The Effect of Using Micro-Clustered Water as a Polymer Medium
Abstract
:1. Introduction
2. Results and Discussion
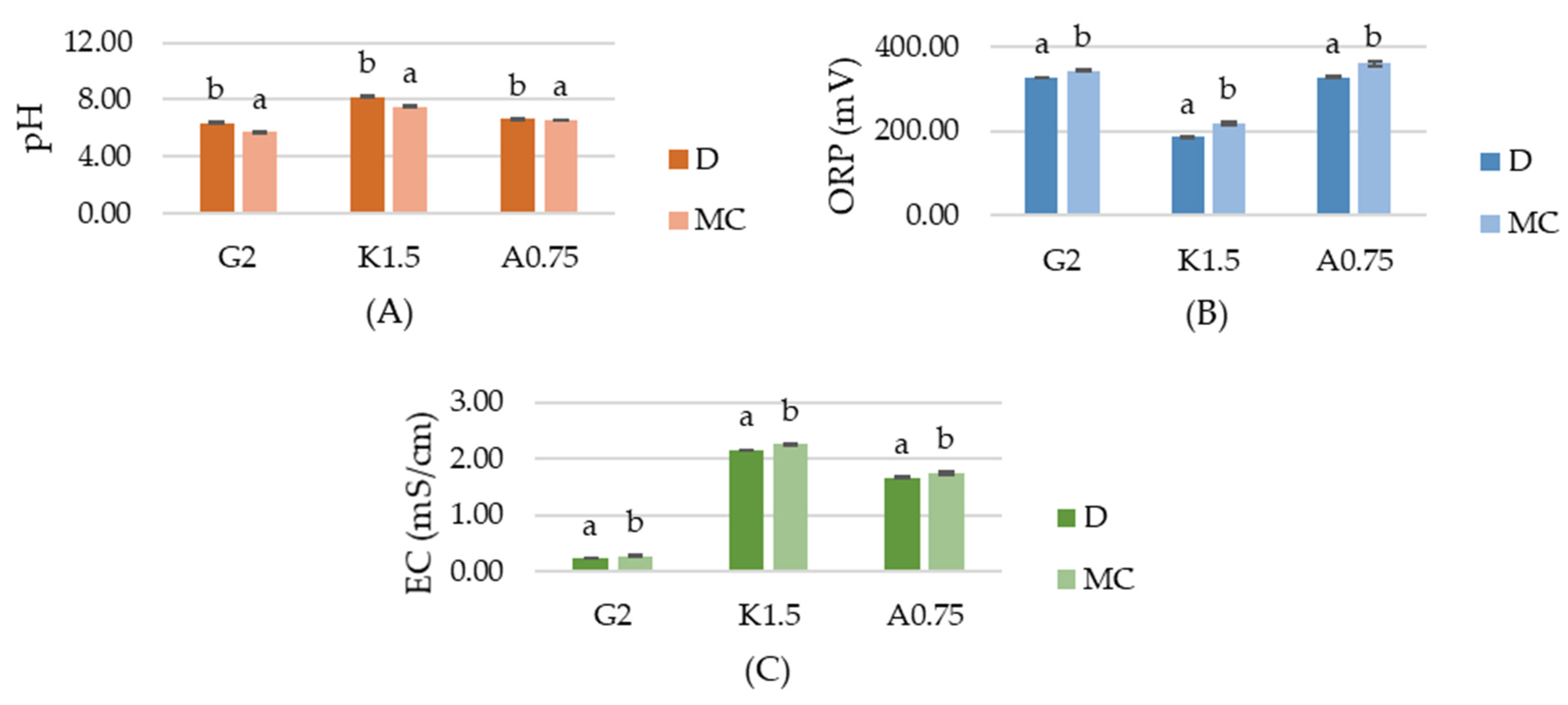
2.1. Physiochemical Properties of Hydrosols
2.2. Rheological Measurements
2.2.1. Flow Property
2.2.2. Time-Dependent Thixotropic Behavior
2.2.3. Determination of the Viscoelastic Properties
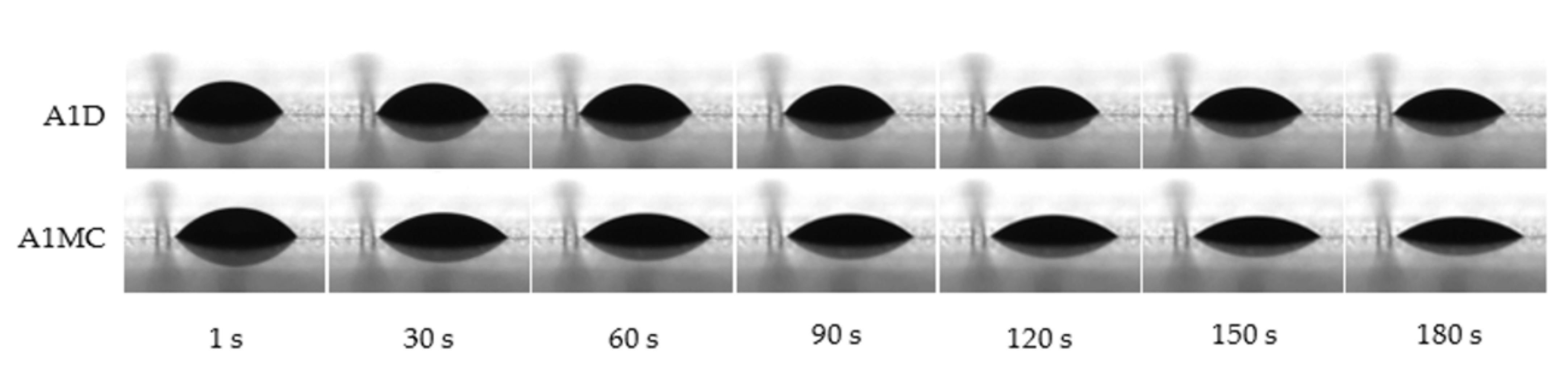
2.3. Contact Angle Measurement
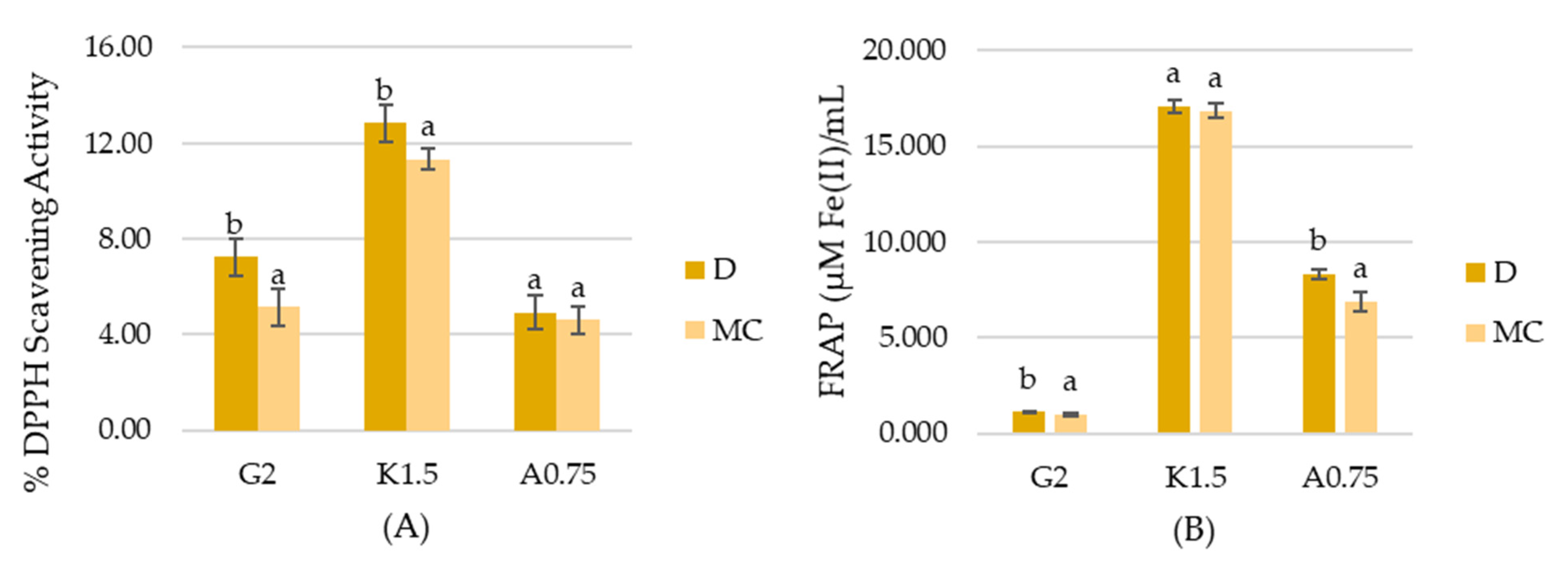
2.4. Antioxidant Activity
3. Materials and Methods
3.1. Material
3.2. Plasma Reatment
3.3. Preparation of Hydrosols
3.4. Hydrosols Characterization
3.4.1. Physiochemical Properties of Hydrosols
3.4.2. Rheological Measurements
Flow Property
Time-Dependent Thixotropic Behavior
Determination of the Viscoelastic Properties
3.4.3. Contact Angle Measurements
3.4.4. Antioxidant Activity
Free Radical Scavenging Activity (DPPH)
Ferric Reducing Ion Antioxidant Power (FRAP)
3.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 77, ISBN 9122336125. [Google Scholar]
- Shaw, P.; Kumar, N.; Kwak, H.S.; Park, J.H.; Uhm, H.S.; Bogaerts, A.; Choi, E.H.; Attri, P. Bacterial inactivation by plasma treated water enhanced by reactive nitrogen species. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vaka, M.R.; Sone, I.; Álvarez, R.G.; Walsh, J.L.; Prabhu, L.; Sivertsvik, M.; Fernández, E.N. Towards the next-generation disinfectant: Composition, storability and preservation potential of plasma activated water on baby spinach leaves. Foods 2019, 8, 692. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.N.; Patil, S.; Moiseev, T.; Bourke, P.; Mosnier, J.P.; Keener, K.M.; Cullen, P.J. In-package atmospheric pressure cold plasma treatment of strawberries. J. Food Eng. 2014, 125, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Chwastowski, J.; Ciesielska, K.; Ciesielski, W.; Khachatryan, K.; Kołoczek, H.; Kulawik, D.; Oszczeda, Z.; Tomasik, P.; Witczak, M. Structure and physicochemical properties of water treated under nitrogen with low-temperature glow plasma. Water 2020, 12, 1314. [Google Scholar] [CrossRef]
- Yelkin, I.; Reszke, E.; Schroeder, G. Glow discharge plasma as a cause of changes in aqueous solutions: The mass spectrometry study of solvation processes of ions. Asian J. Chem. 2021, 33, 220–230. [Google Scholar] [CrossRef]
