Strategy to Establish Embryo-Derived Pluripotent Stem Cells in Cattle
Abstract
1. Introduction
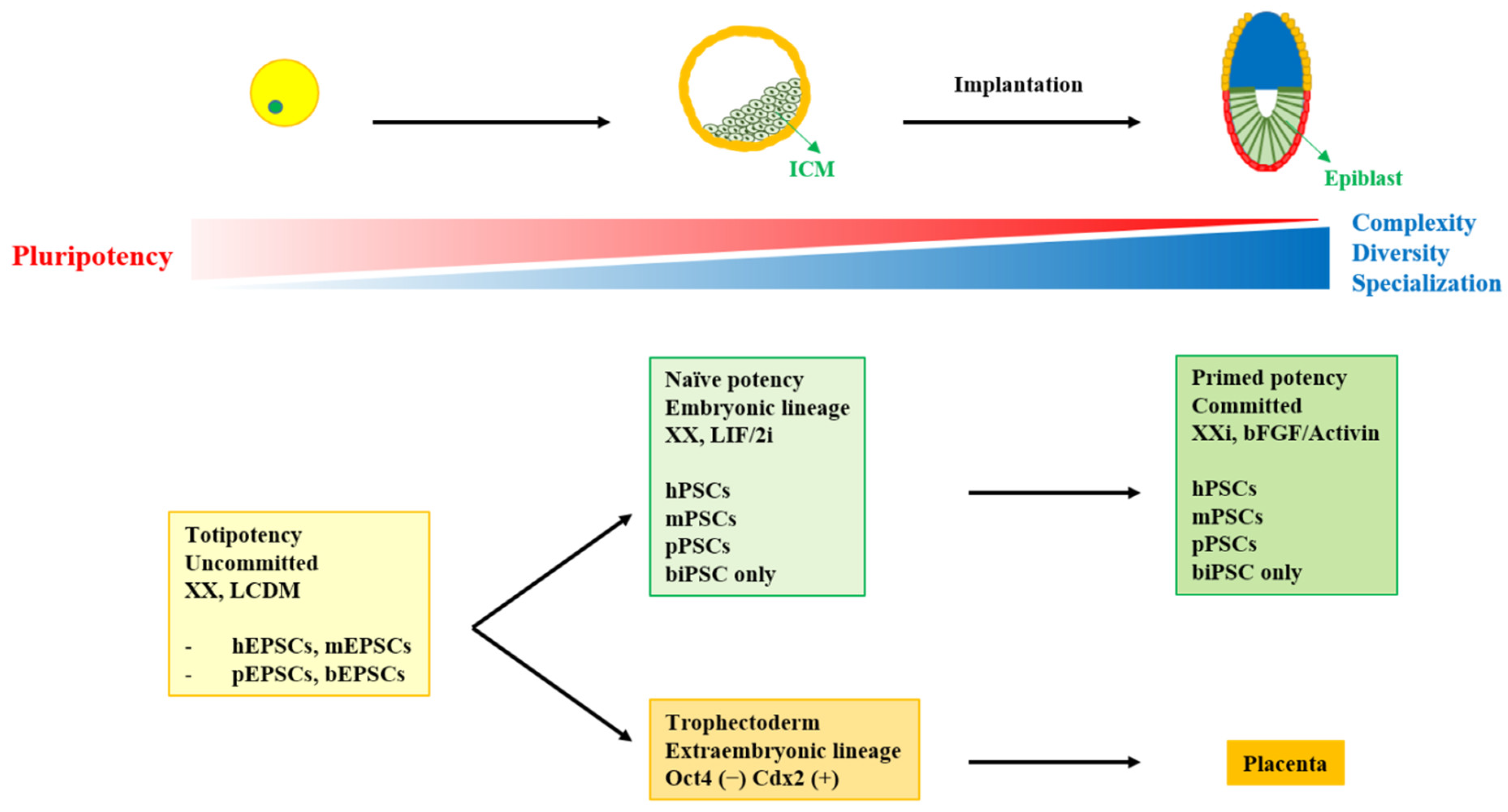
2. Overview of Pluripotent Stem Cells
3. Bovine Embryonic Stem Cells
4. Small Molecules for Capturing Bovine Pluripotency
5. Other Pluripotent Stem Cells in Cattle
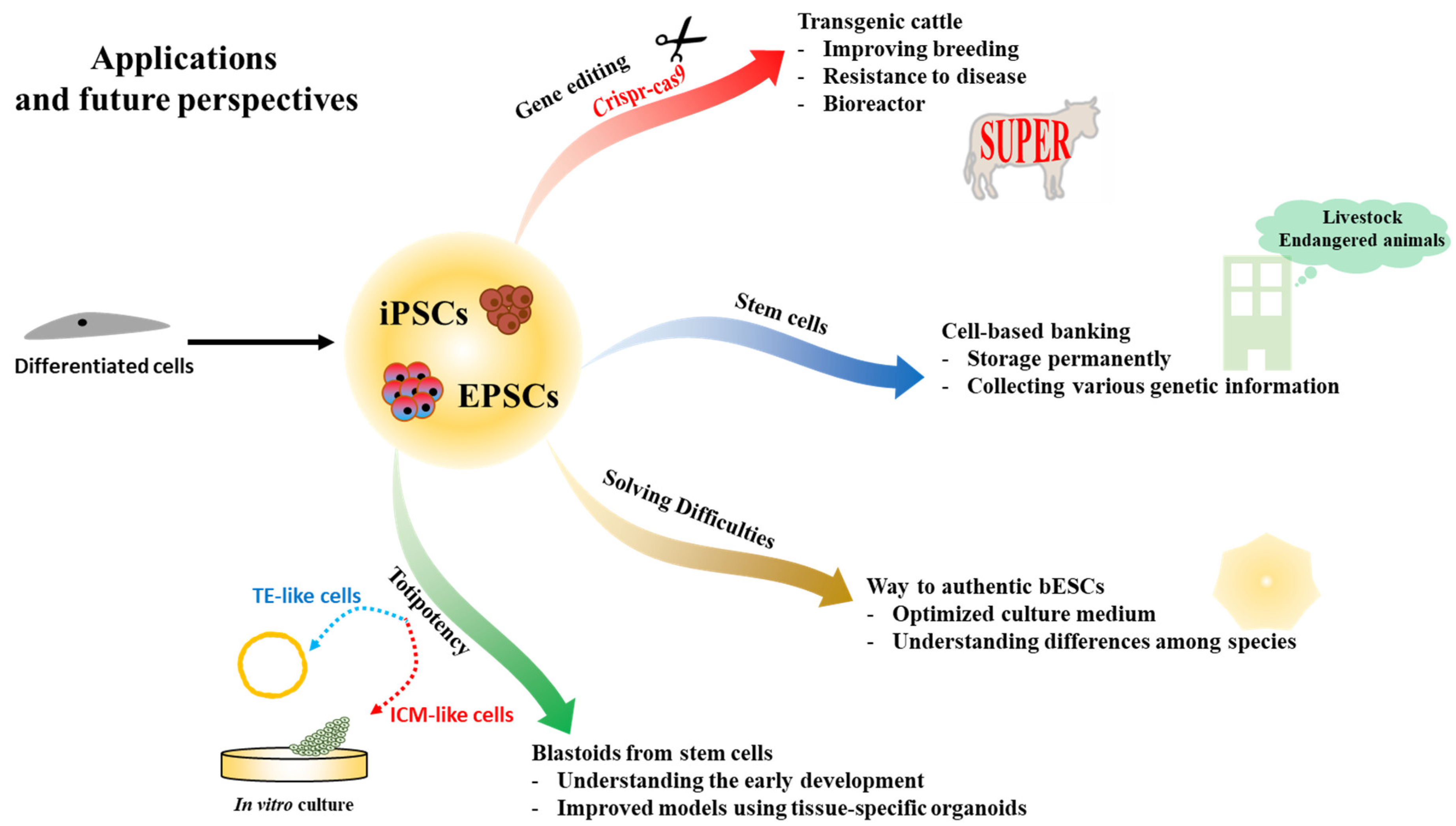
6. Applications and Future Perspectives
6.1. Transgenic Cattle by Genetic Editing
6.2. Cell-Based Banking in Animals
6.3. Difficulties in Generating Bovine Embryonic Stem Cells
6.4. Blastoids from Stem Cells
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurdon, J.B. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol. 1962, 10, 622–640. [Google Scholar]
- Wilmut, I.; Schnieke, A.E.; McWhir, J.; Kind, A.J.; Campbell, K.H.S. Viable offspring derived from fetal and adult mammalian cells. Nature 1997, 385, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, T.; Perry, A.C.F.; Zuccotti, M.; Johnson, K.R.; Yanagimachi, R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998, 394, 369–374. [Google Scholar] [CrossRef]
- Polejaeva, I.A.; Chen, S.H.; Vaught, T.D.; Page, R.L.; Mullins, J.; Ball, S.; Dal, Y.; Boone, J.; Walker, S.; Ayares, D.L.; et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 2000, 407, 86–90. [Google Scholar] [CrossRef]
- Shin, T.; Kraemer, D.; Pryor, J.; Liu, L.; Rugila, J.; Howe, L.; Buck, S.; Murphy, K.; Lyons, L.; Westhusin, M. A cat cloned by nuclear transplantation. Nature 2002, 415, 859. [Google Scholar] [CrossRef]
- Galli, C.; Lagutina, I.; Crotti, G.; Colleoni, S.; Turini, P.; Ponderato, N.; Duchi, R.; Lazzari, G. A cloned horse born to its dam twin. Nature 2003, 424, 635. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Kim, M.K.; Jang, G.; Oh, H.J.; Yuda, F.; Kim, H.J.; Shamim, M.H.; Kim, J.J.; Kang, S.K.; Schatten, G.; et al. Developmemtal technology: Dogs cloned from adult somatic cells. Nature 2005, 436, 641. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, Y.; Wang, Y.; Nie, Y.; Zhang, C.; Xu, Y.; Zhang, X.; Lu, Y.; Wang, Z.; Poo, M.; et al. Cloning of Macaque Monkeys by Somatic Cell Nuclear Transfer. Cell 2018, 172, 881–887.e7. [Google Scholar] [CrossRef]
- Matoba, S.; Zhang, Y. Somatic Cell Nuclear Transfer Reprogramming: Mechanisms and Applications. Cell Stem Cell 2018. [Google Scholar] [CrossRef]
- Kato, Y.; Tani, T.; Sotomaru, Y.; Kurokawa, K.; Kato, J.Y.; Doguchi, H.; Yasue, H.; Tsunoda, Y. Eight calves cloned from somatic cells of a single adult. Science 1998, 282, 2095–2098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Xiong, X.R.; An, Z.X.; Wang, L.J.; Liu, J.; Quan, F.S.; Hua, S.; Zhang, Y. Production of cloned calves by combination treatment of both donor cells and early cloned embryos with 5-aza-2/-deoxycytidine and trichostatin A. Theriogenology 2011, 75, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Iager, A.E.; Ragina, N.P.; Ross, P.J.; Beyhan, Z.; Cunniff, K.; Rodriguez, R.M.; Cibelli, J.B. Trichostatin A improves histone acetylation in bovine somatic cell nuclear transfer early embryos. Cloning Stem Cells 2008, 10, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, P.; Tang, B.; Sun, X.; Zhang, R.; Guo, C.; Gong, G.; Liu, Y.; Li, R.; Zhang, L.; et al. Expression and characterization of bioactive recombinant human α-lactalbumin in the milk of transgenic cloned cows. J. Dairy Sci. 2008, 91, 4466–4476. