Adipocyte Biology from the Perspective of In Vivo Research: Review of Key Transcription Factors
Abstract
:1. Introduction
2. Known Transcriptional Regulators of Adipogenesis in the Light of In Vivo Studies
2.1. CREB
2.2. C/EBPβ and C/CEBPδ
2.3. C/EBPα
2.4. PPARγ
3. Discussion and Conclusions
3.1. Transcription Factor Summary
3.2. The Role of the Transcription Factor Imbalance in the Development of Metabolic Disorders
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef] [Green Version]
- WHO. Obesity, Overweight. Fact sheet 2016. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 25 November 2021).
- Ferrannini, E. Is insulin resistance the cause of the metabolic syndrome? Ann. Med. 2006, 38, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Kaaks, R.; Vainio, H. Overweight, obesity, and cancer risk. Lancet. Oncol. 2002, 3, 565–574. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome: Connecting and reconciling cardiovascular and diabetes worlds. J. Am. Coll. Cardiol. 2006, 47, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Haider, N.; Larose, L. Harnessing adipogenesis to prevent obesity. Adipocyte 2019, 8, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Cawthorn, W.P.; Scheller, E.L.; MacDougald, O.A. Adipose tissue stem cells meet preadipocyte commitment: Going back to the future. J. Lipid. Res. 2012, 53, 227–246. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Gurmaches, J.; Hung, C.M.; Guertin, D.A. Emerging Complexities in Adipocyte Origins and Identity. Trends Cell Biol. 2016, 26, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Park, A.; Kim, W.K.; Bae, K.H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem. Cells 2014, 6, 33–42. [Google Scholar] [CrossRef]
- Brandão, B.B.; Poojari, A.; Rabiee, A. Thermogenic Fat: Development, Physiological Function, and Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 5906. [Google Scholar] [CrossRef]
- Elattar, S.; Satyanarayana, A. Can Brown Fat Win the Battle Against White Fat? J. Cell Physiol. 2015, 230, 2311–2317. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, F.W. The significance of beige and brown fat in humans. Endocr Connect. 2017, 6, R70–R79. [Google Scholar] [CrossRef] [Green Version]
- Schulz, T.J.; Tseng, Y.H. Brown adipose tissue: Development, metabolism and beyond. Biochem J. 2013, 453, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Shinde, A.B.; Song, A.; Wang, Q.A. Brown Adipose Tissue Heterogeneity, Energy Metabolism, and Beyond. Front. Endocrinol. 2021, 12, 651763. [Google Scholar] [CrossRef]
- Mota de Sá, P.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Ntambi, J.M. and K. Young-Cheul, Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [Green Version]
- Brun, R.P.; Tontonoz, P.; Forman, B.M.; Ellis, R.; Chen, J.; Evans, R.M.; Spiegelman, B.M. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 1996, 10, 974–984. [Google Scholar] [CrossRef] [Green Version]
- Chapman, A.B.; Knight, D.M.; Dieckmann, B.S.; Ringold, G.M. Analysis of gene expression during differentiation of adipogenic cells in culture and hormonal control of the developmental program. J. Biol. Chem. 1984, 259, 15548–15555. [Google Scholar] [CrossRef]
- Christy, R.J.; Kaestner, K.H.; Geiman, D.E.; Lane, M.D. CCAAT/enhancer binding protein gene promoter: Binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 1991, 88, 2593–2597. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S.L.; Robinson, C.E.; Gimble, J.M. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor gamma 2 promoter. Biochem. Biophys. Res. Commun. 1997, 240, 99–103. [Google Scholar] [CrossRef]
- Cowherd, R.M.; Lyle, R.E.; McGehee, R.E., Jr. Molecular regulation of adipocyte differentiation. Semin. Cell Dev. Biol. 1999, 10, 3–10. [Google Scholar] [CrossRef]
- Kim, J.B.; Wright, H.M.; Wright, M.; Spiegelman, B.M. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc. Natl. Acad. Sci. USA 1998, 95, 4333–4337. [Google Scholar] [CrossRef] [Green Version]
