Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD)
Abstract
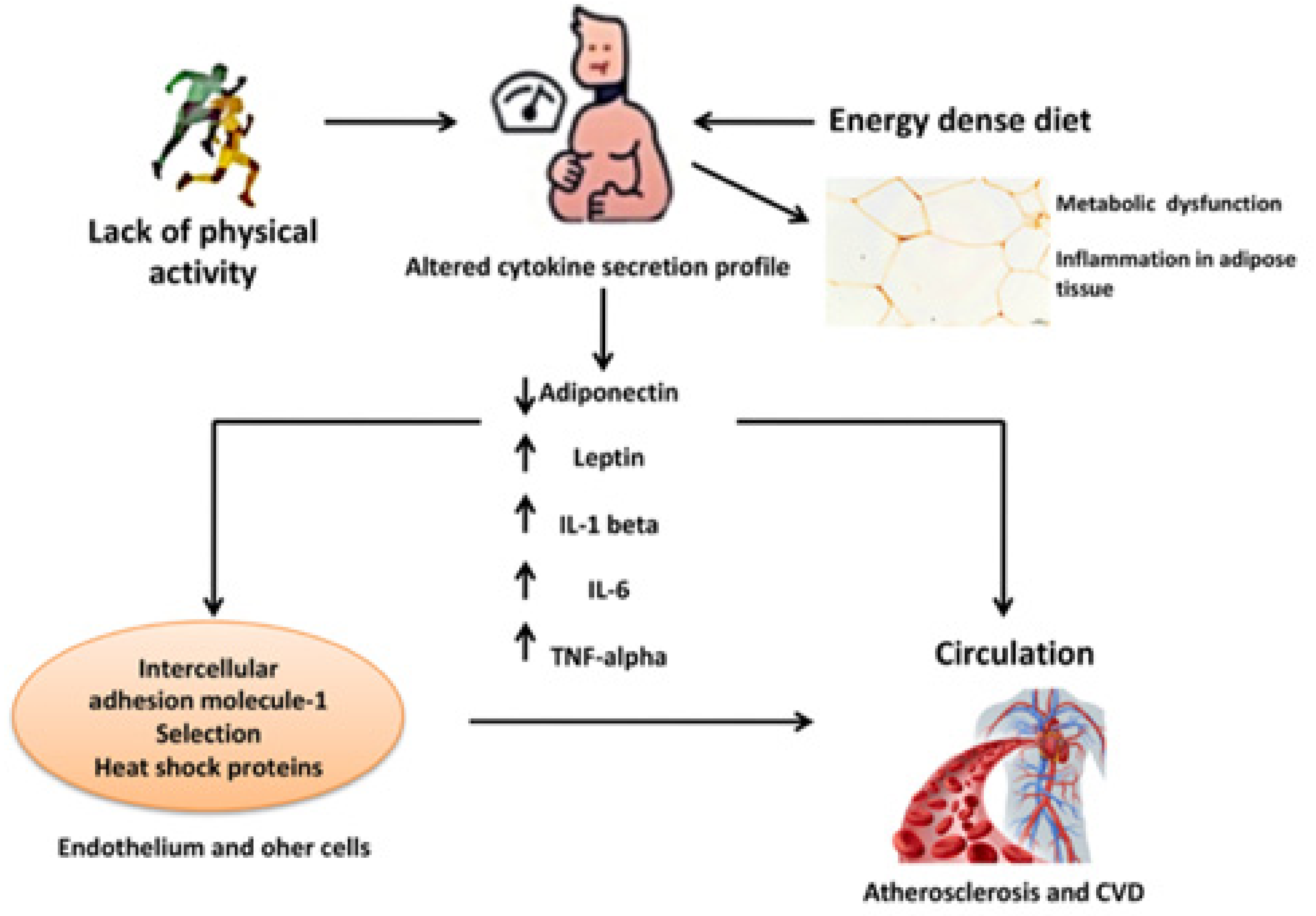
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Quality Evaluation
3. Results
3.1. Search Outcomes
3.2. Study Characteristics
3.2.1. Obesity and Inflammation
3.2.2. Obesity in Cardiovascular Diseases (CVD): Low-Grade Inflammation Effects
4. Discussion
5. Therapeutical Strategies
5.1. Pharmacological Intervention
5.2. Non-Pharmacological Interventions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hruby, A.; Manson, J.E.; Qi, L.; Malik, V.S.; Rimm, E.B.; Sun, Q.; Willett, W.C.; Hu, F.B. Determinants and Consequences of Obesity. Am. J. Public Health 2016, 106, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Bertapelli, F.; Pitetti, K.; Agiovlasitis, S.; Guerra-Junior, G. Overweight and obesity in children and adolescents with Down syndrome—Prevalence, determinants, consequences, and interventions: A literature review. Res. Dev. Disabil. 2016, 57, 181–192. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Palavra, F.; Almeida, L.; Ambrósio, A.F.; Reis, F. Obesity and brain inflammation: A focus on multiple sclerosis. Obes. Rev. 2016, 17, 211–224. [Google Scholar] [CrossRef]
- Krupa-Kotara, K.; Dakowska, D. Impact of obesity on risk of cancer. Central Eur. J. Public Health 2021, 29, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, L.; Torres, A.; Maciejewski, R.; Torres, K. Obesity and Obese-related Chronic Low-grade Inflammation in Promotion of Colorectal Cancer Development. Asian Pac. J. Cancer Prev. 2015, 16, 4161–4168. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, Inflammation and Diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Neyrinck, A.M.; Delzenne, N.M. Changes in gut microbiota control metabolic diet–induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Kodl, C.T.; Seaquist, E.R. Cognitive Dysfunction and Diabetes Mellitus. Endocr. Rev. 2008, 29, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.; Banks, W.; Shah, G.; Gu, Z.; Sowers, J. Cardiorenal Metabolic Syndrome and Diabetic Cognopathy. Cardiorenal Med. 2013, 3, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, J.L.; Coffey, T.; Cole, R.; Lei, C.; Wittmer, C.; Walsh, B.; Weyer, C.; Koda, J.; Baron, A.D.; Parkes, D.G.; et al. Amylin-Mediated Restoration of Leptin Responsiveness in Diet-Induced Obesity: Magnitude and Mechanisms. Endocrinology 2008, 149, 5679–5687. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Gustafson, D.R. Adiposity, Type 2 Diabetes, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 16, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Role of the blood–brain barrier in the evolution of feeding and cognition. Ann. N. Y. Acad. Sci. 2012, 1264, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2006, 8, 21–34. [Google Scholar] [CrossRef]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ Br. Med. J. 2015, 349, g7647. [Google Scholar] [CrossRef]
- El-Wakkad, A.; Hassan, N.E.-M.; Sibaii, H.; El-Zayat, S.R. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013, 61, 682–687. [Google Scholar] [CrossRef]
- Borges, M.D.; Franca, E.L.; Fujimori, M.; Silva, S.M.; De Marchi, P.G.; Deluque, A.L.; Honorio-Franca, A.C.; De Abreu, L.C. Relationship between Proinflammatory Cytokines/Chemokines and Adipokines in Serum of Young Adults with Obesity. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 260–267. [Google Scholar] [CrossRef]