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Tang, B.; Liu, Y.; Guo, C.; Yang, P.; Yu, T.; Li, R.; Zhao, J.; Zhang, L.; et al. Characterization of bioactive recombinant human lysozyme expressed in milk of cloned transgenic cattle. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Carvalho, B.P.; Cunha, A.T.M.; Silva, B.D.M.; Sousa, R.V.; Leme, L.O.; Dode, M.A.N.; Melo, E.O. Production of transgenic cattle by somatic cell nuclear transfer (SCNT) with the human granulocyte colony-stimulation factor (hG-CSF). J. Anim. Sci. Technol. 2019, 61, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Krimpenfort, P.; Rademakers, A.; Eyestone, W.; van der Schans, A.; van den Broek, S.; Kooiman, P.; Kootwijk, E.; Platenburg, G.; Pieper, F.; Strijker, R.; et al. Generation of transgenic dairy cattle using ‘in vitro’ embryo production. Bio/Technology 1991, 9, 844–847. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Z.; Yu, T.; Ding, F.; Li, L.; Wang, X.; Fu, M.; Wang, H.; Huang, J.; Li, N.; et al. Large-scale production of recombinant human lactoferrin from high-expression, marker-free transgenic cloned cows. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Al-Ghobashy, M.A.; Elmeshad, A.N.; Abdelsalam, R.M.; Nooh, M.M.; Al-Shorbagy, M.; Laible, G. Development and Pre-Clinical Evaluation of Recombinant Human Myelin Basic Protein Nano Therapeutic Vaccine in Experimental Autoimmune Encephalomyelitis Mice Animal Model. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Thomson, J.A. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Fang, Z.F.; Gai, H.; Huang, Y.Z.; Li, S.G.; Chen, X.J.; Shi, J.J.; Wu, L.; Liu, A.; Xu, P.; Sheng, H.Z. Rabbit embryonic stem cell lines derived from fertilized, parthenogenetic or somatic cell nuclear transfer embryos. Exp. Cell Res. 2006, 312, 3669–3682. [Google Scholar] [CrossRef]
- Buehr, M.; Meek, S.; Blair, K.; Yang, J.; Ure, J.; Silva, J.; McLay, R.; Hall, J.; Ying, Q.L.; Smith, A. Capture of Authentic Embryonic Stem Cells from Rat Blastocysts. Cell 2008, 135, 1287–1298. [Google Scholar] [CrossRef]
- Nichols, J.; Smith, A. Naive and Primed Pluripotent States. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef]
- Tesar, P.J.; Chenoweth, J.G.; Brook, F.A.; Davies, T.J.; Evans, E.P.; Mack, D.L.; Gardner, R.L.; McKay, R.D.G. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007, 448, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Nichols, J.; Chambers, I.; Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003, 115, 281–292. [Google Scholar] [CrossRef]
- Weinberger, L.; Ayyash, M.; Novershtern, N.; Hanna, J.H. Dynamic stem cell states: Naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 2016, 17, 155–169. [Google Scholar] [CrossRef]
- Chia, N.Y.; Chan, Y.S.; Feng, B.; Lu, X.; Orlov, Y.L.; Moreau, D.; Kumar, P.; Yang, L.; Jiang, J.; Lau, M.S.; et al. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature 2010, 468, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Stice, S.L.; Strelchenko, N.S.; Keefer, C.L.; Matthews, L. Pluripotent bovine embryonic cell lines direct embryonic development following nuclear transfer. Biol. Reprod. 1996, 54, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.L.; Vassiliev, I.; Richings, N.M.; Firsova, A.B.; Zhang, C.; Verma, P.J. A novel, efficient method to derive bovine and mouse embryonic stem cells with in vivo differentiation potential by treatment with 5-azacytidine. Theriogenology 2011, 76, 133–142. [Google Scholar] [CrossRef]
- Mitalipova, M.; Beyhan, Z.; First, N.L. Pluripotency of bovine embryonic cell line derived from precompacting embryos. Cloning 2001, 3, 59–67. [Google Scholar] [CrossRef]
