Mesenchymal Stem Cells Antagonize IFN-Induced Proinflammatory Changes and Growth Inhibition Effects via Wnt/β-Catenin and JAK/STAT Pathway in Human Outer Root Sheath Cells and Hair Follicles
Abstract
1. Introduction
2. Results
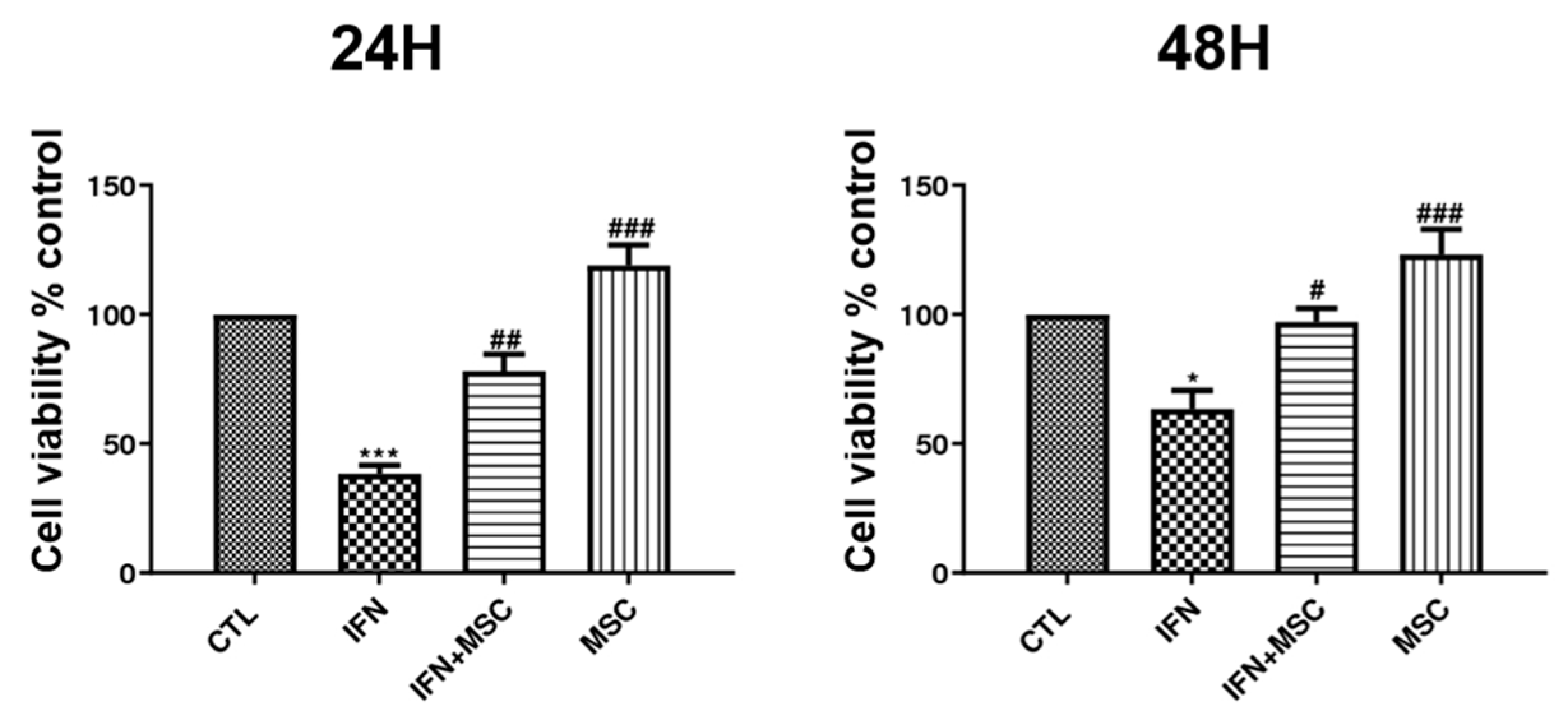
2.1. Effect of hHMSC Treatment on the Cell Viability of hORSCs
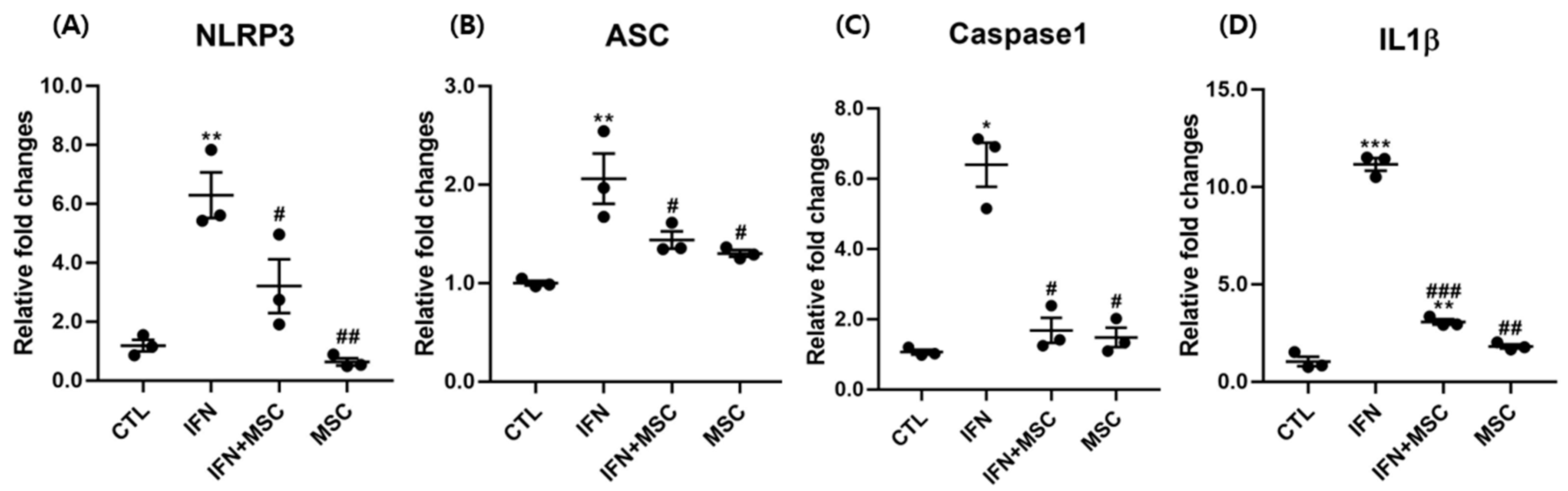
2.2. Effect of hHMSC Treatment on NLRP3 Inflammasome Components
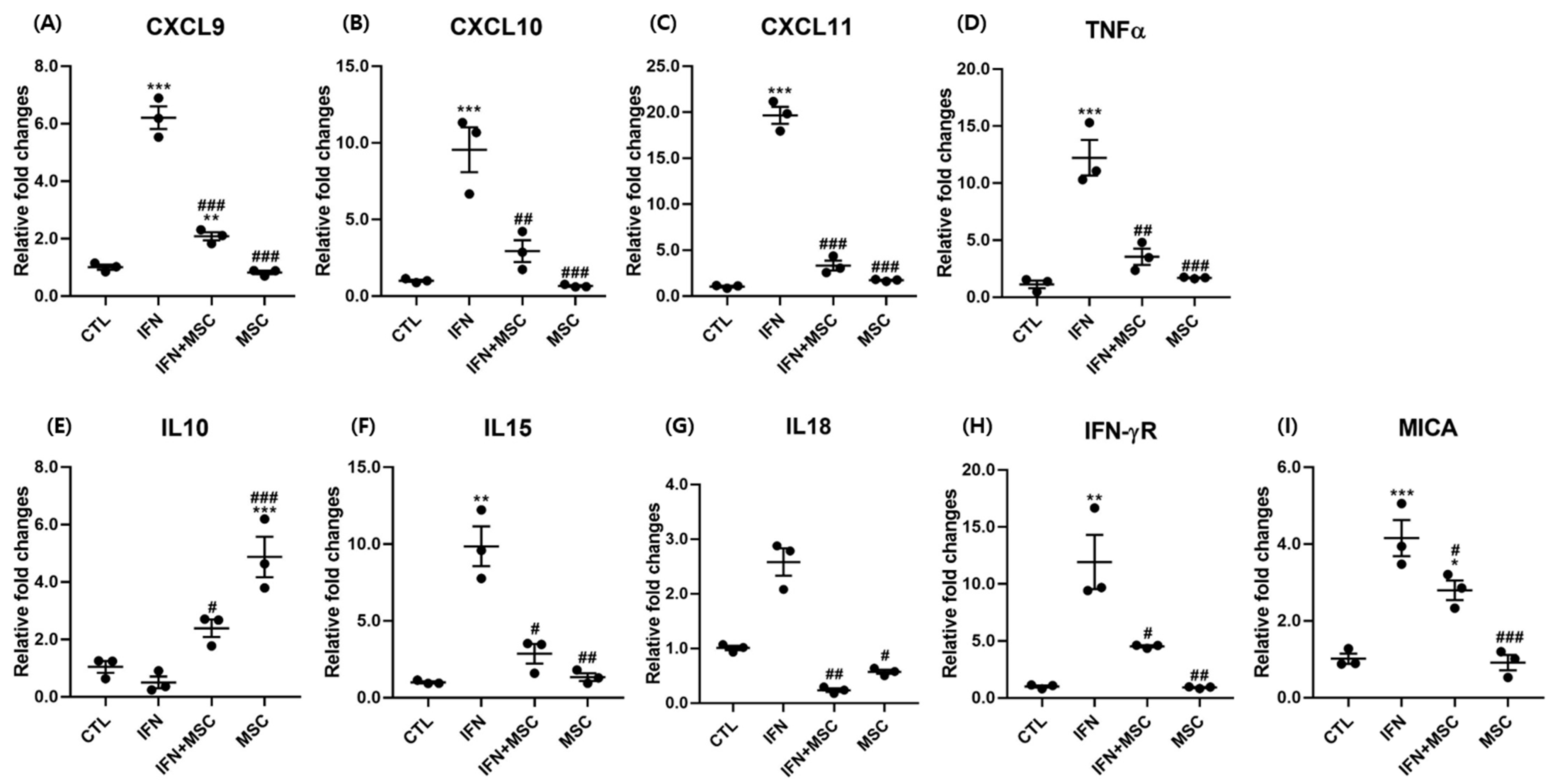
2.3. Effect of hHMSC Treatment on HF-IP Collapse-Related Genes
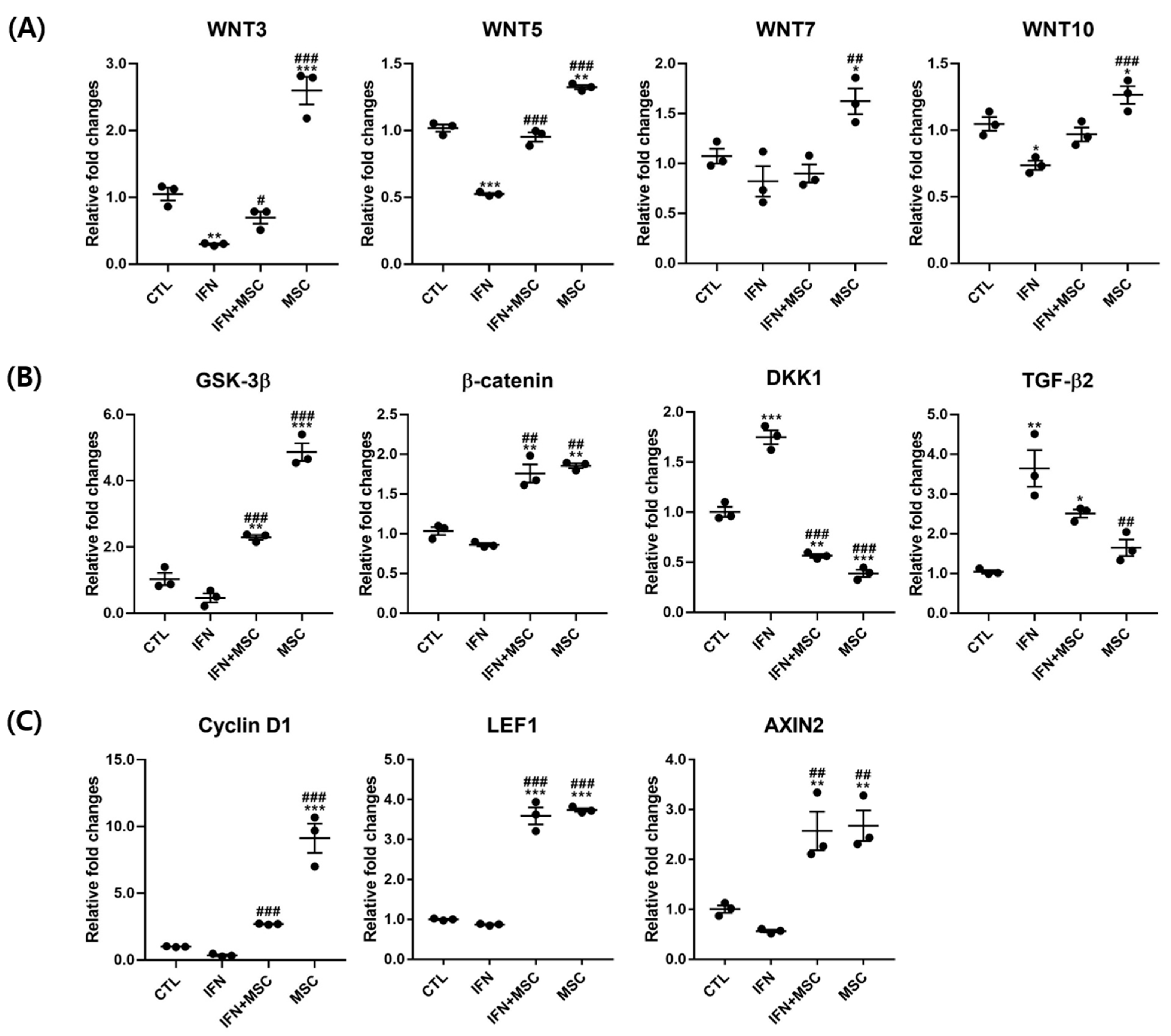
2.4. Effect of hHMSC Treatment on Wnt/β-Catenin Signaling Pathway Molecules
