MicroRNAs in Acute ST Elevation Myocardial Infarction—A New Tool for Diagnosis and Prognosis: Therapeutic Implications
Abstract
1. Introduction
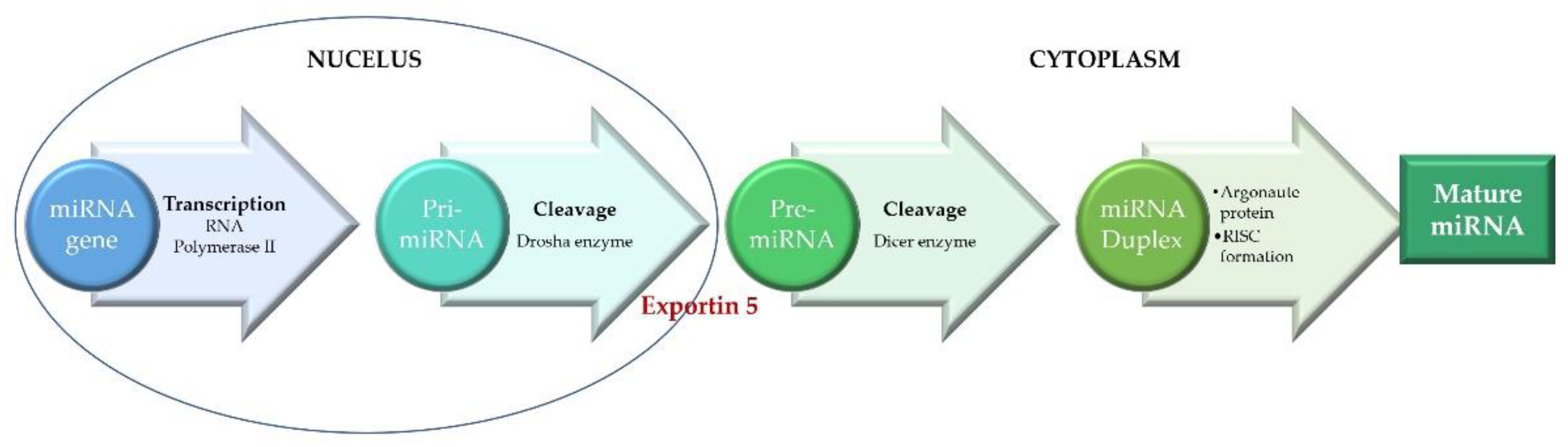
2. miRNAs in Pathophysiology of Acute Myocardial Infarction
2.1. Atherosclerosis
2.2. Thrombosis
2.3. Cardiomyocyte Death
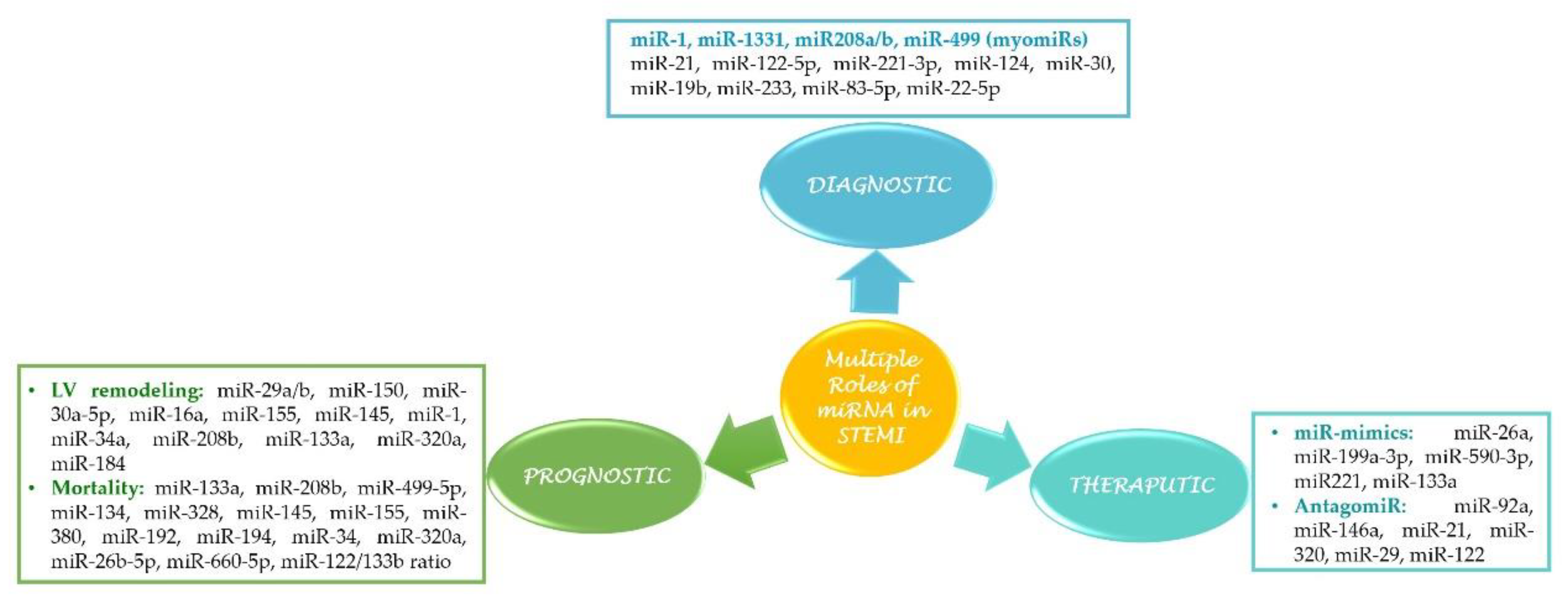
3. miRNAs as Biomarkers in STEMI
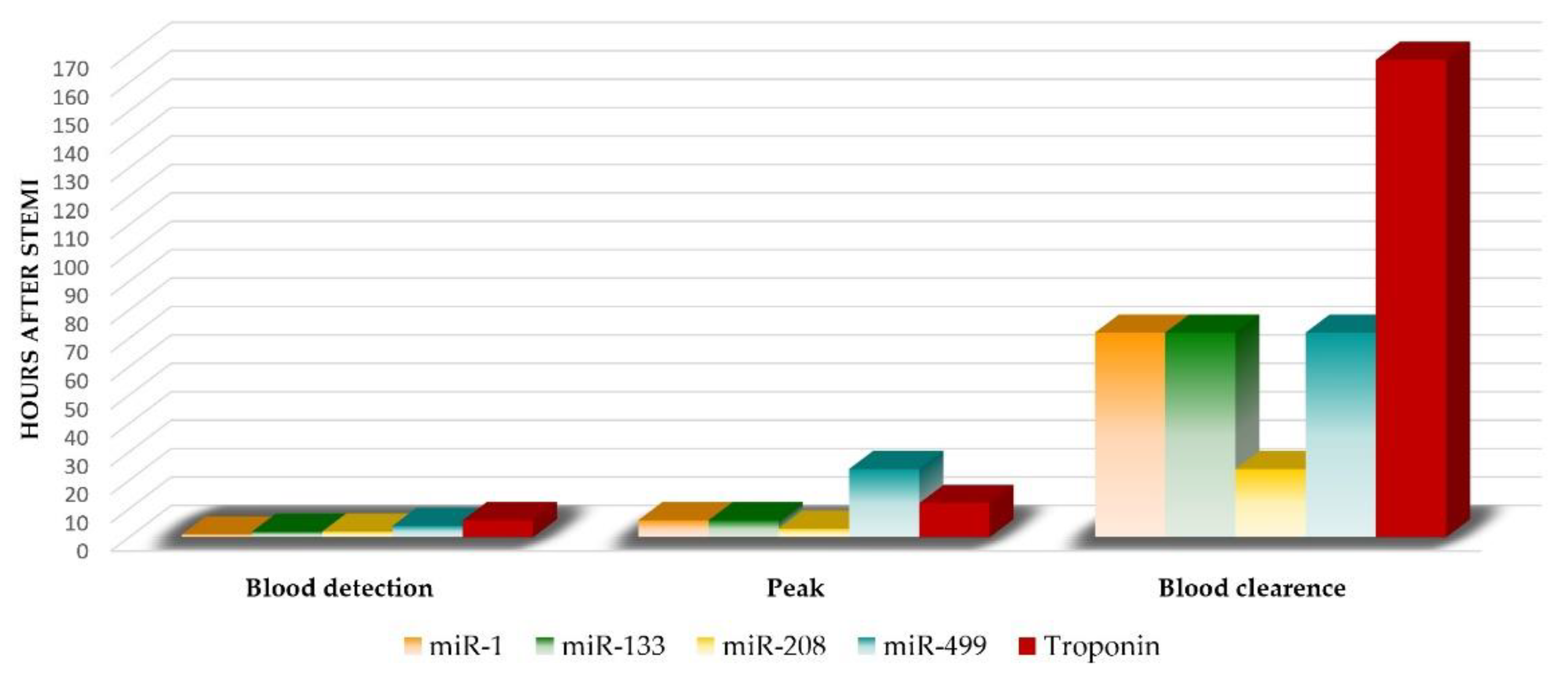
3.1. Cardiac-Specific miRNAs (myomiRs)
3.1.1. miR-499
3.1.2. miR-133a
3.1.3. miR-208
3.1.4. miR-1
3.2. Non-Cardiac miRNAs in STEMI
4. miRNA as a Prognostic Biomarker
4.1. Left Ventricular Remodeling
4.2. Mortality
5. Therapeutic Potential of miRNAs in STEMI
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndromes |
| AMI | acute myocardial infarction |
| ARC | activity-dependent cytoskeleton associated protein |
| ATM | ataxia telangiectasia mutated |
| Bcl2 | B-cell lymphoma 2 regulator protein |
| CPCs | cardiac progenitor cells |
| CTGF | connective tissue growth factor |
| ECM protein | extracellular matrix protein |
| eNOS | endothelial nitric oxide synthase |
| HF | heart failure |
| ICAM | intracellular adhesion molecule 1 |
| IFN-g | interferon gamma |
| IGF | insulin growth factor |
| I/R injury | ischemia/reperfusion injury |
| LV | left ventricle |
| LVEF | LV ejection fraction |
| LVR | left ventricular remodeling |
| MACE | major acute cardiovascular events |
| MI | myocardial infarction |
| miRNAs | microRNAs |
| MSCs | mesenchymal stem cells |
| MYH7 gene | beta myosin heavy chain 7 gene |
| NTproBNP | NTproBtype natriuretic peptide |
| PCI | percutaneous coronary intervention |
| PI3k/AKT pathway | phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) |
| PTEN | phosphatase and tensin homolog |
| pMLKL | phospho mixed lineage kinase domain-like protein |
| pri-miRNAs | precursor miRNAs |
| RIPK | Receptor-interacting serine/threonine protein kinase |
| RISC | RNA-induced silencing complex |
| RTqPCR | quantitative reverse transcription PCR |
| SMC | smooth muscle cell |
| SRCIN1 | SRC signaling inhibitor 1 |
| STEMI | ST elevation myocardial infarction |
| VEGF | vascular endothelial growth factor |
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Li, R.; Hou, K.; Chen, J.; Alzogool, M.; Hu, Y.; Zhang, J.; Zhang, Y.; Wang, L.; Zhang, R.; et al. Value of Blood-Based microRNAs in the Diagnosis of Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. Front. Physiol. 2020, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Karakas, M.; Zeller, T. microRNAs in cardiovascular disease—Clinical application. Clin. Chem. Lab. Med. 2017, 55, 687–704. [Google Scholar] [CrossRef]
- Andreou, I.; Sun, X.; Stone, P.H.; Edelman, E.R.; Feinberg, M.W. miRNAs in atherosclerotic plaque initiation, progression, and rupture. Trends Mol. Med. 2015, 21, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology; American College of Cardiology; American Heart Association; et al. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Khalil, A.; Kamar, A.; Nemer, G. MicroRNAs and Myocardial Infarct: Investigating the Controversial Role of Second Generation Biomarkers. J. Cholest. Heart Dis. 2017, 1, 8–13. [Google Scholar]
- Schulte, C.; Zeller, T. microRNA-based diagnostics and therapy in cardiovascular disease-Summing up the facts. Cardiovasc. Diagn. Ther. 2015, 5, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ye, C.; Ramirez, D.; Manjunath, N. Alternative processing of primary microRNA transcripts by Drosha generates 5’ end variation of mature microRNA. PLoS ONE 2009, 4, e7566. [Google Scholar] [CrossRef]
- Cavarretta, E.; Frati, G. MicroRNAs in Coronary Heart Disease: Ready to Enter the Clinical Arena? BioMed Res. Int. 2016, 2016, 2150763. [Google Scholar] [CrossRef]
- Paiva, S.; Agbulut, O. MiRroring the Multiple Potentials of MicroRNAs in Acute Myocardial Infarction. Front. Cardiovasc. Med. 2017, 4, 73. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Terrinoni, A.; Calabrese, C.; Basso, D.; Aita, A.; Caporali, S.; Plebani, M.; Bernardini, S. The circulating miRNAs as diagnostic and prognostic markers. Clin. Chem. Lab. Med. 2019, 57, 932–953. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, H.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, P. Circulating MicroRNAs: Biogenesis and Clinical Significance in Acute Myocardial Infarction. Front. Physiol. 2020, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Xue, S.; Ding, H.; Wang, Y.; Qi, H.; Wang, Y.; Zhu, W.; Li, P. Clinical significance of circulating microRNAs as diagnostic biomarkers for coronary artery disease. J. Cell Mol. Med. 2020, 24, 1146–1150. [Google Scholar] [CrossRef]
