ceRNA Network of lncRNA/miRNA as Circulating Prognostic Biomarkers in Non-Hodgkin Lymphomas: Bioinformatic Analysis and Assessment of Their Prognostic Value in an NHL Cohort
Abstract
:1. Introduction
2. Results
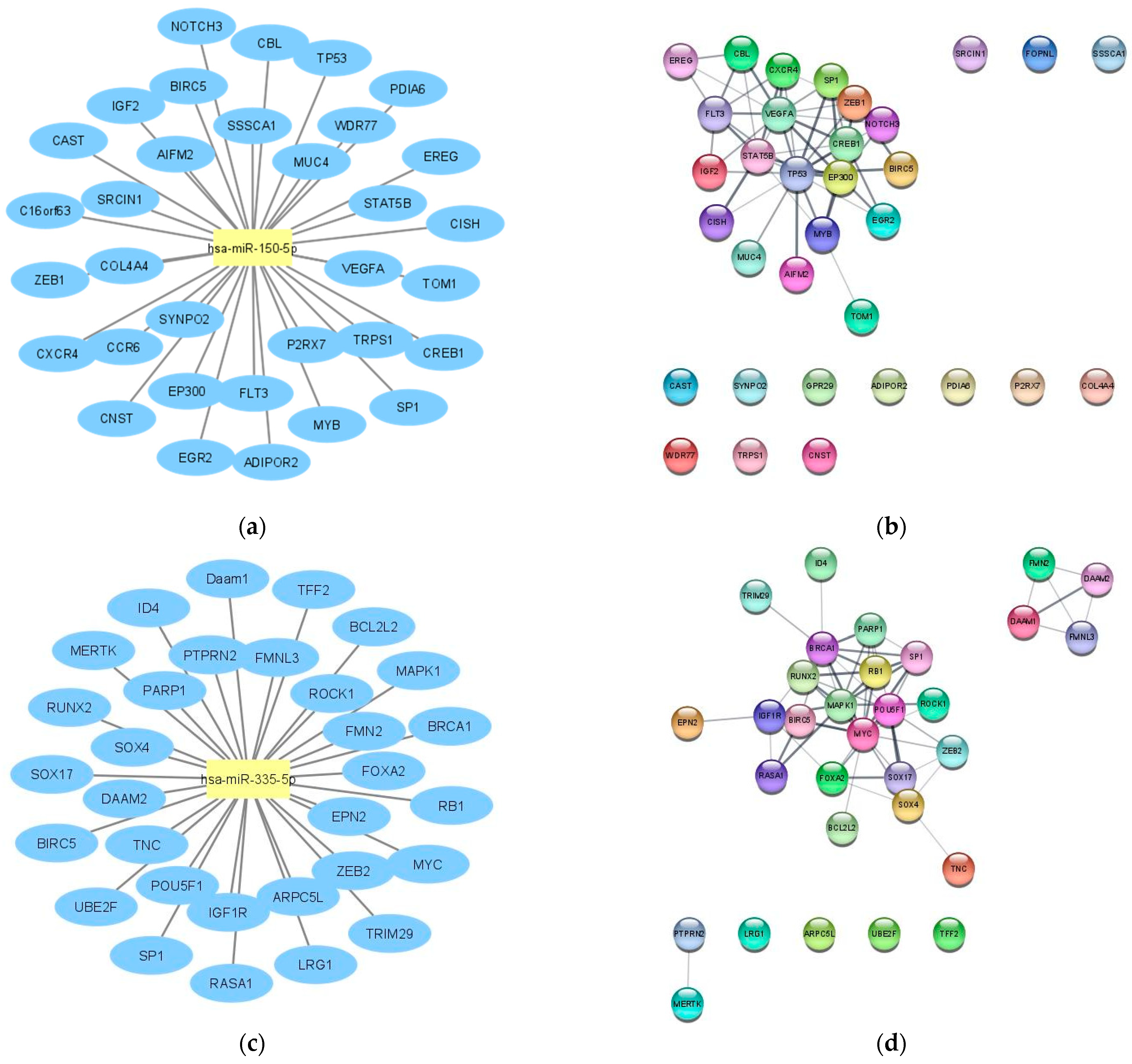
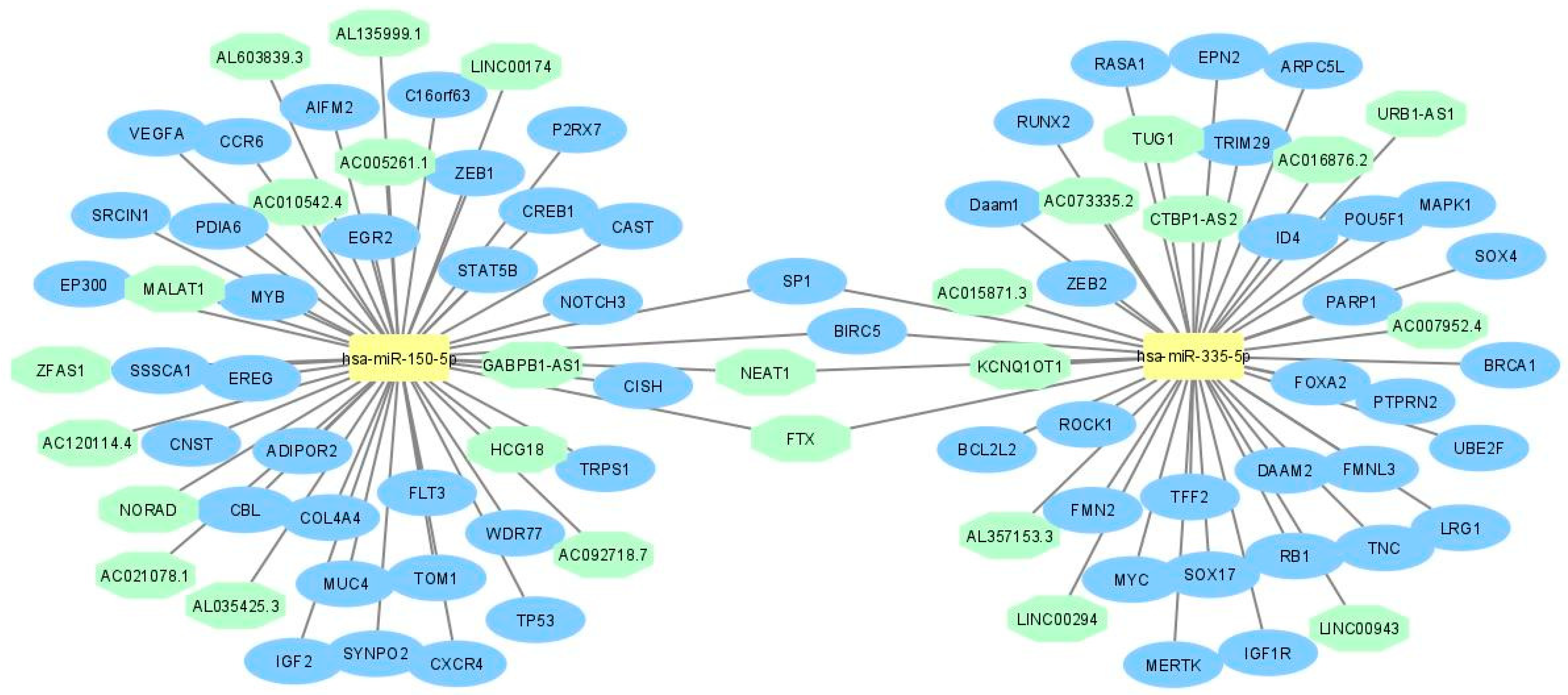
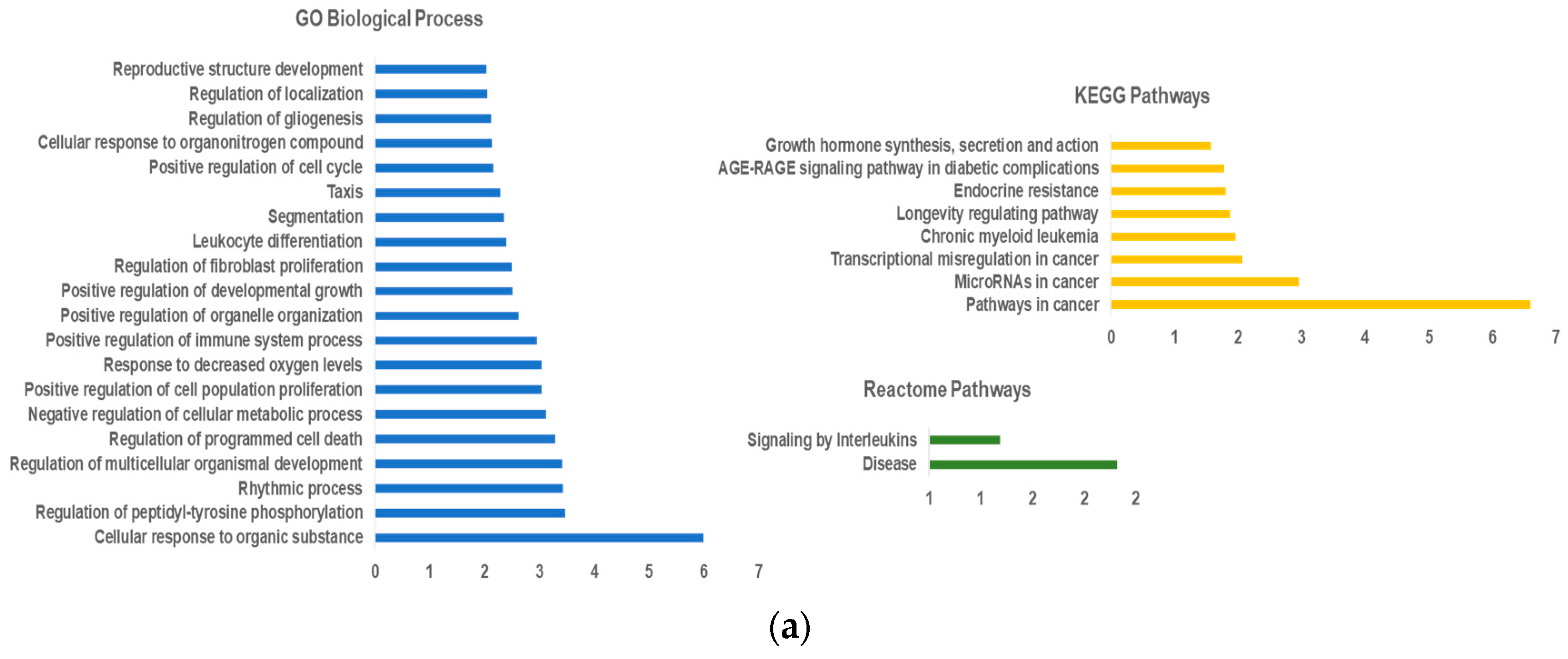
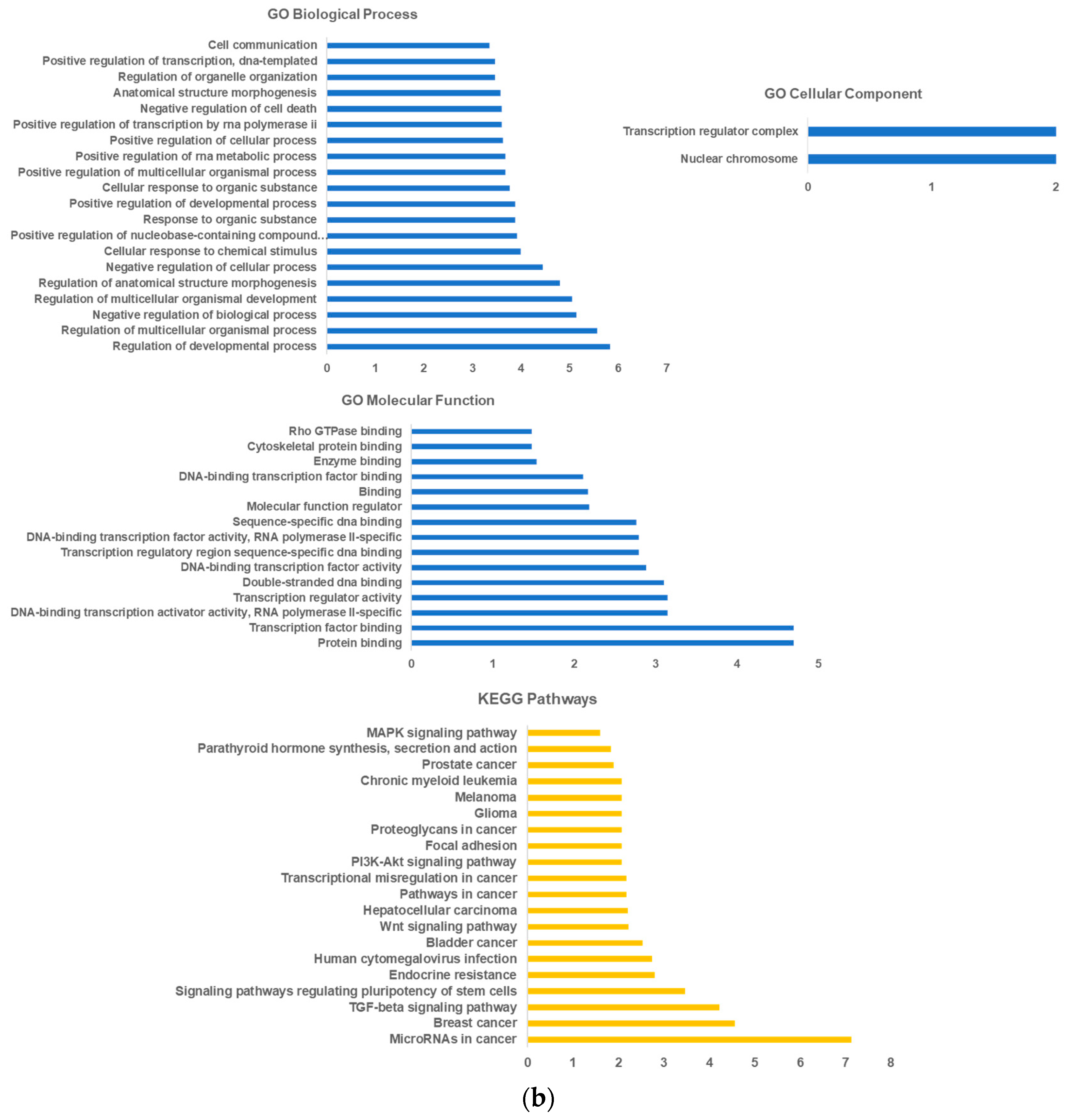
2.1. LncRNA-miRNA—mRNA Network Construction
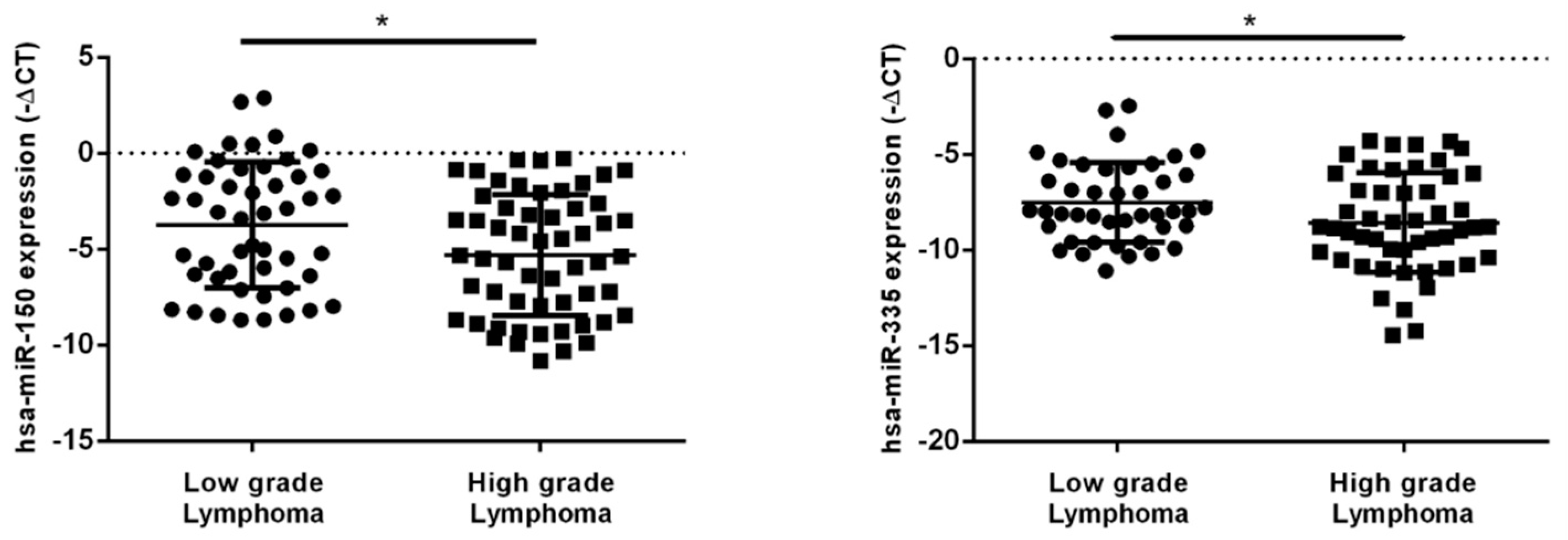
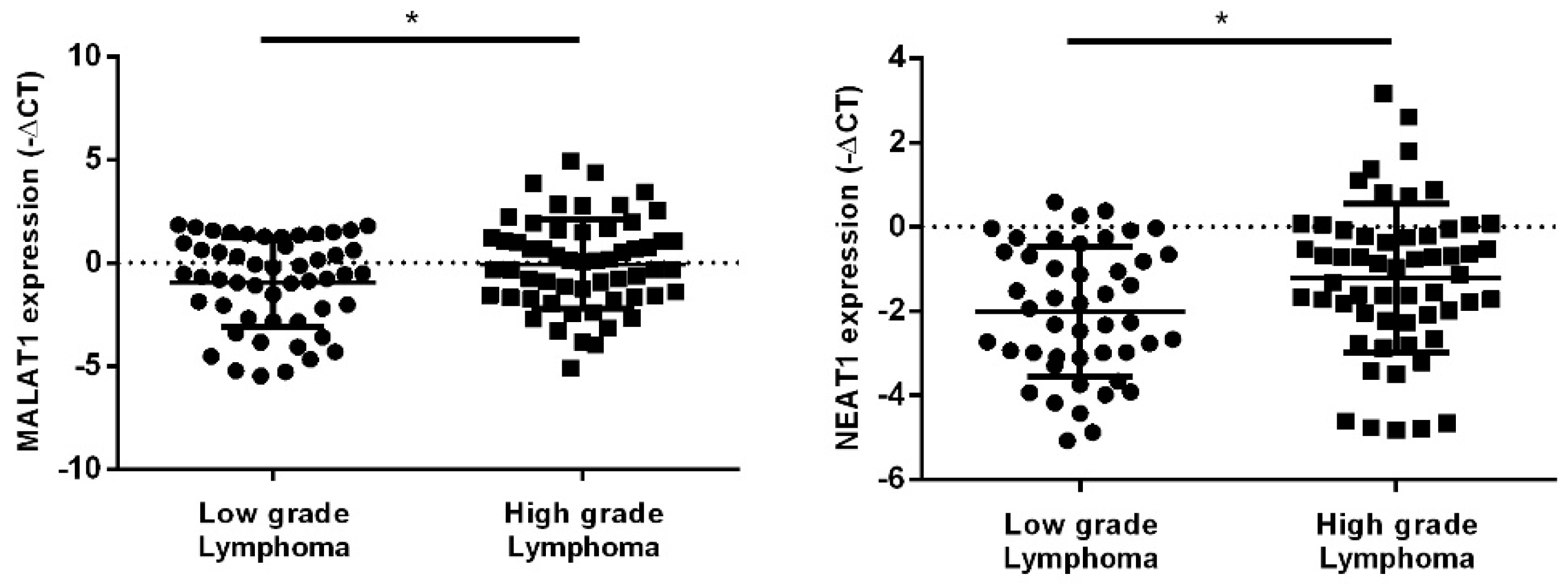
2.2. hsa-miR-150-5p, hsa-miR-335-5p, MALAT1 and NEAT1 Expression Levels in NHL Patients’ Plasma Samples
2.3. Association of miRNAs and lncRNAs Plasmatic Levels and the Clinicopathological Characteristics of NHL Patients
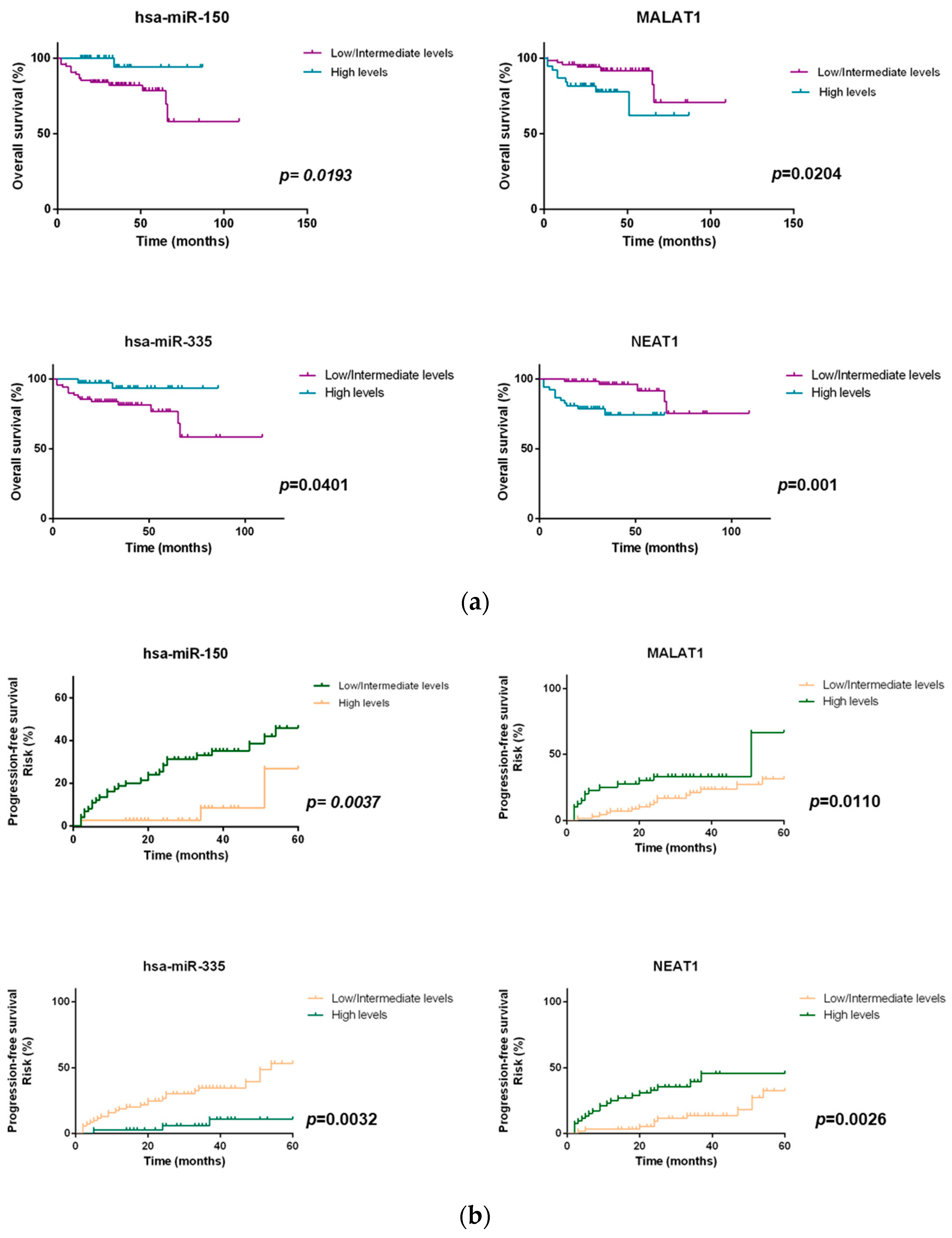
2.4. miRNAs and lncRNAs Impact on Overall Survival and Progression-Free Survival of NHL Patients
3. Discussion
4. Materials and Methods
4.1. Construction of CeRNA Regulatory Network and Functional Analysis
4.2. Study Population
4.3. RNA Extractions and qPCR
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klener, P.; Klanova, M. Drug Resistance in Non-Hodgkin Lymphomas. Int. J. Mol. Sci. 2020, 21, 2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovira, J.; Valera, A.; Colomo, L.; Setoain, X.; Rodríguez, S.; Martínez-Trillos, A.; Giné, E.; Dlouhy, I.; Magnano, L.; Gaya, A. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann. Hematol. 2015, 94, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Arzuaga-Mendez, J.; Lopez-Santillan, M.; Garcia-Ruiz, J.C.; Lopez-Lopez, E.; Martin-Guerrero, I. Systematic review of the potential of MicroRNAs in the management of patients with follicular lymphoma. Crit. Rev. Oncol. Hematol. 2021, 159, 103247. [Google Scholar] [CrossRef] [PubMed]
- Larrabeiti-Etxebarria, A.; Lopez-Santillan, M.; Santos-Zorrozua, B.; Lopez-Lopez, E.; Garcia-Orad, A. Systematic Review of the Potential of MicroRNAs in Diffuse Large B Cell Lymphoma. Cancers 2019, 11, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafouri-Fard, S.; Esmaeili, M.; Taheri, M. Expression of non-coding RNAs in hematological malignancies. Eur. J. Pharmacol. 2020, 875, 172976. [Google Scholar] [CrossRef]