- Larsson, A.; Carlsson, L.; Lind, A.-L.; Gordh, T.; Bodolea, C.; Kamali-Moghaddam, M.; Thulin, M. The body mass index (BMI) is significantly correlated with levels of cytokines and chemokines in cerebrospinal fluid. Cytokine 2015, 76, 514–518. [Google Scholar] [CrossRef]
- Peres, A.; Dorneles, G.P.; Dias, A.S.; Vianna, P.; Chies, J.A.B.; Monteiro, M.B. T-cell profile and systemic cytokine levels in overweight-obese patients with moderate to very-severe COPD. Respir. Physiol. Neurobiol. 2018, 247, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Rość, D.; Adamczyk, P.; Boinska, J.; Szafkowski, R.; Ponikowska, I.; Stankowska, K.; Góralczyk, B.; Ruszkowska-Ciastek, B. CRP, but not TNF-α or IL-6, decreases after weight loss in patients with morbid obesity exposed to intensive weight reduction and balneological treatment. J. Zhejiang Univ. Sci. B 2015, 16, 404–411. [Google Scholar] [CrossRef]
- Pearson, M.J.; Herndler-Brandstetter, D.; Tariq, M.A.; Nicholson, T.A.; Philp, A.M.; Smith, H.L.; Davis, E.T.; Jones, S.W.; Lord, J.M. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci. Rep. 2017, 7, 3451. [Google Scholar] [CrossRef]
- Kang, Y.E.; Kim, J.M.; Joung, K.H.; Lee, J.H.; You, B.R.; Choi, M.J.; Ryu, M.J.; Ko, Y.B.; Lee, M.A.; Lee, J.; et al. The Roles of Adipokines, Proinflammatory Cytokines, and Adipose Tissue Macrophages in Obesity-Associated Insulin Resistance in Modest Obesity and Early Metabolic Dysfunction. PLoS ONE 2016, 11, e0154003. [Google Scholar] [CrossRef]
- Engeli, S.; Feldpausch, M.; Gorzelniak, K.; Hartwig, F.; Heintze, U.; Janke, J.; Möhlig, M.; Pfeiffer, A.F.; Luft, F.C.; Sharma, A.M. Association Between Adiponectin and Mediators of Inflammation in Obese Women. Diabetes 2003, 52, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Anandaraj, A.A.; Ismail, P.M.S.; Namis, S.M.; Bajnaid, Y.J.; Shetty, S.B.; Almutairi, K.M. Association of Selected Adipocytokines and Inflammatory Markers on Body Mass Index in Type 2 Diabetes Patients in Saudi Arabia and as Risk Factors to Cardiovascular Disease. Curr. Diabetes Rev. 2017, 13, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, E.; Tengblad, A.; Länne, T.; Clinchy, B.; Ernerudh, J.; Nystrom, F.; Östgren, C. Abdominal obesity and low-grade systemic inflammation as markers of subclinical organ damage in type 2 diabetes. Diabetes Metab. 2014, 40, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Benbaibeche, H.; Hichami, A.; Oudjit, B.; Haffaf, E.M.; Kacimi, G.; Koceïr, E.A.; Khan, N.A. Circulating mir-21 and mir-146a are associated with increased cytokines and CD36 in Algerian obese male participants. Arch. Physiol. Biochem. 2020, 1–6. [Google Scholar] [CrossRef]
- Garvey, W.T.; Van Gaal, L.; Leiter, L.A.; Vijapurkar, U.; List, J.; Cuddihy, R.; Ren, J.; Davies, M.J. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism 2018, 85, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Massaro, J.M.; Rosito, G.A.; Levy, D.; Murabito, J.M.; Wolf, P.A.; O’Donnell, C.J.; Fox, C.S.; Hoffmann, U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The Framingham Heart Study. Eur. Heart J. 2008, 30, 850–856. [Google Scholar] [CrossRef]