- Oishi, Y.; Manabe, I.; Tobe, K.; Tsushima, K.; Shindo, T.; Fujiu, K.; Nishimura, G.; Maemura, K.; Yamauchi, T.; Kubota, N.; et al. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005, 1, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.K.; Wang, L.; Giampietro, A.; Lai, B.; Lee, J.E.; Ge, K. Distinct Roles of Transcription Factors KLF4, Krox20, and Peroxisome Proliferator-Activated Receptor γ in Adipogenesis. Mol. Cell Biol. 2017, 37, e00554-16. [Google Scholar] [CrossRef] [Green Version]
- Stewart, W.C.; Pearcy, L.A.; Floyd, Z.E.; Stephens, J.M. STAT5A expression in Swiss 3T3 cells promotes adipogenesis in vivo in an athymic mice model system. Obesity 2011, 19, 1731–1734. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.J.; Roesler, W.J.; Boras, T.; Colton, L.A.; Felder, K.; Reusch, J.E. Insulin stimulates cAMP-response element binding protein activity in HepG2 and 3T3-L1 cell lines. J. Biol. Chem. 1998, 273, 917–923. [Google Scholar] [CrossRef] [Green Version]
- Yeh, W.C.; Cao, Z.; Classon, M.; McKnight, S.L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995, 9, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Siersbæk, R.; Nielsen, R.; Mandrup, S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol. Metab. 2012, 23, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Reusch, J.E.; Colton, L.A.; Klemm, D.J. CREB activation induces adipogenesis in 3T3-L1 cells. Mol. Cell Biol. 2000, 20, 1008–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, K.E.; Fankell, D.M.; Erickson, P.F.; Majka, S.M.; Crossno, J.T., Jr.; Klemm, D.J. Depletion of cAMP-response element-binding protein/ATF1 inhibits adipogenic conversion of 3T3-L1 cells ectopically expressing CCAAT/enhancer-binding protein (C/EBP) alpha, C/EBP beta, or PPAR gamma. J. Biol. Chem. 2006, 281, 40341–40353. [Google Scholar] [CrossRef] [Green Version]
- Niehof, M.; Manns, M.P.; Trautwein, C. CREB controls LAP/C/EBP beta transcription. Mol. Cell Biol. 1997, 17, 3600–3613. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.W.; Klemm, D.J.; Vinson, C.; Lane, M.D. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J. Biol. Chem. 2004, 279, 4471–4478. [Google Scholar] [CrossRef] [Green Version]
- Bourtchuladze, R.; Frenguelli, B.; Blendy, J.; Cioffi, D.; Schutz, G.; Silva, A.J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 1994, 79, 59–68. [Google Scholar] [CrossRef]
- Rudolph, D.; Tafuri, A.; Gass, P.; Hämmerling, G.J.; Arnold, B.; Schütz, G. Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc. Natl. Acad. Sci. USA 1998, 95, 4481–4486. [Google Scholar] [CrossRef] [Green Version]
- Lee, D. The Role of CREB in the Liver and Adipose Tissue. Publicly Access. Penn Diss. 2014, 1342. [Google Scholar]
- Lee, D.; Le Lay, J.; Kaestner, K.H. The transcription factor CREB has no non-redundant functions in hepatic glucose metabolism in mice. Diabetologia 2014, 57, 1242–1248. [Google Scholar] [CrossRef]
- Eguchi, J.; Wang, X.; Yu, S.; Kershaw, E.E.; Chiu, P.C.; Dushay, J.; Estall, J.L.; Klein, U.; Maratos-Flier, E.; Rosen, E.D. Transcriptional control of adipose lipid handling by IRF. Cell Metab. 2011, 13, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Russell, S.J.; Ussar, S.; Boucher, J.; Vernochet, C.; Mori, M.A.; Smyth, G.; Rourk, M.; Cederquist, C.; Rosen, E.D.; et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 2013, 62, 864–874. [Google Scholar] [CrossRef] [Green Version]
- Lekstrom-Himes, J.; Xanthopoulos, K.G. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J. Biol. Chem. 1998, 273, 28545–28548. [Google Scholar] [CrossRef] [Green Version]
- Hamm, J.K.; Park, B.H.; Farmer, S.R. A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J. Biol. Chem. 2001, 276, 18464–18471. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Yoshida, N.; Kishimoto, T.; Akira, S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997, 16, 7432–7443. [Google Scholar] [CrossRef] [Green Version]