- Šimečková, J.; Krčma, F.; Klofáč, D.; Dostál, L.; Kozáková, Z. Influence of plasma-activated water on physical and physical-Chemical soil properties. Water 2020, 12, 2357. [Google Scholar] [CrossRef]
- Hoeben, W.F.L.M.; van Ooij, P.P.; Schram, D.C.; Huiskamp, T.; Pemen, A.J.M.; Lukeš, P. On the Possibilities of Straightforward Characterization of Plasma Activated Water. Plasma Chem. Plasma Process. 2019, 39, 597–626. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Su, Y.; Liu, D.; Chen, S.; Hu, Y.; Ye, X. Application of atmospheric cold plasma-activated water (PAW) ice for preservation of shrimps (Metapenaeus ensis). Food Control 2018, 94, 307–314. [Google Scholar] [CrossRef]
- Xinyu, L.; Xiang, Q.; Cullen, P.J.; Su, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. LWT-Food Science and Technology Plasma-activated water (PAW) and slightly acidic electrolyzed water (SAEW) as beef thawing media for enhancing microbiological safety. LWT 2020, 117, 108649. [Google Scholar] [CrossRef]
- López, M. Evaluation of Cold Atmospheric Pressure Plasma (CAPP) and Plasma-Activated Water (PAW) as alternative non-thermal decontamination technologies for tofu: Impact on microbiological, sensorial and functional quality attributes. Food Res. Int. 2019, 129, 108859. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene expression in Tomato seedlings. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Saul, J.M.; Williams, D.F. Hydrogels in Regenerative Medicine. In Principle of Regenerative Medicine, 2nd ed.; Atala, A.A., Robert, L.B., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 637–661. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Ding, W.; Zhou, J.; Zeng, Y.; Wang, Y.N.; Shi, B. Preparation of oxidized sodium alginate with different molecular weights and its application for crosslinking collagen fiber. Carbohydr. Polym. 2017, 157, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Development of silver sulfadiazine loaded bacterial cellulose/sodium alginate composite films with enhanced antibacterial property. Carbohydr. Polym. 2015, 132, 351–358. [Google Scholar] [CrossRef]