- Sole, C.; Arnaiz, E.; Manterola, L.; Otaegui, D.; Lawrie, C.H. The circulating transcriptome as a source of cancer liquid biopsy biomarkers. Semin. Cancer Biol. 2019, 58, 100–108. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.; Tagawa, H. Dysregulation of microRNAs and their association in the pathogenesis of T-cell lymphoma/leukemias. Int. J. Hematol. 2014, 99, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Solé, C.; Arnaiz, E.; Lawrie, C.H. MicroRNAs as Biomarkers of B-cell Lymphoma. Biomark. Insights 2018, 13, 1177271918806840. [Google Scholar] [CrossRef] [Green Version]
- Karstensen, K.T.; Schein, A.; Petri, A.; Bøgsted, M.; Dybkær, K.; Uchida, S.; Kauppinen, S. Long Non-Coding RNAs in Diffuse Large B-Cell Lymphoma. Non-Coding RNA 2021, 7, 1. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Lin, Y.; Song, X.; Wu, D. LINC00857 contributes to proliferation and lymphomagenesis by regulating miR-370-3p/CBX3 axis in diffuse large B-cell lymphoma. Carcinogenesis 2021, 42, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, B.; Wu, Z.; Wu, C.; Ling, J.; Gao, X.; Zeng, H. LncRNA SNHG8 Promotes Proliferation and Inhibits Apoptosis of Diffuse Large B-Cell Lymphoma via Sponging miR-335-5p. Front. Oncol. 2021, 11, 650287. [Google Scholar] [CrossRef] [PubMed]
- Baytak, E.; Gong, Q.; Akman, B.; Yuan, H.; Chan, W.C.; Kucuk, C. Whole transcriptome analysis reveals dysregulated oncogenic lncRNAs in natural killer/T-cell lymphoma and establishes MIR155HG as a target of PRDM1. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39, 1010428317701648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Jiao, C.; Lin, Y.; Chen, M.; Zhang, J.; Wang, J.; Zhang, Z. lncRNA UCA1 Contributes to Imatinib Resistance by Acting as a ceRNA Against miR-16 in Chronic Myeloid Leukemia Cells. DNA Cell Biol. 2017, 36, 18–25. [Google Scholar] [CrossRef]
- Xiao, C.; Calado, D.P.; Galler, G.; Thai, T.-H.; Patterson, H.C.; Wang, J.; Rajewsky, N.; Bender, T.P.; Rajewsky, K. MiR-150 Controls B Cell Differentiation by Targeting the Transcription Factor c-Myb. Cell 2007, 131, 146–159. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kan, Y.; Chen, L.; Ge, P.; Ding, T.; Zhai, Q.; Yu, Y.; Wang, X.; Zhao, Z.; Yang, H.; et al. miR-150 is a negative independent prognostic biomarker for primary gastrointestinal diffuse large B-cell lymphoma. Oncol. Lett. 2020, 19, 3487–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zeng, F.; Wu, J.-Y.; Li, H.-Y.; Fan, J.-J.; Mai, L.; Zhang, J.; Ma, D.-M.; Li, Y.; Song, F.