- Van Stekelenburg-Hamers, A.E.; Van Achterberg, T.A.; Rebel, H.G.; Flechon, J.E.; Campbell, K.H.; Weima, S.M.; Mummery, C.L. Isolation and characterization of permanent cell lines from inner cell mass cells of bovine blastocysts. Mol. Reprod. Dev. 1995, 40, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, S.; Jung, Y.G.Y.-G.G.; Roh, S. in vitro culture of stem-like cells derived from somatic cell nuclear transfer bovine embryos of the Korean beef cattle species, HanWoo. Reprod. Fertil. Dev. 2016, 28, 1762–1780. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, M.; Yang, X.; Liu, X.; Liu, K.; Jiao, C.; Wang, J.; Bai, C.; Su, G.; Liu, X.; et al. Establishment of bovine embryonic stem cells after knockdown of CDX2. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Jung, Y.G.; Roh, S. Thiazovivin, a Rho kinase inhibitor, improves stemness maintenance of embryo-derived stem-like cells under chemically defined culture conditions in cattle. Anim. Reprod. Sci. 2015. [Google Scholar] [CrossRef]
- Niwa, H.; Toyooka, Y.; Shimosato, D.; Strumpf, D.; Takahashi, K.; Yagi, R.; Rossant, J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 2005, 123, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yabuuchi, A.; Eminli, S.; Takeuchi, A.; Lu, C.W.; Hochedlinger, K.; Daley, G.Q. Cross-regulation of the nanog and Cdx2 promoters. Cell Res. 2009, 19, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Strumpf, D.; Mao, C.A.; Yamanaka, Y.; Ralston, A.; Chawengsaksophak, K.; Beck, F.; Rossant, J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 2005, 132, 2093–2102. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Dufort, I.; Caballero, J.; Moulavi, F.; Ghanaei, H.R.; Sirard, M.A. Transcriptome profiling of bovine inner cell mass and trophectoderm derived from in vivo generated blastocysts. BMC Dev. Biol. 2015, 15. [Google Scholar] [CrossRef]
- Madeja, Z.E.; Hryniewicz, K.; Orsztynowicz, M.; Pawlak, P.; Perkowska, A. WNT/β-catenin signaling affects cell lineage and pluripotency-specific gene expression in bovine blastocysts: Prospects for bovine embryonic stem cell derivation. Stem Cells Dev. 2015, 24, 2437–2454. [Google Scholar] [CrossRef]
- Kirchhof, N.; Carnwath, J.W.; Lemme, E.; Anastassiadis, K.; Scholer, H.; Niemann, H. Expression pattern of Oct-4 in preimplantation embryos of different species. Biol. Reprod. 2000, 63, 1698–1705. [Google Scholar] [CrossRef]
- Ozawa, M.; Sakatani, M.; Yao, J.; Shanker, S.; Yu, F.; Yamashita, R.; Wakabayashi, S.; Nakai, K.; Dobbs, K.B.; Sudano, M.J.; et al. Global gene expression of the inner cell mass and trophectoderm of the bovine blastocyst. BMC Dev. Biol. 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Keefer, C.L.; Pant, D.; Blomberg, L.; Talbot, N.C. Challenges and prospects for the establishment of embryonic stem cell lines of domesticated ungulates. Anim. Reprod. Sci. 2007, 98, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.K.; Smith, C.S.; Pearton, D.J.; Wells, D.N.; Broadhurst, R.; Donnison, M.; Pfeffer, P.L. Trophectoderm Lineage Determination in Cattle. Dev. Cell 2011, 20, 244–255. [Google Scholar] [CrossRef]
- Kim, D.; Jung, Y.-G.; Roh, S. Microarray analysis of embryo-derived bovine pluripotent cells: The vulnerable state of bovine embryonic stem cells. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Bogliotti, Y.S.; Wu, J.; Vilarino, M.; Okamura, D.; Soto, D.A.; Zhong, C.; Sakurai, M.; Sampaio, R.V.; Suzuki, K.; Izpisua Belmonte, J.C.; et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2090–2095. [Google Scholar] [CrossRef]