- Cheneval, D.; Christy, R.J.; Geiman, D.; Cornelius, P.; Lane, M.D. Cell-free transcription directed by the 422 adipose P2 gene promoter: Activation by the CCAAT/enhancer binding protein. Proc. Natl. Acad. Sci. USA 1991, 88, 8465–8469. [Google Scholar] [CrossRef] [Green Version]
- Christy, R.J.; Yang, V.W.; Ntambi, J.M.; Geiman, D.E.; Landschulz, W.H.; Friedman, A.D.; Nakabeppu, Y.; Kelly, T.J.; Lane, M.D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989, 3, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- Cornelius, P.; MacDougald, O.A.; Lane, M.D. Regulation of adipocyte development. Annu. Rev. Nutr. 1994, 14, 99–129. [Google Scholar] [CrossRef]
- Hwang, C.S.; Mandrup, S.; MacDougald, O.A.; Geiman, D.E.; Lane, M.D. Transcriptional activation of the mouse obese (ob) gene by CCAAT/enhancer binding protein alpha. Proc. Natl. Acad. Sci. USA 1996, 93, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Kaestner, K.H.; Christy, R.J.; Lane, M.D. Mouse insulin-responsive glucose transporter gene: Characterization of the gene and trans-activation by the CCAAT/enhancer binding protein. Proc. Natl. Acad. Sci. USA 1990, 87, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.T.; Lane, M.D. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992, 6, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.T.; Lane, M.D. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc. Natl. Acad. Sci. USA 1994, 91, 8757–8761. [Google Scholar] [CrossRef] [Green Version]
- MacDougald, O.A.; Lane, M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef]
- Wang, N.D.; Finegold, M.J.; Bradley, A.; Ou, C.N.; Abdelsayed, S.V.; Wilde, M.D.; Taylor, L.R.; Wilson, D.R.; Darlington, G.J. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 1995, 269, 1108–1112. [Google Scholar] [CrossRef]
- Flodby, P.; Barlow, C.; Kylefjord, H.; Ahrlund-Richter, L.; Xanthopoulos, K.G. Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein alpha. J. Biol. Chem. 1996, 271, 24753–24760. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Christoffels, V.M.; Chowdhury, S.; Iwase, K.; Matsuzaki, H.; Mori, M.; Lamers, W.H.; Darlington, G.J.; Takiguchi, M. Hypoglycemia-associated hyperammonemia caused by impaired expression of ornithine cycle enzyme genes in C/EBPalpha knockout mice. J. Biol. Chem. 1998, 273, 27505–27510. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.; Austen, D.E.; Wilde, M.D.; Darlington, G.J.; Brownlee, G.G. Clotting factor IX levels in C/EBP alpha knockout mice. Br. J. Haematol. 1997, 99, 578–579. [Google Scholar] [CrossRef]
- Zhang, D.E.; Zhang, P.; Wang, N.D.; Hetherington, C.J.; Darlington, G.J.; Tenen, D.G. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Linhart, H.G.; Ishimura-Oka, K.; DeMayo, F.; Kibe, T.; Repka, D.; Poindexter, B.; Bick, R.J.; Darlington, G.J. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc. Natl. Acad. Sci. USA 2001, 98, 12532–12537. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Croniger, C.M.; Lekstrom-Himes, J.; Zhang, P.; Fenyus, M.; Tenen, D.G.; Darlington, G.J.; Hanson, R.W. Metabolic response of mice to a postnatal ablation of CCAAT/enhancer-binding protein alpha. J. Biol. Chem. 2005, 280, 38689–38699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.A.; Tao, C.; Jiang, L.; Shao, M.; Ye, R.; Zhu, Y.; Gordillo, R.; Ali, A.; Lian, Y.; Holland, W.L.; et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat. Cell Biol. 2015, 17, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Graves, R.A.; Budavari, A.I.; Spiegelman, B.M. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, A.; Schwarz, E.J.; Dimaculangan, D.D.; Lazar, M.A. Peroxisome proliferator-activated receptor (PPAR) gamma: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 1994, 135, 798–800. [Google Scholar] [CrossRef]
