The AEROPILs Generation: Novel Poly(Ionic Liquid)-Based Aerogels for CO2 Capture
Abstract
:1. Introduction
2. Results and Discussion
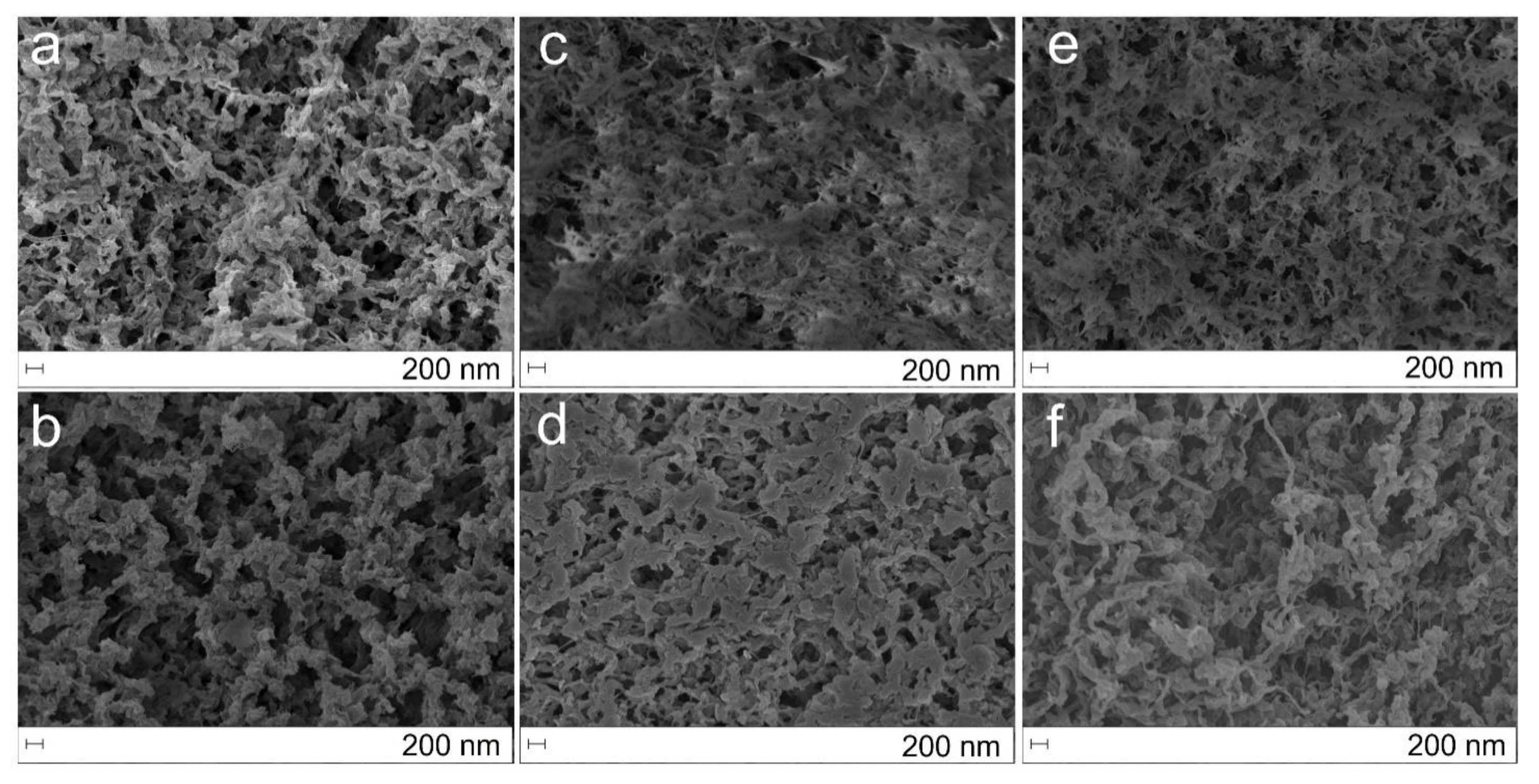
2.1. Morphological and Textural Properties of the Chitosan Aerogels
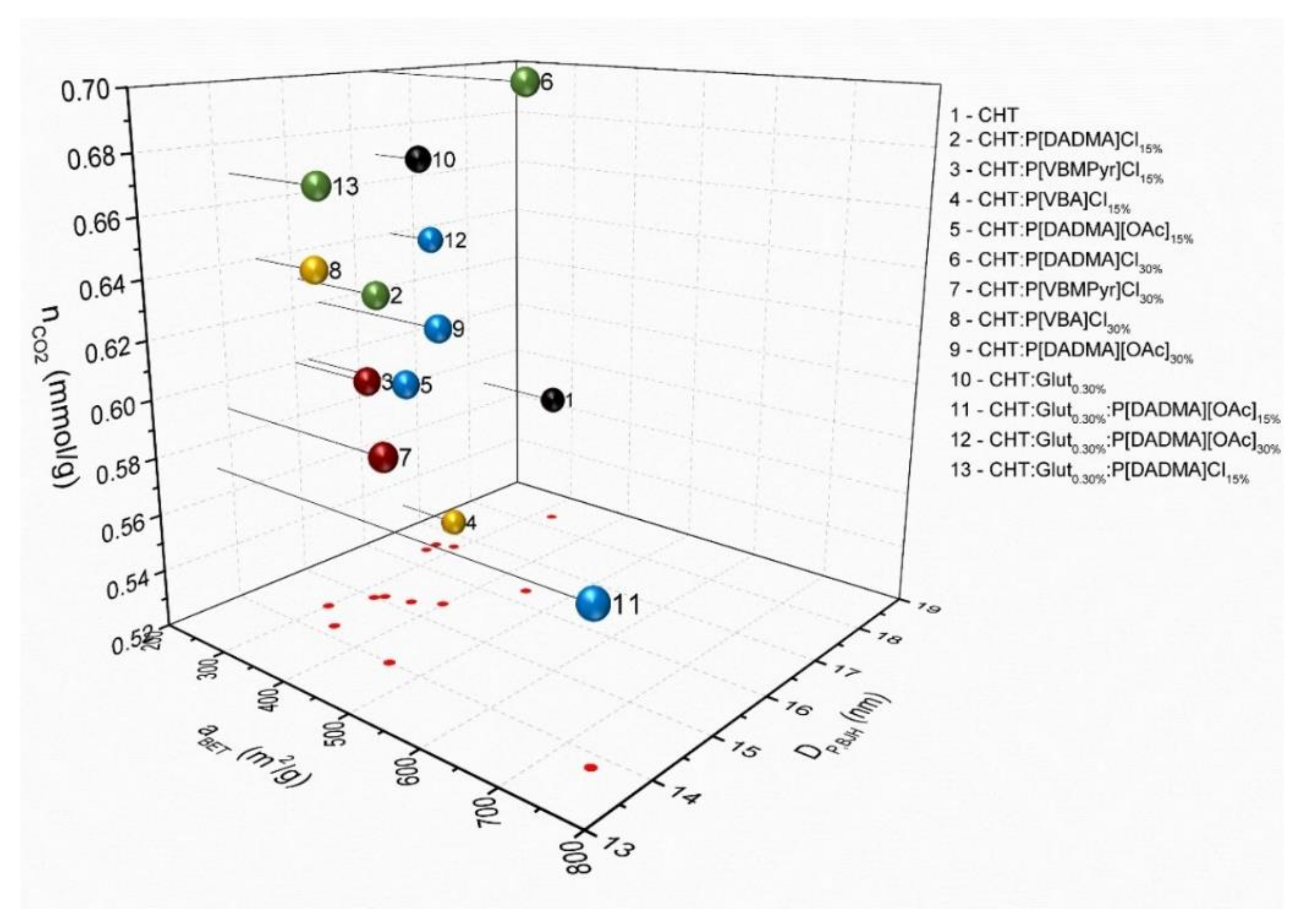
2.2. CO2 Capture
3. Materials and Methods
3.1. Materials
3.2. IL and PIL Synthesis
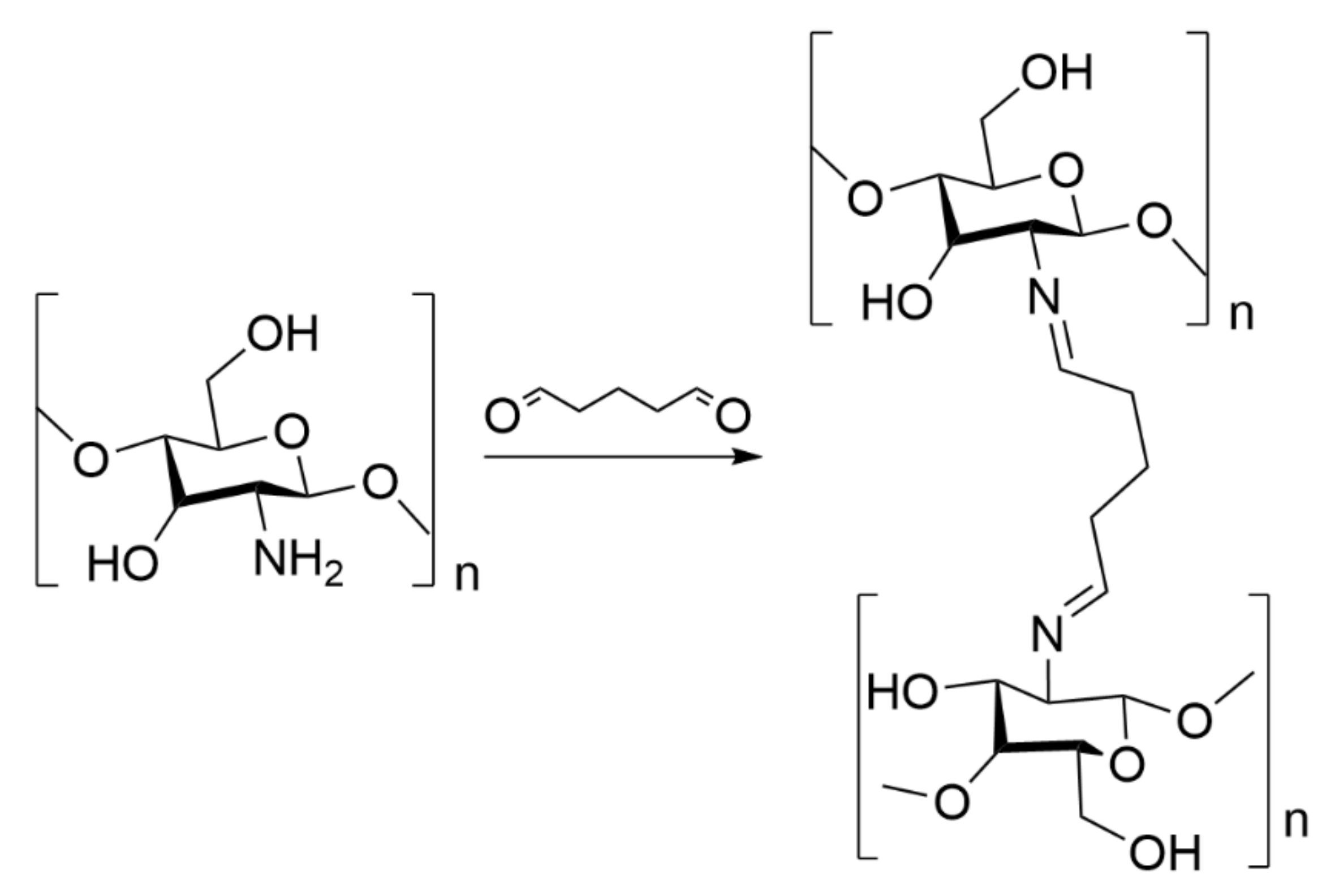
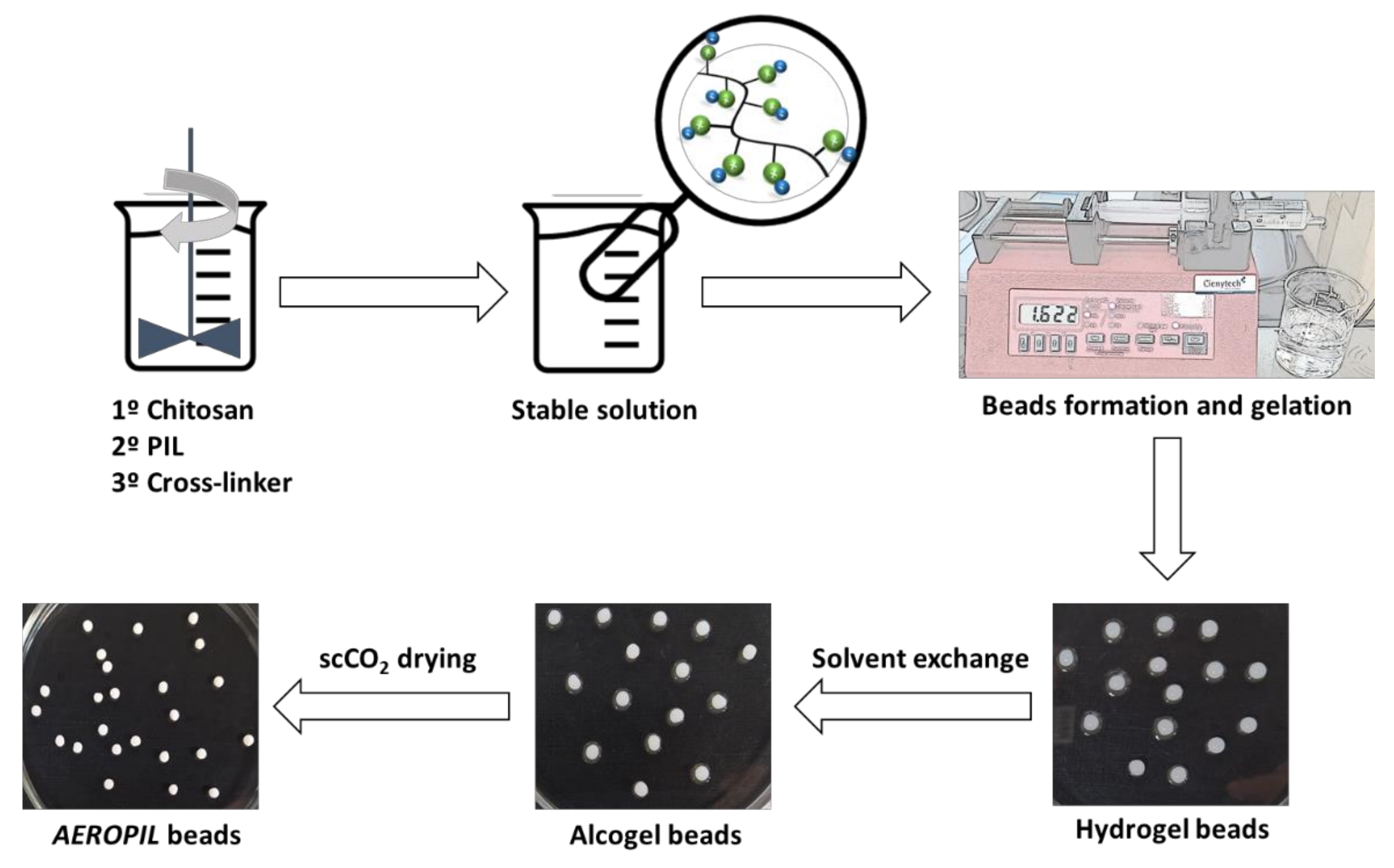
3.3. Preparation of Chitosan Aerogel Beads
3.3.1. Preparation of Chitosan Hydrogel
3.3.2. Solvent Exchange
3.3.3. Supercritical Extraction of the Gel Solvent
3.4. Morphology and Textural Properties
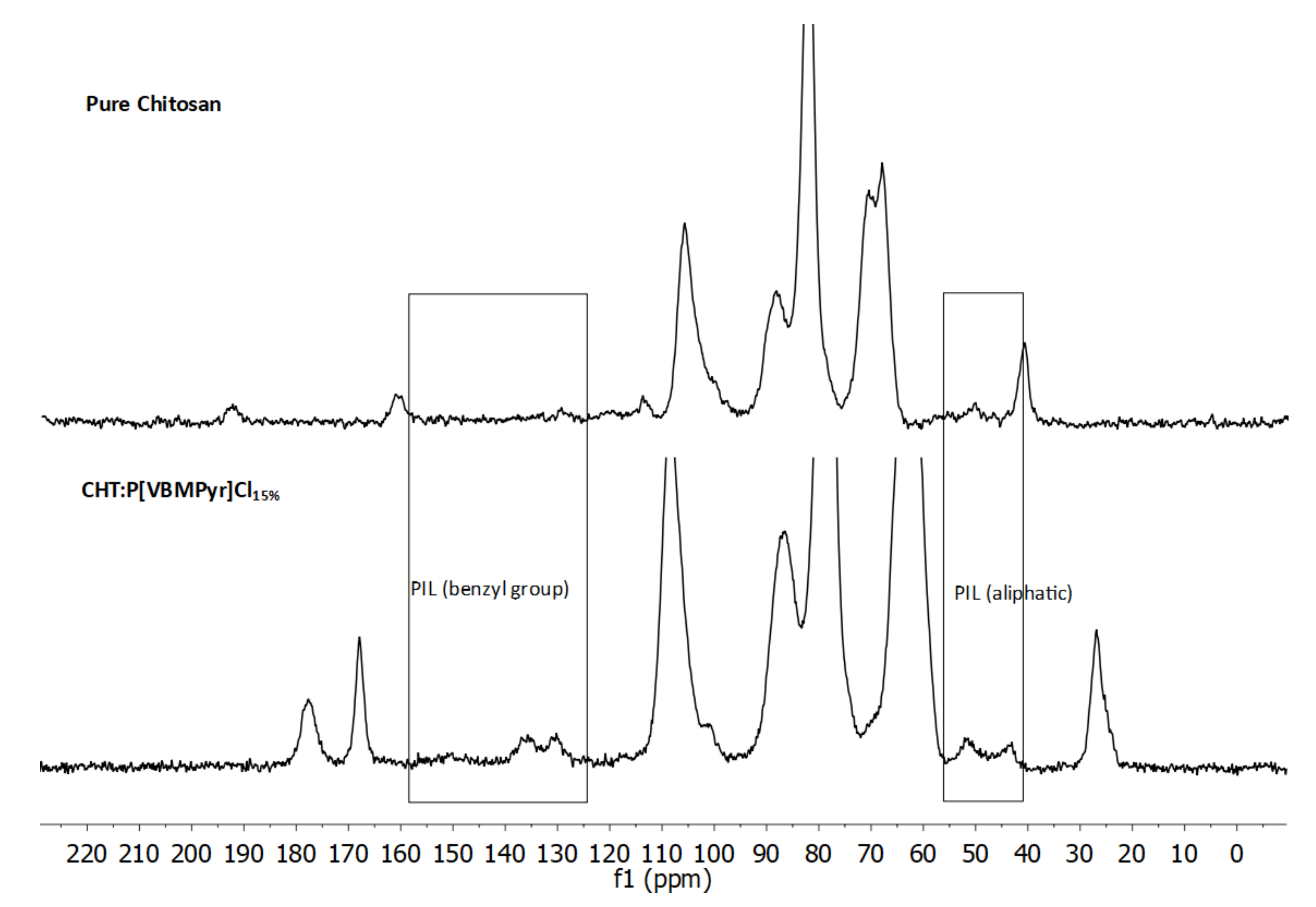
3.5. Solid-State NMR Spectroscopy
3.6. Thermogravimetric Analysis and CO2 Capture Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spigarelli, B.P.; Kawatra, S.K. Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 2013, 1, 69–87. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Zhou, X.; Weber, J.; Yuan, J. Poly(ionic liquid)s: Platform for CO2 capture and catalysis. Curr. Opin. Green Sustain. Chem. 2019, 16, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Simon, N.M.; Zanatta, M.; Neumann, J.; Girard, A.-L.; Marin, G.; Stassen, H.; Dupont, J. Cation−Anion−CO2 Interactions in Imidazolium-Based Ionic Liquid Sorbents. ChemPhysChem 2018, 19, 2879–2884. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasulu, B.; Gayatri, D.; Sreedhar, I.; Raghavan, K. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Gurkan, B.E. Ionic Liquids for CO2 Capture and Emission Reduction. J. Phys. Chem. Lett. 2010, 1, 3459–3464. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef] [PubMed]
- Barrulas, R.V.; Zanatta, M.; Casimiro, T.; Corvo, M.C. Advanced porous materials from poly(ionic liquid)s: Challenges, applications and opportunities. Chem. Eng. J. 2021, 411, 128528. [Google Scholar] [CrossRef]