- Saito, S.; Strelchenko, N.; Niemann, H. Bovine embryonic stem cell-like cell lines cultured over several passages. Roux’s Arch. Dev. Biol. 1992, 201, 134–141. [Google Scholar] [CrossRef]
- Iwasaki, S.; Campbell, K.H.; Galli, C.; Akiyama, K. Production of live calves derived from embryonic stem-like cells aggregated with tetraploid embryos. Biol. Reprod. 2000, 62, 470–475. [Google Scholar] [CrossRef][Green Version]
- Saito, S.; Sawai, K.; Ugai, H.; Moriyasu, S.; Minamihashi, A.; Yamamoto, Y.; Hirayama, H.; Kageyama, S.; Pan, J.; Murata, T.; et al. Generation of cloned calves and transgenic chimeric embryos from bovine embryonic stem-like cells. Biochem. Biophys. Res. Commun. 2003, 309, 104–113. [Google Scholar] [CrossRef]
- Jin, M.; Wu, A.; Dorzhin, S.; Yue, Q.; Ma, Y.; Liu, D. Culture conditions for bovine embryonic stem cell-like cells isolated from blastocysts after external fertilization. Cytotechnology 2012, 64, 379–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verma, V.; Huang, B.; Kallingappa, P.K.; Oback, B. Dual kinase inhibition promotes pluripotency in finite bovine embryonic cell lines. Stem Cells Dev. 2013, 22, 1728–1742. [Google Scholar] [CrossRef]
- Ying, Q.L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef]
- Batlle-Morera, L.; Smith, A.; Nichols, J. Parameters influencing derivation of embryonic stem cells from murine embryos. Genesis 2008, 46, 758–767. [Google Scholar] [CrossRef]
- Pereira, L.; Yi, F.; Merrill, B.J. Repression of Nanog Gene Transcription by Tcf3 Limits Embryonic Stem Cell Self-Renewal. Mol. Cell. Biol. 2006, 26, 7479–7491. [Google Scholar] [CrossRef]
- Takao, Y.; Yokota, T.; Koide, H. β-Catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem. Biophys. Res. Commun. 2007, 353, 699–705. [Google Scholar] [CrossRef]
- Aubert, J.; Dunstan, H.; Chambers, I.; Smith, A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat. Biotechnol. 2002, 20, 1240–1245. [Google Scholar] [CrossRef]
- Anton, R.; Kestler, H.A.; Kühl, M. β-Catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 2007, 581, 5247–5254. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef]
- Ware, C.B.; Nelson, A.M.; Mecham, B.; Hesson, J.; Zhou, W.; Jonlin, E.C.; Jimenez-Caliani, A.J.; Deng, X.; Cavanaugh, C.; Cook, S.; et al. Derivation of naïve human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4484–4489. [Google Scholar] [CrossRef]
- Duggal, G.; Warrier, S.; Ghimire, S.; Broekaert, D.; Van der Jeught, M.; Lierman, S.; Deroo, T.; Peelman, L.; Van Soom, A.; Cornelissen, R.; et al. Alternative Routes to Induce Naïve Pluripotency in Human Embryonic Stem Cells. Stem Cells 2015, 33, 2686–2698. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Yasuda, S.; Kahn, M. Wnt/β-catenin Signaling in Embryonic Stem Cell Self-renewal and Somatic Cell Reprogramming. Stem Cell Rev. Rep. 2011, 7, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Ng, J.H.; Heng, J.C.D.; Ng, H.H. Molecules that Promote or Enhance Reprogramming of Somatic Cells to Induced Pluripotent Stem Cells. Cell Stem Cell 2009, 4, 301–312. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Liang, G.; Zhang, Y. Embryonic stem cell and induced pluripotent stem cell: An epigenetic perspective. Cell Res. 2013, 23, 49–69. [Google Scholar] [CrossRef]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.W.; Markoulaki, S.; Hanna, J.H.; Faddah, D.A.; Buganim, Y.; Kim, J.; Ganz, K.; Steine, E.J.; Cassady, J.P.; Creyghton, M.P.; et al. Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell Stem Cell 2011, 9, 588–598. [Google Scholar] [CrossRef]