- Madina, B.R.; Kuppan, G.; Vashisht, A.A.; Liang, Y.H.; Downey, K.M.; Wohlschlegel, J.A.; Ji, X.; Sze, S.H.; Sacchettini, J.C.; Read, L.K.; et al. Guide RNA biogenesis involves a novel RNase III family endoribonuclease in Trypanosoma brucei. RNA 2011, 17, 1821–1830. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Siomi, H.; Siomi, M.C. Posttranscriptional regulation of microRNA biogenesis in animals. Mol. Cell 2010, 38, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheng, Y.J.; Sara, J.D.; Liu, L.J.; Liu, L.P.; Zhao, X.; Gao, H. Circulating MicroRNA-145 is Associated with Acute Myocardial Infarction and Heart Failure. Chin. Med. J. 2017, 130, 51–56. [Google Scholar] [CrossRef]
- Park, J.E.; Heo, I.; Tian, Y.; Simanshu, D.K.; Chang, H.; Jee, D.; Patel, D.J.; Kim, V.N. Dicer recognizes the 5’ end of RNA for efficient and accurate processing. Nature 2011, 475, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Lima, J., Jr.; Batty, J.A.; Sinclair, H.; Kunadian, V. MicroRNAs in Ischemic Heart Disease: From Pathophysiology to Potential Clinical Applications. Cardiol. Rev. 2017, 25, 117–125. [Google Scholar] [CrossRef]
- Montecucco, F.; Carbone, F.; Schindler, T.H. Pathophysiology of ST-segment elevation myocardial infarction: Novel mechanisms and treatments. Eur. Heart J. 2016, 37, 1268–1283. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M.W.; Moore, K.J. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Romaine, S.P.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in cardiovascular disease: An introduction for clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, Y.; Sheng, Z.; Zhou, P.; Liu, C.; Zhao, H.; Song, L.; Zhou, J.; Chen, R.; Chen, Y.; et al. RNA-seq identifies circulating miRNAs as potential biomarkers for plaque rupture in patients with ST-segment elevation myocardial infarction. Genomics 2021, 113, 1–10. [Google Scholar] [CrossRef]
- Di Gregoli, K.; Jenkins, N.; Salter, R.; White, S.; Newby, A.C.; Johnson, J.L. MicroRNA-24 regulates macrophage behavior and retards atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1990–2000. [Google Scholar] [CrossRef]
- Ma, F.; Xu, S.; Liu, X.; Zhang, Q.; Xu, X.; Liu, M.; Hua, M.; Li, N.; Yao, H.; Cao, X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat. Immunol. 2011, 12, 861–869. [Google Scholar] [CrossRef]
- Ulrich, V.; Rotllan, N.; Araldi, E.; Luciano, A.; Skroblin, P.; Abonnenc, M.; Perrotta, P.; Yin, X.; Bauer, A.; Leslie, K.L.; et al. Chronic miR-29 antagonism promotes favorable plaque remodeling in atherosclerotic mice. EMBO Mol. Med. 2016, 8, 643–653. [Google Scholar] [CrossRef]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Koppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S. microRNA-150 inhibits the formation of macrophage foam cells through targeting adiponectin receptor 2. Biochem. Biophys. Res. Commun. 2016, 476, 218–224. [Google Scholar] [CrossRef]
- Cipollone, F.; Felicioni, L.; Sarzani, R.; Ucchino, S.; Spigonardo, F.; Mandolini, C.; Malatesta, S.; Bucci, M.; Mammarella, C.; Santovito, D.; et al. A unique microRNA signature associated with plaque instability in humans. Stroke 2011, 42, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, H.; Sun, B. MicroRNA-145 is involved in endothelial cell dysfunction and acts as a promising biomarker of acute coronary syndrome. Eur. J. Med. Res. 2020, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Hu, S.; Sun, R.; Wang, C.; Dai, J.; Pan, W.; Xing, L.; Liu, X.; Wu, J.; Liu, J.; et al. High Levels of Circulating MicroRNA-3667-3p Are Associated with Coronary Plaque Erosion in Patients with ST-Segment Elevation Myocardial Infarction. Int. Heart J. 2019, 60, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Carneiro, F.D.; Almeida, K.C.; Fernandes-Santos, C. Role of miRNAs on the Pathophysiology of Cardiovascular Diseases. Arq. Bras. Cardiol. 2018, 111, 738–746. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Contribution of neovascularization and intraplaque haemorrhage to atherosclerotic plaque progression and instability. Acta Physiol. 2015, 213, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, T.; Imanishi, T. Akasaka, MicroRNAs and cardiovascular diseases. BioMed Res. Int. 2015, 2015, 682857. [Google Scholar] [CrossRef]