- Qian, L.; Yang, M.; Chen, H.; Xu, Y.; Zhang, S.; Zhou, Q.; He, B.; Bai, Y.; Song, W. Preparation of a poly(ionic liquid)-functionalized cellulose aerogel and its application in protein enrichment and separation. Carbohydr. Polym. 2019, 218, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Miao, Y.; Luo, H.; Pudukudy, M.; Zhi, Y.; Zhao, W.; Shan, S.; Jia, Q.; Ni, Y. CO2 capture performance and characterization of cellulose aerogels synthesized from old corrugated containers. Carbohydr. Polym. 2020, 227, 115380. [Google Scholar] [CrossRef]
- Soorbaghi, F.P.; Isanejad, M.; Salatin, S.; Ghorbani, M.; Jafari, S.; Derakhshankhah, H. Bioaerogels: Synthesis approaches, cellular uptake, and the biomedical applications. Biomed. Pharmacother. 2019, 111, 964–975. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Rana, A.; Sharma, G.; Sharma, S.; Naushad, M.; Mola, G.T.; Dhiman, P.; Stadler, F.J. Aerogels and metal-organic frameworks for environmental remediation and energy production. Environ. Chem. Lett. 2018, 16, 797–820. [Google Scholar] [CrossRef]
- Keshavarz, L.; Ghaani, M.R.; English, N.J. The Importance of Precursors and Modification Groups of Aerogels in CO2 Capture. Molecules 2021, 26, 5023. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, L.; Ghaani, M.R.; MacElroy, J.D.; English, N.J. A comprehensive review on the application of aerogels in CO2-adsorption: Materials and characterisation. Chem. Eng. J. 2021, 412, 128604. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Cui, S.; Cheng, W.; Shen, X.; Fan, M.; Russell, A.; Wu, Z.; Yi, X. Mesoporous amine-modified SiO2 aerogel: A potential CO2 sorbent. Energy Environ. Sci. 2011, 4, 2070–2074. [Google Scholar] [CrossRef]
- Linneen, N.; Pfeffer, R.; Lin, Y. CO2 capture using particulate silica aerogel immobilized with tetraethylenepentamine. Microporous Mesoporous Mater. 2013, 176, 123–131. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Estevez, L.; Duan, X.; Anako, N.; Park, A.-H.A.; Li, W.; Jones, C.W.; Giannelis, E.P. High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. Energy Environ. Sci. 2011, 4, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Thomas, A.; Liao, Y. Ultra-High Surface Area Nitrogen-Doped Carbon Aerogels Derived From a Schiff-Base Porous Organic Polymer Aerogel for CO2 Storage and Supercapacitors. Adv. Funct. Mater. 2019, 29, 1904785. [Google Scholar] [CrossRef]