-z. MiR-335 inhibits migration of breast cancer cells through targeting oncoprotein c-Met. Tumor Biol. 2015, 36, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bian, T.; Feng, J.; Qian, L.; Zhang, J.; Jiang, D.; Zhang, Q.; Li, X.; Liu, Y.; Shi, J. miR-335 inhibited cell proliferation of lung cancer cells by target Tra2β. Cancer Sci. 2018, 109, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhang, Z.; Liu, Z.; Qiu, B.; Liu, K.; Dong, G. MicroRNA-335 inhibits invasion and metastasis of colorectal cancer by targeting ZEB2. Med. Oncol. 2014, 31, 982. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, H.; Lu, S. MiR-335-5p restores cisplatin sensitivity in ovarian cancer cells through targeting BCL2L2. Cancer Med. 2018, 7, 4598–4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Cai, J.; Huang, D.; Han, Q.; Yang, Q.; Li, T.; Ding, H.; Wang, Z. miR-335 represents an invasion suppressor gene in ovarian cancer by targeting Bcl-w. Oncol. Rep. 2013, 30, 701–706. [Google Scholar] [CrossRef]
- Adams, C.M.; Mitra, R.; Gong, J.Z.; Eischen, C.M. Non-Hodgkin and Hodgkin Lymphomas Select for Overexpression of BCLW. Clin. Cancer Res. 2017, 23, 7119–7129. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lin, J.; Liu, T.; Chen, T.; Pan, S.; Huang, W.; Li, S. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer 2014, 85, 110–115. [Google Scholar] [CrossRef]
- Chen, H.; Xu, J.; Hong, J.; Tang, R.; Zhang, X.; Fang, J.-Y. Long noncoding RNA profiles identify five distinct molecular subtypes of colorectal cancer with clinical relevance. Mol. Oncol. 2014, 8, 1393–1403. [Google Scholar] [CrossRef]
- Su, X.; Malouf, G.G.; Chen, Y.; Zhang, J.; Yao, H.; Valero, V.; Weinstein, J.N.; Spano, J.-P.; Meric-Bernstam, F.; Khayat, D. Comprehensive analysis of long non-coding RNAs in human breast cancer clinical subtypes. Oncotarget 2014, 5, 9864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.-Y.; Wang, J.-H.; Wang, J.-L.; Ma, C.X.; Wang, X.-C.; Liu, F.-S. Malat1 as an evolutionarily conserved lncRNA, plays a positive role in regulating proliferation and maintaining undifferentiated status of early-stage hematopoietic cells. BMC Genom. 