Janus Kinase Inhibitors Ameliorated Gastrointestinal Amyloidosis and Hypoalbuminemia in Persistent Dermatitis Mouse Model
Abstract
:1. Introduction
2. Results
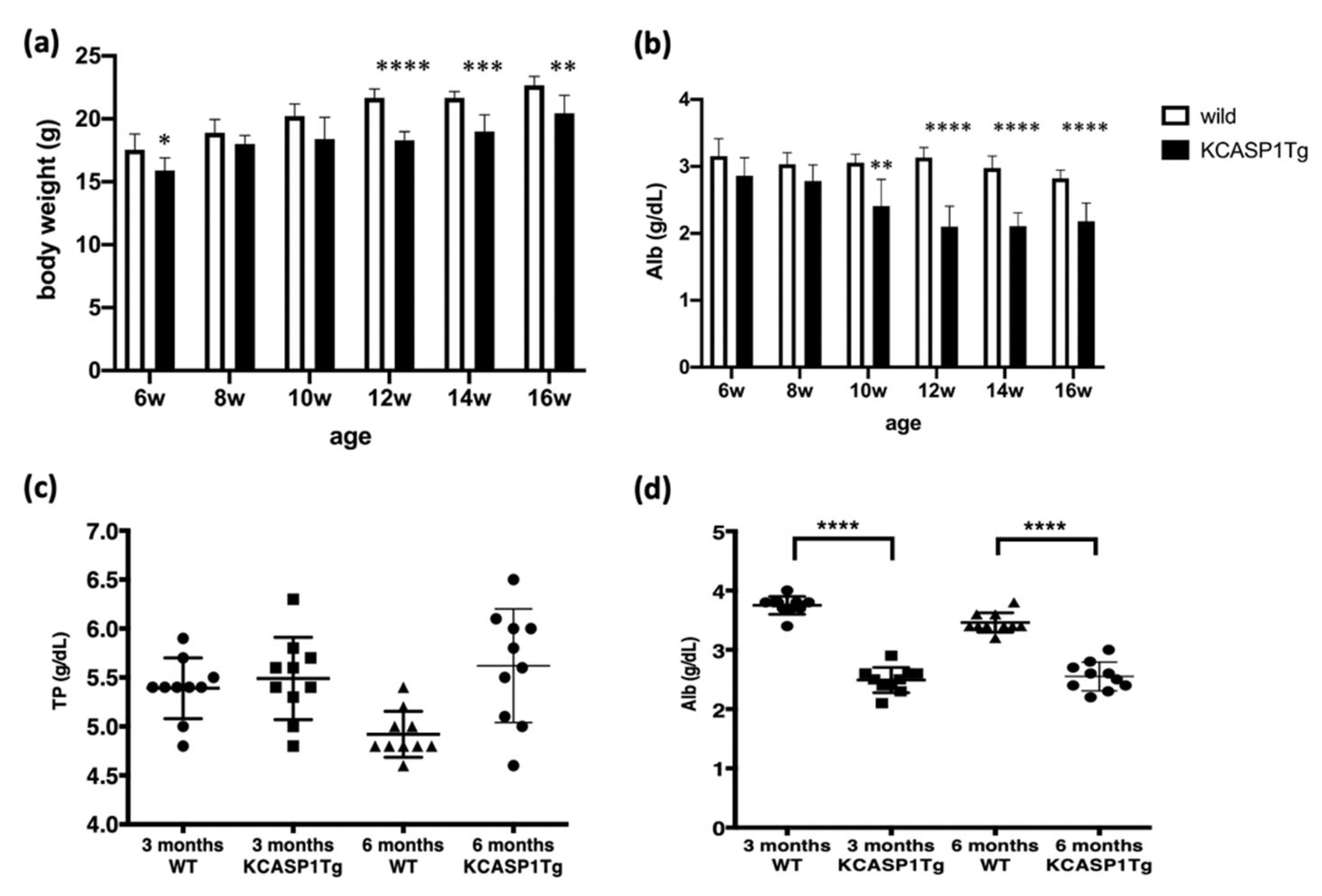
2.1. Human Caspase-1 Gene with Keratin 14 Promoter (KCASP1Tg) Mouse Showed Emaciation
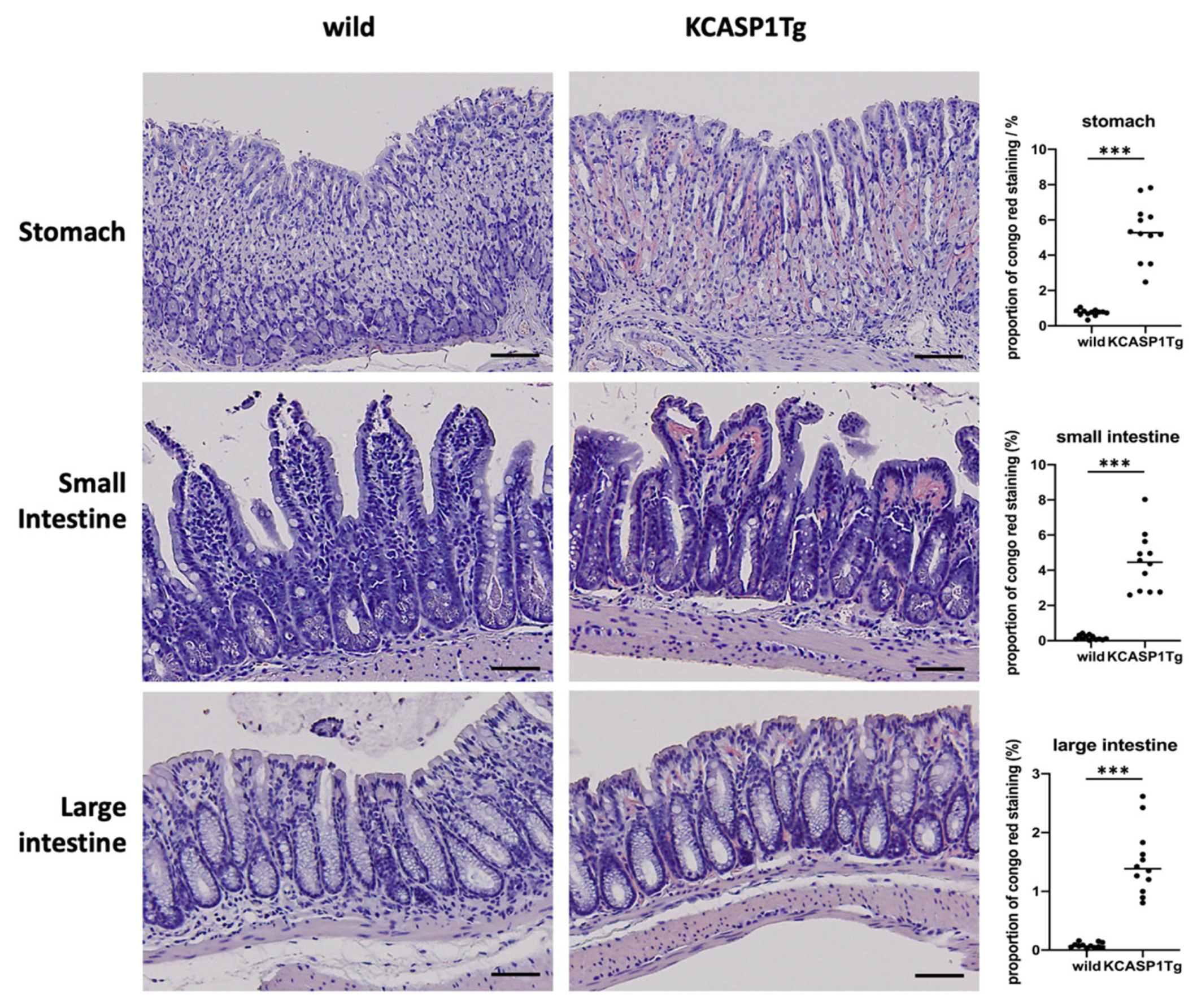
2.2. Histological Analysis Showed Amyloid Deposition in the KCASP1Tg Mice
2.3. Gastrointestinal Tract Barrier Function
2.4. SAA Levels in Tissue and Plasma
2.5. The Effect of Anti-TNF-α and IL-1α/β in the KCASP1Tg Mice
2.6. The Effect of Administration of Janus Kinase (JAK) Inhibitors in KCASP1Tg Mice
3. Discussion
4. Materials and Methods
4.1. Mouse Study
4.2. Blood Sampling and Clinical Chemistry Parameters
4.3. Gastrointestinal Tract Sampling and Congo-Red Stain
4.4. Gastrointestinal Tract Barrier Function
4.5. Real-Time Polymerase Chain Reaction (Real-Time PCR) and ELISA for SAA
4.6. Administration of Anti-TNF-α and IL-1α/β in the KCASP1Tg Mice
4.7. Oral Administration of JAK Inhibitors
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Real de Asua, D.; Costa, R.; Galvan, J.M.; Filigheddu, M.T.; Trujillo, D.; Cadinanos, J. Systemic AA amyloidosis: Epidemiology, diagnosis, and management. Clin. Epidemiol. 2014, 6, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Couderc, E.; Morel, F.; Levillain, P.; Buffiere-Morgado, A.; Camus, M.; Paquier, C.; Bodet, C.; Jegou, J.F.; Pohin, M.; Favot, L.; et al. Interleukin-17A-induced production of acute serum amyloid A by keratinocytes contributes to psoriasis pathogenesis. PLoS ONE 2017, 12, e0181486. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sugaya, M.; Nakajima, R.; Oka, T.; Takahashi, N.; Nakao, M.; Miyagaki, T.; Asano, Y.; Sato, S. Serum amyloid A levels in the blood of patients with atopic dermatitis and cutaneous T-cell lymphoma. J. Dermatol. 2018, 45, 1440–1443. [Google Scholar] [CrossRef]