- Corrales, P.; Vidal-Puig, A.; Medina-Gómez, G. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef] [Green Version]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Duan, S.Z.; Ivashchenko, C.Y.; Whitesall, S.E.; D’Alecy, L.G.; Duquaine, D.C.; Brosius, F.C., 3rd; Gonzalez, F.J.; Vinson, C.; Pierre, M.A.; Milstone, D.S.; et al. Hypotension, lipodystrophy, and insulin resistance in generalized PPARgamma-deficient mice rescued from embryonic lethality. J. Clin. Invest. 2007, 117, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Koutnikova, H.; Cock, T.A.; Watanabe, M.; Houten, S.M.; Champy, M.F.; Dierich, A.; Auwerx, J. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc. Natl. Acad. Sci. USA 2003, 100, 14457–14462. [Google Scholar] [CrossRef] [Green Version]
- Rosen, E.D.; Sarraf, P.; Troy, A.E.; Bradwin, G.; Moore, K.; Milstone, D.S.; Spiegelman, B.M.; Mortensen, R.M. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 1999, 4, 611–617. [Google Scholar] [CrossRef]
- García-Alonso, V.; López-Vicario, C.; Titos, E.; Morán-Salvador, E.; González-Périz, A.; Rius, B.; Párrizas, M.; Werz, O.; Arroyo, V.; Clària, J. Coordinate functional regulation between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in the conversion of white-to-brown adipocytes. J. Biol. Chem. 2013, 288, 28230–28242. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Barak, Y.; Hevener, A.; Olson, P.; Liao, D.; Le, J.; Nelson, M.; Ong, E.; Olefsky, J.M.; Evans, R.M. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Proc. Natl. Acad. Sci. USA 2003, 100, 15712–15717. [Google Scholar] [CrossRef] [Green Version]
- Imai, T.; Takakuwa, R.; Marchand, S.; Dentz, E.; Bornert, J.M.; Messaddeq, N.; Wendling, O.; Mark, M.; Desvergne, B.; Wahli, W.; et al. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc. Natl. Acad. Sci. USA 2004, 101, 4543–4547. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.R.; Barrick, C.; Kim, K.A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Mullican, S.E.; DiSpirito, J.R.; Peed, L.C.; Lazar, M.A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc. Natl. Acad. Sci. USA 2013, 110, 18656–18661. [Google Scholar] [CrossRef] [Green Version]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.A.; Zhang, F.; Jiang, L.; Ye, R.; An, Y.; Shao, M.; Tao, C.; Gupta, R.K.; Scherer, P.E. Peroxisome Proliferator-Activated Receptor γ and Its Role in Adipocyte Homeostasis and Thiazolidinedione-Mediated Insulin Sensitization. Mol. Cell Biol. 2018, 38, e00677-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J., Jr.; Liu, X.S.; et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Sakaue, H.; Iguchi, H.; Gomi, H.; Okada, Y.; Takashima, Y.; Nakamura, K.; Nakamura, T.; Yamauchi, T.; Kubota, N.; et al. Role of Krüppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J. Biol. Chem. 2005, 280, 12867–12875. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.M.; He, A.; Tan, M.; Wang, J.; Lu, D.; Razani, B.; Lodhi, I.J. MED19 Regulates Adipogenesis and Maintenance of White Adipose Tissue Mass by Mediating PPARγ-Dependent Gene Expression. Cell Rep. 2020, 33, 108228. [Google Scholar] [CrossRef]
- Liu, W.; Ji, Y.; Zhang, B.; Chu, H.; Yin, C.; Xiao, Y. Stat5a promotes brown adipocyte differentiation and thermogenic program through binding and transactivating the Kdm6a promoter. Cell Cycle 2020, 19, 895–905. [Google Scholar] [CrossRef]
- Seong, S.; Kim, J.H.; Kim, K.; Kim, I.; Koh, J.T.; Kim, N. Alternative regulatory mechanism for the maintenance of bone homeostasis via STAT5-mediated regulation of the differentiation of BMSCs into adipocytes. Exp. Mol. Med. 2021, 53, 848–863. [Google Scholar] [CrossRef]
- Chusyd, D.E.; Wang, D.; Huffman, D.M.; Nagy, T.R. Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front. Nutr. 2016, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Manolio, T.A. Genomewide association studies and assessment of the risk of disease. N. Engl. J. Med. 2010, 363, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Xiao, T.; Guo, J.; Su, Z. Complex Relationship between Obesity and the Fat Mass and Obesity Locus. Int. J. Biol. Sci. 2017, 13, 615–629. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Zhou, H.; Sahay, K.; Xu, W.; Yang, J.; Zhang, W.; Chen, W. Obesity-associated family with sequence similarity 13, member A (FAM13A) is dispensable for adipose development and insulin sensitivity. Int. J. Obes. 2019, 43, 1269–1280. [Google Scholar] [CrossRef] [Green Version]
