travis, M.A. Regulation of TGFβ in the immune system: An emerging role for integrins and dendritic cells. Immunobiology 2012, 217, 1259–1265. [Google Scholar] [CrossRef]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Anderson, D.E.; Baecher-Allan, C.; Hastings, W.D.; Bettelli, E.; Oukka, M.; Kuchroo, V.K.; Hafler, D.A. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature 2008, 454, 350–352. [Google Scholar] [CrossRef]
- Jin, W.; Dong, C. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect. 2013, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dong, C. TH17 cells in development: An updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008, 8, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Good, S.R.; Thieu, V.T.; Mathur, A.N.; Yu, Q.; Stritesky, G.L.; Yeh, N.; O’Malley, J.T.; Perumal, N.B.; Kaplan, M.H. Temporal Induction Pattern of STAT4 Target Genes Defines Potential for Th1 Lineage-Specific Programming. J. Immunol. 2009, 183, 3839–3847. [Google Scholar] [CrossRef] [Green Version]
- Mori, G.; D’Amelio, P.; Faccio, R.; Brunetti, G. The Interplay between the Bone and the Immune System. Clin. Dev. Immunol. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, J.M.W.; Sims, N.; Saleh, H.; Mirosa, D.; Thompson, K.; Bouralexis, S.; Walker, E.C.; Martin, T.J.; Gillespie, M. IL-23 Inhibits Osteoclastogenesis Indirectly through Lymphocytes and Is Required for the Maintenance of Bone Mass in Mice. J. Immunol. 2008, 181, 5720–5729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet, S.; Pozzi, S.; Patel, K.; Vaghela, N.; Fulciniti, M.; Veiby, P.; Hideshima, T.; Santo, L.; Cirstea, D.; Scadden, D.T.; et al. A novel role for CCL3 (MIP-1α) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia 2011, 25, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Dimopoulos, M.A. Pathogenesis of bone disease in multiple myeloma: From bench to bedside. Blood Cancer J. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, R.; Liu, H.; Zhao, S.; Wang, Y.; Li, L.; Gao, S.; Ruan, E.; Wang, G.; Wang, H.; Song, J.; et al. Osteoblast inhibition by chemokine cytokine ligand3 in myeloma-induced bone disease. Cancer Cell Int. 2014, 14, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergholt, N.L.; Olesen, M.L.; Foldager, C.B. Age-Dependent Systemic Effects of a Systemic Intermittent Hypoxic Therapy in Vivo. High Alt. Med. Biol. 2019, 20, 221–230. [Google Scholar] [CrossRef]
- Ding, M. Microarchitectural adaptations in aging and osteoarthrotic subchondral bone issues. Acta Orthop. 2010, 81, 1–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehl, K.-H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.-M.; Van De Vorstenbosch, C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergholt, N.L.; Demirel, A.; Pedersen, M.; Ding, M.; Kragstrup, T.W.; Andersen, T.; Deleuran, B.W.; Foldager, C.B. Intermittent Hypoxic Therapy Inhibits Allogenic Bone-Graft Resorption by Inhibition of Osteoclastogenesis in a Mouse Model. Int. J. Mol. Sci. 2022, 23, 323. https://doi.org/10.3390/ijms23010323
Bergholt NL, Demirel A, Pedersen M, Ding M, Kragstrup TW, Andersen T, Deleuran BW, Foldager CB. Intermittent Hypoxic Therapy Inhibits Allogenic Bone-Graft Resorption by Inhibition of Osteoclastogenesis in a Mouse Model. International Journal of Molecular Sciences. 2022; 23(1):323. https://doi.org/10.3390/ijms23010323
Chicago/Turabian StyleBergholt, Natasja Leth, Ari Demirel, Michael Pedersen, Ming Ding, Tue Wenzel Kragstrup, Thomas Andersen, Bent Winding Deleuran, and Casper Bindzus Foldager. 2022. "Intermittent Hypoxic Therapy Inhibits Allogenic Bone-Graft Resorption by Inhibition of Osteoclastogenesis in a Mouse Model" International Journal of Molecular Sciences 23, no. 1: 323. https://doi.org/10.3390/ijms23010323
APA StyleBergholt, N. L., Demirel, A., Pedersen, M., Ding, M., Kragstrup, T. W., Andersen, T., Deleuran, B. W., & Foldager, C. B. (2022). Intermittent Hypoxic Therapy Inhibits Allogenic Bone-Graft Resorption by Inhibition of Osteoclastogenesis in a Mouse Model. International Journal of Molecular Sciences, 23(1), 323. https://doi.org/10.3390/ijms23010323