- Landry, P.; Plante, I.; Ouellet, D.L.; Perron, M.P.; Rousseau, G.; Provost, P. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 2009, 16, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Ge, L.; Zhou, X.; Ji, W.J.; Lu, R.Y.; Zhang, Y.Y.; Zeng, S.; Liu, X.; Zhao, J.H.; Zhang, W.C.; et al. Decreased platelet miR-223 expression is associated with high on-clopidogrel platelet reactivity. Thromb Res. 2013, 131, 508–513. [Google Scholar] [CrossRef]
- Gidlof, O.; van der Brug, M.; Ohman, J.; Gilje, P.; Olde, B.; Wahlestedt, C.; Erlinge, D. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood 2013, 121, 3908–3917. [Google Scholar] [CrossRef]
- Buja, L.M. The pathobiology of acute coronary syndromes: Clinical implications and central role of the mitochondria. Tex. Heart Inst. J. 2013, 40, 221–228. [Google Scholar]
- Sun, T.; Dong, Y.H.; Du, W.; Shi, C.Y.; Wang, K.; Tariq, M.A.; Wang, J.X.; Li, P.F. The Role of MicroRNAs in Myocardial Infarction: From Molecular Mechanism to Clinical Application. Int. J. Mol. Sci. 2017, 18, 745. [Google Scholar] [CrossRef]
- Zhao, H.; Jaffer, T.; Eguchi, S.; Wang, Z.; Linkermann, A.; Ma, D. Role of necroptosis in the pathogenesis of solid organ injury. Cell Death Dis. 2015, 6, e1975. [Google Scholar] [CrossRef]
- Wang, K.; Liu, F.; Liu, C.Y.; An, T.; Zhang, J.; Zhou, L.Y.; Wang, M.; Dong, Y.H.; Li, N.; Gao, J.N.; et al. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 2016, 23, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, F.; Zhou, L.Y.; Ding, S.L.; Long, B.; Liu, C.Y.; Sun, T.; Fan, Y.Y.; Sun, L.; Li, P.F. miR-874 regulates myocardial necrosis by targeting caspase-8. Cell Death Dis. 2013, 4, e709. [Google Scholar] [CrossRef] [PubMed]
- Song, C.L.; Liu, B.; Diao, H.Y.; Shi, Y.F.; Zhang, J.C.; Li, Y.X.; Liu, N.; Yu, Y.P.; Wang, G.; Wang, J.P.; et al. Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget 2016, 7, 39740–39757. [Google Scholar] [CrossRef]
- Ke, Z.P.; Xu, P.; Shi, Y.; Gao, A.M. MicroRNA-93 inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by targeting PTEN. Oncotarget 2016, 7, 28796–28805. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liu, W.; Zhang, J.; Xiang, D. miR-153 regulates apoptosis and autophagy of cardiomyocytes by targeting Mcl-1. Mol. Med. Rep. 2016, 14, 1033–1039. [Google Scholar] [CrossRef]
- Liang, W.; Guo, J.; Li, J.; Bai, C.; Dong, Y. Downregulation of miR-122 attenuates hypoxia/reoxygenation (H/R)-induced myocardial cell apoptosis by upregulating GATA-4. Biochem. Biophys. Res. Commun. 2016, 478, 1416–1422. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wang, B.J.; Ma, M.; Yu, K.; Zhang, Q.; Zhang, X.W. MicroRNA-325-3p protects the heart after myocardial infarction by inhibiting RIPK3 and programmed necrosis in mice. BMC Mol. Biol. 2019, 20, 17. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Braniewska, A.; Kozar-Kaminska, K. MicroRNA in cardiovascular biology and disease. Adv. Clin. Exp. Med. 2017, 26, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, X.; Lu, Y.; Yang, B. miRNAs at the heart of the matter. J. Mol. Med. 2008, 86, 771–783. [Google Scholar] [CrossRef]
- Bauersachs, J.; Thum, T. Biogenesis and regulation of cardiovascular microRNAs. Circ. Res. 2011, 109, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, Q.; You, W.; Chen, M.; Xia, J. MiRNAs as biomarkers of myocardial infarction: A meta-analysis. PLoS ONE 2014, 9, e88566. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Jiang, Z.; Wu, F.; Ping, J.; Ming, L. Identification of microRNAs as diagnostic biomarkers for acute myocardial infarction in Asian populations: A systematic review and meta-analysis. Medicine 2017, 96, e7173. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Vechetti, I., Jr.; Wen, Y.; Chaillou, T.; Murach, K.A.; Alimov, A.P.; Figueiredo, V.C.; Dal-Pai-Silva, M.; McCarthy, J.J. Life-long reduction in myomiR expression does not adversely affect skeletal muscle morphology. Sci. Rep. 2019. [Google Scholar] [CrossRef]
- Gidlof, O.; Andersson, P.; van der Pals, J.; Gotberg, M.; Erlinge, D. Cardiospecific microRNA plasma levels correlate with troponin and cardiac function in patients with ST elevation myocardial infarction, are selectively dependent on renal elimination, and can be detected in urine samples. Cardiology 2011, 118, 217–226. [Google Scholar] [CrossRef]