- Motie, M.; Evangelista, L.S.; Horwich, T.; Lombardo, D.; Zaldivar, F.; Hamilton, M.; Fonarow, G.C. Association between inflammatory biomarkers and adiposity in obese patients with heart failure and metabolic syndrome. Exp. Ther. Med. 2014, 8, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of Weight Loss and Lifestyle Changes on Vascular Inflammatory Markers in Obese Women. JAMA 2003, 289, 1799–1804. [Google Scholar] [CrossRef]
- Protein, R.C. Energy Restriction and Weight Loss on Very-Low-Fat Diets Healthy Women. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 968–971. [Google Scholar]
- Lopez-Sandoval, J. Cardiovascular Risk Factors in Adolescents: Role of Insulin Resistance and Obesity. Acta Endocrinol. (Bucharest) 2018, 14, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castro, E.; Rodríguez-Yáñez, M.; Arias-Rivas, S.; Santamaría-Cadavid, M.; López-Dequidt, I.; Hervella, P.; López, M.; Campos, F.; Sobrino, T.; Castillo, J. Obesity Paradox in Ischemic Stroke: Clinical and Molecular Insights. Transl. Stroke Res. 2019, 10, 639–649. [Google Scholar] [CrossRef]
- Mirza, S.S.; Bruijn, R.F.A.G.D.; Koudstaal, P.J.; Meiracker, A.H.V.D.; Franco, O.H.; Hofman, A.; Tiemeier, H.; Ikram, M.A. The N-terminal pro B-type natriuretic peptide, and risk of dementia and cognitive decline: A 10-year follow-up study in the general population. J. Neurol. Neurosurg. Psychiatry 2015, 87, 356–362. [Google Scholar] [CrossRef]
- Gustafson, B.; Smith, U. Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis 2015, 241, 27–35. [Google Scholar] [CrossRef]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.-P. Overview of Epidemiology and Contribution of Obesity to Cardiovascular Disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef]
- Iulita, M.F.; De La Colina, A.N.; Girouard, H. Arterial stiffness, cognitive impairment and dementia: Confounding factor or real risk? J. Neurochem. 2018, 144, 527–548. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Silveira, E.A.; Vaseghi, G.; Santos, A.S.D.C.; Kliemann, N.; Masoudkabir, F.; Noll, M.; Mohammadifard, N.; Sarrafzadegan, N.; De Oliveira, C. Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments. Int. J. Mol. Sci. 2020, 21, 9042. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the Risk of Heart Failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics—2015 Update. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef]
- Tsuji, H.; Larson, M.G.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of Reduced Heart Rate Variability on Risk for Cardiac Events. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Alpert, M.A.; Terry, B.E.; Mulekar, M.; Cohen, M.V.; Massey, C.V.; Fan, T.; Panayiotou, H.; Mukerji, V. Cardiac Morphology and Left Ventricular Function in Normotensive Morbidly Obese Patients With and Without Congestive Heart Failure, and Effect of Weight Loss. Am. J. Cardiol. 1997, 80, 736–740. [Google Scholar] [CrossRef]
- Braunwald, E. Heart Failure. JACC Heart Fail. 2013, 1, 1–20. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes from Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef]
