Fundamental Clock of Biological Aging: Convergence of Molecular, Neurodegenerative, Cognitive and Psychiatric Pathways: Non-Equilibrium Thermodynamics Meet Psychology
Abstract
1. Introduction
“The roots of stress research lie in the belief that stress can accelerate biological aging”[1]
1.1. Aging, Entropy, and Aging Defense System
“It is by avoiding the rapid decay into the inert state of ‘equilibrium’ that an organism appears so enigmatic”[9]
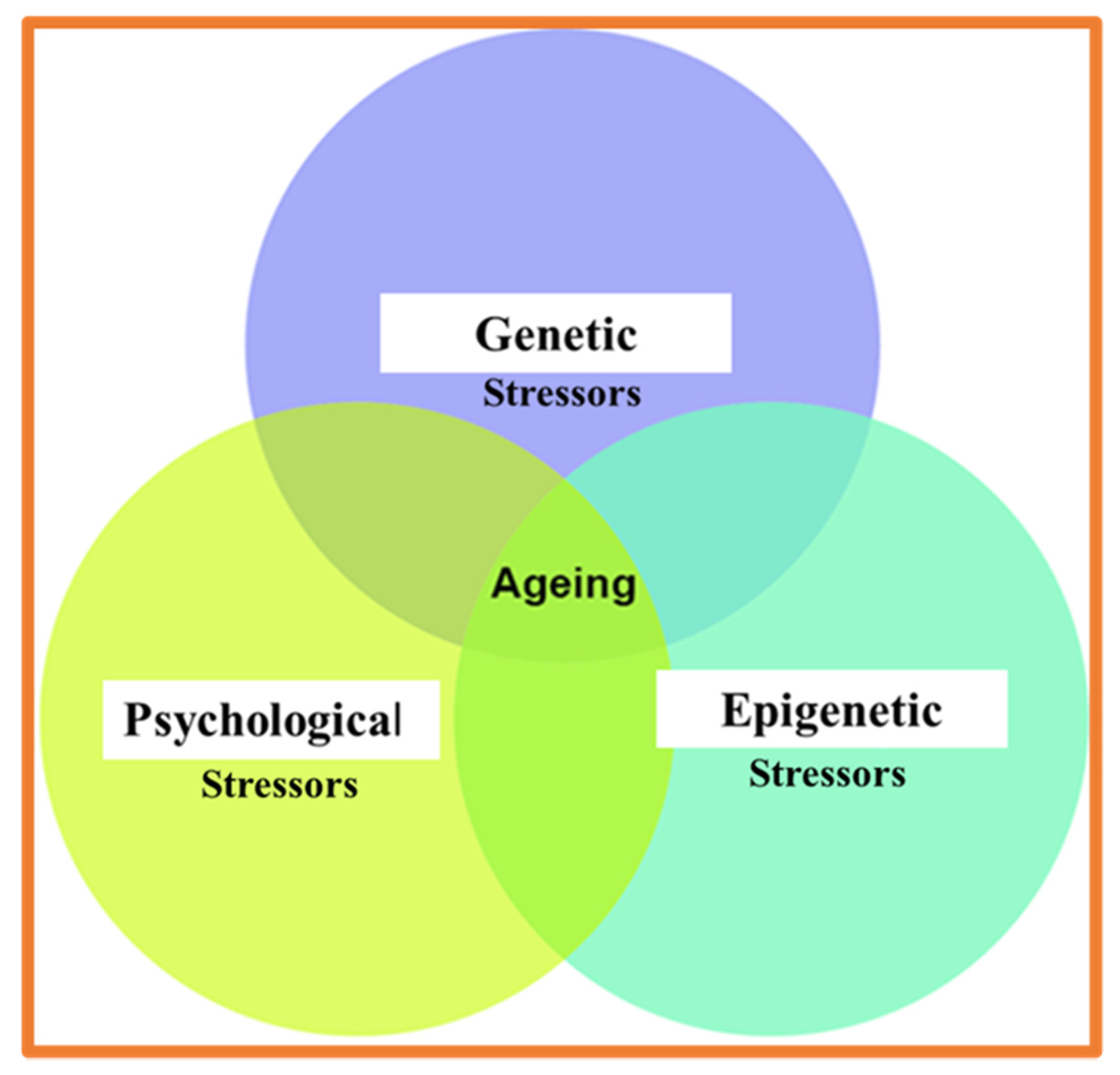
1.2. Psychological and Physical Stressor
1.3. Biochirality, Spontaneous Reactions and Aging
“Chirality is a fundamental, persistent, but often overlooked feature of all living organisms on the molecular level as well as on the macroscopic scale.”
2. Crosstalk of Physiological and Psychological States
2.1. Molecular Level
2.2. System Level
2.3. Organism Level
2.4. Chiral Psychotherapy
3. Universal Biological of Aging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| AAs | Amino acids |
| ATP | Adenosine triphosphate |
| D-AAs | D-amino acids |
| AD | Alzheimer disease |
| A-b | Amyloid-beta |
| LAC | Acetyl-L-carnitine |
| DNA | Deoxyribonucleic acid |
| EPK | Eukaryotic protein kinase |
| LLPs | Long-lived proteins |
| mTOR | Mammalian TOR |
| MA | Mutation accumulation theory |
| AP | hypotheses. Antagonistic pleiotropy |
| NS | hypotheses. Native state |
| NS | Natural selection |
| NE | Non-equilibrium |
| NTs | Nucleotides |
| PTM | Post-translational modification |
| RHA | Racemization hypothesis of aging |
| RNAs | Ribonucleic acids |
| PTMs SP | Spontaneous PTMs |
| Ser | Serine |
| SRS | Stress response systems |
| Tyr | Tyrosine |
References
- Entringer, S.; Epel, E.S. The stress field ages: A close look into cellular aging processes. Psychoneuroendocrinology 2020, 113, 104537. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Bürkle, A. Molecular consequences of psychological stress in human aging. Exp. Gerontol. 2015, 68, 39–42. [Google Scholar] [CrossRef]
- Lavretsky, H.; Newhouse, P.A. Stress, Inflammation, and Aging. Am. J. Geriatr. Psychiatry 2012, 20, 729–733. [Google Scholar] [CrossRef]
- Guillot, M. Thinking of oneself as the thinker: The concept of self and the phenomenology of intellection. Conscious Thinking and Cognitive Phenomenology. Philos. Explor. 2016, 19, 138–160. [Google Scholar] [CrossRef]
- Maier, S.F.; Watkins, L.R. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 1998, 105, 83–107. [Google Scholar] [CrossRef]
- Brugada, V.; De Polavieja, G.G.; Román, Á.-C. Toward a Molecular Profile of Self-Representation. Front. Hum. Neurosci. 2016, 10, 602. [Google Scholar] [CrossRef][Green Version]
- Koseska, A.; Bastiaens, P.I. Cell signaling as a cognitive process. EMBO J. 2017, 36, 568–582. [Google Scholar] [CrossRef]
- Schrödinger, E. What is Life—The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944; ISBN 978-0-521-42708-1. [Google Scholar]
- Prigogine, I.; Nicolis, G. On Symmetry-Breaking Instabilities in Dissipative Systems. J. Chem. Phys. 1967, 46, 3542–3550. [Google Scholar] [CrossRef]
- Teschendorff, A.E.; Sollich, P.; Kuehn, R. Signalling entropy: A novel network-theoretical framework for systems analysis and interpretation of functional omic data. Methods 2014, 67, 282–293. [Google Scholar] [CrossRef]
- Gnesotto, F.; Mura, F.; Gladrow, J.; Broedersz, C. Broken detailed balance and non-equilibrium dynamics in living systems: A review. Rep. Prog. Phys. 2018, 81, 066601. [Google Scholar] [CrossRef]
- Michaelian, K. The Dissipative Photochemical Origin of Life: UVC Abiogenesis of Adenine. Entropy 2021, 23, 217. [Google Scholar] [CrossRef] [PubMed]
- Galzitskaya, O.V. Influence of Conformational Entropy on the Protein Folding Rate. Entropy 2010, 12, 961–982. [Google Scholar] [CrossRef]
- Marques, B.S.; Stetz, M.A.; Jorge, C.; Valentine, K.G.; Wand, A.J.; Nucci, N.V. Protein conformational entropy is not slaved to water. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Popov, E.M. Protein folding as a nonlinear nonequilibrium thermodynamic process. Biochem. Mol. Boil. Int. 1999, 47, 443–453. [Google Scholar] [CrossRef]
- Pickett, S.; Sternberg, M. Empirical Scale of Side-Chain Conformational Entropy in Protein Folding. J. Mol. Biol. 1993, 231, 825–839. [Google Scholar] [CrossRef]
- Rajitha Rajeshwar, T.; Saharay, M.; Smith, J.C.; Krishnan, M. Correlated Response of Protein Side-Chain Fluctuations and Conformational Entropy to Ligand Binding. J. Phys. Chem. B 2021, 125, 9641–9651. [Google Scholar] [CrossRef]
- Banik, S.D.; Nandi, N. Chirality and Protein Biosynthesis. Top. Curr. Chem. 2012, 333, 255–305. [Google Scholar] [CrossRef]
- Baxa, M.C.; Haddadian, E.J.; Jumper, J.M.; Freed, K.F.; Sosnick, T.R. Loss of conformational entropy in protein folding calculated using realistic ensembles and its implications for NMR-based calculations. Proc. Natl. Acad. Sci. USA 2014, 111, 15396–15401. [Google Scholar] [CrossRef]
- Trbovic, N.; Cho, J.-H.; Abel, R.; Friesner, R.A.; Rance, M.; Palmer, I.A.G. Protein Side-Chain Dynamics and Residual Conformational Entropy. J. Am. Chem. Soc. 2009, 131, 615–622. [Google Scholar] [CrossRef]