- Liu, K.-K.; Jin, B.; Meng, L.-Y. Glucose/Graphene-Based Aerogels for Gas Adsorption and Electric Double Layer Capacitors. Polymers 2018, 11, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.; Le, V.-D.; Maiti, U.N.; Hwang, J.O.; Park, W.J.; Lim, J.; Lee, K.E.; Bae, Y.-S.; Kim, Y.-H.; Kim, S.O. Selective and Regenerative Carbon Dioxide Capture by Highly Polarizing Porous Carbon Nitride. ACS Nano 2015, 9, 9148–9157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, Y.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydr. Polym. 2019, 226, 115242. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Thakur, S.; Goel, G.; Raj, J.; Gupta, V.K.; Roberts, D.; Thakur, V.K. Bio-based sustainable aerogels: New sensation in CO2 capture. Curr. Res. Green Sustain. Chem. 2020, 3, 100027. [Google Scholar] [CrossRef]
- Sneddon, G.; Ganin, A.Y.; Yiu, H.H.P. Sustainable CO2 Adsorbents Prepared by Coating Chitosan onto Mesoporous Silicas for Large-Scale Carbon Capture Technology. Energy Technol. 2015, 3, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Hsan, N.; Dutta, P.K.; Kumar, S.; Bera, R.; Das, N. Chitosan grafted graphene oxide aerogel: Synthesis, characterization and carbon dioxide capture study. Int. J. Biol. Macromol. 2019, 125, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan hybrid aerogels with superior adsorption: Role of graphene oxide in CO2 capture. RSC Adv. 2013, 3, 16011. [Google Scholar] [CrossRef]
- Song, J.; Liu, J.; Zhao, W.; Chen, Y.; Xiao, H.; Shi, X.; Liu, Y.; Chen, X. Quaternized Chitosan/PVA Aerogels for Reversible CO2 Capture from Ambient Air. Ind. Eng. Chem. Res. 2018, 57, 4941–4948. [Google Scholar] [CrossRef]
- Kumar, S.; de Silva, J.A.E.; Wani, M.Y.; Gil, J.; Sobral, A.J. Carbon dioxide capture and conversion by an environmentally friendly chitosan based meso-tetrakis(4-sulfonatophenyl) porphyrin. Carbohydr. Polym. 2017, 175, 575–583. [Google Scholar] [CrossRef]
- Ding, L.-G.; Yao, B.-J.; Li, F.; Shi, S.-C.; Huang, N.; Yin, H.-B.; Guan, Q.; Dong, Y.-B. Ionic liquid-decorated COF and its covalent composite aerogel for selective CO2 adsorption and catalytic conversion. J. Mater. Chem. A 2019, 7, 4689–4698. [Google Scholar] [CrossRef]