- Mathieu, P.; Lemieux, I.; Després, J.-P. Obesity, Inflammation, and Cardiovascular Risk. Clin. Pharmacol. Ther. 2010, 87, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Scherer, P.E. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, Inflammation, and Cardiovascular Disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.S.; Catenacci, V.A.; Wyatt, H.R.; Hill, J.O. Obesity: Overview of an Epidemic. Psychiatr. Clin. N. Am. 2011, 34, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Dabkowski, E.R.; Ribeiro, R.F.; O’Connell, K.A. Dietary Fat and Heart Failure: Moving From Lipotoxicity to Lipoprotection. Circ. Res. 2012, 110, 764–776. [Google Scholar] [CrossRef]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef]
- Ouwens, D.M.; Sell, H.; Greulich, S.; Eckel, J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J. Cell. Mol. Med. 2010, 14, 2223–2234. [Google Scholar] [CrossRef]
- Kim, M.; Oh, J.K.; Sakata, S.; Liang, I.; Park, W.; Hajjar, R.J.; Lebeche, D. Role of resistin in cardiac contractility and hypertrophy. J. Mol. Cell. Cardiol. 2008, 45, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Greulich, S.; De Wiza, D.H.; Preilowski, S.; Ding, Z.; Mueller, H.; Langin, M.; Jaquet, K.; Ouwens, D.M.; Eckel, J. Secretory products of guinea pig epicardial fat induce insulin resistance and impair primary adult rat cardiomyocyte function. J. Cell. Mol. Med. 2011, 15, 2399–2410. [Google Scholar] [CrossRef]
- Joo, J.K.; Lee, K.S. Pharmacotherapy for Obesity. J. Menopausal Med. 2014, 20, 90–96. [Google Scholar] [CrossRef]
- Abbas, A.; Blandon, J.; Rude, J.; Elfar, A.; Mukherjee, D. PPAR-γ Agonist in Treatment of Diabetes: Cardiovascular Safety Considerations. Cardiovasc. Hematol. Agents Med. Chem. 2012, 10, 124–134. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Farb, M.G.; Tiwari, S.; Karki, S.; Ngo, D.T.; Carmine, B.; Hess, D.T.; Zuriaga, M.A.; Walsh, K.; Fetterman, J.L.; Hamburg, N.M.; et al. Cyclooxygenase inhibition improves endothelial vasomotor dysfunction of visceral adipose arterioles in human obesity. Obesity 2013, 22, 349–355. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.; Wang, K.; Niu, T.; Huang, D. Moderate- and Low-Dose of Atorvastatin Alleviate Cognition Impairment Induced by High-Fat Diet via Sirt1 Activation. Neurochem. Res. 2019, 44, 1065–1078. [Google Scholar] [CrossRef]
- Wu, H.; Lv, W.; Pan, Q.; Kalavagunta, P.K.; Liu, Q.; Qin, G.; Cai, M.; Zhou, L.; Wang, T.; Xia, Z.; et al. Simvastatin therapy in adolescent mice attenuates HFD-induced depression-like behavior by reducing hippocampal neuroinflammation. J. Affect. Disord. 2019, 243, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Dohi, Y.; Okado, T.; Hashimoto, T.; Goto, Y.; Kimura, G. Effects of ezetimibe on visceral fat in the metabolic syndrome: A randomised controlled study. Eur. J. Clin. Investig. 2012, 42, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Zhao, L.; Chang, Y.; Liu, B.-S.; Xu, F.; Zhang, C.; Ji, X.-P.; Chen, Y.-G.; Li, C.-B. Ezetimibe prevents myocardial remodeling in an obese rat model by inhibiting inflammation. Acta Biochim. Pol. 2018, 65, 465–470. [Google Scholar] [CrossRef]
- Hamulka, J.; Jeruszka-Bielak, M.; Górnicka, M.; Drywień, M.E.; Zielinska-Pukos, M.A. Dietary Supplements during COVID-19 Outbreak. Results of Google Trends Analysis Supported by PLifeCOVID-19 Online Studies. Nutrients 2020, 13, 54. [Google Scholar] [CrossRef]