- van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007, 316, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Ren, X.P.; Chen, J.; Liu, H.; Yang, J.; Medvedovic, M.; Hu, Z.; Fan, G.C. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 2010, 122, 1308–1318. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, H.; Yan, P.; Zhou, X.; Wang, Y.; Yao, Y. Circulating MicroRNA-499 as a Diagnostic Biomarker for Acute Myocardial Infarction: A Meta-analysis. Dis. Markers 2019, 2019, 6121696. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Su, T.; Li, H.; Huang, Q.; Wu, D.; Yang, C.; Han, Z. Kinetics of plasma microRNA-499 expression in acute myocardial infarction. J. Thorac. Dis. 2015, 7, 890–896. [Google Scholar] [CrossRef]
- Olivieri, F.; Rippo, M.R.; Procopio, A.D.; Fazioli, F. Circulating inflamma-miRs in aging and age-related diseases. Front. Genet. 2013, 4, 121. [Google Scholar] [CrossRef]
- Abdellatif, M. The role of microRNA-133 in cardiac hypertrophy uncovered. Circ. Res. 2010, 106, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Alavi-Moghaddam, M.; Chehrazi, M.; Alipoor, S.D.; Mohammadi, M.; Baratloo, A.; Mahjoub, M.P.; Movasaghi, M.; Garssen, J.; Adcock, I.M.; Mortaz, E. A Preliminary Study of microRNA-208b after Acute Myocardial Infarction: Impact on 6-Month Survival. Dis. Markers 2018, 2018, 2410451. [Google Scholar] [CrossRef]
- Kondkar, A.A.; Abu-Amero, K.K. Utility of circulating microRNAs as clinical biomarkers for cardiovascular diseases. BioMed Res. Int. 2015, 2015, 821823. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Z.; Zhao, T.; Cao, W.; Zhang, L.; Li, H.; Xie, Q.; Tian, Y.; Wang, B. Plasma miR-1, miR-208, miR-499 as potential predictive biomarkers for acute myocardial infarction: An independent study of Han population. Exp. Gerontol. 2015, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Bialek, S.; Gorko, D.; Zajkowska, A.; Koltowski, L.; Grabowski, M.; Stachurska, A.; Kochman, J.; Sygitowicz, G.; Malecki, M.; Opolski, G.; et al. Release kinetics of circulating miRNA-208a in the early phase of myocardial infarction. Kardiol. Pol. 2015, 73, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Nakanishi, M.; Otsuka, Y.; Nishimura, K.; Hirokawa, G.; Goto, Y.; Nonogi, H.; Iwai, N. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin. Chem. 2010, 56, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, R.; Li, Y.; Pu, J.; Lu, Y.; Jiao, J.; Li, K.; Yu, B.; Li, Z.; Wang, R.; et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2010, 391, 73–77. [Google Scholar] [CrossRef]
- Long, G.; Wang, F.; Duan, Q.; Chen, F.; Yang, S.; Gong, W.; Wang, Y.; Chen, C.; Wang, D.W. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. Int. J. Biol. Sci. 2012, 8, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Zhu, J.Q.; Zhang, J.T.; Li, Q.; Li, Y.; He, J.; Qin, Y.W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tan, N.; Yang, J.; Liu, X.; Cao, X.; He, P.; Dong, X.; Qin, S.; Zhang, C. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin. Sci. 2010, 119, 87–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.J.; Liu, T.; Zhang, H.; Yang, S.J. Plasma microRNA-21 is a potential diagnostic biomarker of acute myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 323–329. [Google Scholar]
- Yao, X.L.; Lu, X.L.; Yan, C.Y.; Wan, Q.L.; Cheng, G.C.; Li, Y.M. Circulating miR-122-5p as a potential novel biomarker for diagnosis of acute myocardial infarction. Int. J. Clin. Exp. Pathol. 2015, 8, 16014–16019. [Google Scholar]
- Coskunpinar, E.; Cakmak, H.A.; Kalkan, A.K.; Tiryakioglu, N.O.; Erturk, M.; Ongen, Z. Circulating miR-221-3p as a novel marker for early prediction of acute myocardial infarction. Gene 2016, 591, 90–96. [Google Scholar] [CrossRef]
- Guo, M.L.; Guo, L.L.; Weng, Y.Q. Implication of peripheral blood miRNA-124 in predicting acute myocardial infarction. Eur Rev. Med. Pharmacol. Sci. 2017, 21, 1054–1059. [Google Scholar] [PubMed]
- Bye, A.; Rosjo, H.; Nauman, J.; Silva, G.J.; Follestad, T.; Omland, T.; Wisloff, U. Circulating microRNAs predict future fatal myocardial infarction in healthy individuals—The HUNT study. J. Mol. Cell Cardiol. 2016, 97, 162–168. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Wu, M.; Chi, C.; Hu, D.; Cui, Y.; Song, J.; Lee, C.; Chen, H. Early diagnostic value of circulating microRNAs in patients with suspected acute myocardial infarction. J. Cell Physiol. 2019, 234, 13649–13658. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, W.; Zhang, Y.; Zhang, L.; Ding, H.; Qi, H.; Xue, S.; Yu, H.; Hu, L.; Liu, D.; et al. Circulating miR-22-5p and miR-122-5p are promising novel biomarkers for diagnosis of acute myocardial infarction. J. Cell Physiol. 2019, 234, 4778–4786. [Google Scholar] [CrossRef]
