Abstract
Deep eutectic solvents (DESs) have been widely used to capture CO2 in recent years. Understanding CO2 mechanisms by DESs is crucial to the design of efficient DESs for carbon capture. In this work, we studied the CO2 absorption mechanism by DESs based on ethylene glycol (EG) and protic ionic liquid ([MEAH][Im]), formed by monoethanolamine (MEA) with imidazole (Im). The interactions between CO2 and DESs [MEAH][Im]-EG (1:3) are investigated thoroughly by applying 1H and 13 C nuclear magnetic resonance (NMR), 2-D NMR, and Fourier-transform infrared (FTIR) techniques. Surprisingly, the results indicate that CO2 not only binds to the amine group of MEA but also reacts with the deprotonated EG, yielding carbamate and carbonate species, respectively. The reaction mechanism between CO2 and DESs is proposed, which includes two pathways. One pathway is the deprotonation of the [MEAH]+ cation by the [Im]− anion, resulting in the formation of neutral molecule MEA, which then reacts with CO2 to form a carbamate species. In the other pathway, EG is deprotonated by the [Im]−, and then the deprotonated EG, HO-CH2-CH2-O−, binds with CO2 to form a carbonate species. The absorption mechanism found by this work is different from those of other DESs formed by protic ionic liquids and EG, and we believe the new insights into the interactions between CO2 and DESs will be beneficial to the design and applications of DESs for carbon capture in the future.
1. Introduction
Recently, deep eutectic solvents have attracted much attention because of their unique properties, including a negligible vapor pressure, non-flammability, ease of synthesis, and tailorable polarity, which make them a promising alternative to traditional organic solvents [1,2]. Most of DESs are obtained by mixing appropriate hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs). The applications of DESs in many fields have been investigated so far, such as organic synthesis, extractions processes, catalytic reactions and electrochemistry [3,4,5,6]. Moreover, DESs have been widely studied as absorbents for CO2 capture [7,8,9,10,11]. Among them, functional DESs which could chemically absorb CO2 have received much attention because of their high capacities. The chemical absorption of CO2 by DESs depends on the chemical reactions between CO2 and functional groups of DESs [12,13,14,15,16]. Therefore, understanding the reaction mechanisms between CO2 and functional groups of DESs is important to the design of efficient DESs for CO2 capture.
Trivedi et al. studied the CO2 absorption mechanism by DESs employing ethylenediamine (EDA) as an HBD [17] and the results indicated that a carbamate species was formed between the amine group of EDA and CO2. In our previous work, we found that CO2 was attached to ethylene glycol (EG) with the formation of a carbonate species when CO2 was captured by DESs consisting of azolide-based ILs and EG [18]. Zhang et al. studied the interactions between CO2 and ternary DESs consisting of ionic liquid (IL) 1-butyl-3-methylimidazolium chloride ([Bmim][Cl]), imidazole (Im), and super base 1,5-diazabicyclo [4.3.0] non-5-ene (DBN) [19]. The results indicated that CO2 was bonded to the C2-position of the imidazolium ring of the cation [Bmim]+. Zeng et al. investigated the reaction pathway between CO2 and DESs composed of protic IL 1,8-diazabicyclo-[0,4,5]undec-7-ene imidazolate ([DBUH][Im]) and EG [20] and it was found that CO2 reacted both with the anion [Im]− and EG. Another reaction mechanism between CO2 and DESs consisting of ionic liquid 1-ethyl-3-methylimidazolium 2-cyanopyrrolide ([Emim][2-CNpyr]) and EG was reported by Gurkan and co-authors [21], and the results revealed that CO2 was attached to the anion [2-CNpyr]-, the imidazolium cation and EG, by forming carbamate, carboxylate and carbonate species, respectively.
In a recent paper, Mukesh et al. reported the CO2 capture by DESs based on EG and protic ILs [22]. The protic ILs they used were obtained by mixing polyamines or monoethanolamine (MEA) with imidazole (Im). These DESs showed a high absorption capacity for CO2. The authors also investigated the reactions between CO2 and the DESs used in their work. On the basis of the NMR and FTIR results, they believed that CO2 was bonded to the amine group of polyamines or MEA, and EG or anion [Im]− did not react with CO2.