- Lail, H.L.; Feresin, R.; Hicks, D.; Stone, B.; Price, E.; Wanders, D. Berries as a Treatment for Obesity-Induced Inflammation: Evidence from Preclinical Models. Nutrients 2021, 13, 334. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Martin, K.R.; Burrell, L.; Bopp, J. Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: A randomized, crossover pilot study. Food Funct. 2018, 9, 5290–5300. [Google Scholar] [CrossRef]
- Di Bonaventura, M.V.M.; Martinelli, I.; Moruzzi, M.; Di Bonaventura, E.M.; Giusepponi, M.E.; Polidori, C.; Lupidi, G.; Tayebati, S.K.; Amenta, F.; Cifani, C.; et al. Brain Alterations in High Fat Diet Induced Obesity: Effects of Tart Cherry Seeds and Juice. Nutrients 2020, 12, 623. [Google Scholar] [CrossRef]
- Martinelli, I.; Di Bonaventura, M.V.M.; Moruzzi, M.; Amantini, C.; Maggi, F.; Gabrielli, M.G.; Fruganti, A.; Marchegiani, A.; Dini, F.; Marini, C.; et al. Effects of Prunus cerasus L. Seeds and Juice on Liver Steatosis in an Animal Model of Diet-Induced Obesity. Nutrients 2020, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Cocci, P.; Moruzzi, M.; Martinelli, I.; Maggi, F.; Di Bonaventura, M.V.M.; Cifani, C.; Mosconi, G.; Tayebati, S.K.; Damiano, S.; Lupidi, G.; et al. Tart cherry (Prunus cerasus L.) dietary supplement modulates visceral adipose tissue CB1 mRNA levels along with other adipogenesis-related genes in rat models of diet-induced obesity. Eur. J. Nutr. 2021, 1–13. [Google Scholar] [CrossRef]
- Moruzzi, M.; Klöting, N.; Blüher, M.; Martinelli, I.; Tayebati, S.; Gabrielli, M.; Roy, P.; Di Bonaventura, M.M.; Cifani, C.; Lupidi, G.; et al. Tart Cherry Juice and Seeds Affect Pro-Inflammatory Markers in Visceral Adipose Tissue of High-Fat Diet Obese Rats. Molecules 2021, 26, 1403. [Google Scholar] [CrossRef]
- Vendrame, S.; Del Bo’, C.; Ciappellano, S.; Riso, P.; Klimis-Zacas, D. Berry Fruit Consumption and Metabolic Syndrome. Antioxidants 2016, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2019, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124 086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef]
- Ellulu, M.S. Obesity, cardiovascular disease, and role of vitamin C on inflammation: A review of facts and underlying mechanisms. Inflammopharmacology 2017, 25, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, D.F.; Lopez-Legarrea, P.; Quintero, P.; Martinez, J.A. Vitamin C in the Treatment and/or Prevention of Obesity. J. Nutr. Sci. Vitaminol. 2014, 60, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Brown, R.; Keenan, P.D.; Willis, N.D.; Siervo, M.; Mathers, J.C. Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. Nutr. Res. 2019, 61, 1–12. [Google Scholar] [CrossRef]
- Silva, P.; Sureda, A.; Tur, J.A.; Andreoletti, P.; Cherkaoui-Malki, M.; Latruffe, N. How efficient is resveratrol as an antioxidant of the Mediterranean diet, towards alterations during the aging process? Free. Radic. Res. 2019, 53, 1101–1112. [Google Scholar] [CrossRef]
- Cho, S.-J.; Jung, U.J.; Choi, M.-S. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br. J. Nutr. 2012, 108, 2166–2175. [Google Scholar] [CrossRef]