- Faraggi, E.; Dunker, A.K.; Jernigan, R.L.; Kloczkowski, A. Entropy, Fluctuations, and Disordered Proteins. Entropy 2019, 21, 764. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L. Amino acid racemization dating of fossil bones. Annu. Rev. Earth Planet. Sci. 1985, 13, 241–268. [Google Scholar] [CrossRef]
- Dyakin, V.V.; Lajtha, A.; Dyakina-Fagnano, N.V. Racemization hypothesis of neurodegeneration (RHND). Alzheimer’s Dement. 2020, 16, e047697. [Google Scholar] [CrossRef]
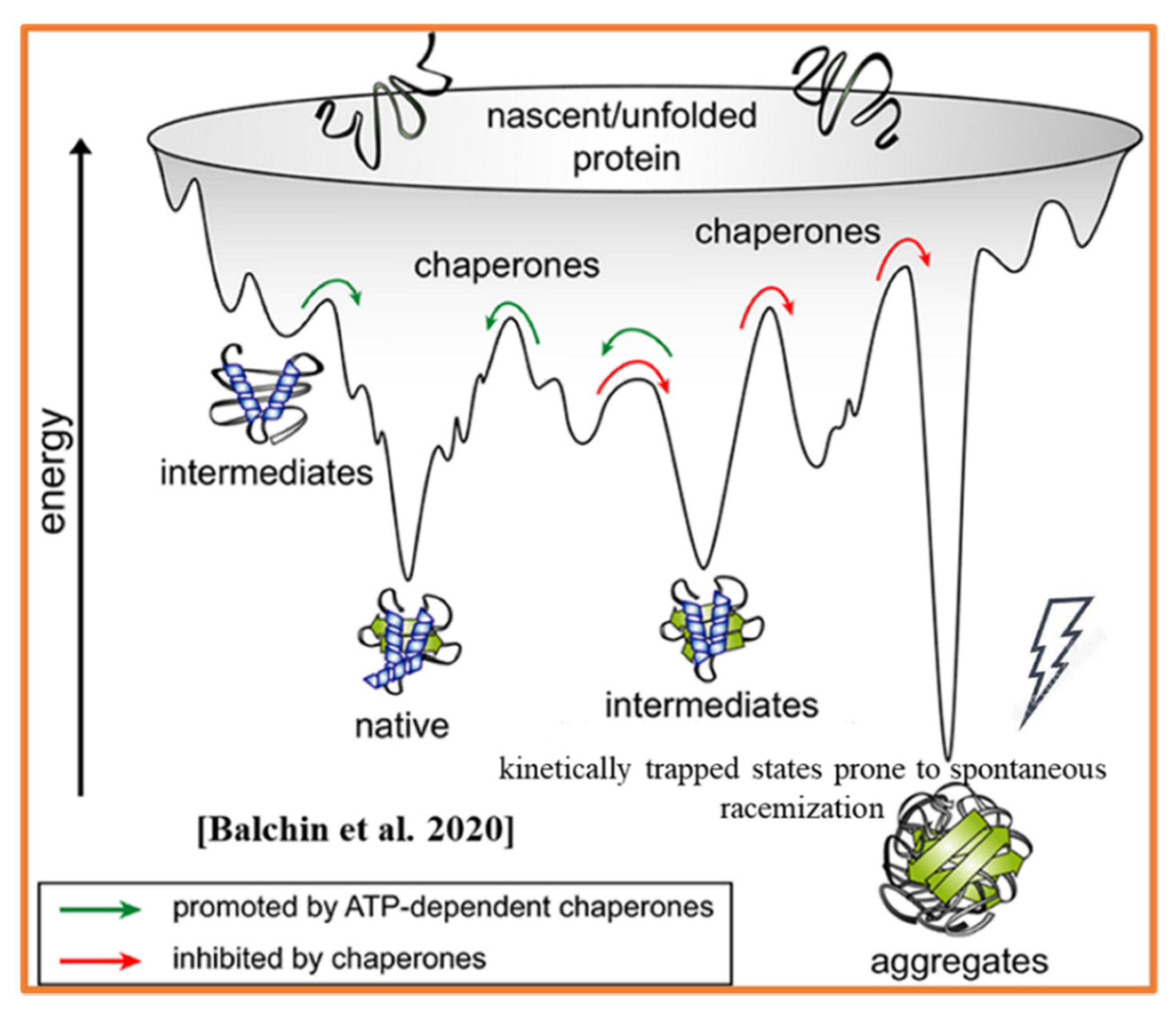
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. Recent advances in understanding catalysis of protein folding by molecular chaperones. FEBS Lett. 2020, 594, 2770–2781. [Google Scholar] [CrossRef]
- Lester, A.W.; Moffat, S.D.; Wiener, J.; Barnes, C.A.; Wolbers, T. The Aging Navigational System. Neuron 2017, 95, 1019–1035. [Google Scholar] [CrossRef]
- Yu, S.; Boone, A.P.; He, C.; Davis, R.C.; Hegarty, M.; Chrastil, E.R.; Jacobs, E.G. Age-Related Changes in Spatial Navigation Are Evident by Midlife and Differ by Sex. Psychol. Sci. 2021, 32, 692–704. [Google Scholar] [CrossRef]
- Eturgeon, M.; Elustig, C.; Meck, W.H. Cognitive Aging and Time Perception: Roles of Bayesian Optimization and Degeneracy. Front. Aging Neurosci. 2016, 8, 102. [Google Scholar] [CrossRef]
- Adamo, D.E.; Briceño, E.M.; Sindone, J.A.; Alexander, N.B.; Moffat, S.D. Age differences in virtual environment and real world path integration. Front. Aging Neurosci. 2012, 4, 26. [Google Scholar] [CrossRef]
- Flatt, T.; Schmidt, P.S. Integrating evolutionary and molecular genetics of aging. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 951–962. [Google Scholar] [CrossRef]
- Flatt, T.; Partridge, L. Horizons in the evolution of aging. BMC Biol. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Johnson, A.A.; Shokhirev, M.N.; Shoshitaishvili, B. Revamping the evolutionary theories of aging. Ageing Res. Rev. 2019, 55, 100947. [Google Scholar] [CrossRef]
- Turan, Z.G.; Parvizi, P.; Dönertaş, H.M.; Tung, J.; Khaitovich, P.; Somel, M. Molecular footprint of Medawar’s mutation accumulation process in mammalian aging. Aging Cell 2019, 18, e12965. [Google Scholar] [CrossRef]
- Medawar, P. An Unsolved Problem of Biology; H.K Lewis: London, UK, 1952. [Google Scholar]
- Williams, G.C. Pleiotropy, natural selection, and the evolution of senescence. Sci. Aging Knowl. Environ. 1957, 11, 398–411. [Google Scholar] [CrossRef]
- Graham, J.E.; Christian, L.; Kiecolt-Glaser, J.K. Stress, Age, and Immune Function: Toward a Lifespan Approach. J. Behav. Med. 2006, 29, 389–400. [Google Scholar] [CrossRef]
- Uhart, M.; Oswald, L.; McCaul, M.E.; Chong, R.; Wand, G.S. Hormonal Responses to Psychological Stress and Family History of Alcoholism. Neuropsychopharmacology 2006, 31, 2255–2263. [Google Scholar] [CrossRef]
- Clark, B.L.; Thomas, P.G. A Cell for the Ages: Human γδ T Cells across the Lifespan. Int. J. Mol. Sci. 2020, 21, 8903. [Google Scholar] [CrossRef]
- Riboni, F.V.; Belzung, C. Stress and psychiatric disorders: From categorical to dimensional approaches. Curr. Opin. Behav. Sci. 2017, 14, 72–77. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B.; Fricchione, G.L.; Benson, H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol. Lett. 2002, 23, 199–208. [Google Scholar]
- Dayas, C.V.; Buller, K.M.; Crane, J.; Xu, Y.; Day, T. Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur. J. Neurosci. 2001, 14, 1143–1152. [Google Scholar] [CrossRef]
- Carter, J.R.; Goldstein, D.S. Sympathoneural and Adrenomedullary Responses to Mental Stress. Compr. Physiol. 2014, 5, 119–146. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef]
- Shonkoff, J.P.; Boyce, W.T.; McEwen, B.S. Neuroscience, Molecular Biology, and the Childhood Roots of Health Disparities: Building a new framework for health promotion and disease prevention. JAMA 2009, 301, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Parker, K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011, 137, 959–997. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Caspi, A.; Williams, B.; Ambler, A.; Sugden, K.; Mika, J.; Werts, H.; Freeman, J.; Pariante, C.M.; Moffitt, T.; et al. Biological embedding of stress through inflammation processes in childhood. Mol. Psychiatry 2010, 16, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, K.R.; Gutièrrez-Mecinas, M.; Trollope, A.F.; Collins, A.; Saunderson, E.A.; Reul, J.M. Epigenetic mechanisms in stress and adaptation. Brain Behav. Immun. 2011, 25, 1305–1315. [Google Scholar] [CrossRef]
- Eachus, H.; Cunliffe, V. Biological Embedding of Psychosocial Stress over the Life Course. Epigenetics Aging Longev. 2018, 14, 251–270. [Google Scholar] [CrossRef]
- Santos, A.C.L.; Muniz, C.R.; Oliveira, L.T.; Souza, J.T. Contributions of a modified electrodynamics to the molecular biochirality. Chirality 2020, 32, 1186–1190. [Google Scholar] [CrossRef]
- Manhas, R.; Rath, P.C. Ribosome, protein synthesis, and aging. In Models, Molecules and Mechanisms in Biogerontology; Springer: Singapore, 2020; pp. 67–87. [Google Scholar]
- McCudden, C.R.; Kraus, V.B. Biochemistry of amino acid racemization and clinical application to musculoskeletal disease. Clin. Biochem. 2006, 39, 1112–1130. [Google Scholar] [CrossRef]