- Hamdan, M.A.; Ramli, N.A.; Othman, N.A.; Mohd Amin, K.N.; Adam, F. Characterization and property investigation of microcrystalline cellulose (MCC) and carboxymethyl cellulose (CMC) filler on the carrageenan-based biocomposite film. Mater. Today Proc. 2020, 42, 56–62. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Y.; Wang, W.; Zhang, K.; Tian, X.; Zhao, K.; Duan, S.; Li, S.; Guo, Y. Incorporation of gelatin improves toughness of collagen film with a homo-hierarchical structure. Food Chem. 2020, 345, 128802. [Google Scholar] [CrossRef]
- Watthanaphanit, A.; Saito, N. Effect of polymer concentration on the depolymerization of sodium alginate by the solution plasma process. Polym. Degrad. Stab. 2013, 98, 1072–1080. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Ciesielska, A.; Ciesielski, W.; Khachatryan, K.; Koloczek, H.; Kulawik, D.; Oszczeda, Z.; Soroka, J.; Tomasik, P. Structure and physicochemical properties of water treated under carbon dioxide with low-temperature low-pressure glow plasma of low frequency. Water 2020, 12, 1920. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Król, Z.; Malik, M.; Marycz, K.; Jarmoluk, A. Characteristic of gelatine, carrageenan and sodium alginate hydrosols treated by direct electric current. Polymers 2016, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Subhash, G.; Kwon, J.; Mei, R.; Moore, D.F. Non-Newtonian Behavior of Ballistic Gelatin at High Shear Rates. Exp. Mech. 2012, 52, 551–560. [Google Scholar] [CrossRef]
- Gómez-Díaz, D.; Navaza, J.M. Rheology of aqueous solutions of food additives. J. Food Eng. 2003, 56, 387–392. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Kulig, D.; Jarmoluk, A.; Marycz, K.; Matuszczak, W. Study of enzymatically treated alginate/chitosan hydrosols in sponges formation process. Polymers 2016, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Król, Z.; Kulig, D.; Marycz, K.; Zimoch-Korzycka, A.; Jarmoluk, A. The effects of using sodium alginate hydrosols treated with direct electric current as coatings for sausages. Polymers 2017, 9, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A. Cross-linked alginate/chitosan polyelectrolytes as carrier of active compound and beef color stabilizer. Meat Sci. 2017, 123, 219–228. [Google Scholar] [CrossRef]