- Lv, Z.-M.; Wang, Q.; Chen, Y.-H.; Wang, S.-H.; Huang, D.-Q. Resveratrol attenuates inflammation and oxidative stress in epididymal white adipose tissue: Implications for its involvement in improving steroidogenesis in diet-induced obese mice. Mol. Reprod. Dev. 2015, 82, 321–328. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Wong, R.; Howe, P.; Buckley, J.; Coates, A.; Kunz, I.; Berry, N. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Namkoong, K.; Shin, M.; Park, J.; Yang, E.; Ihm, J.; Thu, V.T.; Kim, H.K.; Han, J. Cardiovascular Protective Effects and Clinical Applications of Resveratrol. J. Med. Food 2017, 20, 323–334. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Arinno, A.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. The roles of resveratrol on cardiac mitochondrial function in cardiac diseases. Eur. J. Nutr. 2021, 60, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Josse, A.R. Fish oil and omega-3 fatty acids. Can. Med. Assoc. J. 2008, 178, 150. [Google Scholar] [CrossRef][Green Version]
- Brenna, J.T.; Salem, N.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Khaza’Ai, H.; Abed, Y.; Rahmat, A.; Ismail, P.; Ranneh, Y. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacology 2015, 23, 79–89. [Google Scholar] [CrossRef]
- Battson, M.L.; Lee, D.M.; Weir, T.L.; Gentile, C.L. The gut microbiota as a novel regulator of cardiovascular function and disease. J. Nutr. Biochem. 2018, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Thushara, R.M.; Gangadaran, S.; Solati, Z.; Moghadasian, M.H. Cardiovascular benefits of probiotics: A review of experimental and clinical studies. Food Funct. 2016, 7, 632–642. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.; Robertson, K.; Yung, A.; Que, M.; Randall, H.; Wellalagodage, D.; Cox, T.; Robertson, D.; Chi, C.; Sun, J. Efficacy of Probiotics in Patients of Cardiovascular Disease Risk: A Systematic Review and Meta-analysis. Curr. Hypertens. Rep. 2020, 22, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ogino, E.; Manly, J.J.; Schupf, N.; Mayeux, R.; Gu, Y. Current and past leisure time physical activity in relation to risk of Alzheimer’s disease in older adults. Alzheimer’s Dement. 2019, 15, 1603–1611. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-Y.; Heo, J.-W.; Ko, J.R.; Kwak, H.-B. Exercise and Neuroinflammation in Health and Disease. Int. Neurourol. J. 2019, 23, S82–S92. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, B.J.; Marko, D.M.; Fenech, R.K.; Yang, A.J.; MacPherson, R.E. Healthy brain, healthy life: A review of diet and exercise interventions to promote brain health and reduce Alzheimer’s disease risk. Appl. Physiol. Nutr. Metab. 2020, 45, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017, 2, e000143. [Google Scholar] [CrossRef]
- Quindry, J.C.; Franklin, B.A. Cardioprotective Exercise and Pharmacologic Interventions as Complementary Antidotes to Cardiovascular Disease. Exerc. Sport Sci. Rev. 2018, 46, 5–17. [Google Scholar] [CrossRef] [PubMed]


| No | Study Design | Sample Size | Main Outcomes | Ref. |
|---|---|---|---|---|
| 1 | Cross-sectional | 86 patients | Positive correlation between waist-to-hip ratio and tumor necrosis factor-alpha (TNF-α) (r = 0.559, p < 0.001), interleukin-1beta (IL-1-β) (r = 0.435, p < 0.004), IL-4 ((r = 0.509, p < 0.001), IL-5 (r = 0.550, p < 0.005), leptin ((r = 0.331, p < 0.03), and negative correlation with adiponectin (r = −0.410, p < 0.006) in adolescents with central obesity. | [19] |