- Bostan, M.M.; Statescu, C.; Anghel, L.; Serban, I.L.; Cojocaru, E.; Sascau, R. Post-Myocardial Infarction Ventricular Remodeling Biomarkers-The Key Link between Pathophysiology and Clinic. Biomolecules 2020, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, X.; Ma, K.; Li, G.; Liu, Z.; Rong, W.; Miao, H.; Zhu, F.; Cui, Q.; Wu, S.; et al. Circulating miRNAs Related to Long-term Adverse Cardiovascular Events in STEMI Patients: A Nested Case-Control Study. Can. J. Cardiol. 2021, 37, 77–85. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Y.; Wang, X.; Li, S.; Yu, T.; Duan, W.; Wu, J.; Wen, Z.; Jiao, Y.; Sun, Z.; et al. Circulating miR-1 as a potential predictor of left ventricular remodeling following acute ST-segment myocardial infarction using cardiac magnetic resonance. Quant. Imaging Med. Surg. 2020, 10, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Danielson, K.M.; Shah, R.; Yeri, A.; Liu, X.; Camacho Garcia, F.; Silverman, M.; Tanriverdi, K.; Das, A.; Xiao, C.; Jerosch-Herold, M.; et al. Plasma Circulating Extracellular RNAs in Left Ventricular Remodeling Post-Myocardial Infarction. EBioMedicine 2018, 32, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Menees, D.S.; Peterson, E.D.; Wang, Y.; Curtis, J.P.; Messenger, J.C.; Rumsfeld, J.S.; Gurm, H.S. Door-to-balloon time and mortality among patients undergoing primary PCI. N. Engl. J. Med. 2013, 369, 901–909. [Google Scholar] [CrossRef]
- Galeano-Otero, I.; Del Toro, R.; Guisado, A.; Diaz, I.; Mayoral-Gonzalez, I.; Guerrero-Marquez, F.; Gutierrez-Carretero, E.; Casquero-Dominguez, S.; Diaz-de la Llera, L.; Baron-Esquivias, G.; et al. Circulating miR-320a as a Predictive Biomarker for Left Ventricular Remodelling in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2020, 9, 1051. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Maciejak-Jastrzebska, A.; Sitkiewicz, D. MicroRNAs in the development of left ventricular remodeling and postmyocardial infarction heart failure. Pol. Arch. Intern. Med. 2020, 130, 59–65. [Google Scholar] [CrossRef]
- Lakhani, H.V.; Khanal, T.; Gabi, A.; Yousef, G.; Alam, M.B.; Sharma, D.; Aljoudi, H.; Puri, N.; Thompson, E.; Shapiro, J.I.; et al. Developing a panel of biomarkers and miRNA in patients with myocardial infarction for early intervention strategies of heart failure in West Virginian population. PLoS ONE 2018, 13, e0205329. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; McCann, G.P.; Kelly, D.; Collignon, O.; Ng, L.L.; Wagner, D.R.; Squire, I.B. A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction. PLoS ONE 2013, 8, e70644. [Google Scholar] [CrossRef]
- Dutka, M.; Bobinski, R.; Korbecki, J. The relevance of microRNA in post-infarction left ventricular remodelling and heart failure. Heart Fail. Rev. 2019, 24, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, Y.; Park, K.M.; Hu, Q.; Teoh, J.P.; Broskova, Z.; Ranganathan, P.; Jayakumar, C.; Li, J.; Su, H.; et al. MicroRNA-150 protects the mouse heart from ischaemic injury by regulating cell death. Cardiovasc. Res. 2015, 106, 387–397. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; McCann, G.P.; Zangrando, J.; Kelly, D.; Razvi, N.; Zhang, L.; Ng, L.L.; Wagner, D.R.; Squire, I.B. MicroRNA-150: A novel marker of left ventricular remodeling after acute myocardial infarction. Circ. Cardiovasc. Genet. 2013, 6, 290–298. [Google Scholar] [CrossRef]
- Desjarlais, M.; Dussault, S.; Dhahri, W.; Mathieu, R.; Rivard, A. MicroRNA-150 Modulates Ischemia-Induced Neovascularization in Atherosclerotic Conditions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.L.; Guo, W.L.; Chen, X.M. Overexpressing microRNA-150 attenuates hypoxia-induced human cardiomyocyte cell apoptosis by targeting glucose-regulated protein-94. Mol. Med. Rep. 2018, 17, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Latet, S.C.; Van Herck, P.L.; Claeys, M.J.; Van Craenenbroeck, A.H.; Haine, S.E.; Vandendriessche, T.R.; Van Hoof, V.O.; Fransen, E.; De Winter, B.Y.; Van Craenenbroeck, E.M.; et al. Failed Downregulation of Circulating MicroRNA-155 in the Early Phase after ST Elevation Myocardial Infarction Is Associated with Adverse Left Ventricular Remodeling. Cardiology 2017, 138, 91–96. [Google Scholar] [CrossRef]