However, in our study, we found that CO2 not only binds with the amine group but also reacts with EG in DESs (Scheme 1), suggesting the CO2 absorption mechanism reported by Mukesh et al. is inaccurate [22]. The absorption mechanism found by this work is different from those of other DESs formed by protic ionic liquids and EG. For example, CO2 reacted both with the anion [Im]− and EG, but did not react with the cation [DBUH]+, when CO2 was captured by [DBUH][Im]-EG DESs. The CO2 absorption mechanism by [DBUH][MLU] (MLU: Methyl urea)-EG presented a similar phenomenon, i.e., CO2 reacted with the anion [MLU]− and EG rather than reacting with the cation [DBUH]+ [16]. However, the mechanism found by our work indicates that CO2 binds to both the amino group on the cation [MEAH]+ and EG, but CO2 is not bonded to the anion [Im]− in [MEAH][Im]-EG (1:3) DESs. We believe that the findings of our work provide new insights about the interactions between CO2 and DESs, which will be useful to design new, efficient DESs for carbon capture in the future.
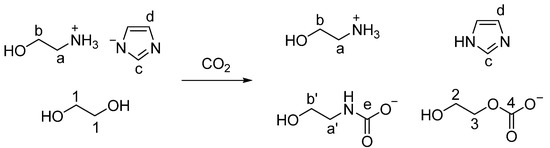
Scheme 1.
The reaction between CO2 and [MEAH][Im]-EG (1:3).
2. Results and Discussion
At first, we prepared the DESs [MEAH][Im]-EG (1:3) following the procedures reported by Mukesh and co-authors [22]. The formation of [MEAH][Im] was confirmed by the 1H NMR results (Figure S1). As shown in Figure S1, the -NH3+ peak can be observed at 5.54 ppm, suggesting the IL [MEAH][Im] was obtained. The CO2 capacity by the [MEAH][Im]-EG (1:3) is 8.94 wt% (Figure S2), which is similar to that reported by Mukesh’s paper, and the diagram of the CO2 absorption apparatus is shown in Figure S3. The 1H (Figure 1a) and 13C (Figure 1b) NMR spectra of [MEAH][Im]-EG(1:3) before and after CO2 capture were carefully studied. As shown in Figure 1a, the hydrogen peaks of -CH2- group (H-a′ and H-b′) of MEA-based carbamate can be found after CO2 absorption, [23] suggesting the CO2 is bonded to the amine group. However, two other new peaks at 3.05 (H-2) and 3.35 (H-3) ppm can also be found in Figure 1a. As seen in Figure 1b, the carbon signals of -CH2- group related to the MEA-based carbamate can be found at 42.7 (C-a′) and 60.6 (C-b′) ppm, and the carbonyl signal of MEA-based carbamate appears at 163.3 (C-e) ppm. However, there are three other new peaks at 60.7 (C-2), 65.9 (C-3), and 158.2 (C-4) ppm.
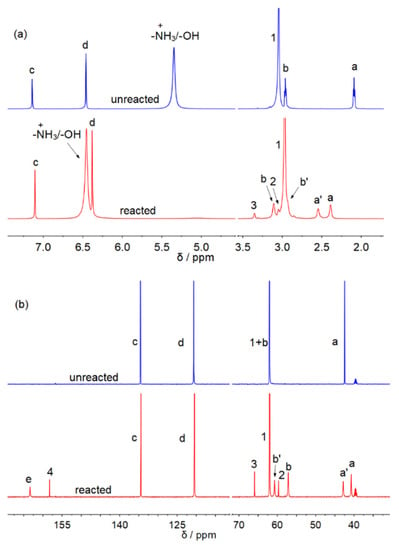
Figure 1.
1H (a) and 13C (b) NMR spectra of [MEAH][Im]-EG (1:3) before and after CO2 absorption.