2015, 16, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, L.; Begum, N.A.; Burroughs, A.M.; Doi, T.; Kawai, J.; Daub, C.O.; Kawaguchi, T.; Matsuda, F.; Hayashizaki, Y.; Honjo, T. Nonimmunoglobulin target loci of activation-induced cytidine deaminase (AID) share unique features with immunoglobulin genes. Proc. Natl. Acad. Sci. USA 2012, 109, 2479–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Sehgal, L.; Jain, N.; Khashab, T.; Mathur, R.; Samaniego, F. LncRNA MALAT1 promotes development of mantle cell lymphoma by associating with EZH2. J. Transl. Med. 2016, 14, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, K.; Adams, P.D.; Copland, M. EZH2 in normal and malignant hematopoiesis. Leukemia 2014, 28, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Musilova, K.; Devan, J.; Cerna, K.; Seda, V.; Pavlasova, G.; Sharma, S.; Oppelt, J.; Pytlik, R.; Prochazka, V.; Prouzova, Z.; et al. miR-150 downregulation contributes to the high-grade transformation of follicular lymphoma by upregulating FOXP1 levels. Blood 2018, 132, 2389–2400. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.-F.; Hong, L.-L.; Li, H.; Huang, F.-Y.; Wen, Q.; Zhuang, H.-F. Long non-coding RNA MALAT1 modulate cell migration, proliferation and apoptosis by sponging microRNA-146a to regulate CXCR4 expression in acute myeloid leukemia. Hematology 2021, 26, 43–52. [Google Scholar] [CrossRef]
- Su, K.; Wang, N.; Shao, Q.; Liu, H.; Zhao, B.; Ma, S. The role of a ceRNA regulatory network based on lncRNA MALAT1 site in cancer progression. Biomed. Pharmacother. 2021, 137, 111389. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Chen, G.; He, R.; Yang, L. LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci. 2019, 9, 54. [Google Scholar] [CrossRef]
- Vimalraj, S.; Subramanian, R.; Dhanasekaran, A. LncRNA MALAT1 Promotes Tumor Angiogenesis by Regulating MicroRNA-150-5p/VEGFA Signaling in Osteosarcoma: In-Vitro and In-Vivo Analyses. Front. Oncol. 2021, 11, 742789. [Google Scholar] [CrossRef]
- Wang, X.; Angelis, N.; Thein, S.L. MYB—A regulatory factor in hematopoiesis. Gene 2018, 665, 6–17. [Google Scholar] [CrossRef]
- Quintana, A.M.; Liu, F.; O’Rourke, J.P.; Ness, S.A. Identification and regulation of c-Myb target genes in MCF-7 cells. BMC Cancer 2011, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Salomoni, P.; Perrotti, D.; Martinez, R.; Franceschi, C.; Calabretta, B. Resistance to apoptosis in CTLL-2 cells constitutively expressing c-Myb is associated with induction of BCL-2 expression and Myb-dependent regulation of bcl-2 promoter activity. Proc. Natl. Acad. Sci. USA 1997, 94, 3296–3301. [Google Scholar] [CrossRef] [Green Version]