- Yamanaka, K.; Nakanishi, T.; Saito, H.; Maruyama, J.; Isoda, K.; Yokochi, A.; Imanaka-Yoshida, K.; Tsuda, K.; Kakeda, M.; Okamoto, R.; et al. Persistent release of IL-1s from skin is associated with systemic cardio-vascular disease, emaciation and systemic amyloidosis: The potential of anti-IL-1 therapy for systemic inflammatory diseases. PLoS ONE 2014, 9, e104479. [Google Scholar] [CrossRef]
- Kato, S.; Matsushima, Y.; Mizutani, K.; Kawakita, F.; Fujimoto, M.; Okada, K.; Kondo, M.; Habe, K.; Suzuki, H.; Mizutani, H.; et al. The Stenosis of Cerebral Arteries and Impaired Brain Glucose Uptake by Long-Lasting Inflammatory Cytokine Release from Dermatitis Is Rescued by Anti-IL-1 Therapy. J. Investig. Dermatol. 2018, 138, 2280–2283. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, K.; Tanaka, M.; Tsutsui, H.; Kupper, T.S.; Asahi, K.; Okamura, H.; Nakanishi, K.; Suzuki, M.; Kayagaki, N.; Black, R.A.; et al. Skin-specific caspase-1-transgenic mice show cutaneous apoptosis and pre-endotoxin shock condition with a high serum level of IL-18. J. Immunol. 2000, 165, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, K.; Shirakami, E.; Ichishi, M.; Matsushima, Y.; Umaoka, A.; Okada, K.; Yamaguchi, Y.; Watanabe, M.; Morita, E.; Yamanaka, K. Systemic Dermatitis Model Mice Exhibit Atrophy of Visceral Adipose Tissue and Increase Stromal Cells via Skin-Derived Inflammatory Cytokines. Int. J. Mol. Sci. 2020, 21, 3367. [Google Scholar] [CrossRef]
- Andersen, Y.M.F.; Egeberg, A.; Gislason, G.H.; Hansen, P.R.; Skov, L.; Thyssen, J.P. Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 310–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascott, A.; Mulick, A.; Yu, A.M.; Prieto-Merino, D.; Schmidt, M.; Abuabara, K.; Smeeth, L.; Roberts, A.; Langan, S.M. Atopic eczema and major cardiovascular outcomes: A systematic review and meta-analysis of population-based studies. J. Allergy Clin. Immunol. 2019, 143, 1821–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.C.; Lan, C.E. Psoriasis and Cardiovascular Comorbidities: Focusing on Severe Vascular Events, Cardiovascular Risk Factors and Implications for Treatment. Int. J. Mol. Sci. 2017, 18, 2211. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, J.M.; Dommasch, E.D.; Shin, D.B.; Azfar, R.S.; Kurd, S.K.; Wang, X.; Troxel, A.B. The risk of stroke in patients with psoriasis. J. Investig. Dermatol. 2009, 129, 2411–2418. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, K.; Isono, K.; Matsushima, Y.; Okada, K.; Umaoka, A.; Iida, S.; Habe, K.; Hagimori, K.; Yamazaki, H.; Yamanaka, K. Inflammatory Skin-Derived Cytokines Accelerate Osteoporosis in Mice with Persistent Skin Inflammation. Int. J. Mol. Sci. 2020, 21, 3620. [Google Scholar] [CrossRef] [PubMed]
- Umaoka, A.; Takeuchi, H.; Mizutani, K.; Seo, N.; Matsushima, Y.; Habe, K.; Hagimori, K.; Yamaguchi, Y.; Ikeda, T.; Yamanaka, K. Skin Inflammation and Testicular Function: Dermatitis Causes Male Infertility via Skin-Derived Cytokines. Biomedicines 2020, 8, 293. [Google Scholar] [CrossRef]
- Yamanaka, K.; Mizutani, H. “Inflammatory skin march”: IL-1—mediated skin inflammation, atopic dermatitis, and psoriasis to cardiovascular events. J. Allergy Clin. Immunol. 2015, 136, 823–824. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.B.; Aherne, C.M.; Ehrentraut, S.F.; Gerich, M.E.; McNamee, E.N.; McManus, M.C.; Lebsack, M.D.; Jedlicka, P.; Azam, T.; de Zoeten, E.F.; et al. Alpha-1-antitrypsin therapy ameliorates acute colitis and chronic murine ileitis. Inflamm. Bowel Dis. 2013, 19, 1964–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Ahsan, M.H.; Purchio, A.F.; West, D.B. Serum amyloid A-luciferase transgenic mice: Response to sepsis, acute arthritis, and contact hypersensitivity and the effects of proteasome inhibition. J. Immunol. 2005, 174, 8125–8134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlar, C.M.; Whitehead, A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999, 265, 501–523. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta. 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Y.; Wu, L.; Xiao, S.; Ji, Y.; Tan, Y.; Jiang, C.; Zhang, G. Dysregulation of the gut-brain-skin axis and key overlapping inflammatory and immune mechanisms of psoriasis and depression. Biomed. Pharmacother. 2021, 137, 111065. [Google Scholar] [CrossRef]