- Pohako-Esko, K.; Bahlmann, M.; Schulz, P.S.; Wasserscheid, P. Chitosan Containing Supported Ionic Liquid Phase Materials for CO2 Absorption. Ind. Eng. Chem. Res. 2016, 55, 7052–7059. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Chen, N.; Dai, S.; Jiang, H.; Wang, S. Effects of amine loading on the properties of cellulose nanofibrils aerogel and its CO2 capturing performance. Carbohydr. Polym. 2018, 194, 252–259. [Google Scholar] [CrossRef]
- Zhu, W.; Yao, Y.; Zhang, Y.; Jiang, H.; Wang, Z.; Chen, W.; Xue, Y. Preparation of an Amine-Modified Cellulose Nanocrystal Aerogel by Chemical Vapor Deposition and Its Application in CO2 Capture. Ind. Eng. Chem. Res. 2020, 59, 16660–16668. [Google Scholar] [CrossRef]
- Wei, J.; Geng, S.; Hedlund, J.; Oksman, K. Lightweight, flexible, and multifunctional anisotropic nanocellulose-based aerogels for CO2 adsorption. Cellulose 2020, 27, 2695–2707. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jia, P.; Xu, J.; Wu, Y.; Jiang, H.; Li, Z. The Aminosilane Functionalization of Cellulose Nanofibrils and the Mechanical and CO2 Adsorption Characteristics of Their Aerogel. Ind. Eng. Chem. Res. 2020, 59, 2874–2882. [Google Scholar] [CrossRef]
- Wang, C.; Okubayashi, S. Polyethyleneimine-crosslinked cellulose aerogel for combustion CO2 capture. Carbohydr. Polym. 2019, 225, 115248. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Feng, J.; Han, S.; Ji, X.; Li, C.; Feng, J.; Sun, H.; Fan, J. Poly(ionic liquid)-hybridized silica aerogel for solid-phase microextraction of polycyclic aromatic hydrocarbons prior to gas chromatography-flame ionization detection. Microchim. Acta 2021, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Concilio, S.; Cardea, S.; de Marco, I.; Reverchon, E. Complete glutaraldehyde elimination during chitosan hydrogel drying by SC-CO2 processing. J. Supercrit. Fluids 2015, 103, 70–76. [Google Scholar] [CrossRef]