- Down, C.F.; Millour, J.; Lam, E.W.-F.; Watson, R.J. Binding of FoxM1 to G2/M gene promoters is dependent upon B-Myb. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 855–862. [Google Scholar] [CrossRef]
- Nakata, Y.; Shetzline, S.; Sakashita, C.; Kalota, A.; Rallapalli, R.; Rudnick, S.I.; Zhang, Y.; Emerson, S.G.; Gewirtz, A.M. c-Myb contributes to G2/M cell cycle transition in human hematopoietic cells by direct regulation of cyclin B1 expression. Mol. Cell. Biol. 2007, 27, 2048–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, D.; Wen, S.-C.; Engelhard, A.; Nicolaides, N.; Lipson, K.; Marino, T.; Calabretta, B. c-myb transactivates cdc2 expression via Myb binding sites in the 5′-flanking region of the human cdc2 gene. J. Biol. Chem. 1993, 268, 2255–2259. [Google Scholar] [CrossRef]
- Lanzós, A.; Carlevaro-Fita, J.; Mularoni, L.; Reverter, F.; Palumbo, E.; Guigó, R.; Johnson, R. Discovery of Cancer driver long noncoding RNAs across 1112 tumour genomes: New candidates and distinguishing features. Sci. Rep. 2017, 7, 41544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Gu, W.; Zhu, X. NEAT1 mediates paclitaxel-resistance of non-small cell of lung cancer through activation of Akt/mTOR signalling pathway. J. Drug Target. 2019, 27, 1061–1067. [Google Scholar] [CrossRef]
- Yu, H.-M.; Wang, C.; Yuan, Z.; Chen, G.-L.; Ye, T.; Yang, B.-W. LncRNA NEAT1 promotes the tumorigenesis of colorectal cancer by sponging miR-193a-3p. Cell Prolif. 2019, 52, e12526. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, W.; Zhang, Y.; Liang, B. Long Non-Coding RNA-NEAT1 Promotes Cell Migration and Invasion via Regulating miR-124/NF-κB Pathway in Cervical Cancer. OncoTargets Ther. 2020, 13, 3265–3276. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.-Y.; Wang, C.; Liu, G.; Zhou, X. Long noncoding RNA NEAT1-modulated miR-506 regulates gastric cancer development through targeting STAT3. J. Cell. Biochem. 2019, 120, 4827–4836. [Google Scholar] [CrossRef]
- Shin, V.Y.; Chen, J.; Cheuk, I.W.Y.; Siu, M.-T.; Ho, C.-W.; Wang, X.; Jin, H.; Kwong, A. Long non-coding RNA NEAT1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Taiana, E.; Favasuli, V.; Ronchetti, D.; Todoerti, K.; Pelizzoni, F.; Manzoni, M.; Barbieri, M.; Fabris, S.; Silvestris, I.; Gallo Cantafio, M.E.; et al. Long non-coding RNA NEAT1 targeting impairs the DNA repair machinery and triggers anti-tumor activity in multiple myeloma. Leukemia 2020, 34, 234–244. [Google Scholar] [CrossRef]