- Chin, M.H.; Mason, M.J.; Xie, W.; Volinia, S.; Singer, M.; Peterson, C.; Ambartsumyan, G.; Aimiuwu, O.; Richter, L.; Zhang, J.; et al. Induced Pluripotent Stem Cells and Embryonic Stem Cells Are Distinguished by Gene Expression Signatures. Cell Stem Cell 2009, 5, 111–123. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.V.; Kei, T.G.; Reddy, S.E.; Das, M.; Abratte, C.; Cheong, S.H.; Selvaraj, V. Induced pluripotent stem cell generation from bovine somatic cells indicates unmet needs for pluripotency sustenance. Anim. Sci. J. 2019, 90, 1149–1160. [Google Scholar] [CrossRef]
- Fang, R.; Liu, K.; Xu, J.; Deng Correspondence, H. Generation of Naive Induced Pluripotent Stem Cells from Rhesus Monkey Fibroblasts. Stem Cell 2014, 15, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Wainger, B.J.; Kiskinis, E.; Mellin, C.; Wiskow, O.; Han, S.S.W.; Sandoe, J.; Perez, N.P.; Williams, L.A.; Lee, S.; Boulting, G.; et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014, 7, 1–11. [Google Scholar] [CrossRef]
- Huang, B.; Li, T.; Alonso-Gonzalez, L.; Gorre, R.; Keatley, S.; Green, A.; Turner, P.; Kallingappa, P.K.; Verma, V.; Oback, B. A Virus-Free Poly-Promoter Vector Induces Pluripotency in Quiescent Bovine Cells under Chemically Defined Conditions of Dual Kinase Inhibition. PLoS ONE 2011, 6, e24501. [Google Scholar] [CrossRef][Green Version]
- Sumer, H.; Liu, J.; Malaver-Ortega, L.F.; Lim, M.L.; Khodadadi, K.; Verma, P.J. NANOG is a key factor for induction of pluripotency in bovine adult fibroblasts1. J. Anim. Sci. 2011, 89, 2708–2716. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Xu, J.; Wang, J.; Wu, J.; Shi, C.; Xu, Y.; Dong, J.; Wang, C.; Lai, W.; et al. Derivation of Pluripotent Stem Cells with in vivo Embryonic and Extraembryonic Potency. Cell 2017, 169, 243–257.e25. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, H.; Zhang, Y.; Wang, J.; Liu, F.; Han, X.; Lu, Z.; Li, C.; Li, Z.; Gao, Y.; et al. LCDM medium supports the derivation of bovine extended pluripotent stem cells with embryonic and extraembryonic potency in bovine-mouse chimeras from iPSCs and bovine fetal fibroblasts. FEBS J. 2021, 1–18. [Google Scholar] [CrossRef]
- Yang, J.; Ryan, D.J.; Wang, W.; Tsang, J.C.H.; Lan, G.; Masaki, H.; Gao, X.; Antunes, L.; Yu, Y.; Zhu, Z.; et al. Establishment of mouse expanded potential stem cells. Nature 2017, 550, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Nowak-Imialek, M.; Chen, X.; Chen, D.; Herrmann, D.; Ruan, D.; Chen, A.C.H.; Eckersley-Maslin, M.A.; Ahmad, S.; Lee, Y.L.; et al. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 2019, 21, 687–699. [Google Scholar] [CrossRef]
- Han, X.; Han, J.; Ding, F.; Cao, S.; Lim, S.S.; Dai, Y.; Zhang, R.; Zhang, Y.; Lim, B.; Li, N. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. Cell Res. 2011, 21, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yang, P.; Pu, Y.; Sun, X.; Yin, H.; Zhang, Y.; Zhang, Y.; Li, Y.; Liu, Y.; Fang, F.; et al. Characterization of bovine induced pluripotent stem cells by lentiviral transduction of reprogramming factor fusion proteins. Int. J. Biol. Sci. 2012, 8, 498–511. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, S.S.W.; Wu, D.C.; Lin, Y.C.; Ku, C.C.; Wu, C.C.; Chai, C.Y.; Lee, J.N.; Tsai, E.M.; Lin, C.L.S.; et al. Androgen receptor-mediated apoptosis in bovine testicular induced pluripotent stem cells in response to phthalate esters. Cell Death Dis. 2013, 4, e907. [Google Scholar] [CrossRef] [PubMed]