2.5. Effect of hHMSC Treatment on Hair Stem Cell Markers
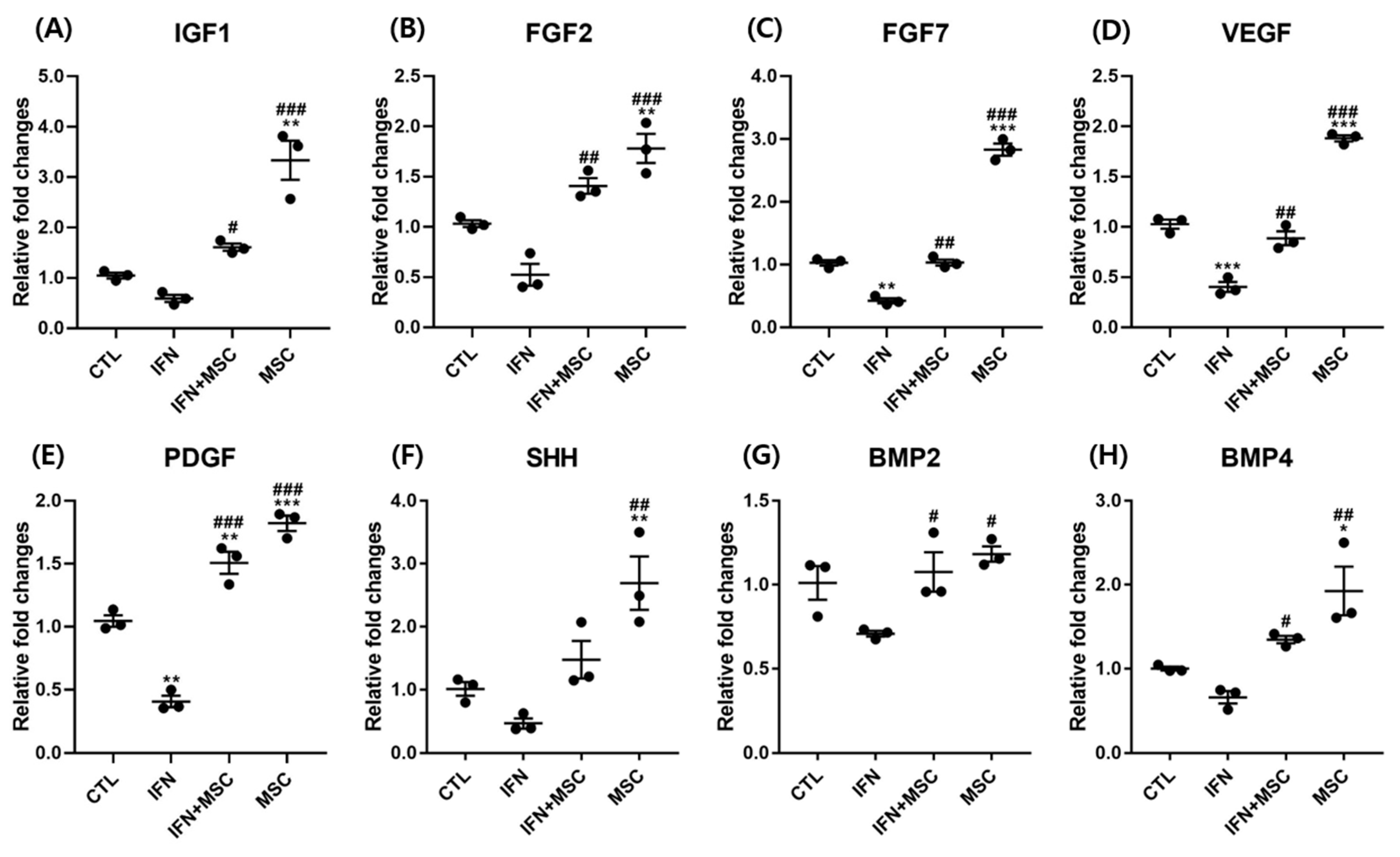
2.6. Effect of hHMSC Treatment on Growth Factors
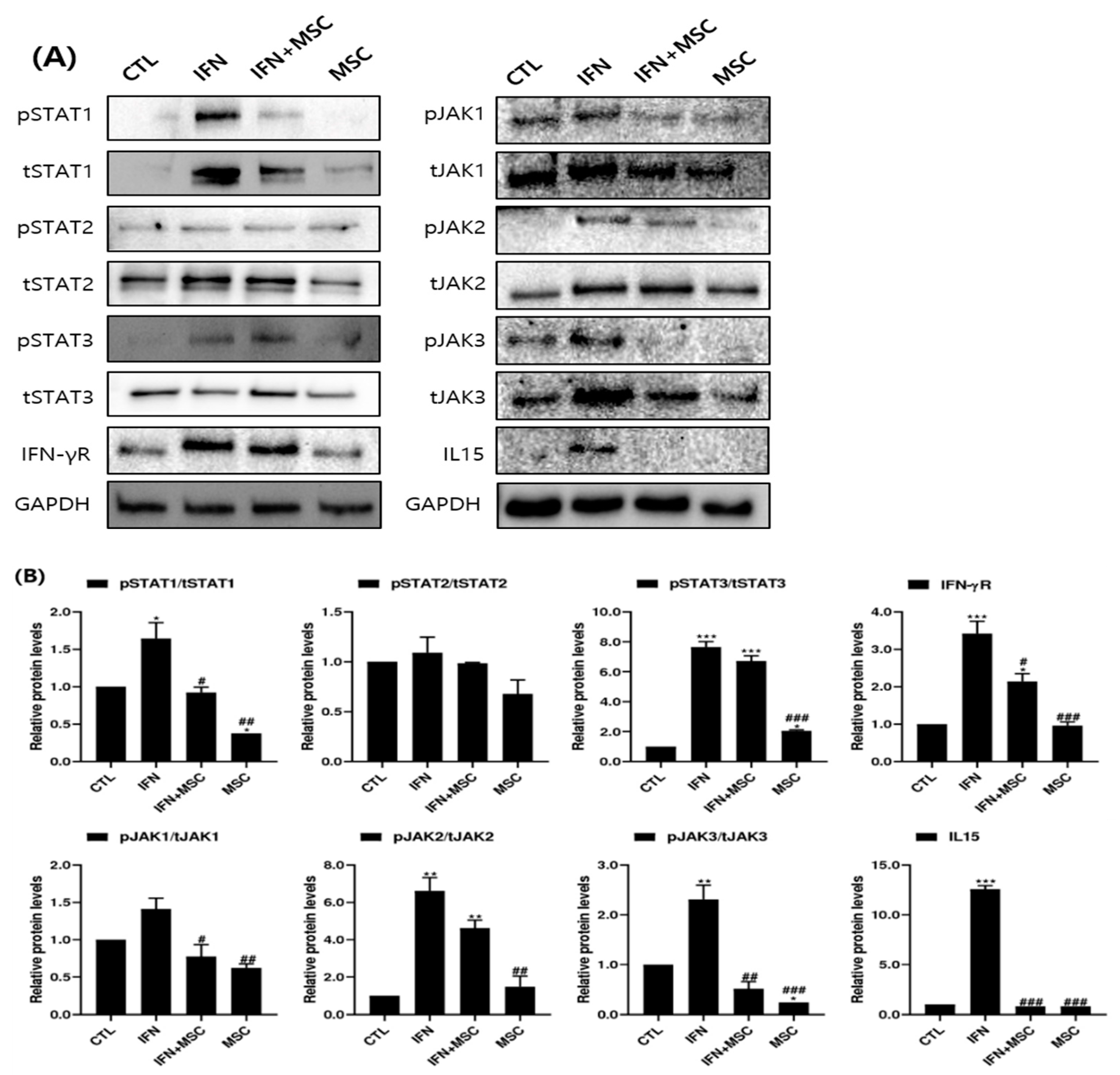
2.7. Effect of hHMSC Treatment on the JAK/STAT Pathway
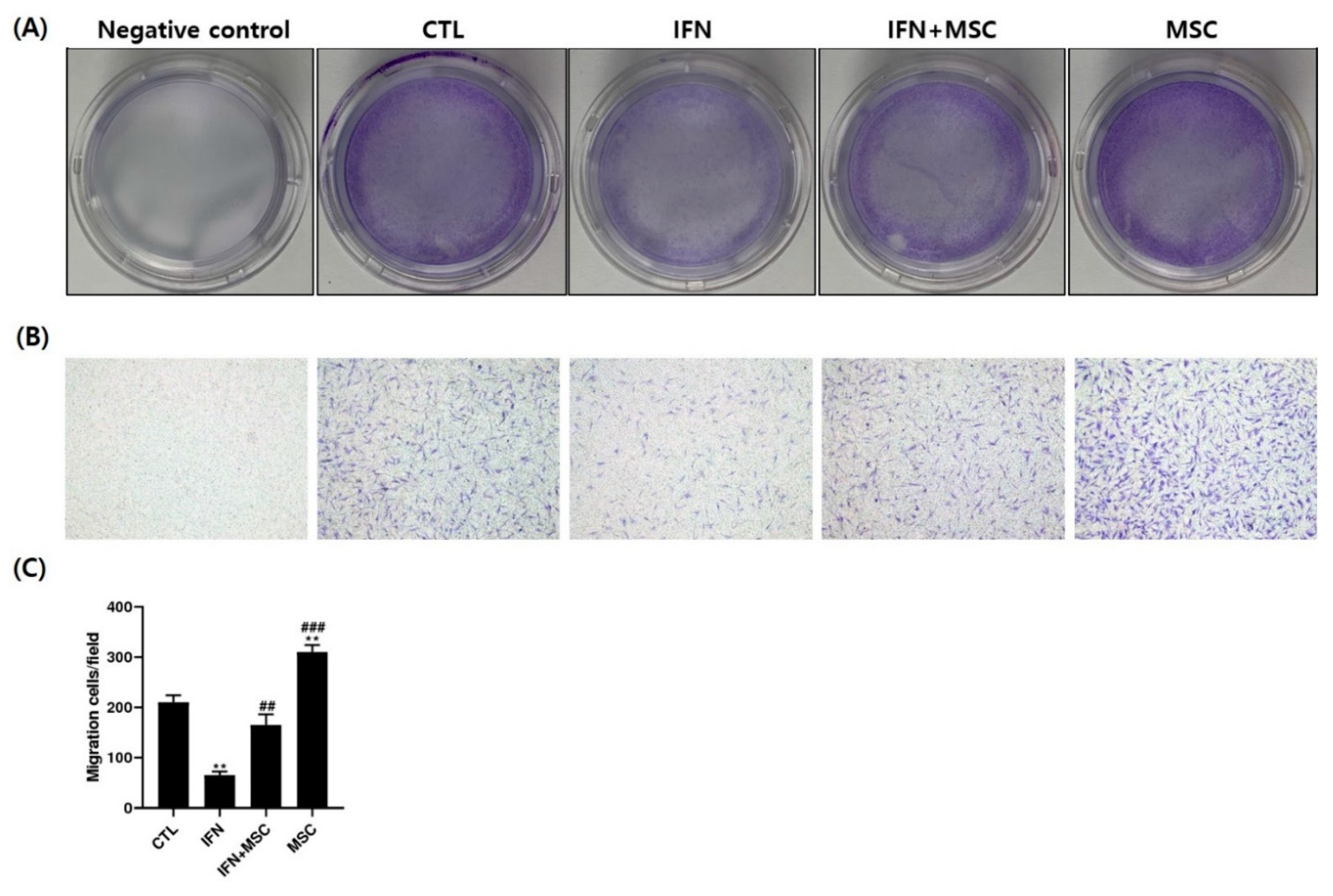
2.8. Effect of hHMSC Treatment on hORSC Migration Ability
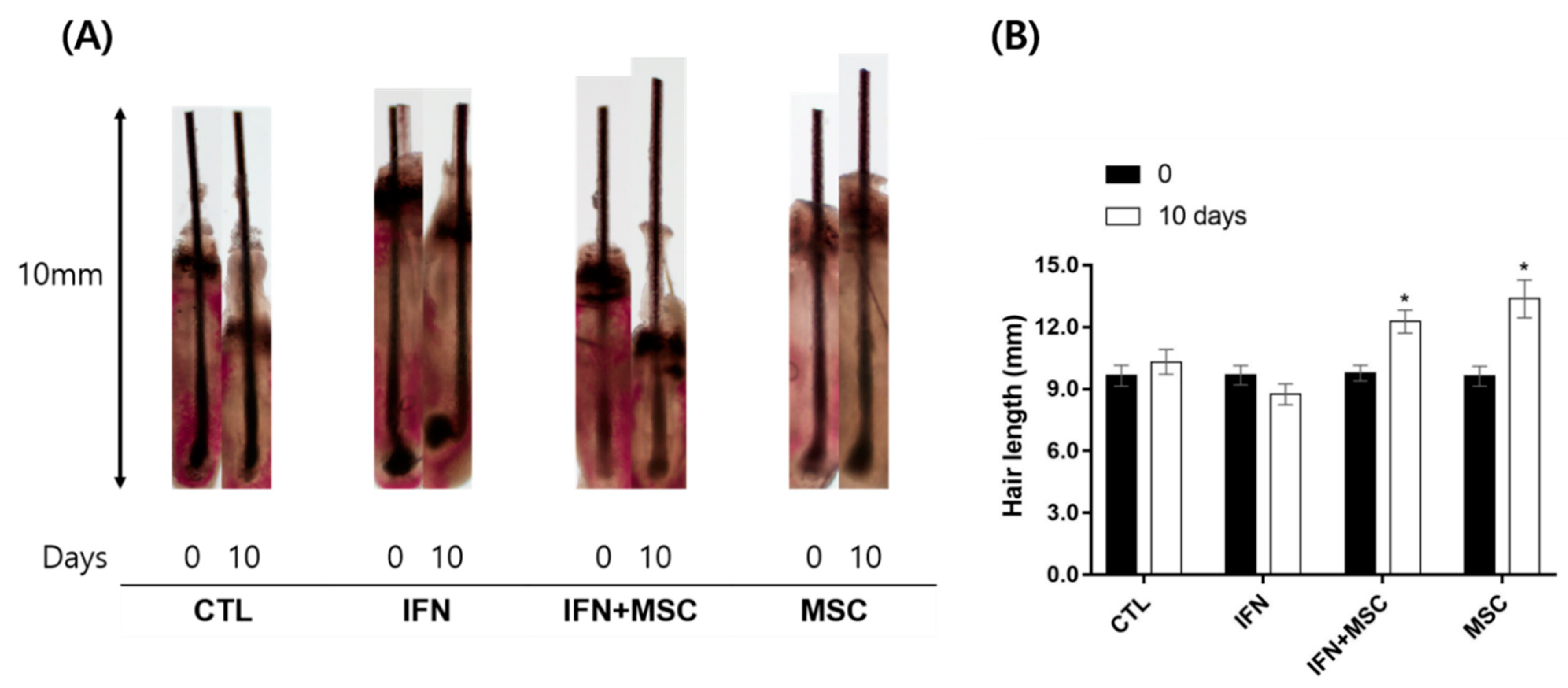
2.9. Effects of hHMSCs Treatment on the Mouse Vibrissa HF Organ Culture
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Co-Culture
4.3. Cell Viability Assay (MTT Assay)
4.4. Real-Time PCR
4.5. Western Blot Analysis
4.6. Transwell Migration Assay
4.7. Hair Follicle Organ Culture Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Alopecia areata |