- Liu, K.; Lei, S.; Kuang, Y.; Jin, Q.; Long, D.; Liu, C.; Jiang, Y.; Zhao, H.; Yao, H. A Novel Mechanism of the c-Myc/NEAT1 Axis Mediating Colorectal Cancer Cell Response to Photodynamic Therapy Treatment. Front. Oncol. 2021, 11, 2135. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, H.; Yuan, Z.; Zhang, Q.; Xiong, H.; Hu, Z.; Wu, H.; Huang, R.; Wang, G.; Tang, Q. LncRNA NEAT1 remodels chromatin to promote the 5-Fu resistance by maintaining colorectal cancer stemness. Cell Death Dis. 2020, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, M.; Li, X.; Cui, F. The long noncoding RNA NEAT1 targets miR-34a-5p and drives nasopharyngeal carcinoma progression via Wnt/β-catenin signaling. Yonsei Med. J. 2019, 60, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Wu, H.; Tao, T.; Peng, E. NEAT1 regulates cell proliferation and apoptosis of ovarian cancer by miR-34a-5p/BCL2. OncoTargets Ther. 2017, 10, 4905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Zhou, Y.; Sun, A.-J.; Xue, J.-L. NEAT1 contributes to breast cancer progression through modulating miR-448 and ZEB1. J. Cell. Physiol. 2018, 233, 8558–8566. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.-Y.; Zhou, M.; Lv, H.; Qin, X.; Zhou, J.; Mao, X.; Li, X.; Xu, Y.; Liu, Y.; Xing, H. Involvement of NEAT1/miR-133a axis in promoting cervical cancer progression via targeting SOX4. J. Cell. Physiol. 2019, 234, 18985–18993. [Google Scholar] [CrossRef] [PubMed]
- Che, F.; Ye, X.; Wang, Y.; Ma, S.; Wang, X. Lnc NEAT1/miR-29b-3p/Sp1 form a positive feedback loop and modulate bortezomib resistance in human multiple myeloma cells. Eur. J. Pharmacol. 2021, 891, 173752. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Li, M.; Wang, S.; Liu, Y. Advances of target therapy on NOTCH1 signaling pathway in T-cell acute lymphoblastic leukemia. Exp. Hematol. Oncol. 2020, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, P.; Li, W.-J.; Zhang, J.; Wang, G.-P.; Jiang, D.-F.; Chen, F.-P. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7-H3 in multiple myeloma. Mol. Immunol. 2020, 117, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xia, L.; Zha, B.; Zuo, C.; Deng, D.; Chen, M.; Hu, L.; He, Y.; Dai, F.; Wu, J. miR-335-5p targeting ICAM-1 inhibits invasion and metastasis of thyroid cancer cells. Biomed. Pharmacother. 