- Morrow, S.M.; Bissette, A.; Fletcher, S.P. Transmission of chirality through space and across length scales. Nat. Nanotechnol. 2017, 12, 410–419. [Google Scholar] [CrossRef]
- Pályi, G.; Zucchi, C.; Caglioti, L. Advances in BioChirality; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Inaki, M.; Liu, J.; Matsuno, K. Cell chirality: Its origin and roles in left–right asymmetric development. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150403. [Google Scholar] [CrossRef]
- Dyakin, V.V.; Lucas, J.; Dyakina-Fagnano, N.V.; Posner, E.V. The Chain of Chirality Transfer as Determinant of Brain Functional Laterality. Breaking the Chirality Silence: Search for New Generation of Biomarkers; Relevance to Neurodegenerative Diseases, Cognitive Psychology, and Nutrition Science. Neurol. Neurosci. Res. 2017, 1, 2. [Google Scholar] [CrossRef]
- Liu, B.; Pappas, C.; Ottelé, J.; Schaeffer, G.; Jurissek, C.; Pieters, P.F.; Altay, M.; Marić, I.; Stuart, M.C.A.; Otto, S. Spontaneous Emergence of Self-Replicating Molecules Containing Nucleobases and Amino Acids. J. Am. Chem. Soc. 2020, 142, 4184–4192. [Google Scholar] [CrossRef]
- Plasson, R.; Brandenburg, A. Homochirality and the Need for Energy. Orig. Life Evol. Biosph. 2009, 40, 93–110. [Google Scholar] [CrossRef]
- Lambeth, T.R.; Riggs, D.L.; Talbert, L.E.; Tang, J.; Coburn, E.; Kang, A.S.; Noll, J.; Augello, C.; Ford, B.D.; Julian, R.R. Spontaneous Isomerization of Long-Lived Proteins Provides a Molecular Mechanism for the Lysosomal Failure Observed in Alzheimer’s Disease. ACS Central Sci. 2019, 5, 1387–1395. [Google Scholar] [CrossRef]
- Hinterholzer, A.; Stanojlovic, V.; Regl, C.; Huber, C.G.; Cabrele, C.; Schubert, M. Detecting aspartate isomerization and backbone cleavage after aspartate in intact proteins by NMR spectroscopy. J. Biomol. NMR 2021, 75, 71–82. [Google Scholar] [CrossRef]
- Lehmann, J.; Ye, S. D Amino Acids Highlight the Catalytic Power of the Ribosome. Cell Chem. Biol. 2019, 26, 1639–1641. [Google Scholar] [CrossRef] [PubMed]
- Englander, M.T.; Avins, J.L.; Fleisher, R.; Liu, B.; Effraim, P.R.; Wang, J.; Schulten, K.; Leyh, T.S.; Gonzalez, R.L.; Cornish, V.W. The ribosome can discriminate the chirality of amino acids within its peptidyl-transferase center. Proc. Natl. Acad. Sci. USA 2015, 112, 6038–6043. [Google Scholar] [CrossRef]
- Huang, P.-Y.; Wang, F.; Narasimhan, K.; Chatman, K.; Aach, J.; Trauger, S.A.; Spoering, R.; Church, G.M. Toward D-Peptide Biosyn-thesis: Elongation Factor P Enables Ribosomal Incorporation f Consecutive D-Amino Acids. bioRxiv 2017, 125930. [Google Scholar] [CrossRef]
- Raghunathan, S.; Yadav, K.; Rojisha, V.C.; Jaganade, T.; Prathyusha, V.; Bikkina, S.; Lourderaj, U.; Priyakum, U.D. Transition between [R]- and [S]-stereoisomers without bond breakng. Phys. Chem. Chem. Phys. 2020, 22, 14983–14991. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Cabrele, C. Susceptibility of protein therapeutics to spontaneous chemical modifications by oxidation, cyclization, and elimination reactions. Amino Acids 2019, 51, 1409–1431. [Google Scholar] [CrossRef]
- Schneiderman, N.; Ironson, G.; Siegel, S.D. Stress and Health: Psychological, Behavioral, and Biological Determinants. Annu. Rev. Clin. Psychol. 2005, 1, 607–628. [Google Scholar] [CrossRef]
- Dziechciaż, M.; Filip, R. Biological psychological and social determinants of old age: Bio-psycho-social aspects of human aging. Ann. Agric. Environ. Med. 2014, 21, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Kohda, M.; Hotta, T.; Takeyama, T.; Awata, S.; Tanaka, H.; Asai, J.-Y.; Jordan, A.L. If a fish can pass the mark test, what are the implications for consciousness and self-awareness testing in animals? PLoS Biol. 2019, 17, e3000021. [Google Scholar] [CrossRef] [PubMed]
- Poplin, L.; Delong, R. Accelerated Aging due to Enzymatic Racemization. Gerontology 1978, 24, 365–368. [Google Scholar] [CrossRef]
- Fujii, N.; Ishibashi, Y.; Satoh, K.; Fujino, M.; Harada, K. Simultaneous racemization and isomerization at specific aspartic acid residues in αB-crystallin from the aged human lens. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1994, 1204, 157–163. [Google Scholar] [CrossRef]
- Mori, H.; Ishii, K.; Tomiyama, T.; Furiya, Y.; Sahara, N.; Asano, S.; Endo, N.; Shirasawa, T.; Takio, K. Racemization: Its Biological Significance on Neuropathogenesis of Alzheimer’s Disease. Tohoku J. Exp. Med. 1994, 174, 251–262. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Nair, K.S. Muscle Protein Turnover: Methodological Issues and the Effect of Aging. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1995, 50, 107–112. [Google Scholar] [CrossRef]
- Harding, J.J.; Beswick, H.T.; Ajiboye, R.; Huby, R.; Blakytny, R.; Rixon, K.C. Non-enzymic post-translational modification of proteins in aging. A review. Mech. Ageing Dev. 1989, 50, 7–16. [Google Scholar] [CrossRef]
- Maddox, J.L. The Encyclopedia of Aging: A Comprehensive Resource in Gerontology and Geriatrics, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 568, 854–856, 1117. [Google Scholar]