- Mirzaei, B.E.; Ramazani, A.; Shafiee, M.; Danaei, M. Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 605–611. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.; Alvarez-Lorenzo, C.; Gomez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Runge, T.; Wang, L.; Li, R.; Feng, J.; Shu, X.-L.; Shi, Q.-S. Hydrogen bonding impact on chitosan plasticization. Carbohydr. Polym. 2018, 200, 115–121. [Google Scholar] [CrossRef]
- Ayral, A.; Phalippou, J.; Woignier, T. Skeletal density of silica aerogels determined by helium pycnometry. J. Mater. Sci. 1992, 27, 1166–1170. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Leng, C.H.; Razali, M.A.A.; Rosdi, M.R.H.; Ariffin, A. Composite flocculants based on magnesium salt–polydiallyldimethylammonium chloride: Characterization and flocculation behaviour. RSC Adv. 2015, 5, 53462–53470. [Google Scholar] [CrossRef]
- Sun, L.; Luo, J.; Gao, M.; Tang, S. Bi-functionalized ionic liquid porous copolymers for CO2 adsorption and conversion under ambient pressure. React. Funct. Polym. 2020, 154, 104636. [Google Scholar] [CrossRef]
- Eftaiha, A.F.; Qaroush, A.K.; Hasan, A.K.; Assaf, K.I.; Al-Qaisi, F.M.; Melhem, M.E.; Al-Maythalony, B.A.; Usman, M. Cross-linked, porous imidazolium-based poly(ionic liquid)s for CO2 capture and utilisation. New J. Chem. 2021, 45, 16452–16460. [Google Scholar] [CrossRef]
- Kildeeva, N.R.; Perminov, P.A.; Vladimirov, L.V.; Novikov, V.V.; Mikhailov, S.N. About mechanism of chitosan cross-linking with glutaraldehyde. Russ. J. Bioorg. Chem. 2009, 35, 360–369. [Google Scholar] [CrossRef]
- Xie, J.-N.; Yu, B.; Zhou, Z.-H.; Fu, H.-C.; Wang, N.; He, L.-N. Copper(I)-based ionic liquid-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure. Tetrahedron Lett. 2015, 56, 7059–7062. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sun, W.; Radosz, M.; Shen, Y. Low-pressure CO2 sorption in ammonium-based poly(ionic liquid)s. Polymer 2005, 46, 12460–12467. [Google Scholar] [CrossRef]
- Zajac, A.; Szpecht, A.; Zielinski, D.; Rola, K.; Hoppe, J.; Komorowska, K.; Smiglak, M. Synthesis and characterization of potentially polymerizable amine-derived ionic liquids bearing 4-vinylbenzyl group. J. Mol. Liq. 2019, 283, 427–439. [Google Scholar] [CrossRef]