In order to assign the new peaks in the 1H and 13C NMR spectra after CO2 capture, the 1H-13C HMBC NMR spectra of [MEA][Im]-EG (1:3) after CO2 capture were investigated (Figure 2). As shown in Figure 2, C-e correlates with the H-a′, suggesting the formation of the MEA-based carbamate. Moreover, the carbonyl carbon C-4 correlates with the H-3, and the correlation between C-4 and the hydrogens on the imidazole ring also could not be seen. The 1H-13C HMBC suggested that the carbon C-4 should be the carbonyl carbon of the carbonate formed by CO2 and EG. The carbons at 60.7 (C-2) and 65.9 (C-3) ppm are the -CH2- carbons of HO-CH2-CH2-OCOO− [20,21]. Similar carbon peaks could also be found in the Mukesh and co-authors’ paper [22]. As shown in Figure S4 of Mukesh and co-authors’ paper, the -CH2- carbons of HO-CH2-CH2-OCOO− could be clearly observed in the 13C NMR spectra of [TEPA]2[Im]:EG-4 after CO2 absorption, which disappeared after CO2 desorption. In Figure S10a of their paper, the -CH2- carbons of HO-CH2-CH2-OCOO− could also be found in the 13C NMR spectra of TPEA-EG solution after CO2 capture, and the carbonyl carbon of EG-based carbonate appeared at 159.67 ppm. In the 13C NMR spectra of [TEPA]2[Im]:EG-4 after CO2 absorption (Figure S10c of their paper), the carbonyl carbon of EG-based carbonate appeared at 159.79 ppm. Therefore, in our opinion, the 13C NMR results of [TEPA]2[Im]:EG-4 after carbon capture indicated that CO2 reacted with EG by forming carbonate species. Interestingly, as seen in Figure 1a, the peaks H-c, H-d, and H-1 shift slightly to upfield, and the H-a peak shift to downfield after carbon capture. These shifts may be due to the change in the hydrogen bonds network in the DESs because of the formations of carbamate and carbonate species after capture.
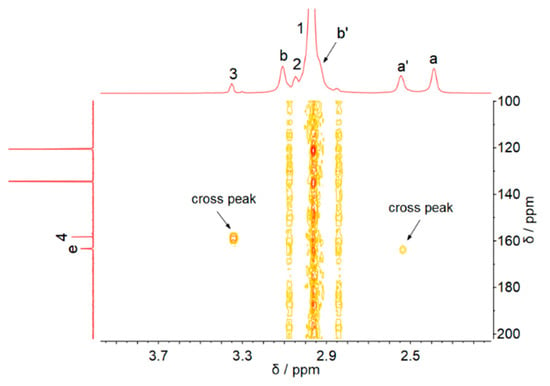
Figure 2.
The 1H-13C HMBC spectra of [MEAH][Im]-EG (1:3) after CO2 capture.
Although Mukesh and co-authors investigated the HMBC spectra of [TEPA]2[Im]:EG-4 after CO2 capture (Figure S11 of their paper) and they believed that CO2 did not react with EG on the basis of the HMBC results, we think their HMBC results are not accurate because the 13C peaks in the HMBC spectra are not consistent with those in the 13C NMR spectra of Figure S10c of their paper. In the 13C NMR spectra of Figure S10c, the carbamate signals were in the range of 164 to 165 ppm and the strong carbonate signal was at 159.79 ppm. In their HMBC spectra, the carbamate signals can be found near 165 ppm, but the peak near 159 ppm could not be observed, so the HMBC spectra they obtained may be incorrect.
Our findings were further confirmed by the FTIR spectra. The FTIR spectra of [MEAH][Im]-EG (1:3) before and after CO2 capture were shown in Figure 3. The peak at 1635 cm−1 is associated with the combined C=O stretching band of carbonate and carbamate species, and the peak at 1573 cm−1 corresponds to COO− asymmetric stretching of carbamate -NHCOO−. The new peak at 1296 cm−1 is ascribed to the stretching band of RO-COO− [21]. The FTIR results suggested again that both carbonate and carbamate species are formed after CO2 capture.