| hORSCs | Human outer root sheath cells |
| hHMSCs | Human hematopoietic mesenchymal stem cells |
| hDPCs | Human derma papilla cells |
| MSCT | Mesenchymal stem cell therapy |
| JAK | Janus kinase |
| STAT | Signal transducers and activators of transcription protein |
| CXCL | C-X-C motif chemokines |
| IL | Interleukin |
| IFN | Interferon |
| FBS | Fetal bovine serum |
| DMEM | Dulbecco Modified Eagle Medium |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| RT-PCR | Real time-polymerase chain reaction |
| HFs | Hair follicles |
| HF-IP | Hair follicle-immune privilege |
| NLRP3 | NLR family pyrin domain containing 3 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| MICA | Major histocompatibility complex (MHC) class I chain-related protein A |
| NK cell | Natural killer cell |
| LEF1 | Lymphoid enhancer binding factor 1 |
| AXIN2 | Axis inhibition protein 2 |
| BMP2 | Bone morphogenetic protein |
| SHH | Sonic hedgehog |
| ALP | Alkaline phosphatase |
| CRABP1 | Cellular retinoic acid binding protein 1 |
| SOX | SRY-box transcription factor |
| DP | Dermal papilla |
References
- Gilhar, A.; Etzioni, A.; Paus, R. Alopecia Areata. N. Engl. J. Med. 2012, 366, 1515–1525. [Google Scholar] [CrossRef]
- Alexis, A.F.; Dudda-Subramanya, R.; Sinha, A.A. Alopecia areata: Autoimmune basis of hair loss. Eur. J. Dermatol. 2004, 14, 364–370. [Google Scholar] [PubMed]