- Grabmaier, U.; Clauss, S.; Gross, L.; Klier, I.; Franz, W.M.; Steinbeck, G.; Wakili, R.; Theiss, H.D.; Brenner, C. Diagnostic and prognostic value of miR-1 and miR-29b on adverse ventricular remodeling after acute myocardial infarction—The SITAGRAMI-miR analysis. Int. J. Cardiol. 2017, 244, 30–36. [Google Scholar] [CrossRef]
- Lv, P.; Zhou, M.; He, J.; Meng, W.; Ma, X.; Dong, S.; Meng, X.; Zhao, X.; Wang, X.; He, F. Circulating miR-208b and miR-34a are associated with left ventricular remodeling after acute myocardial infarction. Int. J. Mol. Sci. 2014, 15, 5774–5788. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Adams, V.; Dieterich, P.; Fuernau, G.; de Waha, S.; Desch, S.; Schuler, G.; Thiele, H. Relation of circulating MicroRNA-133a concentrations with myocardial damage and clinical prognosis in ST-elevation myocardial infarction. Am. Heart J. 2012, 164, 706–714. [Google Scholar] [CrossRef]
- Coelho-Lima, J.; Mohammed, A.; Cormack, S.; Jones, S.; Ali, A.; Panahi, P.; Barter, M.; Bagnall, A.; Ali, S.; Young, D.; et al. Kinetics Analysis of Circulating MicroRNAs Unveils Markers of Failed Myocardial Reperfusion. Clin. Chem. 2020, 66, 247–256. [Google Scholar] [CrossRef]
- Liu, Z.H.; Sun, X.P.; Zhou, S.L.; Wang, H.X. Research on the relations between the variation of miRNA-184 before and after treatment of acute myocardial infarction and prognosis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 843–847. [Google Scholar] [PubMed]
- Widera, C.; Gupta, S.K.; Lorenzen, J.M.; Bang, C.; Bauersachs, J.; Bethmann, K.; Kempf, T.; Wollert, K.C.; Thum, T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J. Mol. Cell Cardiol. 2011, 51, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Gidlof, O.; Smith, J.G.; Miyazu, K.; Gilje, P.; Spencer, A.; Blomquist, S.; Erlinge, D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc. Disord. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Goretti, E.; Vausort, M.; Wagner, D.R.; Devaux, Y. Association between circulating microRNAs, cardiovascular risk factors and outcome in patients with acute myocardial infarction. Int. J. Cardiol. 2013, 168, 4548–4550. [Google Scholar] [CrossRef]
- Olivieri, F.; Antonicelli, R.; Spazzafumo, L.; Santini, G.; Rippo, M.R.; Galeazzi, R.; Giovagnetti, S.; D’Alessandra, Y.; Marcheselli, F.; Capogrossi, M.C.; et al. Admission levels of circulating miR-499-5p and risk of death in elderly patients after acute non-ST elevation myocardial infarction. Int. J. Cardiol. 2014, 172, e276–e278. [Google Scholar] [CrossRef]
- He, F.; Lv, P.; Zhao, X.; Wang, X.; Ma, X.; Meng, W.; Meng, X.; Dong, S. Predictive value of circulating miR-328 and miR-134 for acute myocardial infarction. Mol. Cell Biochem. 2014, 394, 137–144. [Google Scholar] [CrossRef]
- Dong, Y.M.; Liu, X.X.; Wei, G.Q.; Da, Y.N.; Cha, L.; Ma, C.S. Prediction of long-term outcome after acute myocardial infarction using circulating miR-145. Scand. J. Clin. Lab. Invest. 2015, 75, 85–91. [Google Scholar] [CrossRef]
- Matsumoto, S.; Sakata, Y.; Suna, S.; Nakatani, D.; Usami, M.; Hara, M.; Kitamura, T.; Hamasaki, T.; Nanto, S.; Kawahara, Y.; et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ. Res. 2013, 113, 322–326. [Google Scholar] [CrossRef]
- Matsumoto, S.; Sakata, Y.; Nakatani, D.; Suna, S.; Mizuno, H.; Shimizu, M.; Usami, M.; Sasaki, T.; Sato, H.; Kawahara, Y.; et al. A subset of circulating microRNAs are predictive for cardiac death after discharge for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2012, 427, 280–284. [Google Scholar] [CrossRef]
- Mirzavi, F.; Ebrahimi, S.; Ghazvini, K.; Hasanian, S.M.; Hashemy, S.I. Diagnostic, Prognostic, and Therapeutic Potencies of Circulating miRNAs in Acute Myocardial Infarction. Crit. Rev. Eukaryot. Gene Expr. 2019, 29, 333–342. [Google Scholar] [CrossRef]
- Jakob, P.; Kacprowski, T.; Briand-Schumacher, S.; Heg, D.; Klingenberg, R.; Stahli, B.E.; Jaguszewski, M.; Rodondi, N.; Nanchen, D.; Raber, L.; et al. Profiling and validation of circulating microRNAs for cardiovascular events in patients presenting with ST-segment elevation myocardial infarction. Eur. Heart J. 2017, 38, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Dias, N.; Costa, M.C.; Carrilho-Ferreira, P.; Silva, D.; Jorge, C.; Calisto, C.; Pessoa, T.; Robalo Martins, S.; de Sousa, J.C.; da Silva, P.C.; et al. Circulating miR-122-5p/miR-133b Ratio Is a Specific Early Prognostic Biomarker in Acute Myocardial Infarction. Circ. J. 2016, 80, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, B.; Zhang, X.; Lock, R.; Nash, T.; Vunjak-Novakovic, G. Cell type-specific microRNA therapies for myocardial infarction. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Sun, X.; Shan, H.; Wang, N.; Wang, J.; Ren, J.; Feng, S.; Xie, L.; Lu, C.; Yuan, Y.; et al. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-beta1 pathway. Circulation 2012, 126, 840–850. [Google Scholar] [CrossRef]