- Ritz-Timme, S.; Collins, M.J. Racemization of aspartic acid in human proteins. Ageing Res. Rev. 2002, 1, 43–59. [Google Scholar] [CrossRef]
- Inoue, K.; Hosaka, D.; Mochizuki, N.; Akatsu, H.; Tsutsumiuchi, K.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’Oka, T. Simultaneous Determination of Post-Translational Racemization and Isomerization of N-Terminal Amyloid-β in Alzheimer’s Brain Tissues by Covalent Chiral Derivatized Ultraperformance Liquid Chromatography Tandem Mass Spectrometry. Anal. Chem. 2013, 86, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I.; Cuervo, A.M. Protein Homeostasis and Aging: Taking Care of Proteins from the Cradle to the Grave. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2009, 64, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Salmonowicz, H.; Gladyshev, V.N. Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell 2019, 18, e12841. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Bommarius, A.S.; Champion, J.A.; Chernoff, Y.O.; Lynn, D.G.; Paravastu, A.K.; Liang, C.; Hsieh, M.-C.; Heemstra, J.M. Biomolecular Assemblies: Moving from Observation to Predictive Design. Chem. Rev. 2018, 118, 11519–11574. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Villanueva, J.F.; Díaz-Molina, R.; García-González, V. Protein Folding and Mechanisms of Proteostasis. Int. J. Mol. Sci. 2015, 16, 17193–17230. [Google Scholar] [CrossRef]
- Bulvik, B.E.; Berenshtein, E.; Konijn, A.M.; Grinberg, L.; Vinokur, V.; Eliashar, R.; Chevion, M. Aging is an organ-specific process: Changes in homeostasis of iron and redox proteins in the rat. Age 2012, 34, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Ori, A.; Toyama, B.H.; Harris, M.S.; Bock, T.K.C.; Iskar, M.; Bork, P.; Ingolia, N.T.; Hetzer, M.W.; Beck, M. Integrated Transcriptome and Proteome Analyses Reveal Organ-Specific Proteome Deterioration in Old Rats. Cell Syst. 2015, 1, 224–237. [Google Scholar] [CrossRef]
- Dyakin, V.V.; Wisniewski, T.M.; Lajtha, A. Chiral Interface of Amyloid Beta (Aβ): Relevance to Protein Aging, Aggregation and Neurodegeneration. Symmetry 2020, 12, 585. [Google Scholar] [CrossRef]
- Dyakin, V.; Wisniewski, T.; Lajtha, A. Racemization in Post-Translational Modifications Relevance to Protein Aging, Aggregation and Neurodegeneration: Tip of the Iceberg. Symmetry 2021, 13, 455. [Google Scholar] [CrossRef]
- Herhausa, B.; Joistenb, N.; Wessels, I.; Zimmer, P.; Petrowski, K. How acute physical and psychological stress differentially influence the kynurenine pathway: A randomized cross-over trial. Psychoneuroendocrinology 2021, 134, 105433. [Google Scholar] [CrossRef]
- Bonneau, R.H.; Hunzeker, J.T. Stress-induced Modulation of the Immune Response to Herpes Simplex Virus Infections. 2. Animal Model Studies. In Psychoneuroimmunology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1077–1096. [Google Scholar]
- Bashour, H.; Salam, A.A. Psychological stress and spontaneous abortion. Int. J. Gynecol. Obstet. 2001, 73, 179–181. [Google Scholar] [CrossRef]
- Karl, J.P.; Hatch-McChesney, A.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef]
- Lehman, B.J.; David, D.M.; Gruber, J.A. Rethinking the biopsychosocial model of health: Understanding health as a dynamic system. Soc. Pers. Psychol. Compass 2017, 11, e12328. [Google Scholar] [CrossRef]
- Johnson, A.K.; Hayes, S.N.; Sawchuk, C.; Johnson, M.P.; Best, P.J.; Gulati, R.; Tweet, M.S. Analysis of Posttraumatic Stress Disorder, Depression, Anxiety, and Resiliency Within the Unique Population of Spontaneous Coronary Artery Dissection Survivors. J. Am. Hear. Assoc. 2020, 9, e014372. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, F.; Xin, Y.; Chanrion, B.; O’Donnell, A.H.; Ge, Y.; Dwork, A.J.; Arango, V.; Mann, J.J. Increased DNA methylation in the suicide brain. Dialogues Clin. Neurosci. 2014, 16, 430–438. [Google Scholar] [PubMed]
- Ising, M.; Holsboer, F. Genetics of stress response and stress-related disorders. Dialogues Clin. Neurosci. 2006, 8, 433–444. [Google Scholar]
- Harrison, P.J.; Weinberger, D.R. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Psychiatry 2004, 10, 40–68. [Google Scholar] [CrossRef] [PubMed]
- Bettens, K.; Sleegers, K.; Van Broeckhoven, C. Genetic insights in Alzheimer’s disease. Lancet Neurol. 2013, 12, 92–104. [Google Scholar] [CrossRef]
- Gan, L.; Cookson, M.R.; Petrucelli, L.; La Spada, A.R. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 2018, 21, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Wang, C.; Shou, H.; Schultz, M.D.; Chiarenza, S.; Nussaume, L.; Ecker, J.; Whelan, J.; Lister, R. Stress induced gene expression drives transient DNA methylation changes at adjacent repetitive elements. eLife 2015, 4, e09343. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, I. Genetic insights into dementia disorders. Nat. Rev. Neurol. 2021, 17, 193. [Google Scholar] [CrossRef]