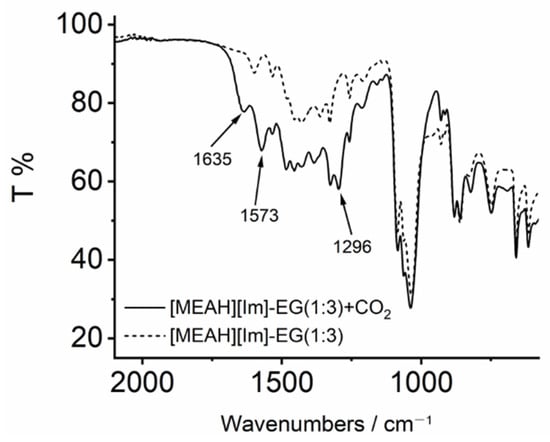
Figure 3.
The FTIR spectra of [MEAH][Im]-EG (1:3) before and after CO2 capture.
Based on the above discussion, the possible absorption mechanism by [MEAH][Im]-EG (1:3) is presented in Scheme 2. Pathway 1 is the deprotonation of the [MEAH]+ cation by [Im]− anion, resulting in the formation of the neutral molecule MEA, which then reacts with CO2 to form a carbamate species. Pathway 2 is that EG is deprotonated by [Im]−, forming the HO-CH2-CH2-O− anion, which then binds with CO2 to form a carbonate species. The absorption mechanism by [MEAH][Im]-EG (1:3) is different from that of [DBUH][Im]-EG, which may be due to that DBU is a much stronger base than MEA. In other words, [DBUH]+ is a much weaker acid than [MEAH]+, rendering the deprotonation of [DBUH]+ by [Im]− extremely difficult, so CO2 is not found to be bonded to DBU in the [DBUH][Im]-EG DESs after CO2 capture [20]. In our previous work, the steric hindrance of functional groups is also found to be a factor that affects the CO2 absorption mechanism. [24,25]. Therefore, we suggest that the factors, which may include the basicity, acidity, and the steric hindrance of functional groups of DESs, should be considered to modulate the CO2 absorption mechanism by DESs.
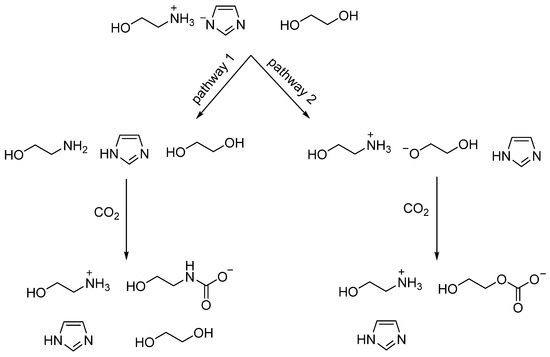
Scheme 2.
The proposed reaction mechanism between CO2 and [MEAH][Im]-EG (1:3).
3. Materials and Methods
3.1. Materials and Characterizations
Imidazole (Im, 98%), ethylene glycol (EG, 99.5%), and monoethanolamine (MEA, 99%) were obtained from J&K Scientific Ltd. (Beijing, China). CO2 (≥99.99%) was supplied by Beijing ZG Special Gases Sci. and Tech. Co. Ltd. The 1H NMR (600 MHz) and 13C NMR (151 MHz) spectra were recorded on a Bruker spectrometer using DMSO-d6 as an external solvent. FTIR spectra were recorded on a PerkinElmer Frontier spectrometer with an attenuated total reflection (ATR) accessory. The water content of the DESs was determined using Karl Fisher titration by V20 Volumetric KF titrator (Mettler Toledo, Zaventem, Belgium).
3.2. Synthesis of [MEAH][Im] and DESs
The procedure to prepare [MEAH][Im] followed the methods reported by Mukesh et al. MEA and Im were mixed in a flask at a 1:1 molar ratio, and then the mixtures were stirred at 80 °C for 2 h. The obtained [MEAH][Im] was stored in a glass vial.