- Mogha, N.K.; Yadav, N.; Sindhu, A.; Venkatesu, P. Does poly(ionic liquid) modulate the non-covalent interactions of chicken egg white lysozyme? Elucidation of biomolecular interactions between biomolecules and macromolecular solvents. New J. Chem. 2019, 43, 16759–16766. [Google Scholar] [CrossRef]
- Zanatta, M.; Lopes, M.; Cabrita, E.J.; Bernardes, C.E.; Corvo, M.C. Handling CO2 sorption mechanism in PIL@IL composites. J. CO2 Util. 2020, 41, 101225. [Google Scholar] [CrossRef]
- Zanatta, M.; Girard, A.-L.; Marin, G.; Ebeling, G.; dos Santos, F.P.; Valsecchi, C.; Stassen, H.; Livotto, P.R.; Lewis, W.; Dupont, J. Confined water in imidazolium based ionic liquids: A supramolecular guest@host complex case. Phys. Chem. Chem. Phys. 2016, 18, 18297–18304. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.; Camino-Rey, M.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/solid Systems with Special Reference to the Determination of Surface area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]


| Entry | Material | Modifications | nCO2 (mmol g−1) | SBET (m2/g) | P (bar) | T (°C) | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | Pure chitosan | - | 0.02 | nd | nd | nd | [30] |
| 2 | CHT-GO aerogels | CHT grafted GO | 0.26 | 33.32 | 1.00 | 25 | [31] |
| 3 | CHT-GO-20% | CHT-GO aerogels | 4.15 | 412.00 | 1.00 | 25 | [32] |
| 4 | QCHT/PVA aerogels | Quaternized CHT+ PVA | 0.18 | nd | nd | 20 | [33] |
| 5 | CHT-TPPS | Ionic complexation | 0.90 | 26.75 | 5.00 | 25 | [34] |
| 6 | COF-IL@CHT aerogel | COF-CHT aerogel + allylimidazolium IL | 1.05 * | 103.30 | 1.00 | 25 | [35] |
| 7 | 40%([EMIM][OAc] + 5%CHT) +60% silica | SILP—encapsulation of ionogel with nanoporous fumed silica | 0.71 | 53.00 | 1.00 | 40 | [36] |
| 8 | 40%([BMIM]Cl + 5%CHT) +60% silica | 0.11 | 52.00 | 1.00 | 40 | ||
| 9 | CNF + APS | Cellulose nanofibril aerogel grafted with aminosilane | 1.91 | 51.80 | 1.00 | 25 | [37] |
| 10 | CNC + APS | CNC aerogel grafted APS | 1.50 | 29.14 | 1.00 | 25 | [38] |
| 11 | CNF-X-a-CNC | Acetylated cellulose nanocrystals aerogels | 1.14 | 21.04 | 1.00 | 0 | [39] |
| 12 | APMDS-CNF | APMDS-modified CNF aerogel | 1.01 | nd | 0.15 | 25 | [40] |