- Li, Q.; Xie, J.; Li, R.; Shi, J.; Sun, J.; Gu, R.; Ding, L.; Wang, L.; Xu, B. Overexpression of microRNA-99a attenuates heart remodelling and improves cardiac performance after myocardial infarction. J. Cell Mol. Med. 2014, 18, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Calway, T.; Kim, G.H. Harnessing the Therapeutic Potential of MicroRNAs for Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 131–143. [Google Scholar] [CrossRef]
- Olson, E.N. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci. Transl. Med. 2014, 6, 239ps3. [Google Scholar] [CrossRef]
- Wang, Z. The guideline of the design and validation of MiRNA mimics. Methods Mol. Biol. 2011, 676, 211–223. [Google Scholar] [CrossRef]
- Caroli, A.; Cardillo, M.T.; Galea, R.; Biasucci, L.M. Potential therapeutic role of microRNAs in ischemic heart disease. J. Cardiol. 2013, 61, 315–320. [Google Scholar] [CrossRef]
- Krutzfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Oliveira-Carvalho, V.; Carvalho, V.O.; Silva, M.M.; Guimaraes, G.V.; Bocchi, E.A. MicroRNAs: A new paradigm in the treatment and diagnosis of heart failure? Arq. Bras. Cardiol. 2012, 98, 362–369. [Google Scholar] [CrossRef]
- Yao, L.; Lv, X.; Wang, X. MicroRNA 26a inhibits HMGB1 expression and attenuates cardiac ischemia-reperfusion injury. J. Pharmacol. Sci. 2016, 131, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, Q.; Liang, D.; Shi, J. MiRNA-26a inhibits myocardial infarction-induced apoptosis by targeting PTEN via JAK/STAT pathways. Cell Dev. 2021, 165, 203661. [Google Scholar] [CrossRef]
- Leeper, N.J.; Raiesdana, A.; Kojima, Y.; Chun, H.J.; Azuma, J.; Maegdefessel, L.; Kundu, R.K.; Quertermous, T.; Tsao, P.S.; Spin, J.M. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J. Cell Physiol. 2011, 226, 1035–1043. [Google Scholar] [CrossRef]
- Chiang, M.H.; Liang, C.J.; Lin, L.C.; Yang, Y.F.; Huang, C.C.; Chen, Y.H.; Kao, H.L.; Chen, Y.C.; Ke, S.R.; Lee, C.W.; et al. miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia-telangiectasia mutated in myocardial infarction. J. Cell Physiol. 2020, 235, 6085–6102. [Google Scholar] [CrossRef]
- Xing, X.; Guo, S.; Zhang, G.; Liu, Y.; Bi, S.; Wang, X.; Lu, Q. miR-26a-5p protects against myocardial ischemia/reperfusion injury by regulating the PTEN/PI3K/AKT signaling pathway. Braz. J. Med. Biol. Res. 2020, 53, e9106. [Google Scholar] [CrossRef]
- Lesizza, P.; Prosdocimo, G.; Martinelli, V.; Sinagra, G.; Zacchigna, S.; Giacca, M. Single-Dose Intracardiac Injection of Pro-Regenerative MicroRNAs Improves Cardiac Function After Myocardial Infarction. Circ. Res. 2017, 120, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef]
- Peters, L.J.F.; Biessen, E.A.L.; Hohl, M.; Weber, C.; van der Vorst, E.P.C.; Santovito, D. Small Things Matter: Relevance of MicroRNAs in Cardiovascular Disease. Front. Physiol. 2020, 11, 793. [Google Scholar] [CrossRef]
- Zhou, Y.; Richards, A.M.; Wang, P. MicroRNA-221 Is Cardioprotective and Anti-fibrotic in a Rat Model of Myocardial Infarction. Mol. Ther. Nucleic. Acids 2019, 17, 185–197. [Google Scholar] [CrossRef]
- Nakano, K.; Vousden, K.H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 2001, 7, 683–694. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.; Tuazon, J.P.; Borlongan, C.V.; Yu, G. MicroRNA-133a and Myocardial Infarction. Cell Transplant. 2019, 28, 831–838. [Google Scholar] [CrossRef]
- Izarra, A.; Moscoso, I.; Levent, E.; Canon, S.; Cerrada, I.; Diez-Juan, A.; Blanca, V.; Nunez-Gil, I.J.; Valiente, I.; Ruiz-Sauri, A.; et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Rep. 2014, 3, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Chen, W.; Xie, L.; Zhao, Z.A.; Yang, J.; Chen, Y.; Lei, W.; Shen, Z. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res. Ther. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Morelli, M.B.; Matarese, A.; Sardu, C.; Santulli, G. Cardiomyocyte-derived exosomal microRNA-92a mediates post-ischemic myofibroblast activation both in vitro and ex vivo. ESC Heart Fail. 2020, 7, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef]
- Dong, S.; Cheng, Y.; Yang, J.; Li, J.; Liu, X.; Wang, X.; Wang, D.; Krall, T.J.; Delphin, E.S.; Zhang, C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J. Biol. Chem. 2009, 284, 29514–29525. [Google Scholar] [CrossRef]