- Ng, S.P.; Lai, O.M.; Abas, F.; Lim, H.K.; Tan, C.P. Stability of a concentrated oil-in-water emulsion model prepared using palm olein-based diacylglycerol/virgin coconut oil blends: Effects of the rheological properties, droplet size distribution and microstructure. Food Res. Int. 2014, 64, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173–1180. [Google Scholar] [CrossRef]
- Warner, E.L.; Norton, I.T.; Mills, T.B. Comparing the viscoelastic properties of gelatin and different concentrations of kappa-carrageenan mixtures for additive manufacturing applications. J. Food Eng. 2019, 246, 58–66. [Google Scholar] [CrossRef]
- Jiang, Y.; De La Cruz, J.A.; Ding, L.; Wang, B.; Feng, X.; Mao, Z.; Xu, H.; Sui, X. Rheology of regenerated cellulose suspension and influence of sodium alginate. Int. J. Biol. Macromol. 2020, 148, 811–816. [Google Scholar] [CrossRef]
- Thomas, Y. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Chen, L.; Bonaccurso, E. Effects of surface wettability and liquid viscosity on the dynamic wetting of individual drops. Phys. Rev. E 2014, 90, 022401. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.F.; Islam, M.A.; Khorshidi, B.; Sadrzadeh, M. Prediction of surface charge properties on the basis of contact angle titration models. Mater. Chem. Phys. 2021, 258, 123933. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; James Feng, L.X.; Bang, W.S.; Yuk, H.G. Inactivation of Listeria monocytogenes and natural microbiota on raw salmon fillets using acidic electrolyzed water, ultraviolet light or/and ultrasounds. Food Control 2017, 74, 54–60. [Google Scholar] [CrossRef]
- Xuan, X.T.; Ding, T.; Li, J.; Ahn, J.H.; Zhao, Y.; Chen, S.G.; Ye, X.Q.; Liu, D.H. Estimation of growth parameters of Listeria monocytogenes after sublethal heat and slightly acidic electrolyzed water (SAEW) treatment. Food Control 2017, 71, 17–25. [Google Scholar] [CrossRef]
- Luo, K.; Kim, S.Y.; Wang, J.; Oh, D.H. A combined hurdle approach of slightly acidic electrolyzed water simultaneous with ultrasound to inactivate Bacillus cereus on potato. LWT 2016, 73, 615–621. [Google Scholar] [CrossRef]