- Howes, O.; McCutcheon, R.; Ston, J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 2015, 29, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Religa, D.; Laudon, H.; Styczynska, M.; Winblad, B.; Näslund, J.; Haroutunian, V. Amyloid β Pathology in Alzheimer’s Disease and Schizophrenia. Am. J. Psychiatry 2003, 160, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Longinetti, E.; Mariosa, D.; Larsson, H.; Ye, W.; Ingre, C.; Almqvist, C.; Lichtenstein, P.; Piehl, F.; Fang, F. Neuro-degenerative and psychiatric diseases among families with amyotrophic lateral sclerosis. Neurology 2017, 89, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nat. Cell Biol. 2008, 455, 903–911. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Logan, J.G.; Barksdale, D.J. Allostasis and allostatic load: Expanding the discourse on stress and cardiovascular disease. J. Clin. Nurs. 2008, 17, 201–208. [Google Scholar] [CrossRef]
- McEwen, B.S.; Gray, J.D.; Nasca, C. Redefining neuroendocrinology: Stress, sex and cognitive and emotional regulation. J. Endocrinol. 2015, 226, T67–T83. [Google Scholar] [CrossRef]
- Hannibal, K.E.; Bishop, M. Chronic Stress, Cortisol Dysfunction, and Pain: A Psychoneuroendocrine Rationale for Stress Management in Pain Rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H.; Davidson, R.J.; Coe, C.L.; Wheeler, R.E.; Tomarken, A.J.; Ershler, W.B. Frontal brain asymmetry and immune function. Behav. Neurosci. 1991, 105, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, J.; Almeida, O.F.; Sousa, N. The stressed prefrontal cortex. Left? Right! Brain Behav. Immun. 2008, 22, 630–638. [Google Scholar] [CrossRef]
- Gerendai, I.; Halász, B. Neuroendocrine Asymmetry. Front. Neuroendocr. 1997, 18, 354–381. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, Z.; Decheva, L.; Pashalieva, I.; Nikolova, P. Brain asymmetry, immunity, handedness. Open Med. 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Dyakin, V.V.; Lucas, J. Non-equilibrium phase transition in biochemical—Systems. Chain of chirality transfer as Determinant of Brain Functional Laterality. Relevance to Alzheimer disease and cognitive psychology. In Proceedings of the Alzheimer’s Association International Conference (AAIC-2017), London, UK, 16–20 July 2017. [Google Scholar]
- Sasabe, J.; Suzuki, M. Distinctive Roles of D-Amino Acids in the Homochiral World: Chirality of Amino Acids Modulates Mammalian Physiology and Pathology. Keio J. Med. 2018, 68, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hawbaker, N.A.; Blackmond, D.G. Energy threshold for chiral symmetry breaking in molecular self-replication. Nat. Chem. 2019, 11, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Zach, P.; Valeš, K.; Stuchlik, A.; Čermáková, P. Effect of stress on structural bran asymmetry. Project: Memory Research in Neuropsychiatric Disorders. Neuro Endocrinol. Lett. 2016, 37, 253–264. [Google Scholar] [CrossRef]
- Shalev, I.; Hastings, W.J. Psychological Stress and Cellular Aging; Oxford University Press (OUP): Oxford, UK, 2017. [Google Scholar]
- Leander, M.; Yuan, Y.; Meger, A.; Cui, Q.; Raman, S. Functional plasticity and evolutionary adaptation of allosteric regulation. Proc. Natl. Acad. Sci. USA 2020, 117, 25445–25454. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Social Foundations of Thought and Action: A Social Cognitive Theory; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1986; ISBN 978-0-13-815614-5. [Google Scholar]
- Bandura, A. Toward a Psychology of Human Agency. Perspect. Psychol. Sci. 2006, 1, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Van Bokhoven, H. Genetic and Epigenetic Networks in Intellectual Disabilities. Annu. Rev. Genet. 2011, 45, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Toulopoulou, T.; Van Haren, N.; Zhang, X.; Sham, P.C.; Cherny, S.; Campbell, D.; Picchioni, M.; Murray, R.; Boomsma, D.I.; Pol, H.H.; et al. Reciprocal causation models of cognitive vs volumetric cerebral intermediate phenotypes for schizophrenia in a pan-European twin cohort. Mol. Psychiatry 2014, 20, 1386–1396. [Google Scholar] [CrossRef]
- Martin, P.; McEwen, B.S. Review. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosom. Med. 2018, 80, 126–140. [Google Scholar] [CrossRef]
- Martino, G.; Langher, V.; Cazzato, V.; Vicario, C.M. Editorial: Psychological Factors as Determinants of Medical Conditions. Front. Psychol. 2019, 10, 2502. [Google Scholar] [CrossRef]
- Tao, Y.; Kang, B.; Petkovich, D.; Bhandari, Y.R.; In, J.; Stein-O’Brien, G.; Kong, X.; Xie, W.; Zachos, N.; Maegawa, S.; et al. Aging-like Spontaneous Epigenetic Silencing Facilitates Wnt Activation, Stemness, and BrafV600E-Induced Tumorigenesis. Cancer Cell 2019, 35, 315–328.e6. [Google Scholar] [CrossRef]
- Nollet, M.; Wisden, W.; Franks, N.P. Sleep deprivation and stress: A reciprocal relationship. Interface Focus 2020, 10, 20190092. [Google Scholar] [CrossRef] [PubMed]
- Park, D.P.; Farrell, M.E. The Aging Mind in Transition: Amyloid Deposition and Progression toward Alzheimer’s Disease. In Handbook of the Psychology of Aging, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 5; pp. 87–103. [Google Scholar]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, A.; Lucca, U.; Menasce, G.; Bandera, L.; Cizza, G.; Forloni, G.; Tettamanti, M.; Frattura, L.; Tiraboschi, P.; Comelli, M.; et al. Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurology 1991, 41, 1726. [Google Scholar] [CrossRef] [PubMed]