2018, 106, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Tang, H.; Lei, Z.; Zhu, J.; Zeng, Y.; Liu, Z.; Huang, J.-A. miR-335-5p inhibits TGF-β1-induced epithelial–mesenchymal transition in non-small cell lung cancer via ROCK1. Respir. Res. 2019, 20, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zeng, X.; Wang, N.; Zhao, W.; Zhang, X.; Teng, S.; Zhang, Y.; Lu, Z. Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma. Mol. Cancer 2018, 17, 89. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, N. MiR-335-5p inhibits cell proliferation, migration and invasion in colorectal cancer through downregulating LDHB. J. Buon 2019, 24, 1128–1136. [Google Scholar]
- Wang, Y.; Yang, T.; Zhang, Z.; Lu, M.; Zhao, W.; Zeng, X.; Zhang, W. Long non-coding RNA TUG1 promotes migration and invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells. Cancer Sci. 2017, 108, 859–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Zhang, C.; Guan, H.; Liu, J.; Cui, Y. LncRNA DANCR promotes cervical cancer progression by upregulating ROCK1 via sponging miR-335-5p. J. Cell. Physiol. 2019, 234, 7266–7278. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, F.; Huang, H.; Zhang, Y.; Xie, H.; Men, T. lncRNA SLCO4A1-AS1 promotes growth and invasion of bladder cancer through sponging miR-335-5p to upregulate OCT4. OncoTargets Ther. 2019, 12, 1351–1358. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, M.; Sun, G. Long non-coding RNA NEAT1 regulates the proliferation, migration and invasion of gastric cancer cells via targeting miR-335-5p/ROCK1 axis. Die Pharm. Int. J. Pharm. Sci. 2018, 73, 150–155. [Google Scholar]
- Chen, S.; Xia, X. Long noncoding RNA NEAT1 suppresses sorafenib sensitivity of hepatocellular carcinoma cells via regulating miR-335–c-Met. J. Cell. Physiol. 2019, 234, 14999–15009. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.Y.; Lin, Y.C.; Li, J.; Huang, K.Y.; Shrestha, S.; Hong, H.C.; Tang, Y.; Chen, Y.G.; Jin, C.N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]

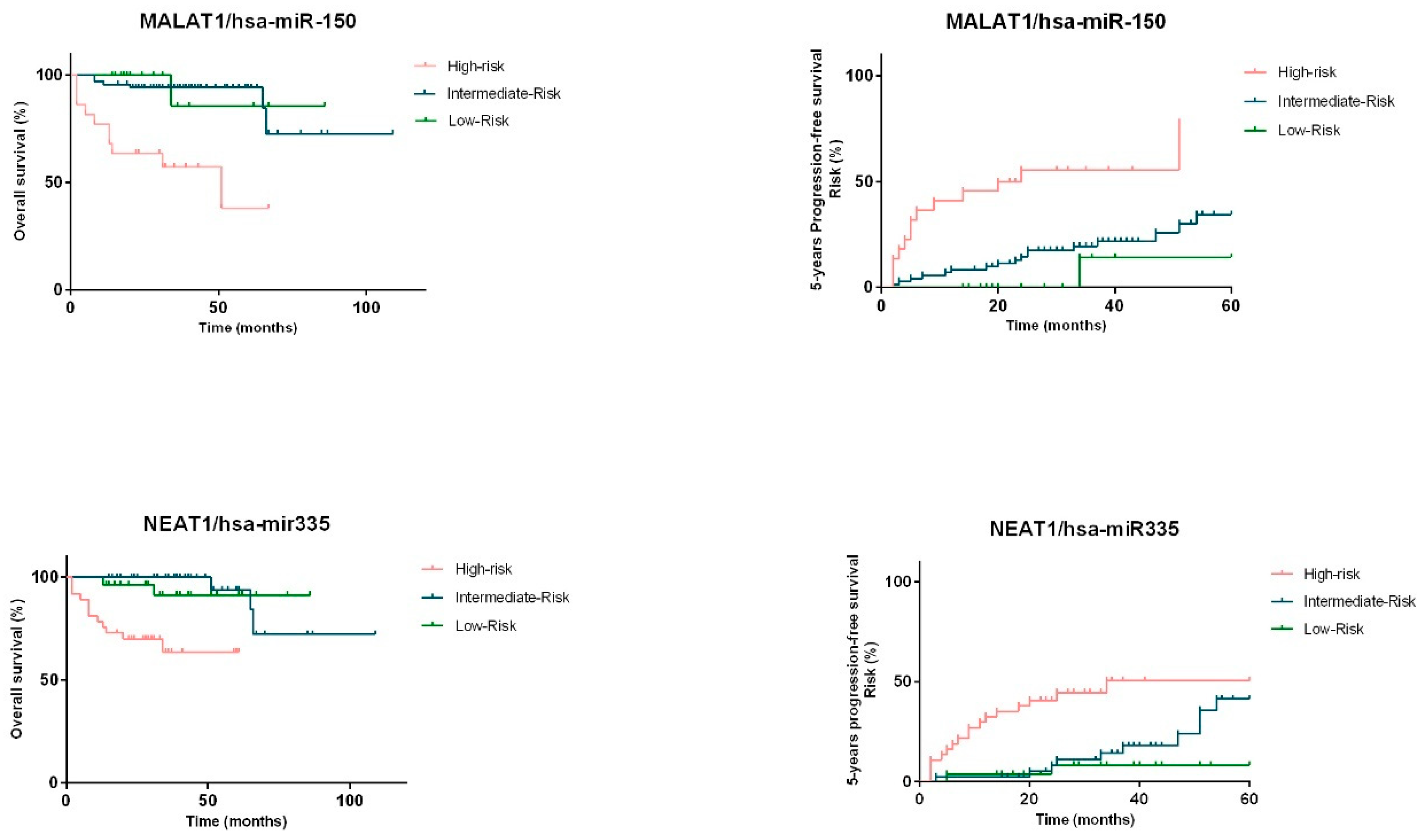
| Groups | hsa-miR-150-5p-MALAT1 | hsa-miR-335-5p-NEAT1 |
|---|---|---|
| Low-risk | ↑hsa-miR-150 +↓MALAT1 | ↑hsa-miR-335 +↓NEAT1 |
| Intermediate-risk | ↑hsa-miR-150 +↑MALAT1 ↓hsa-miR-150 +↓MALAT1 | ↑hsa-miR-335 +↑NEAT1 ↓hsa-miR-335 +↓NEAT1 |
| High-risk | ↓hsa-miR-150 +↑MALAT1 | ↓hsa-miR-335 +↑NEAT1 |
| Clinical-Pathological Characteristics | N (%) N = 113 |