- Wang, X.; Marr, A.K.; Breitkopf, T.; Leung, G.; Hao, J.; Wang, E.; Kwong, N.; Akhoundsadegh, N.; Chen, L.; Mui, A.; et al. Hair follicle mesenchyme-associated PD-L1 regulates T-cell activation induced apoptosis: A potential mechanism of immune privilege. J. Investig. Dermatol. 2014, 134, 736–745. [Google Scholar] [CrossRef]
- Kang, H.; Wu, W.Y.; Lo, B.K.; Yu, M.; Leung, G.; Shapiro, J.; McElwee, K.J. Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J. Investig. Dermatol. 2010, 130, 2677–2680. [Google Scholar] [CrossRef]
- McElwee, K.J.; Gilhar, A.; Tobin, D.J.; Ramot, Y.; Sundberg, J.P.; Nakamura, M.; Bertolini, M.; Inui, S.; Tokura, Y.; King, L.E., Jr.; et al. What causes alopecia areata? Exp. Dermatol. 2013, 22, 609–626. [Google Scholar] [CrossRef]
- Li, Y.; Yan, B.; Wang, H.; Li, H.; Li, Q.; Zhao, D.; Chen, Y.; Zhang, Y.; Li, W.; Zhang, J.; et al. Hair regrowth in alopecia areata patients following Stem Cell Educator therapy. BMC Med. 2015, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Elmaadawi, I.H.; Mohamed, B.M.; Ibrahim, Z.A.S.; Abdou, S.M.; El Attar, Y.A.; Youssef, A.; Shamloula, M.M.; Taha, A.; Metwally, H.G.; El Afandy, M.M.; et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J. Dermatol. Treat. 