- Talluri, T.R.; Kumar, D.; Glage, S.; Garrels, W.; Ivics, Z.; Debowski, K.; Behr, R.; Niemann, H.; Kues, W.A. Derivation and characterization of bovine induced pluripotent stem cells by transposon-mediated reprogramming. Cell. Reprogram. 2015, 17, 131–140. [Google Scholar] [CrossRef]
- Heo, Y.T.; Quan, X.; Xu, Y.N.; Baek, S.; Choi, H.; Kim, N.-H.; Kim, J. CRISPR/Cas9 Nuclease-Mediated Gene Knock-In in Bovine-Induced Pluripotent Cells. Stem Cells Dev. 2015, 24, 393–402. [Google Scholar] [CrossRef]
- Malaver-Ortega, L.F.; Sumer, H.; Liu, J.; Verma, P.J. Inhibition of JAK-STAT ERK/MAPK and Glycogen Synthase Kinase-3 Induces a Change in Gene Expression Profile of Bovine Induced Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.; Zhang, J.; Yang, J.; Gao, X.; Wu, B.; Zhao, G.; Bao, S.; Hu, S.; Liu, P.; et al. Characterization of the single-cell derived bovine induced pluripotent stem cells. Tissue Cell 2017, 49, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Laible, G.; Alonso-González, L. Gene targeting from laboratory to livestock: Current status and emerging concepts. Biotechnol. J. 2009, 4, 1278–1292. [Google Scholar] [CrossRef]
- Goszczynski, D.E.; Cheng, H.; Demyda-Peyrás, S.; Medrano, J.F.; Wu, J.; Ross, P.J. in vitro breeding: Application of embryonic stem cells to animal production. Biol. Reprod. 2019, 100, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Eakin, G.S.; Hadjantonakis, A.K. Production of chimeras by aggregation of embryonic stem cells with diploid or tetraploid mouse embryos. Nat. Protoc. 2006, 1, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Taft, R.A.; Low, B.E.; Byers, S.L.; Murray, S.A.; Kutny, P.; Wiles, M.V. The Perfect Host: A Mouse Host Embryo Facilitating More Efficient Germ Line Transmission of Genetically Modified Embryonic Stem Cells. PLoS ONE 2013, 8, e67826. [Google Scholar] [CrossRef][Green Version]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef]
- Mara, L.; Casu, S.; Carta, A.; Dattena, M. Cryobanking of farm animal gametes and embryos as a means of conserving livestock genetics. Anim. Reprod. Sci. 2013, 138, 25–38. [Google Scholar] [CrossRef]
- Ogonuki, N.; Inoue, K.; Yamamoto, Y.; Noguchi, Y.; Tanemura, K.; Suzuki, O.; Nakayama, H.; Doi, K.; Ohtomo, Y.; Satoh, M.; et al. Early death of mice cloned from somatic cells. Nat. Genet. 2002, 30, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Arat, S.; Caputcu, A.T.; Akkoc, T.; Pabuccuoglu, S.; Sagirkaya, H.; Cirit, U.; Nak, Y.; Koban, E.; Bagis, H.; Demir, K.; et al. Using cell banks as a tool in conservation programmes of native domestic breeds: The production of the first cloned Anatolian Grey cattle. Reprod. Fertil. Dev. 2011, 23, 1012. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Liu, C.L.; Ting, C.Y.; Chiu, Y.T.; Cheng, Y.C.; Nicholson, M.W.; Hsieh, P.C.H. Human iPSC banking: Barriers and opportunities. J. Biomed. Sci. 2019, 26, 1–14. [Google Scholar] [CrossRef]
- Friedrich Ben-Nun, I.; Montague, S.C.; Houck, M.L.; Tran, H.T.; Garitaonandia, I.; Leonardo, T.R.; Wang, Y.C.; Charter, S.J.; Laurent, L.C.; Ryder, O.A.; et al. Induced pluripotent stem cells from highly endangered species. Nat. Methods 2011, 8, 829–831. [Google Scholar] [CrossRef]