- Król, Ż.; Marycz, K.; Kulig, D.; Marędziak, M.; Jarmoluk, A. Cytotoxicity, bactericidal, and antioxidant activity of sodium alginate hydrosols treated with direct electric current. Int. J. Mol. Sci. 2017, 18, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolova, E.V.; Barabanova, A.O.; Bogdanovich, R.N.; Khomenko, V.A.; Solov’eva, T.F.; Yermak, I.M. In vitro antioxidant properties of red algal polysaccharides. Biomed. Prev. Nutr. 2011, 1, 161–167. [Google Scholar] [CrossRef]
- Chen, J.C.; Yeh, J.Y.; Chen, P.C.; Hsu, C.K. Phenolic Content and DPPH Radical Scavenging Activity of Yam-containing Surimi Gels Influenced by Salt and Heating. Asian J. Health Inf. Sci. 2007, 2, 1–11. [Google Scholar]
- Olugbami, J.O.; Gbadegesin, M.A.; Odunola, O.A. In vitro free radical scavenging and antioxidant properties of ethanol extract of Terminalia glaucescens. Pharmacogn. Res. 2015, 7, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variant | Ostwald De Waele Model | Herschel–Bulkley Model | Shear Stress τ5fl (Pa) | Shear Stress τ7760 (Pa) | Apparent Viscosity η5 (Pa∙s) | Apparent Viscosity η7760 (Pa∙s) | |
|---|---|---|---|---|---|---|---|
| Consistency Index k (Pa∙s) | Flow Behavior Index n (-) | Yield Stress τ0 (Pa) | |||||
| G2D | 0.13 a ± 0.08 | 1.33 c ± 0.07 | 0.05 a ± 0.03 | 0.04 a,b ± 0.02 | 19.05 a ± 5.65 | 0.008 a,b ± 0.00 | 0.002 a ± 0.00 |
| G4D | 0.88 a,b ± 0.01 | 1.16 b ± 0.01 | 0.05 a ± 0.00 | 0.04 a,b ± 0.02 | 28.10 b ± 1.57 | 0.009 a,b ± 0.00 | 0.004 b ± 0.00 |
| G8D | 7.45 c ± 1.00 | 1.03 a ± 0.01 | 0.03 a ± 0.00 | 0.07 b ± 0.00 | 78.44 c ± 3.85 | 0.014 b ± 0.00 | 0.010 c ± 0.00 |
| G2MC | 0.15 a ± 0.01 | 1.31 c ± 0.00 | 0.04 a ± 0.00 | 0.01 a ± 0.00 | 18.76 a ± 1.30 | 0.002 a ± 0.00 | 0.002 a ± 0.00 |
| G4MC | 1.93 b ± 0.31 | 1.08 a ± 0.01 | 0.04 a ± 0.00 | 0.04 a,b ± 0.01 | 31.61 b ± 1.94 | 0.009 a,b ± 0.00 | 0.004 b ± 0.00 |
| G8MC | 6.12 c ± 1.29 | 1.06 a ± 0.02 | 0.04 a ± 0.01 | 0.05 b ± 0.00 | 87.31 d ± 0.23 | 0.010 b ± 0.00 | 0.011 c ± 0.00 |
| C1.5D | 0.82 a,b ± 0.09 | 0.53 b ± 0.03 | −2.25 a ± 0.49 | 0.43 a ± 0.03 | 83.58 a ± 16.06 | 0.085 b ± 0.01 | 0.009 a ± 0.00 |
| C2.0D | 2.16 b ± 0.01 | 0.48 b ± 0.01 | −6.05 a ± 0.07 | 1.28 d ± 0.03 | 162.69 e ± 0.46 | 0.257 d ± 0.01 | 0.015 c ± 0.00 |
| C2.5D | 4.60 c ± 0.12 | 0.39 a ± 0.01 | −33.83 b ± 7.92 | 2.48 e ± 0.67 | 116.20 c ± 2.94 | 0.496 f ± 0.13 | 0.021 e ± 0.00 |
| C1.5MC | 0.73 a ± 0.03 | 0.53 b ± 0.02 | −1.99 a ± 0.29 | 0.41 b ± 0.04 | 80.08 b ± 22.16 | 0.081 a ± 0.01 | 0.012 b ± 0.00 |