- Kepka, A.; Ochocinska, A.; Borzym-Kluczyk, M.; Skorupa, E.; Stasiewicz-Jarocka, B.; Chojnowska, S.; Waszkiewicz, N. Preventive Role of L-Carnitine and Balanced Diet in Alzheimer’s Disease. Nutrients 2020, 12, 1987. [Google Scholar] [CrossRef]
- Nasca, C.; Watson-Lin, K.; Bigio, B.; Robakis, T.K.; Myoraku, A.; Wroolie, T.E.; McEwen, B.S.; Rasgon, N. Childhood trauma and insulin resistance in patients suffering from depressive disorders. Exp. Neurol. 2019, 315, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Cherix, A.; Larrieu, T.; Grosse, J.; Rodrigues, J.; McEwen, B.; Nasca, C.; Gruetter, R.; Sandi, C. Metabolic signature in nucleus accumbens for anti-depressant-like effects of acetyl-L-carnitine. eLife 2020, 9, e50631. [Google Scholar] [CrossRef] [PubMed]
- Nasca, C.; Menard, C.; Hodes, G.; Bigio, B.; Pena, C.; Lorsch, Z.; Zelli, D.; Ferris, A.; Kana, V.; Purushothaman, I.; et al. Multidimensional Predictors of Susceptibility and Resilience to Social Defeat Stress. Biol. Psychiatry 2019, 86, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; McKenna, M.C. l-Carnitine and Acetyl-l-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef]
- Cuccurazzu, B.; Bortolotto, V.; Valente, M.M.; Ubezio, F.; Koverech, A.; Canonico, P.L.; Grilli, M. Upregulation of mGlu2 Receptors via NF-κB p65 Acetylation Is Involved in the Proneurogenic and Antidepressant Effects of Acetyl-L-Carnitine. Neuropsychopharmacology 2013, 38, 2220–2230. [Google Scholar] [CrossRef]
- Nasca, C.; Bigio, B.; Lee, F.S.; Young, S.P.; Kautz, M.M.; Albright, A.; Beasley, J.; Millington, D.S.; Mathé, A.A.; Kocsis, J.H.; et al. Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc. Natl. Acad. Sci. USA 2018, 115, 8627–8632. [Google Scholar] [CrossRef]
- Bigio, B.; Mathé, A.A.; Sousa, V.C.; Zelli, D.; Svenningsson, P.; McEwen, B.S.; Nasca, C. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: Implications for treatment resistance. Proc. Natl. Acad. Sci. USA 2016, 113, 7906–7911. [Google Scholar] [CrossRef]
- Nasca, C.; Dobbin, J.; Bigio, B.; Watson, K.; de Angelis, P.; Kautz, M.; Cochran, A.; Mathé, A.A.; Kocsis, J.H.; Lee, F.S.; et al. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: On the path of creation of biosignatures of central insulin resistance. Mol. Psychiatry 2020, 26, 5140–5149. [Google Scholar] [CrossRef] [PubMed]
- Hullinger, R.; Puglielli, L. Molecular and cellular aspects of age-related cognitive decline and Alzheimer’s disease. Behav. Brain Res. 2017, 322, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.; Naselaris, T.; Holmes, E.A.; Kosslyn, S.M. Mental Imagery: Functional Mechanisms and Clinical Applications. Trends Cogn. Sci. 2015, 19, 590–602. [Google Scholar] [CrossRef]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Jylhävä, J.; Pedersen, N.L.; Hägg, S. Biological Age Predictors. EBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Collino, S.; Montoliu, I.; Martin, F.-P.; Scherer, M.; Mari, D.; Salvioli, S.; Bucci, L.; Ostan, R.; Monti, D.; Biagi, E.; et al. Metabolic Signatures of Extreme Longevity in Northern Italian Centenarians Reveal a Complex Remodeling of Lipids, Amino Acids, and Gut Microbiota Metabolism. PLoS ONE 2013, 8, e56564. [Google Scholar] [CrossRef]
- Slagboom, P.E.; Berg, N.V.D.; Deelen, J. Phenome and genome based studies into human ageing and longevity: An overview. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 2742–2751. [Google Scholar] [CrossRef]
- Levine, M.E. Assessment of Epigenetic Clocks as Biomarkers of Aging in Basic and Population Research. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2020, 75, 463–465. [Google Scholar] [CrossRef]
- Prieto, M.; Folci, A.; Martin, S. Post-translational modifications of the Fragile X Mental Retardation Protein in neuronal function and dysfunction. Mol. Psychiatry 2020, 25, 1688–1703. [Google Scholar] [CrossRef]
- Schaffert, L.-N.; Carter, W.G. Do Post-Translational Modifications Influence Protein Aggregation in Neurodegenerative Diseases: A Systematic Review. Brain Sci. 2020, 10, 232. [Google Scholar] [CrossRef]
- Kostich, M.; English, J.; Madison, V.; Gheyas, F.; Wang, L.; Qiu, P.; Greene, J.; Laz, T.M. Human members of the eukaryotic protein kinase family. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Martin, H.S.; Podolsky, K.A.; Devaraj, N.K. Probing the Role of Chirality in Phospholipid Membranes. ChemBioChem 2021, 22, 3148–3157. [Google Scholar] [CrossRef]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.A.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8, 998. [Google Scholar] [CrossRef]
- Jewell, J.L. RAG-ulating mTORC1 with amino acids. Nat. Rev. Mol. Cell Biol. 2021, 22, 587. [Google Scholar] [CrossRef]
- Yin, S.; Liu, L.; Gan, W. The Roles of Post-Translational Modifications on mTOR Signaling. Int. J. Mol. Sci. 2021, 22, 1784. [Google Scholar] [CrossRef] [PubMed]
- Maiole, F.; Giachero, S.; Fossati, S.M.; Rocchi, A.; Zullo, L. mTOR as a Marker of Exercise and Fatigue in Octopus vulgaris Arm. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.B.; Kemp, B.E. [3] Protein kinase phosphorylation site sequences and consensus specificity motifs: Tabulations. Methods Enzymol. 1991, 200, 62–81. [Google Scholar] [CrossRef]