- Verma, R.; Liu, J.; Holland, M.K.; Temple-Smith, P.; Williamson, M.; Verma, P.J. Nanog Is an Essential Factor for Induction of Pluripotency in Somatic Cells from Endangered Felids. Biores. Open Access 2013, 2, 72–76. [Google Scholar] [CrossRef]
- Piliszek, A.; Madeja, Z.E. Pre-implantation Development of Domestic Animals. In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 128, pp. 267–294. [Google Scholar]
- Płusa, B.; Piliszek, A. Common principles of early mammalian embryo self-organisation. Development 2020, 147, dev183079. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Tsukiyama, T.; Kimura, K.; Matsuyama, S.; Minami, N.; Yamada, M.; Imai, H. Generation of Naïve Bovine Induced Pluripotent Stem Cells Using PiggyBac Transposition of Doxycycline-Inducible Transcription Factors. PLoS ONE 2015, 10, e0135403. [Google Scholar] [CrossRef]
- Moris, N.; Anlas, K.; van den Brink, S.C.; Alemany, A.; Schröder, J.; Ghimire, S.; Balayo, T.; van Oudenaarden, A.; Martinez Arias, A. An in vitro model of early anteroposterior organization during human development. Nature 2020, 582, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Sozen, B.; Cox, A.L.; De Jonghe, J.; Bao, M.; Hollfelder, F.; Glover, D.M.; Zernicka-Goetz, M. Self-Organization of Mouse Stem Cells into an Extended Potential Blastoid. Dev. Cell 2019, 51, 698–712.e8. [Google Scholar] [CrossRef] [PubMed]
- Rivron, N.C.; Frias-Aldeguer, J.; Vrij, E.J.; Boisset, J.C.; Korving, J.; Vivié, J.; Truckenmüller, R.K.; Van Oudenaarden, A.; Van Blitterswijk, C.A.; Geijsen, N. Blastocyst-like structures generated solely from stem cells. Nature 2018, 557, 106–111. [Google Scholar] [CrossRef]
- Li, R.; Zhong, C.; Yu, Y.; Liu, H.; Sakurai, M.; Yu, L.; Min, Z.; Shi, L.; Wei, Y.; Takahashi, Y.; et al. Generation of Blastocyst-like Structures from Mouse Embryonic and Adult Cell Cultures. Cell 2019, 179, 687–702.e18. [Google Scholar] [CrossRef]
- Czerniecki, S.M.; Cruz, N.M.; Harder, J.L.; Menon, R.; Annis, J.; Otto, E.A.; Gulieva, R.E.; Islas, L.V.; Kim, Y.K.; Tran, L.M.; et al. High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell 2018, 22, 929–940.e4. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Huch, M. Disease modelling in human organoids. DMM Dis. Model. Mech. 2019, 12. [Google Scholar] [CrossRef]
- Di Lullo, E.; Kriegstein, A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017, 18, 573–584. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Nashun, B.; Hill, P.W.; Hajkova, P. Reprogramming of cell fate: Epigenetic memory and the erasure of memories past. EMBO J. 2015, 34, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.; Pelizzola, M.; Kida, Y.S.; Hawkins, R.D.; Nery, J.R.; Hon, G.; Antosiewicz-Bourget, J.; Ogmalley, R.; Castanon, R.; Klugman, S.; et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2011, 471, 68–73. [Google Scholar] [CrossRef]
| Medium | Morphology | Pluripotency | Differentiation | Special | Reference |
|---|---|---|---|---|---|
| FCS, heparin, LIF | mES-like cells | X | Epithelial, fibroblastic, neuron-type cells | With trophoblastic cell | [46] |
| FCS | Low cytoplasmic/nuclear ratio | X | in vitro differentiation | Trophecoderm-like cells | [28] |
| FBS, LIF | Monolayer cells | X | X | Tetraploid embryos test Contributing to liver, placenta, and hair roots in chimera | [47] |
| FBS | Small cytoplasmic/nuclear volume ratio | SSEA-1(+), SSEA-3(+), SSEA-4(+) | in vitro differentiation | Long term culture Cystic form observed like TE | [30] |