- Take, K.; Waki, H.; Sun, W.; Wada, T.; Yu, J.; Nakamura, M.; Aoyama, T.; Yamauchi, T.; Kadowaki, T. CDK5 Regulatory Subunit-Associated Protein 1-like 1 Negatively Regulates Adipocyte Differentiation through Activation of Wnt Signaling Pathway. Sci. Rep. 2017, 7, 7326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Deliard, S.; Aziz, A.R.; Grant, S.F. Expression analyses of the genes harbored by the type 2 diabetes and pediatric BMI associated locus on 10q. BMC Med. Genet. 2012, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evseeva, M.N.; Dyikanov, D.T.; Karagyaur, M.N.; Prikazchikova, T.A.; Sheptulina, A.F.; Balashova, M.S.; Zatsepin, T.S.; Rubtsov, Y.P.; Kulebyakin, K.Y. Hematopoietically-expressed homeobox protein HHEX regulates adipogenesis in preadipocytes. Biochimie 2021, 185, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.; Han, P.W. Cellularity of rat adipose tissue: Effects of growth, starvation, and obesity. J. Lipid Res. 1969, 10, 77–82. [Google Scholar] [CrossRef]
- Appleton, S.L.; Seaborn, C.J.; Visvanathan, R.; Hill, C.L.; Gill, T.K.; Taylor, A.W.; Adams, R.J. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: A cohort study. Diabetes Care 2013, 36, 2388–2394. [Google Scholar] [CrossRef] [Green Version]
- Denis, G.V.; Hamilton, J.A. Healthy obese persons: How can they be identified and do metabolic profiles stratify risk? Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Kissebah, A.H.; Krakower, G.R. Regional adiposity and morbidity. Physiol. Rev. 1994, 74, 761–811. [Google Scholar] [CrossRef]
- Sims, E.A. Are there persons who are obese, but metabolically healthy? Metabolism 2001, 50, 1499–1504. [Google Scholar] [CrossRef]
- Krotkiewski, M.; Björntorp, P.; Sjöström, L.; Smith, U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Invest. 1983, 72, 1150–1162. [Google Scholar] [CrossRef]
- Lönn, M.; Mehlig, K.; Bengtsson, C.; Lissner, L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010, 24, 326–331. [Google Scholar] [CrossRef]
- Weyer, C.; Foley, J.E.; Bogardus, C.; Tataranni, P.A.; Pratley, R.E. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000, 43, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Danforth, E., Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat. Genet. 2000, 26, 13. [Google Scholar] [CrossRef]
- Kim, J.K.; Gavrilova, O.; Chen, Y.; Reitman, M.L.; Shulman, G.I. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J. Biol. Chem. 2000, 275, 8456–8460. [Google Scholar] [CrossRef] [Green Version]
- Lotta, L.A.; Gulati, P.; Day, F.R.; Payne, F.; Ongen, H.; van de Bunt, M.; Gaulton, K.J.; Eicher, J.D.; Sharp, S.J.; Luan, J.; et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 2017, 49, 17–26. [Google Scholar] [CrossRef]
- Hue, L.; Taegtmeyer, H. The Randle cycle revisited: A new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.K.; Garg, A. Genetic disorders of adipose tissue development, differentiation, and death. Annu. Rev. Genom. Hum. Genet. 2006, 7, 175–199. [Google Scholar] [CrossRef]
- Hussain, I.; Garg, A. Lipodystrophy Syndromes. Endocrinol. Metab. Clin. North. Am. 2016, 45, 783–797. [Google Scholar] [CrossRef] [Green Version]
- Garg, A. Lipodystrophies. Am. J. Med. 2000, 108, 143–152. [Google Scholar] [CrossRef]
- Tchoukalova, Y.D.; Sarr, M.G.; Jensen, M.D. Measuring committed preadipocytes in human adipose tissue from severely obese patients by using adipocyte fatty acid binding protein. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1132–R1140. [Google Scholar] [CrossRef] [Green Version]