| 2 | Cross-sectional | 30 patients | Inverse correlation between adiponectin with body mass index (BMI), abdominal circumference, cholesterol LDL-C, IL-6, TNF-α, and leptin, and a positive correlation with cholesterol HDL-C in obese individuals. Leptin positively correlated with BMI, abdominal circumference, insulin, IL-6, TNF-α, and LAR, and negatively correlated with cholesterol HDL-C and adiponectin in obese subjects. | [20] |
| 3 | Clinical trial | 89 fluid samples | The negative association between BMI and inflammatory markers. | [21] |
| 4 | Case-control | 40 patients | The obese chronic obstructive pulmonary disease (COPD) group had lower levels of IL-2 (p = 0.01) and higher interferon gamma (INF-γ) levels (p = 0.02) and IL-6 (p = 0.003) than lean COPD. Whereas lean COPD patients had higher CD25+ (p = 0.01), CCr5 (p = 0.04), and HLA-DR (p = 0.007) expression on T cell surface compared to overweight–obese COPD participants. | [22] |
| 5 | Follow up study | 33 patients | Increased levels of C-reactive protein (CRP), TNF-α, triglycerides, homeostatic model assessment for insulin resistance (HOMA-IR), and fasting glucose, and a decreased level of high-density lipoprotein (HDL)-cholesterol were found in obese (BMI > 40 kg/m2) compared with the healthy individuals (BMI < 24.9 kg/m2). | [23] |
| 6 | Cross-sectional | 56 patients | Peripheral blood or local lymphocytes did not differ between obese and normal-weight patients with hip osteoarthritis (OA). However, higher levels of IL-6 and IL-8 (p < 0.05) were detected in the synovial fluid of the obese OA patients. | [24] |
| 7 | Cross-sectional | 51 female patients | Serum levels of adiponectin and leptin were significantly correlated with HOMA-IR and BMI. The levels of expression of monocyte chemoattractant protein-1 (MCP-1) and TNF-α in visceral adipose tissue were higher in the obese group (BMI ≥ 25). Moreover, the expression of mRNA MCP-1 in visceral adipose tissue was positively correlated with BMI (r = 0.428, p = 0.037). | [25] |
| 8 | Observational | 65 postmenopausal women | Adiponectin plasma levels and adipose-tissue gene expression were significantly lower in obese subjects and negatively correlated with obesity-associated variables, including hs-CRP and IL-6. | [26] |
| 9 | Prospective study | 85 patients | There was a negative association observed between obesity and adiponectin. Type 2 diabetes (T2D) patients have shown a significant correlation between plasma insulin, adipocytokines, and other inflammatory markers. | [27] |
| 10 | Cross-sectional | 740 Type 2 diabetic patients | Abdominal obesity was significantly correlated with IL-6 (waist circumference (WC): r = 0.27, p < 0.001; sagittal abdominal diameter (SAD): r = 031, p < 0.001), CRP (WC: r = 0.29, p < 0.001; SAD: r = 0.29, p < 0.001), IMT (WC: r = 0.09, p = 0.013; SAD: r = 0.11, p = 0.003), and PWV (WC: r = 0.18, p < 0.001; SAD: r = 0.21, p < 0.001)). | [28] |
| 11 | Case-control | 42 patients | microRNA-146a (miR-146a) and miR-21 concentrations were negatively correlated to IL-6, TNF-α, and CD36 in obese | [29] |
| 12 | Follow up study | 200 patients | From baseline to Week 52 changes in serum leptin, adiponectin, IL-6, TNFα, CRP, PAI-1, vascular cell adhesion molecule-1(VCAM-1), and MCP-1 were measured in patients with T2D. At weeks 52, there was a 22% reduction in median serum IL-6 (95% CI: −34%, −10%) and a 7% increase in median serum TNFα (95% CI: 1%, 12%) with canagliflozin versus glimepiride. | [30] |