| 13 | PCC-1 | PEI-cross-linked cellulose aerogel | 2.31 | 234.20 | nd | 25 | [41] |
| Entry | Particles | Diameter (mm) | ρskel (g/cm3) | ρenv (g/cm3) | ε (%) | Overall Volume Shrinkage (%) |
|---|---|---|---|---|---|---|
| 1 | CHT | 3.12 (0.1) | 1.414 (0.030) | 0.070 (0.015) | 95.1 (1.0) | n.d. |
| 2 | CHT:P[DADMA]Cl15% | 3.43 (0.1) | 1.281 (0.044) | 0.051 (0.010) | 96.0 (0.8) | 74.1 (5.4) |
| 3 | CHT:P[VBMPyr]Cl15% | 3.44 (0.1) | 1.254 (0.019) | 0.052 (0.010) | 95.9 (0.8) | 66.3 (7.2) |
| 4 | CHT:P[VBA]Cl15% | 3.41 (0.1) | 1.304 (0.018) | 0.057 (0.011) | 95.6 (0.9) | 68.7 (6.6) |
| 5 | CHT:P[DADMA][OAc]15% | 3.27 (0.1) | 1.299 (0.026) | 0.070 (0.014) | 94.6 (1.1) | 69.7 (6.7) |
| 6 | CHT:P[DADMA]Cl30% | 3.18 (0.1) | 1.404 (0.058) | 0.072 (0.015) | 94.9 (1.1) | n.d. |
| 7 | CHT:P[VBMPyr]Cl30% | 3.70 (0.1) | 1.248 (0.026) | 0.068 (0.012) | 94.6 (0.9) | 62.4 (7.5) |
| 8 | CHT:P[VBA]Cl30% | 3.40 (0.1) | 1.391 (0.018) | 0.062 (0.012) | 95.5 (0.9) | 68.5 (6.7) |
| 9 | CHT:P[DADMA][OAc]30% | 3.30 (0.1) | 1.281 (0.043) | 0.062 (0.012) | 95.2 (1.0) | 65.3 (7.7) |
| 10 | CHT:Glut0.30% | 3.33 (0.1) | 1.259 (0.017) | 0.046 (0.010) | 96.3 (0.8) | 74.7 (5.4) |
| 11 | CHT:Glut0.30%:P[DADMA][OAc]15% | 3.30 (0.1) | 1.405 (0.015) | 0.067 (0.013) | 95.2 (0.9) | 67.2 (7.2) |
| 12 | CHT:Glut0.30%:P[DADMA][OAc]30% | 3.61 (0.1) | 1.421 (0.010) | 0.052 (0.010) | 96.4 (0.7) | 63.2 (7.5) |
| 13 | CHT:Glut0.30%:P[DADMA]Cl15% | 3.43 (0.1) | 1.390 (0.030) | 0.055 (0.011) | 96.0 (0.8) | 55.1 (9.9) |
| Entry | Particles | aBET (m2/g) | VP,BJH (cm3/g) | DP,BJH (nm) | Vmes (cm3/g) | VMP (cm3/g) |
| 1 | CHT | 323 | 1.77 | 18.3 | 1.19 | 12.42 |
| 2 | CHT:P[DADMA]Cl15% | 332 | 1.51 | 15.1 | 1.05 | 17.72 |
| 3 | CHT:P[VBMPyr]Cl15% | 324 | 1.46 | 15.0 | 1.03 | 17.51 |
| 4 | CHT:P[VBA]Cl15% | 292 | 1.47 | 16.7 | 0.96 | 15.77 |
| 5 | CHT:P[DADMA][OAc]15% | 366 | 1.67 | 15.2 | 1.19 | 12.27 |
| 6 | CHT:P[DADMA]Cl30% | 449 | 2.23 | 16.3 | 1.40 | 11.85 |
| 7 | CHT:P[VBMPyr]Cl30% | 454 | 1.92 | 14.0 | 1.39 | 12.62 |
| 8 | CHT:P[VBA]Cl30% | 300 | 1.32 | 14.5 | 0.92 | 14.48 |
| 9 | CHT:P[DADMA][OAc]30% | 398 | 1.83 | 15.4 | 1.24 | 14.18 |