- Isakson, P.; Hammarstedt, A.; Gustafson, B.; Smith, U. Impaired preadipocyte differentiation in human abdominal obesity: Role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 2009, 58, 1550–1557. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.; Yamamoto, Y.; Gesta, S.; Kahn, C.R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008, 7, 410–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Li, M.; Zou, Y.; Cao, T. Induction of adipocyte hyperplasia in subcutaneous fat depot alleviated type 2 diabetes symptoms in obese mice. Obesity 2014, 22, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.G.; Heilbronn, L.K.; Smith, S.R.; Albu, J.B.; Kelley, D.E.; Ravussin, E. Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity 2006, 14, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Cabrera-Mulero, A.; Millán-Gómez, M.; Garrido-Sánchez, L.; Cardona, F.; Tinahones, F.J.; Moreno-Santos, I.; Macías-González, M. Transcriptional Analysis of FOXO1, C/EBP-α and PPAR-γ2 Genes and Their Association with Obesity-Related Insulin Resistance. Genes 2019, 10, 706. [Google Scholar] [CrossRef] [Green Version]
- Stumvoll, M.; Häring, H.U. Glitazones: Clinical effects and molecular mechanisms. Ann. Med. 2002, 34, 217–224. [Google Scholar] [CrossRef]
- Barroso, I.; Gurnell, M.; Crowley, V.E.; Agostini, M.; Schwabe, J.W.; Soos, M.A.; Maslen, G.L.; Williams, T.D.; Lewis, H.; Schafer, A.J.; et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999, 402, 880–883. [Google Scholar] [CrossRef]
- Majithia, A.R.; Flannick, J.; Shahinian, P.; Guo, M.; Bray, M.A.; Fontanillas, P.; Gabriel, S.B.; Rosen, E.D.; Altshuler, D. Rare variants in PPARG with decreased activity in adipocyte differentiation are associated with increased risk of type 2 diabetes. Proc. Natl. Acad. Sci. USA 2014, 111, 13127–13132. [Google Scholar] [CrossRef] [Green Version]
- Sugii, S.; Olson, P.; Sears, D.D.; Saberi, M.; Atkins, A.R.; Barish, G.D.; Hong, S.H.; Castro, G.L.; Yin, Y.Q.; Nelson, M.C.; et al. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. USA 2009, 106, 22504–22509. [Google Scholar] [CrossRef] [Green Version]
- White, U.; Fitch, M.D.; Beyl, R.A.; Hellerstein, M.K.; Ravussin, E. Adipose depot-specific effects of 16 weeks of pioglitazone on in vivo adipogenesis in women with obesity: A randomised controlled trial. Diabetologia 2021, 64, 159–167. [Google Scholar] [CrossRef]
- Hansmann, G.; Calvier, L.; Risbano, M.G.; Chan, S.Y. Activation of the Metabolic Master Regulator PPARγ: A Potential PIOneering Therapy for Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2020, 62, 143–156. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [Green Version]
- McGregor, R.A.; Choi, M.S. MicroRNAs in the regulation of adipogenesis and obesity. Curr Mol. Med. 2011, 11, 304–316. [Google Scholar] [CrossRef]
- Lin, Q.; Gao, Z.; Alarcon, R.M.; Ye, J.; Yun, Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009, 276, 2348–2358. [Google Scholar] [CrossRef]
- Tang, Y.F.; Zhang, Y.; Li, X.Y.; Li, C.; Tian, W.; Liu, L. Expression of miR-31, miR-125b-5p, and miR-326 in the adipogenic differentiation process of adipose-derived stem cells. OMICS 2009, 13, 331–336. [Google Scholar] [CrossRef]
- Thomas, A.W.; Davies, N.A.; Moir, H.; Watkeys, L.; Ruffino, J.S.; Isa, S.A.; Butcher, L.R.; Hughes, M.G.; Morris, K.; Webb, R. Exercise-associated generation of PPARγ ligands activates PPARγ signaling events and upregulates genes related to lipid metabolism. J. Appl. Physiol. 2012, 112, 806–815. [Google Scholar] [CrossRef] [Green Version]
- Antunes, B.M.; Rosa-Neto, J.C.; Batatinha, H.A.P.; Franchini, E.; Teixeira, A.M.; Lira, F.S. Physical fitness status modulates the inflammatory proteins in peripheral blood and circulating monocytes: Role of PPAR-gamma. Sci. Rep. 2020, 10, 14094. [Google Scholar] [CrossRef]
- Khalafi, M.; Mohebbi, H.; Symonds, M.E.; Karimi, P.; Akbari, A.; Tabari, E.; Faridnia, M.; Moghaddami, K. The Impact of Moderate-Intensity Continuous or High-Intensity Interval Training on Adipogenesis and Browning of Subcutaneous Adipose Tissue in Obese Male Rats. Nutrients 2020, 12, 925. [Google Scholar] [CrossRef] [Green Version]
- Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γ gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Mirhosseini, N.; Afshar Ebrahimi, F.; Aghadavod, E.; Taghizadeh, M.; Asemi, Z. A Randomized Double-Blinded, Placebo-Controlled Trial Investigating the Effect of Fish Oil Supplementation on Gene Expression Related to Insulin Action, Blood Lipids, and Inflammation in Gestational Diabetes Mellitus-Fish Oil Supplementation and Gestational Diabetes. Nutrients 2018, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Mejía-Barradas, C.M.; Del-Río-Navarro, B.E.; Domínguez-López, A.; Campos-Rodríguez, R.; Martínez-Godínez, M.; Rojas-Hernández, S.; Lara-Padilla, E.; Abarca-Rojano, E.; Miliar-García, Á. The consumption of n-3 polyunsaturated fatty acids differentially modulates gene expression of peroxisome proliferator-activated receptor alpha and gamma and hypoxia-inducible factor 1 alpha in subcutaneous adipose tissue of obese adolescents. Endocrine 2014, 45, 98–105. [Google Scholar] [CrossRef]
- Anderson, E.J.; Thayne, K.A.; Harris, M.; Shaikh, S.R.; Darden, T.M.; Lark, D.S.; Williams, J.M.; Chitwood, W.R.; Kypson, A.P.; Rodriguez, E. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARγ activation? Antioxid. Redox Signal. 2014, 21, 1156–1163. [Google Scholar] [CrossRef] [Green Version]
- Haidari, F.; Shayesteh, F.; Mohammad-Shahi, M.; Jalali, M.T.; Ahmadi-Angali, K. Olive Leaf Extract Supplementation Combined with Calorie-Restricted Diet on Reducing Body Weight and Fat Mass in Obese Women: Result of a Randomized Control Trial. Clin. Nutr. Res. 2021, 10, 314–329. [Google Scholar] [CrossRef]
- Matsubara, T.; Takakura, N.; Urata, M.; Muramatsu, Y.; Tsuboi, M.; Yasuda, K.; Addison, W.N.; Zhang, M.; Matsuo, K.; Nakatomi, C.; et al. Geranylgeraniol Induces PPARγ Expression and Enhances the Biological Effects of a PPARγ Agonist in Adipocyte Lineage Cells. In Vivo 2018, 32, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Sapwarobol, S.; Saphyakhajorn, W.; Astina, J. Biological Functions and Activities of Rice Bran as a Functional Ingredient: A Review. Nutr. Metab. Insights 2021, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
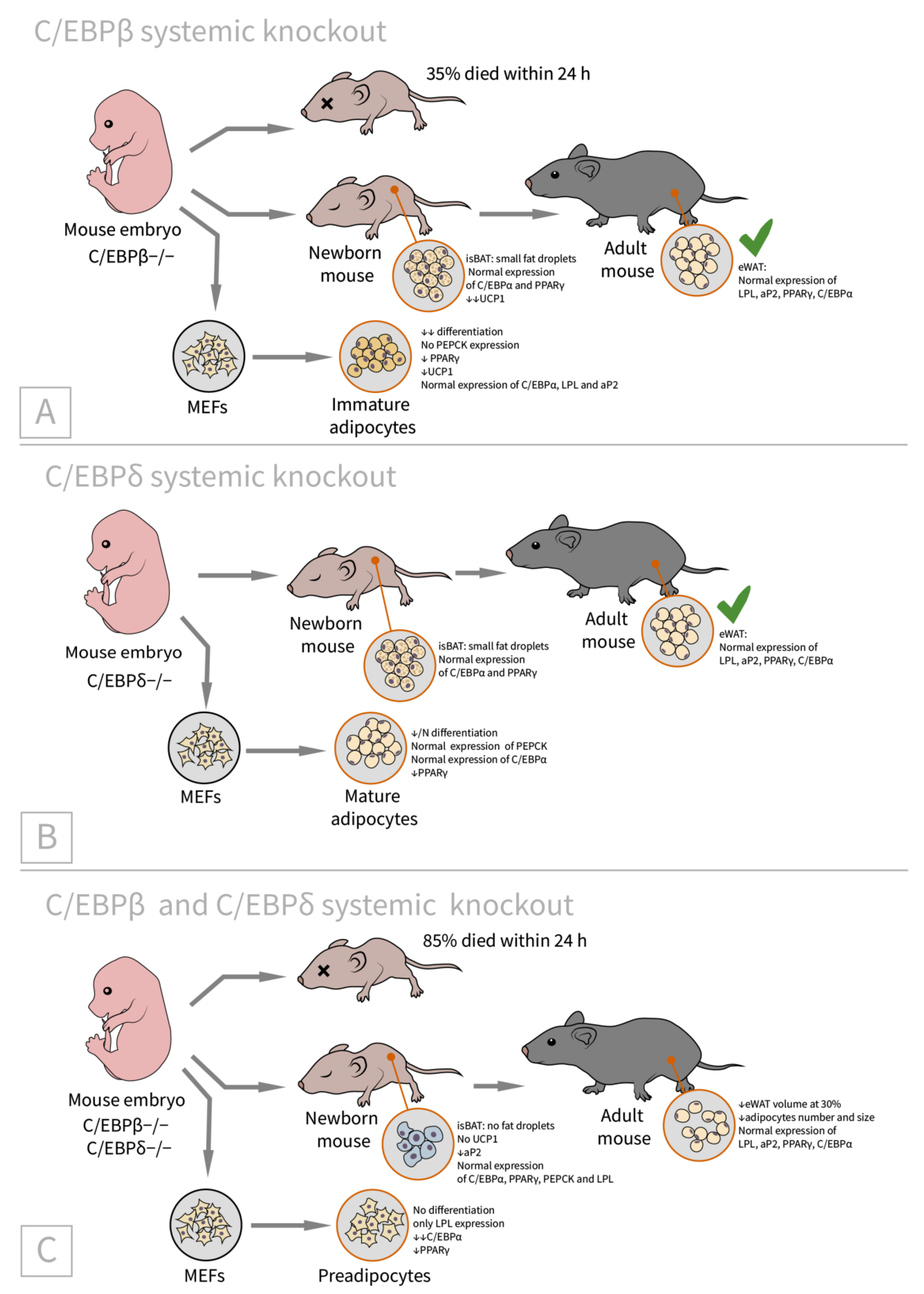


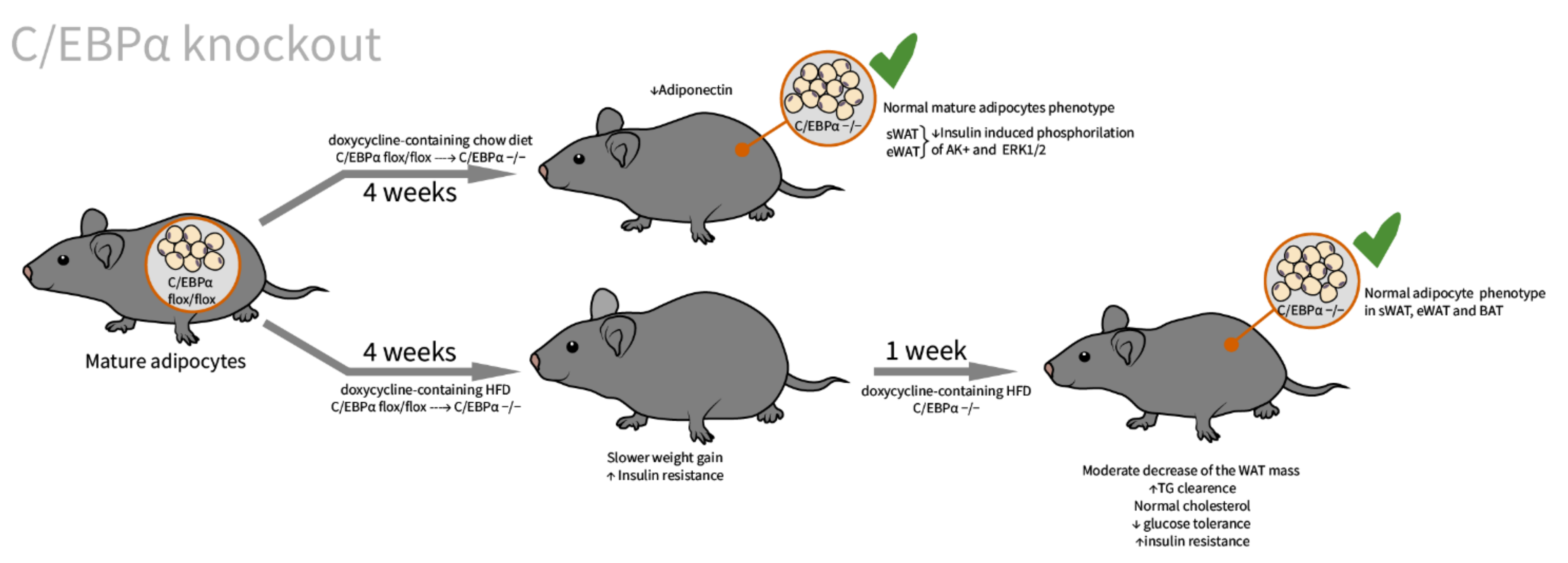
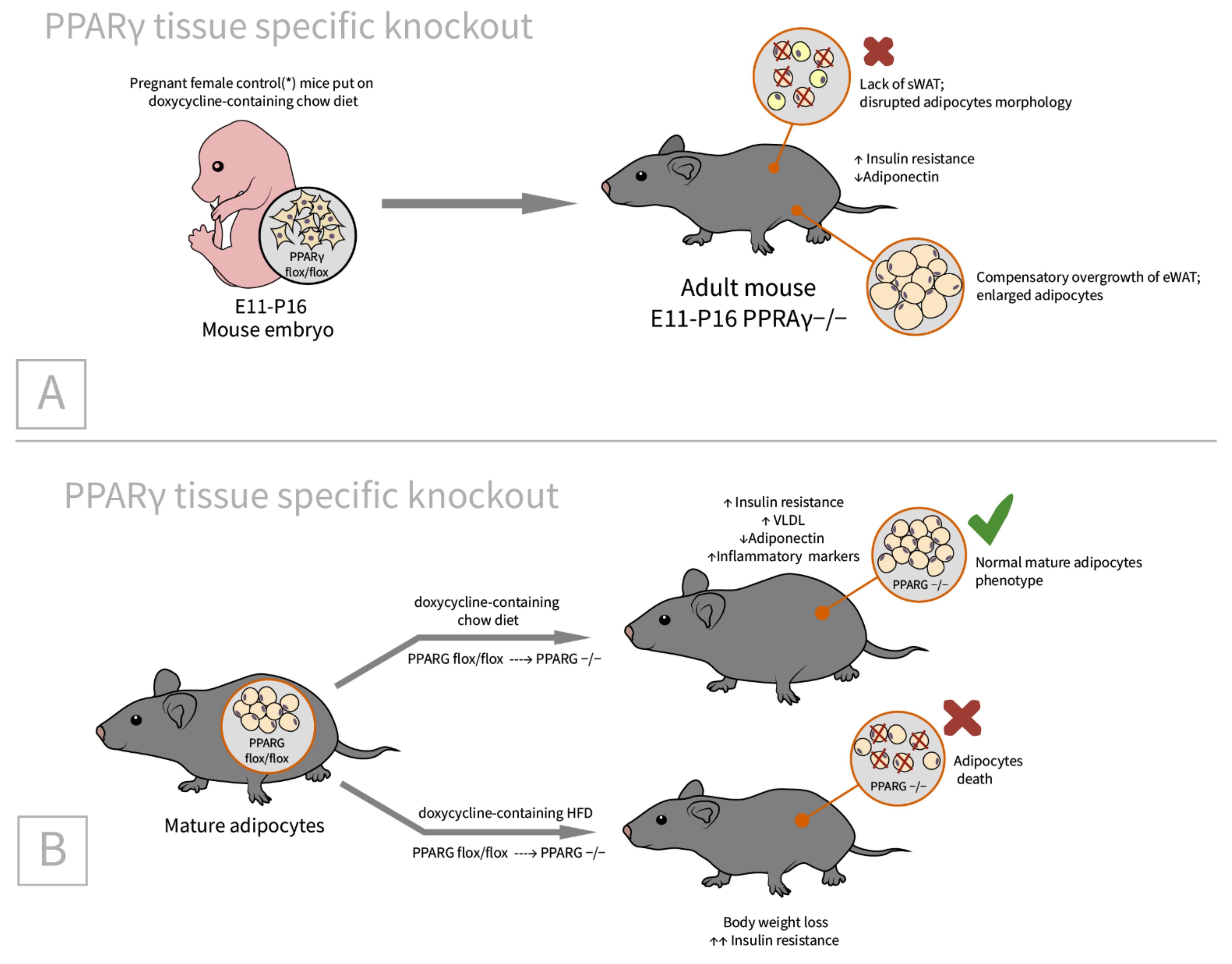

| Transcription Factor Knockout/Transgenic Model/Developmental Stage Analyzed | Adipose Tissue Phenotype | Systemic Phenotype | Comments |
|---|---|---|---|
| CREB [40]/C57BL/6J background, AT-specific CREB(−/−)/adults 3–5 months | Lean-to-fat mass ratio and body weights were comparable with control, moderate decrease in fasting-induced lipolysis | Non-esterified fatty acids (NEFA) levels were 40% lower compared to control | CREB knockout under adiponectin promotor—may be too late to evaluate CREB function |
| CEBP β [46]/C57BL/6 background, systemic C/EBPβ(−/−)/newborns (BAT) adults (WAT) | eWAT: LPL, aP2, PPARγ, and C/EBPα are expressed comparably to control isBAT: Lipid accumulation is only slightly impaired, UCP1 expression is reduced MEFs: differentiation is significantly reduced, cells expressed LPL and aP2 but not PEPCK (immature adipocytes), C/EBPα expression is comparable with WT, and PPARγ is reduced substantially | 35% of knockout animals die within early neonatal period | isBAT: cells with small lipid droplets suggested differentiation block is at the immature adipocyte stage |
| CEBP δ [46]/C57BL/6 background, systemic C/EBPδ(−/−)/newborns (BAT) adults (WAT) | eWAT: LPL, aP2, PPARγ, and C/EBPα are expressed comparably to WT isBAT: Lipid accumulation is only slightly impaired, UCP1 expression is slightly reduced MEFs: differentiation is slightly reduced, cells express PEPCK (mature adipocytes), C/EBPα expression is comparable with WT, and PPARγ is reduced slightly | N/A | isBAT: the adipocytes are the same as the wild-type or slightly reduced in size |