| 13 | Cross-sectional | 1267 patients | Pericardial fat (odds ratio (OR) 1.32, 95% confidence interval (CI) 1.11–1.57; p = 0.002) and visceral adipose tissue (VAT) (OR 1.35, 95% CI 1.11–1.57; p = 0.003) were significantly associated with prevalent CVD in age–sex-adjusted models and after adjustment for BMI and waist circumference. | [31] |
| 14 | Cross-sectional | 36 patients | A significant correlation was observed between CRP and leptin, CRP and BMI (BMI). Patients with the highest BMI quartile (BMI, 40.3–61.2) had higher CRP levels (4.83 μg/mL vs. 3.03 μg/mL; p = 0.033) and higher leptin levels (44.97 ng/mL vs. 24.64 ng/mL; p = 0.042) compared with patients in the lower BMI quartile (BMI, 28.6–32.4). | [32] |
| 15 | Randomized single-blind trial | 120 premenopausal obese women | After 2 years of follow-up of obese women, BMI and serum concentrations of IL-6 (−1.1 pg/mL; p = 0.009), IL-18 (−57 pg/mL; p = 0.02) and CRP (−1.6 mg/L; p = 0.008) decreased, while adiponectin levels increased significantly (2.2 μ g/mL; p = 0.01) in the intervention group compared to controls (−4, 2; p < 0.001). | [33] |
| 16 | Follow up trails | 83 Women | CRP was positively associated with BMI (r = 0.281, p = 0.01) and waist circumference (r = 0.278, p = 0.01). After 12 weeks, weight loss was 7.9+/−0.3 kg. CRP was significantly decreased by 26% (p < 0.001), and a correlation was observed between weight loss and the change in CRP (r = 0.309, p = 0.005). | [34] |
| 17 | Cross-sectional | 83 patients | Obesity, dyslipidemia, IL-6, and CRP were significantly higher in the Insulin resistance (IR) group than in the non-IR group. Increased insulin levels, HOMA-IR, inflammatory markers, and triglycerides; while having lower HDL-C and adiponectin in obese adolescents than normal-weight adolescents. | [35] |
| 18 | Case control | 98 patients | Differences in functional outcomes were not found for three months after stroke between obese and non-obese groups. Obese patients experienced a high reduction of body weight, and pro-inflammatory IL-6 levels were higher after strokes. | [36] |
| 19 | Follow up study | 6040 participants | It was reported that the high value of N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a major risk of dementia, excluding CVD patients and adapting risk factors. Higher NT-proBNP was cross-sectionally connected with more unfortunate executions in different psychological tests. | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. https://doi.org/10.3390/ijms22094798
Battineni G, Sagaro GG, Chintalapudi N, Amenta F, Tomassoni D, Tayebati SK. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). International Journal of Molecular Sciences. 2021; 22(9):4798. https://doi.org/10.3390/ijms22094798
Chicago/Turabian StyleBattineni, Gopi, Getu Gamo Sagaro, Nalini Chintalapudi, Francesco Amenta, Daniele Tomassoni, and Seyed Khosrow Tayebati. 2021. "Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD)" International Journal of Molecular Sciences 22, no. 9: 4798. https://doi.org/10.3390/ijms22094798
APA StyleBattineni, G., Sagaro, G. G., Chintalapudi, N., Amenta, F., Tomassoni, D., & Tayebati, S. K. (2021). Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). International Journal of Molecular Sciences, 22(9), 4798. https://doi.org/10.3390/ijms22094798