The DESs [MEAH][Im]-EG (1:3) was obtained by mixing [MEAH][Im] and EG in a glass vial at a 1:3 molar ratio at room temperature. The water content of [MEAH][Im]-EG (1:3) is 0.23 wt%.
3.3. CO2 Absorption
[MEAH][Im]-EG (1:3) (2.0 g) was added into a glass tube with a diameter of 10 mm. The tube was equipped with a rubber lid, which contained two needles. The tube was partially immersed in a water bath (25 ± 0.3 °C), and CO2 was bubbled into the solvent through one needle at a flow rate of ~50 mL/min, and the other needle was for a CO2 outlet. The weight difference of the tube before and after CO2 absorption was determined by an electronic balance (±0.1 mg).
4. Conclusions
The reaction mechanism between CO2 and DES [MEA][Im]-EG (1:3) is studied carefully by using NMR and FTIR spectroscopy. The CO2 capacity of [MEA][Im]-EG (1:3) is 8.94 wt% at 25 °C and 1.0 atm. The results indicate that CO2 not only binds with the amino group of MEA but also reacts with the deprotonated EG in the solvent. There are two possible reaction pathways that form carbamate and carbonate species. One is the deprotonation of the [MEAH]+ cation by [Im]− anion, forming the neutral molecule MEA, which then reacts with CO2 to form a carbamate species. Another one is the reaction between EG and [Im]−, forming the HO-CH2-CH2-O− anion, which then binds with CO2 to form a carbonate species. We believe the new insights about the interactions between CO2 and DESs will bring benefits to the design and applications of DESs for carbon capture in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23031893/s1.
Author Contributions
Investigation, J.C., W.G., H.L., Y.M. and S.L.; data curation, J.C., D.Y., and C.W.; writing—original draft preparation, D.Y. and C.W.; writing—review and editing, D.Y.; supervision, D.Y.; funding acquisition, D.Y. and C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities (No. 2652019111, 265QZ2022003, and 2652019017) and the National Natural Science Foundation of China (No. 21503196).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Yu, D.; Xue, Z.; Mu, T. Eutectics: Formation, properties, and applications. Chem. Soc. Rev. 2021, 50, 8596–8638. [Google Scholar] [CrossRef]
- Atilhan, M.; Aparicio, S. Review and Perspectives for Effective Solutions to Grand Challenges of Energy and Fuels Technologies via Novel Deep Eutectic Solvents. Energy Fuels 2021, 35, 6402–6419. [Google Scholar] [CrossRef]
- Wu, J.; Liang, Q.; Yu, X.; Lü, Q.-F.; Ma, L.; Qin, X.; Chen, G.; Li, B. Deep Eutectic Solvents for Boosting Electrochemical Energy Storage and Conversion: A Review and Perspective. Adv. Funct. Mater. 2021, 31, 2011102. [Google Scholar] [CrossRef]
- Pelaquim, F.P.; Barbosa Neto, A.M.; Dalmolin, I.A.L.; Costa, M.C. Gas Solubility Using Deep Eutectic Solvents: Review and Analysis. Ind. Eng. Chem. Res. 2021, 60, 8607–8620. [Google Scholar] [CrossRef]
- Dashti, A.; Raji, M.; Amani, P.; Baghban, A.; Mohammadi, A.H. Insight into the Estimation of Equilibrium CO2 Absorption by Deep Eutectic Solvents using Computational Approaches. Sep. Sci. Technol. 2021, 56, 2351–2368. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Z.; Zhang, Z.; Zeng, S.; Li, F.; Zhang, X.; Nie, Y.; Zhang, L.; Zhang, S.; Ji, X. Ionic liquids/deep eutectic solvents for CO2 capture: Reviewing and evaluating. Green Energy Environ. 2021, 6, 314–328. [Google Scholar] [CrossRef]