|---|---|
| Age | |
| ≤60 years | 53 (46.9%) |
| >60 years | 60 (53.1%) |
| Gender | |
| Female | 57 (50.4%) |
| Male | 56 (49.6%) |
| Grade | |
| Low (indolent) | 55 (48.7%) |
| High (aggressive) | 58 (51.3%) |
| Subtype of NHL | |
| Follicular | 40 (35.4%) |
| Diffuse large B-cell | 58 (51.3%) |
| Marginal Zone | 15 (13.3%) |
| Stage | |
| I/II | 43 (38.1%) |
| III/IV | 70 (61.9%) |
| LDH serum levels | |
| Normal | 67 (59.3%) |
| High | 45 (39.8%) |
| Unknown | 1 (0.9%) |
| ECOG | |
| 0–1 | 97 (85.8%) |
| ≥2 | 14 (12.4%) |
| Unknown | 2 (1.8%) |
| B symptoms | |
| Absent | 80 (70.8%) |
| Present | 33 (29.2%) |
| IPI Score (high grade tumors) | |
| Low-risk (0–1) | 17 (29.3%) |
| Intermediate-risk (2–3) | 25 (43.1%) |
| High-risk (4–5) | 14 (24.1%) |
| Unknown | 2 (3.4%) |
| FLIPI score (low grade tumors) | |
| Low-risk (0–1) | 18 (32.7%) |
| Intermediate-risk (2) | 18 (32.7%) |
| High-risk (3, 4, 5) | 19 (34.5%) |
| BM involvement | |
| Negative | 81 (71.7%) |
| Positive | 32 (28.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, M.; Marques, H.; Teixeira, A.L.; Medeiros, R. ceRNA Network of lncRNA/miRNA as Circulating Prognostic Biomarkers in Non-Hodgkin Lymphomas: Bioinformatic Analysis and Assessment of Their Prognostic Value in an NHL Cohort. Int. J. Mol. Sci. 2022, 23, 201. https://doi.org/10.3390/ijms23010201
Fernandes M, Marques H, Teixeira AL, Medeiros R. ceRNA Network of lncRNA/miRNA as Circulating Prognostic Biomarkers in Non-Hodgkin Lymphomas: Bioinformatic Analysis and Assessment of Their Prognostic Value in an NHL Cohort. International Journal of Molecular Sciences. 2022; 23(1):201. https://doi.org/10.3390/ijms23010201
Chicago/Turabian StyleFernandes, Mara, Herlander Marques, Ana Luísa Teixeira, and Rui Medeiros. 2022. "ceRNA Network of lncRNA/miRNA as Circulating Prognostic Biomarkers in Non-Hodgkin Lymphomas: Bioinformatic Analysis and Assessment of Their Prognostic Value in an NHL Cohort" International Journal of Molecular Sciences 23, no. 1: 201. https://doi.org/10.3390/ijms23010201
APA StyleFernandes, M., Marques, H., Teixeira, A. L., & Medeiros, R. (2022). ceRNA Network of lncRNA/miRNA as Circulating Prognostic Biomarkers in Non-Hodgkin Lymphomas: Bioinformatic Analysis and Assessment of Their Prognostic Value in an NHL Cohort. International Journal of Molecular Sciences, 23(1), 201. https://doi.org/10.3390/ijms23010201