| 10 | CHT:Glut0.30% | 272 | 1.30 | 16.4 | 0.86 | 19.93 |
| 11 | CHT:Glut0.30%:P[DADMA][OAc]15% | 744 | 3.10 | 13.8 | 2.29 | 11.94 |
| 12 | CHT:Glut0.30%:P[DADMA][OAc]30% | 270 | 1.33 | 16.6 | 0.92 | 17.72 |
| 13 | CHT:Glut0.30%:P[DADMA]Cl15% | 344 | 1.47 | 14.2 | 1.02 | 16.40 |
| Entry | Particles | nCO2 (mmol g−1) |
|---|---|---|
| 1 | CHT | 0.57 |
| 2 | CHT:P[DADMA]Cl15% | 0.63 |
| 3 | CHT:P[VBMPyr]Cl15% | 0.60 |
| 4 | CHT:P[VBA]Cl15% | 0.53 |
| 5 | CHT:P[DADMA][OAc]15% | 0.60 |
| 6 | CHT:P[DADMA]Cl30% | 0.70 |
| 7 | CHT:P[VBMPyr]Cl30% | 0.59 |
| 8 | CHT:P[VBA]Cl30% | 0.64 |
| 9 | CHT:P[DADMA][OAc]30% | 0.62 |
| 10 | CHT:Glut0.30% | 0.67 |
| 11 | CHT:Glut0.30%:P[DADMA][OAc]15% | 0.57 |
| 12 | CHT:Glut0.30%:P[DADMA][OAc]30% | 0.64 |
| 13 | CHT:Glut0.30%:P[DADMA]Cl15% | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrulas, R.V.; López-Iglesias, C.; Zanatta, M.; Casimiro, T.; Mármol, G.; Carrott, M.R.; García-González, C.A.; Corvo, M.C. The AEROPILs Generation: Novel Poly(Ionic Liquid)-Based Aerogels for CO2 Capture. Int. J. Mol. Sci. 2022, 23, 200. https://doi.org/10.3390/ijms23010200
Barrulas RV, López-Iglesias C, Zanatta M, Casimiro T, Mármol G, Carrott MR, García-González CA, Corvo MC. The AEROPILs Generation: Novel Poly(Ionic Liquid)-Based Aerogels for CO2 Capture. International Journal of Molecular Sciences. 2022; 23(1):200. https://doi.org/10.3390/ijms23010200
Chicago/Turabian StyleBarrulas, Raquel V., Clara López-Iglesias, Marcileia Zanatta, Teresa Casimiro, Gonzalo Mármol, Manuela Ribeiro Carrott, Carlos A. García-González, and Marta C. Corvo. 2022. "The AEROPILs Generation: Novel Poly(Ionic Liquid)-Based Aerogels for CO2 Capture" International Journal of Molecular Sciences 23, no. 1: 200. https://doi.org/10.3390/ijms23010200
APA StyleBarrulas, R. V., López-Iglesias, C., Zanatta, M., Casimiro, T., Mármol, G., Carrott, M. R., García-González, C. A., & Corvo, M. C. (2022). The AEROPILs Generation: Novel Poly(Ionic Liquid)-Based Aerogels for CO2 Capture. International Journal of Molecular Sciences, 23(1), 200. https://doi.org/10.3390/ijms23010200