- Okada, K.; Matsushima, Y.; Mizutani, K.; Yamanaka, K. The Role of Gut Microbiome in Psoriasis: Oral Administration of Staphylococcus aureus and Streptococcus danieliae Exacerbates Skin Inflammation of Imiquimod-Induced Psoriasis-Like Dermatitis. Int. J. Mol. Sci. 2020, 21, 3303. [Google Scholar] [CrossRef]
- Furuya, Y.; Mori, K.; Ninomiya, T.; Tomimori, Y.; Tanaka, S.; Takahashi, N.; Udagawa, N.; Uchida, K.; Yasuda, H. Increased bone mass in mice after single injection of anti-receptor activator of nuclear factor-kappaB ligand-neutralizing antibody: Evidence for bone anabolic effect of parathyroid hormone in mice with few osteoclasts. J. Biol. Chem. 2011, 286, 37023–37031. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, T.; Mizutani, K.; Iida, S.; Matsushima, Y.; Umaoka, A.; Kondo, M.; Habe, K.; Yamanaka, K. Janus Kinase Inhibitors Ameliorated Gastrointestinal Amyloidosis and Hypoalbuminemia in Persistent Dermatitis Mouse Model. Int. J. Mol. Sci. 2022, 23, 28. https://doi.org/10.3390/ijms23010028
Nakanishi T, Mizutani K, Iida S, Matsushima Y, Umaoka A, Kondo M, Habe K, Yamanaka K. Janus Kinase Inhibitors Ameliorated Gastrointestinal Amyloidosis and Hypoalbuminemia in Persistent Dermatitis Mouse Model. International Journal of Molecular Sciences. 2022; 23(1):28. https://doi.org/10.3390/ijms23010028
Chicago/Turabian StyleNakanishi, Takehisa, Kento Mizutani, Shohei Iida, Yoshiaki Matsushima, Ai Umaoka, Makoto Kondo, Koji Habe, and Keiichi Yamanaka. 2022. "Janus Kinase Inhibitors Ameliorated Gastrointestinal Amyloidosis and Hypoalbuminemia in Persistent Dermatitis Mouse Model" International Journal of Molecular Sciences 23, no. 1: 28. https://doi.org/10.3390/ijms23010028
APA StyleNakanishi, T., Mizutani, K., Iida, S., Matsushima, Y., Umaoka, A., Kondo, M., Habe, K., & Yamanaka, K. (2022). Janus Kinase Inhibitors Ameliorated Gastrointestinal Amyloidosis and Hypoalbuminemia in Persistent Dermatitis Mouse Model. International Journal of Molecular Sciences, 23(1), 28. https://doi.org/10.3390/ijms23010028