| C2.0MC | 1.74 a,b ± 0.15 | 0.50 b ±0.00 | −4.83 a ± 0.44 | 1.02 c ± 0.07 | 146.57 d ± 7.19 | 0.204 c ± 0.01 | 0.016 c ± 0.00 |
| C2.5MC | 4.64 c ± 1.08 | 0.41 a ± 0.02 | −28.95 b ± 16.32 | 1.98 f ± 0.22 | 120.62 c ± 6.25 | 0.396 e ± 0.04 | 0.019 d ± 0.00 |
| A0.75D | 0.93 a ± 0.06 | 0.54 a ± 0.00 | −2.49 a ± 0.17 | 0.44 b ± 0.01 | 120.90 b ± 7.56 | 0.09 b ± 0.00 | 0.016 a ± 0.00 |
| A1D | 2.15 b ± 0.17 | 0.49 b ± 0.00 | −6.52 a ± 0.59 | 0.94 d ± 0.00 | 173.25 c ± 0.00 | 0.19 d ± 0.00 | 0.021 b ± 0.00 |
| A1.5D | 3.80 c ± 0.04 | 0.45 c ± 0.00 | −14.36 a ± 0.42 | 1.63 f ± 0.00 | 181.13 c ± 40.09 | 0.33 f ± 0.03 | 0.023 b ± 0.01 |
| A0.75MC | 0.86 a ± 0.02 | 0.55 a ± 0.00 | −2.33 a ± 0.07 | 0.41 a ± 0.01 | 113.24 a,b ± 4.17 | 0.08 a ± 0.01 | 0.009 a ± 0.00 |
| A1MC | 3.90 c ± 0.04 | 0.38 d ± 0.00 | −39.63 a,b ± 1.03 | 0.77 c ± 0.00 | 70.02 a ± 1.59 | 0.15 c ± 0.00 | 0.015 a ± 0.00 |
| A1.5MC | 5.27 d ± 0.25 | 0.39 d ± 0.03 | −63.61 b ± 44.83 | 1.38 e ± 0.02 | 117.95 b ± 27.71 | 0.28 e ± 0.04 | 0.014 a ± 0.00 |
| Variant | Storage Modulus G′5Hz (mPa) | Loss Modulus G″5 Hz (mPa) | Loss Tangent Tanδ5Hz (Pa) | Thixotropy Area (Pa/s) |
|---|---|---|---|---|
| G2D | 3.69 a ± 2.79 | 13.50 a ± 9.20 | 6.45 a ± 7.38 | 0.38 a ± 0.01 |
| G4D | 3.98 a ± 0.35 | 14.95 a ± 0.88 | 3.78 a ± 0.55 | 4.15 d ± 0.72 |
| G8D | 1.43 a ± 0.03 | 55.86 b ± 0.68 | 39.00 b ± 0.43 | 6.17 e ± 2.89 |
| G2MC | 6.47 a ± 0.07 | 8.04 a ± 0.01 | 1.24 a ± 0.01 | 0.70 a ± 2.59 |
| G4MC | 6.16 a ± 2.17 | 16.63 a ± 1.10 | 2.90 a ± 1.21 | 1.49 b ± 0.24 |
| G8MC | 3.65 a ± 3.56 | 119.81 b ± 32.67 | 16.53 b ± 1.18 | 2.76 c ± 0.02 |
| C1.5D | 9.85 a ± 1.97 | 398.73 a ± 5.99 | 41.35 b ± 8.86 | −1.22 a ± 0.07 |
| C2.0D | 58.18 b ± 16.26 | 1071.74 b,c ± 148.97 | 18.80 a ± 2.69 | −6.31 c ± 0.13 |
| C2.5D | 195.80 c ± 17.21 | 2760.00 d ± 213.39 | 14.10 a ± 0.15 | −20.25 d ± 0.74 |
| C1.5MC | 10.49 a ± 2.18 | 476.18 a,b ± 86.67 | 45.50 b ± 1.18 | −5.62 b ± 3.41 |
| C2.0MC | 63.91 b ± 3.91 | 1205.89 c ± 31.74 | 18.92 a ± 1.65 | −6.41 c ± 6.65 |
| C2.5MC | 149.58 c ± 36.17 | 2257.76 d ± 559.69 | 15.08 a ± 0.09 | −30.20 e ± 40.00 |
| A0.75D | 9.88 a ± 7.87 | 560.10 a ± 47.32 | 34.68 b ± 0.79 | 0.76 b ± 0.05 |
| A1D | 29.37 b ± 1.01 | 1144.00 c ± 4.45 | 38.97 b ± 1.19 | 1.71b c ± 0.00 |