- Hamelberg, D.; Shen, T.; McCammon, J.A. Phosphorylation Effects on cis/trans Isomerization and the Backbone Conformation of Serine−Proline Motifs: Accelerated Molecular Dynamics Analysis. J. Am. Chem. Soc. 2005, 127, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Ailshire, J.A.; Crimmins, E.M. Psychosocial Factors Associated with Longevity in the United States: Age Differences between the Old and Oldest-Old in the Health and Retirement Study. Behav. Factors Longev. 2011, 2011, 530534. [Google Scholar] [CrossRef] [PubMed]
- Cloos, P.A.C.; Christgau, S. Post-Translational Modifications of Proteins: Implications for Aging, Antigen Recognition, and Autoimmunity. Biogerontology 2004, 5, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.G.; Lowe, R.; Adams, P.D.; Baccarelli, A.A.; Beck, S.; Bell, J.T.; Christensen, B.C.; Gladyshev, V.N.; Heijmans, B.T.; Horvath, S.; et al. DNA methylation aging clocks: Challenges and recommendations. Genome Biol. 2019, 20, 1–24. [Google Scholar] [CrossRef]
- Aoki, M.; Blazek, E.; Vogt, P.K. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc. Natl. Acad. Sci. USA 2001, 98, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.B.; Prior, T.I.; Coutts, R.T. Chirality and drugs used to treat psychiatric disorders. J. Psychiatry Neurosci. 2002, 27, 401–403. [Google Scholar] [PubMed]
- Howland, R.H. Clinical Implications of Chirality and Stereochemistry in Psychopharmacology. J. Psychosoc. Nurs. Ment. Heal. Serv. 2009, 47, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Saganuwan, S.A. Chirality of Central Nervous System (CNS) Acting Drugs: A Formidable Therapeutic Hurdle against CNS Diseases. Central Nerv. Syst. Agents Med. Chem. 2019, 19, 171–179. [Google Scholar] [CrossRef]
- Hamczyk, M.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef]
- Johnson, F.; Sinclair, D.; Guarente, L. Molecular Biology of Aging. Cell 1999, 96, 291–302. [Google Scholar] [CrossRef]
- Burbank, P.M. Psychosocial theories of aging. Adv. Nurs. Sci. 1986, 9, 73–86. [Google Scholar] [CrossRef]
- Pedersen, J.B. The psychological process of ageing. What does it mean to get old? Nord. J. Psychiatry 1993, 47, 49–51. [Google Scholar] [CrossRef]
- Charles, S.T.; Carstensen, L.L. Social and Emotional Aging. Annu. Rev. Psychol. 2010, 61, 383–409. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Yankner, B.A. The Aging Stress Response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Dues, D.J.; Andrews, E.K.; Schaar, C.E.; Bergsma, A.L.; Senchuk, M.M.; Van Raamsdonk, J.M. Aging causes decreased resistance to multiple stresses and a failure to activate specific stress response pathways. Aging 2016, 8, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Yegorov, Y.; Poznyak, A.; Nikiforov, N.; Sobenin, I.; Orekhov, A. The Link between Chronic Stress and Accelerated Aging. Biomedicines 2020, 8, 198. [Google Scholar] [CrossRef]
- Kustubayeva, A.; Kamzanova, A.; Kudaibergenova, S.; Pivkina, V.; Matthews, G. Major Depression and Brain Asymmetry in a Decision-Making Task with Negative and Positive Feedback. Symmetry 2020, 12, 2118. [Google Scholar] [CrossRef]
- Gur, T.L.; Worly, B.L.; Bailey, M. Stress and the Commensal Microbiota: Importance in Parturition and Infant Neurodevelopment. Front. Psychiatry 2015, 6, 5. [Google Scholar] [CrossRef]
- Velten, J.; Lavallee, K.L.; Scholten, S.; Meyer, A.H.; Zhang, X.C.; Schneider, S.; Margraf, J. Lifestyle choices and mental health: A representative population survey. BMC Psychol. 2014, 2, 58. [Google Scholar] [CrossRef]
- Loriena, Y.; Aldwin, C.M. Does psychosocial stress accelerate the aging process? Aging Am. 2010, 2, 100–118. [Google Scholar]
- Simm, A.; Klotz, L.-O. Stress and biological aging: A double-edged sword. Z. Gerontol. Geriatr. 2015, 48, 505–510. [Google Scholar] [CrossRef]
- Robert, L.; Labat-Robert, J. Stress in biology and medicine, role in aging. Pathol. Biol. 2015, 63, 230–234. [Google Scholar] [CrossRef]
- Morey, J.N.; Boggero, I.A.; Scott, A.B.; Segerstrom, S. Current directions in stress and human immune function. Curr. Opin. Psychol. 2015, 5, 13–17. [Google Scholar] [CrossRef]
- Gues, P.C. Reviews on Biomarker Studies in Aging and Anti-Aging Research, 1st ed.; Springer: Singapore, 2019. [Google Scholar]
- Kashetti, S. Psychosocial Stress: A Cause towards Ageing. J. Gerontol. Geriatr. Res. 2020, 9, 532. [Google Scholar] [CrossRef]
- Mulligan, C.J. Early Environments, Stress, and the Epigenetics of Human Health. Annu. Rev. Anthr. 2016, 45, 233–249. [Google Scholar] [CrossRef]
- Cunliffe, V.T. The epigenetic impacts of social stress: How does social adversity become biologically embedded? Epigenomics 2016, 8, 1653–1669. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J. Alpha crystallin: The quest for a homogeneous quaternary structure. Exp. Eye Res. 2009, 88, 190–194. [Google Scholar] [CrossRef][Green Version]
- Hooi, M.Y.S.; Raftery, M.J.; Truscott, R.J.W. Age-dependent racemization of serine residues in a human chaperone protein. Protein Sci. 2013, 22, 93–100. [Google Scholar] [CrossRef]