| FBS, LIF, EGF | Small cells compact colony | AP(+), SSEA-1(+), STAT3(+), OCT4(+) SSEA-3(−), SSEA-4(−) | in vitro differentiation | Chimeric test Contributing to both lineages | [48] |
| FCS, ITS, LIF, bFGF, EGF, 5-azacytidine | Heterogenetic morphology | REX1(+), OCT4(+), SSEA-4(+) | in vitro differentiation | 5-azacytidine improved pluripotency and ability to differentiate | [29] |
| FBS, bFGF, SCF | Bubble-like or TE-like cell | OCT4(+), SSEA-1(+), SSEA4(+), AP(+) | in vitro differentiation | Stem cell factor (SCF), a cytokine that binds to the c-Kit receptor | [49] |
| PD0325901, CHIR99021 | Flat-shaped | Naïve state markers(+) Primed state markers(−) | in vitro differentiation | GATA6 and CDX2 expression | [50] |
| bFGF, LIF, KSR | Dome-like (early passages) Flat-shaped (late passages) | OCT4(+), SOX2(+), NANOG(+), E-CAD(+), SSEA1(+), SSEA4(+) | in vitro and in vivo differentiation | TE related genes still expressed in CDX2-KD lines | [33] |
| PD18435, SU5402, CHIR99021 | Heterogenetic morphology mixed with TE | Naïve state markers(+) Primed state markers (−) | in vitro and in vivo differentiation | OCT4 or Nanog positive cells without CDX2 negative | [32] |
| BSA, bFGF, IWR1 | Flat-shaped | Primed state markers(+) | in vitro and in vivo differentiation | X | [45] |
| Medium | Cell Source | Morphology | Reprogramming Factors * | Pluripotency | Differentiation | Reference |
|---|---|---|---|---|---|---|
| KSR, bFGF | MEF | Dome-like | bOSKMLN | AP, OCT4, SOX2, NANOG, SSEA1,4 | in vitro and in vivo | [78] |
| PD0325901, CHIR99021, LIF | MEF | Dome-like | bOKSM | AP, OCT4, SOX2, KIF4, SSEA3, 4, TRA-1-60 | in vitro and in vivo | [72] |
| FBS, bFGF, LIF | skin fibroblast | Dome-like | hOKMN | AP, OCT4, SOX2, KLF4, C-MYC, NANOG, SSEA1/4 | in vitro and in vivo | [73] |
| FBS, bFGF, LIF | MEF | Flat-shaped | hO+pSKM | AP, OCT4, SOX2, KLF4, NANOG, SSEA1 | in vitro and in vivo | [79] |
| LIF, FBS | testicular cells | Dome-like | hO | OCT4, SOX2, NANOG, SSEA1, SSEA4 | in vitro and in vivo | [80] |
| KSR, bFGF, hLIF | BFF | Dome-like | hOSKM | OCT4, SSEA1, 3, 4, REX1 | in vitro and in vivo | [81] |
| CHIR99021, PD0325901, Valproic acid | BFF | Dome-like | bOSKM | OCT4, SOX2, NANOG, KLF4, C-MYC, REX1 | in vitro and in vivo | [82] |
| Bio, SC1, 5-AzaC | bAF | Dome-like | hOSKMN | OCT4, NANOG, SSEA-1, SSEA-4, TRA-1-60 | in vitro and in vivo | [83] |
| FBS, LIF, bFGF | BFF | Dome-like | bOSKM | OCT4, NANOG, SOX2, SSEA1, SSEA4, AP | in vitro and in vivo | [84] |
| KSR, hLIF, CHIR99021, (S)-(+)-dimethindene maleate, and minocycline hydrochloride ** | BFF | Dome-like | bOSKM | OCT4, SOX2, NANOG | in vitro and in vivo Interspecies chimeric embryo test Totipotent-like cells | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Roh, S. Strategy to Establish Embryo-Derived Pluripotent Stem Cells in Cattle. Int. J. Mol. Sci. 2021, 22, 5011. https://doi.org/10.3390/ijms22095011
Kim D, Roh S. Strategy to Establish Embryo-Derived Pluripotent Stem Cells in Cattle. International Journal of Molecular Sciences. 2021; 22(9):5011. https://doi.org/10.3390/ijms22095011
Chicago/Turabian StyleKim, Daehwan, and Sangho Roh. 2021. "Strategy to Establish Embryo-Derived Pluripotent Stem Cells in Cattle" International Journal of Molecular Sciences 22, no. 9: 5011. https://doi.org/10.3390/ijms22095011
APA StyleKim, D., & Roh, S. (2021). Strategy to Establish Embryo-Derived Pluripotent Stem Cells in Cattle. International Journal of Molecular Sciences, 22(9), 5011. https://doi.org/10.3390/ijms22095011