| A1.25D | 86.27 d ± 4.44 | 2083.61 e ± 168.92 | 24.14 a ± 0.72 | 1.91 c ± 0.01 |
| A0.75MC | 2.80 a ± 0.16 | 457.13 a ± 3.86 | 63.75 c ± 7.82 | 0.64 a ± 0.00 |
| A1MC | 22.32 b ± 0.87 | 904.22 b ± 30.06 | 40.52 b ± 0.23 | 3.26 d ± 1.29 |
| A1.25MC | 53.08 c ± 2.68 | 1394.64 d ± 51.09 | 26.28 a ± 0.36 | 10.72 e ± 1.63 |
| Variant | Time of Measurement (s) | Water Contact Angle (°) |
|---|---|---|
| A1D | 1 | 49.89 b ± 0.73 |
| A1MC | 44.29 a ± 0.80 | |
| A1D | 30 | 49.89 b ± 0.43 |
| A1MC | 41.46 a ± 0.77 | |
| A1D | 60 | 48.36 b ± 0.55 |
| A1MC | 38.28 a ± 0.41 | |
| A1D | 90 | 46.8 2 b ± 0.55 |
| A1MC | 37.43 a ± 0.46 | |
| A1D | 120 | 45.07 b ± 0.26 |
| A1MC | 36.43 a ± 0.56 | |
| A1D | 150 | 44.51 b ± 0.36 |
| A1MC | 36.01 a ± 0.74 | |
| A1D | 180 | 41.07 b ± 0.11 |
| A1MC | 30.41 a ± 0.55 |
| Run Code Letters | Gelatin (G) [%] | Carrageenan (C) [%] | Sodium Alginate (A) [%] | Water (D or MC) | ||
|---|---|---|---|---|---|---|
| G2D | C1.5D | A0.75D | 2.0 | 1.5 | 0.75 | D |
| G2MC | C1.5MC | A0.75MC | MC | |||
| G4D | C2D | A1D | 4.0 | 2.0 | 1.0 | D |
| G4MC | C2MC | A1MC | MC | |||
| G8D | C2.5D | A1.25D | 8.0 | 2.0 | 1.25 | D |
| G8MC | C2.5MC | A1.25MC | MC | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król-Kilińska, Ż.; Kulig, D.; Yelkin, I.; Zimoch-Korzycka, A.; Bobak, Ł.; Jarmoluk, A. The Effect of Using Micro-Clustered Water as a Polymer Medium. Int. J. Mol. Sci. 2021, 22, 4730. https://doi.org/10.3390/ijms22094730
Król-Kilińska Ż, Kulig D, Yelkin I, Zimoch-Korzycka A, Bobak Ł, Jarmoluk A. The Effect of Using Micro-Clustered Water as a Polymer Medium. International Journal of Molecular Sciences. 2021; 22(9):4730. https://doi.org/10.3390/ijms22094730
Chicago/Turabian StyleKról-Kilińska, Żaneta, Dominika Kulig, Ihar Yelkin, Anna Zimoch-Korzycka, Łukasz Bobak, and Andrzej Jarmoluk. 2021. "The Effect of Using Micro-Clustered Water as a Polymer Medium" International Journal of Molecular Sciences 22, no. 9: 4730. https://doi.org/10.3390/ijms22094730
APA StyleKról-Kilińska, Ż., Kulig, D., Yelkin, I., Zimoch-Korzycka, A., Bobak, Ł., & Jarmoluk, A. (2021). The Effect of Using Micro-Clustered Water as a Polymer Medium. International Journal of Molecular Sciences, 22(9), 4730. https://doi.org/10.3390/ijms22094730