- Altamash, T.; Amhamed, A.; Aparicio, S.; Atilhan, M. Effect of Hydrogen Bond Donors and Acceptors on CO2 Absorption by Deep Eutectic Solvents. Processes 2020, 8, 1533. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.-P.; Ji, X. Carbon Dioxide Capture with Ionic Liquids and Deep Eutectic Solvents: A New Generation of Sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mikkola, J.-P. Unusual temperature-promoted carbon dioxide capture in deep-eutectic solvents: The synergistic interactions. Chem. Commun. 2019, 55, 3939–3942. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Ma, J.; Yang, N.; Huang, Z.; Zhang, N.; Tantai, X.; Sun, Y.; Zhang, L. Superbase/Acylamido-Based Deep Eutectic Solvents for Multiple-Site Efficient CO2 Absorption. Energy Fuels 2019, 33, 7569–7577. [Google Scholar] [CrossRef]
- Cao, L.; Huang, J.; Zhang, X.; Zhang, S.; Gao, J.; Zeng, S. Imidazole tailored deep eutectic solvents for CO2 capture enhanced by hydrogen bonds. Phys. Chem. Chem. Phys. 2015, 17, 27306–27316. [Google Scholar] [CrossRef] [PubMed]
- Belal Haider, M.; Kumar, R. Solubility of CO2 and CH4 in Sterically Hindered Amine-Based Deep Eutectic Solvents. Sep. Purif. Technol. 2020, 248, 117055. [Google Scholar] [CrossRef]
- Fu, H.; Wang, X.; Sang, H.; Liu, J.; Lin, X.; Zhang, L. Highly efficient absorption of carbon dioxide by EG-assisted DBU-based deep eutectic solvents. J. CO2 Util. 2021, 43, 101372. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Jeong, Y.K.; Choi, J.W. Deep eutectic solvents as attractive media for CO2 capture. Green Chem. 2016, 18, 2834–2842. [Google Scholar] [CrossRef]
- Cui, G.; Lv, M.; Yang, D. Efficient CO2 absorption by azolide-based deep eutectic solvents. Chem. Commun. 2019, 55, 1426–1429. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Z.; Zhang, H.; Ma, J.; Jiang, B.; Zhang, L. Highly Efficient and Reversible CO2 Capture by Task-Specific Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2019, 58, 13321–13329. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, L.; Bai, Y.; Li, F.; Dong, H.; Wang, H.; Zhang, X.; Zeng, S. Superbase Ionic Liquid-Based Deep Eutectic Solvents for Improving CO2 Absorption. ACS Sustain. Chem. Eng. 2020, 8, 2523–2530. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Penley, D.; Klemm, A.; Dean, W.; Gurkan, B. Deep Eutectic Solvent Formed by Imidazolium Cyanopyrrolide and Ethylene Glycol for Reactive CO2 Separations. ACS Sustain. Chem. Eng. 2021, 9, 1090–1098. [Google Scholar] [CrossRef]
- Mukesh, C.; Khokarale, S.G.; Virtanen, P.; Mikkola, J.-P. Rapid desorption of CO2 from deep eutectic solvents based on polyamines at lower temperatures: An alternative technology with industrial potential. Sustain. Energy Fuels 2019, 3, 2125–2134. [Google Scholar] [CrossRef]
- Fan, G.-j.; Wee, A.G.H.; Idem, R.; Tontiwachwuthikul, P. NMR Studies of Amine Species in MEA−CO2−H2O System: Modification of the Model of Vapor−Liquid Equilibrium (VLE). Ind. Eng. Chem. Res. 2009, 48, 2717–2720. [Google Scholar] [CrossRef]
- Yang, D.; Lv, M.; Chen, J. Efficient non-aqueous solvent formed by 2-piperidineethanol and ethylene glycol for CO2 absorption. Chem. Commun. 2019, 55, 12483–12486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Chen, J.; Wu, C.; Yang, D. The Influence of Hydrogen Bond Donors on the CO2 Absorption Mechanism by the Bio-Phenol-Based Deep Eutectic Solvents. Molecules 2021, 26, 7167. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).