| CEBP β + δ [46]/C57BL/6 background, systemic C/EBPβ(−/−)C/EBPδ(−/−)/ newborns (BAT), adults (WAT) | eWAT (adult): 30% lower than in control mice, LPL, aP2, PPARγ, and C/EBPα are expressed comparably to WT isBAT (newborn): no fat droplets, UCP1 mRNA almost absent, aP2 mRNA is reduced by half, PEPCK and LPL are expressed normally MEFs: only LPL expression (preadipocytes stage), C/EBPα is reduced markedly, and PPARγ is reduced substantially | No histological abnorm- alities in the liver and lung, no hypoglycemia | All mice develop system growth defects, 85% of double knockout animals die within 24 h, and the remaining phenotypes were analyzed (thus the phenotype may be underestimated) |
| C/EBPα [60]/FvB/N mice, systemic C/EBPα knockout except for the liver/newborn and 7-days old mice | WAT: absent except for mammary fat pad (morphologically similar to control) sBAT: present, enlarged, and contains larger fat vacuoles. UCP mRNA is reduced in newborns; by 7 days of age mRNA levels of UCP, FAT, LPL, and aP2 are comparable with control | Agranulocytosis and pulmonary dysplasia in newborns Postprandial hyperlipidemia, fatty liver, and 60% reduction in serum leptin levels | N/A |
| C/EBPα [61]/mixed background C57BL6, SVE129, and CBA strains/C/EBPα systemic inducible knockout/newborns and 3 months old adults | WAT: decreased in size, triglyceride loss BAT: either larger than or similar to control | fatty liver, hypoglycemia, hypocholesterolemia, hypoinsulinemia, hyperammonemia, and hyperproteinemia | All animals display biphasic changes in phenotype: the first 2 weeks phenotype of transgenic animals are comparable with control, the subsequent 2 weeks is accompanied by severe weight loss, hypophagia and death. |
| C/EBPα [62]/C57BL/6J background/C/EBPα tissue specific doxycycline inducible knockout/embryos, newborns, adults, and MEFs | WAT: adults with embryonically knocked out C/EBPα have comparable tissue mass and normal adipocyte size and morphology; insulin-stimulated phosphorylation of Akt and Erk1/2 is significantly impaired BAT: slightly enlarged adipocytes | Decrease in adiponectin to 14% of control; impaired glucose tolerance, insulin resistant on HFD | indispensable for adipocyte regeneration in adults, and expansion under HFD conditions, not essential in terminal embryonic adipogenesis, and mature adipocyte survival |
| PPARγ [62]/C57BL/6J background/PPARγ tissue specific doxycycline inducible knockout/embryos, newborns, adults, and MEFs | sWAT: small size, disrupted adipocyte morphology in Adn-PPARγ−/−(E11-P16) * male offspring eWAT: compensatory increased (by 36%) | Adiponectin reduction by 24% in Adn-PPARγ−/−(E11-P16) mice. Insulin resistance in adipose tissue and liver (adults) [78] | Indispensable in nearly all circumstances except for short-term knockout in adult mature adipocytes in vivo [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evseeva, M.N.; Balashova, M.S.; Kulebyakin, K.Y.; Rubtsov, Y.P. Adipocyte Biology from the Perspective of In Vivo Research: Review of Key Transcription Factors. Int. J. Mol. Sci. 2022, 23, 322. https://doi.org/10.3390/ijms23010322
Evseeva MN, Balashova MS, Kulebyakin KY, Rubtsov YP. Adipocyte Biology from the Perspective of In Vivo Research: Review of Key Transcription Factors. International Journal of Molecular Sciences. 2022; 23(1):322. https://doi.org/10.3390/ijms23010322
Chicago/Turabian StyleEvseeva, Maria N., Maria S. Balashova, Konstantin Y. Kulebyakin, and Yury P. Rubtsov. 2022. "Adipocyte Biology from the Perspective of In Vivo Research: Review of Key Transcription Factors" International Journal of Molecular Sciences 23, no. 1: 322. https://doi.org/10.3390/ijms23010322
APA StyleEvseeva, M. N., Balashova, M. S., Kulebyakin, K. Y., & Rubtsov, Y. P. (2022). Adipocyte Biology from the Perspective of In Vivo Research: Review of Key Transcription Factors. International Journal of Molecular Sciences, 23(1), 322. https://doi.org/10.3390/ijms23010322