- Uversky, V.N.; Oldfield, C.J.; Dunker, A.K. Intrinsically Disordered Proteins in Human Diseases: Introducing the D2 Concept. Annu. Rev. Biophys. 2008, 37, 215–246. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T. Conversion of psychological stress into cellular stress response: Roles of the sigma-1 receptor in the process. Psychiatry Clin. Neurosci. 2015, 69, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Tsankova, N.; Renthal, W.; Kumar, A.; Nestler, E.J. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 2007, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Zannas, A.S. Editorial Perspective: Psychological stress and epigenetic aging–what can we learn and how can we prevent? J. Child Psychol. Psychiatry 2016, 57, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Schiele, M.; Gottschalk, M.; Domschke, K. The applied implications of epigenetics in anxiety, affective and stress-related disorders–A review and synthesis on psychosocial stress, psychotherapy and prevention. Clin. Psychol. Rev. 2020, 77, 101830. [Google Scholar] [CrossRef] [PubMed]
- Ferracioli, N.G.M. Psychological aspects of aging and psychology’s contributions to gerontology: Theoretical and technical interface. MOJ Gerontol. Geriatr. 2018, 3, 1–2. [Google Scholar] [CrossRef][Green Version]
- Melnikov, S.V.; Khabibullina, N.F.; Mairhofer, E.; Vargas-Rodriguez, O.; Reynolds, N.M.; Micura, R.; Soll, D.; Yury, S. Polikanov 2,Mechanistic insights into the slow peptide bond formation with D-amino acids in the ribosomal active site. Nucleic Acids Res. 2019, 47, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Nanda, V.; Andrianarijaona, A.; Narayanan, C. The role of protein homochirality in shaping the energy landscape of folding. Protein Sci. 2007, 16, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ramakrishnan, V.; Ranbhor, R.; Patel, K.; Durani, S. Homochiral Stereochemistry: The Missing Link of Structure to Energetics in Protein Folding. J. Phys. Chem. B 2009, 113, 16435–16442. [Google Scholar] [CrossRef]
- Grishin, D.V.; Zhdanov, D.D.; Pokrovskaya, M.V.; Sokolov, N.N. D-amino acids in nature, agriculture and biomedicine. All Life 2020, 13, 11–22. [Google Scholar] [CrossRef]
- Fichtner, M.; Schuster, S.; Stark, H. Determination of scoring functions for protein damage susceptibility. Biosystems 2019, 187, 104035. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C.; Dressel, C. Mirror Symmetry Breaking in Liquids and Their Impact on the Development of Homochirality in Abiogenesis: Emerging Proto-RNA as Source of Biochirality? Symmetry 2020, 12, 1098. [Google Scholar] [CrossRef]
- Fujii, N.; Kaji, Y.; Nakamura, T.; Motoie, R.; Mori, Y.; Kinouchi, T. Collapse of Homochirality of Amino Acids in Proteins from Various Tissues during Aging. Chem. Biodivers. 2010, 7, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, A.R.; Markwardt, S.; Thielke, S.; Rostant, O.; Vásquez, E.; Botoseneanu, A. Prospective Disability in Different Combinations of Somatic and Mental Multimorbidity. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2018, 73, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Han, L.K.; Verhoeven, J.E.; Aberg, K.A.; Oord, E.C.V.D.; Milaneschi, Y.; Penninx, B.W. An integrative study of five biological clocks in somatic and mental health. eLife 2021, 10, e59479. [Google Scholar] [CrossRef] [PubMed]
- Carrard, J.; Gallart-Ayala, H.; Infanger, D.; Teav, T.; Wagner, J.; Knaier, R.; Colledge, F.; Streese, L.; Königstein, K.; Hinrichs, T.; et al. Metabolic View on Human Healthspan: A Lipidome-Wide Association Study. Metabolites 2021, 11, 287. [Google Scholar] [CrossRef]
- Mitina, M.; Young, S.; Zhavoronkov, A. Psychological aging, depression, and well-being. Aging 2020, 12, 18765–18777. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyakin, V.V.; Dyakina-Fagnano, N.V.; Mcintire, L.B.; Uversky, V.N. Fundamental Clock of Biological Aging: Convergence of Molecular, Neurodegenerative, Cognitive and Psychiatric Pathways: Non-Equilibrium Thermodynamics Meet Psychology. Int. J. Mol. Sci. 2022, 23, 285. https://doi.org/10.3390/ijms23010285
Dyakin VV, Dyakina-Fagnano NV, Mcintire LB, Uversky VN. Fundamental Clock of Biological Aging: Convergence of Molecular, Neurodegenerative, Cognitive and Psychiatric Pathways: Non-Equilibrium Thermodynamics Meet Psychology. International Journal of Molecular Sciences. 2022; 23(1):285. https://doi.org/10.3390/ijms23010285
Chicago/Turabian StyleDyakin, Victor V., Nuka V. Dyakina-Fagnano, Laura B. Mcintire, and Vladimir N. Uversky. 2022. "Fundamental Clock of Biological Aging: Convergence of Molecular, Neurodegenerative, Cognitive and Psychiatric Pathways: Non-Equilibrium Thermodynamics Meet Psychology" International Journal of Molecular Sciences 23, no. 1: 285. https://doi.org/10.3390/ijms23010285
APA StyleDyakin, V. V., Dyakina-Fagnano, N. V., Mcintire, L. B., & Uversky, V. N. (2022). Fundamental Clock of Biological Aging: Convergence of Molecular, Neurodegenerative, Cognitive and Psychiatric Pathways: Non-Equilibrium Thermodynamics Meet Psychology. International Journal of Molecular Sciences, 23(1), 285. https://doi.org/10.3390/ijms23010285