- Bejerano, T.; Etzion, S.; Elyagon, S.; Etzion, Y.; Cohen, S. Nanoparticle Delivery of miRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018, 18, 5885–5891. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Zhang, J.; Jiao, Z.; Dong, N.; Wang, G.; Wang, Z.; Wang, L. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics 2019, 9, 2346–2360. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, P.; Yang, J.; Liu, X.; Dong, S.; Wang, X.; Chun, B.; Zhuang, J.; Zhang, C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc. Res. 2010, 87, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ha, T.; Liu, L.; Zou, J.; Zhang, X.; Kalbfleisch, J.; Gao, X.; Williams, D.; Li, C. Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2013, 97, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; He, M.; Hong, C.; Gao, S.; Rane, S.; Yang, Z.; Abdellatif, M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 2010, 285, 20281–20290. [Google Scholar] [CrossRef]
- Huang, W.; Tian, S.S.; Hang, P.Z.; Sun, C.; Guo, J.; Du, Z.M. Combination of microRNA-21 and microRNA-146a Attenuates Cardiac Dysfunction and Apoptosis During Acute Myocardial Infarction in Mice. Mol. Ther. Nucleic. Acids 2016, 5, e296. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Q.; Jiang, H.; Lu, Z.B. MiR-320 regulates cardiomyocyte apoptosis induced by ischemia-reperfusion injury by targeting AKIP1. Cell Mol. Biol. Lett. 2018, 23, 41. [Google Scholar] [CrossRef]
- Song, C.L.; Liu, B.; Diao, H.Y.; Shi, Y.F.; Li, Y.X.; Zhang, J.C.; Lu, Y.; Wang, G.; Liu, J.; Yu, Y.P.; et al. The protective effect of microRNA-320 on left ventricular remodeling after myocardial ischemia-reperfusion injury in the rat model. Int. J. Mol. Sci. 2014, 15, 17442–17456. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.P.; Wu, J.; Wang, X.; Sartor, M.A.; Jones, K.; Qian, J.; Nicolaou, P.; Pritchard, T.J.; Fan, G.C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef]
- Ye, Y.; Hu, Z.; Lin, Y.; Zhang, C.; Perez-Polo, J.R. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2010, 87, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J. Cell signaling pathways for the regulation of GATA4 transcription factor: Implications for cell growth and apoptosis. Cell Signal. 2011, 23, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Zhou, Y.F.; Zhao, X.; Jiang, B.; Yang, X.J. GATA-4 protects against hypoxia-induced cardiomyocyte injury: Effects on mitochondrial membrane potential. Can. J. Physiol. Pharmacol. 2014, 92, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Taubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef] [PubMed]
| miRNA Prognostic Value (LVR and Mortality after STEMI) | ||
|---|---|---|
| Favorable | Unfavorable | |
| miRNAs | miR-150 (LVR) miR-145 (LVR) miR-101 (LVR) | miR-27a, miR-16 (LVR) miR-155 (LVR and mortality) miR-1, miR-29b (LVR) miR-208b (LVR and mortality) miR-34 (LVR) miR-133a (LVR and mortality), miR-133b (LVR) miR-320 (LVR and mortality) miR-184 (LVR) miR-499 (mortality) miR-134, miR-328 (mortality) miR-380 (mortality) miR-192, miR-194, and miR-34 (LVR) miR-26b, miR-660 (mortality) miR122-5p/133b ratio (mortality) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scărlătescu, A.I.; Micheu, M.M.; Popa-Fotea, N.-M.; Dorobanțu, M. MicroRNAs in Acute ST Elevation Myocardial Infarction—A New Tool for Diagnosis and Prognosis: Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 4799. https://doi.org/10.3390/ijms22094799
Scărlătescu AI, Micheu MM, Popa-Fotea N-M, Dorobanțu M. MicroRNAs in Acute ST Elevation Myocardial Infarction—A New Tool for Diagnosis and Prognosis: Therapeutic Implications. International Journal of Molecular Sciences. 2021; 22(9):4799. https://doi.org/10.3390/ijms22094799
Chicago/Turabian StyleScărlătescu, Alina Ioana, Miruna Mihaela Micheu, Nicoleta-Monica Popa-Fotea, and Maria Dorobanțu. 2021. "MicroRNAs in Acute ST Elevation Myocardial Infarction—A New Tool for Diagnosis and Prognosis: Therapeutic Implications" International Journal of Molecular Sciences 22, no. 9: 4799. https://doi.org/10.3390/ijms22094799
APA StyleScărlătescu, A. I., Micheu, M. M., Popa-Fotea, N.-M., & Dorobanțu, M. (2021). MicroRNAs in Acute ST Elevation Myocardial Infarction—A New Tool for Diagnosis and Prognosis: Therapeutic Implications. International Journal of Molecular Sciences, 22(9), 4799. https://doi.org/10.3390/ijms22094799