2018, 29, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.W.; Kim, H.J.; Na, K.; Ko, H.S.; Song, H.J.; Song, S.U.; Jeon, M.S.; Choi, G.S. Bone marrow-derived mesenchymal stem cells prevent alopecia areata development through the inhibition of NKG2D expression: A pilot study. Exp. Dermatol. 2017, 26, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Oh, J.H.; Woo, Y.J.; Jung, J.H.; Jeong, K.H.; Kang, H. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models. Exp. Dermatol. 2020, 29, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.M.; Jeong, K.H.; Kim, J.E.; Park, Y.M.; Kang, H. Hair growth-promotion effects of different alternating current parameter settings are mediated by the activation of Wnt/β-catenin and MAPK pathway. Exp. Dermatol. 2015, 24, 958–963. [Google Scholar] [CrossRef]
- Legrand, J.M.D.; Roy, E.; Ellis, J.J.; Francois, M.; Brooks, A.J.; Khosrotehrani, K. STAT5 Activation in the Dermal Papilla Is Important for Hair Follicle Growth Phase Induction. J. Investig. Dermatol. 2016, 136, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Nistico, S.; Tamburi, F.; Bennardo, L.; Dastoli, S.; Schipani, G.; Caro, G.; Fortuna, M.C.; Rossi, A. Treatment of telogen effluvium using a dietary supplement containing Boswellia serrata, Curcuma longa, and Vitis vinifera: Results of an observational study. Dermatol. Ther. 2019, 32, e12842. [Google Scholar] [CrossRef] [PubMed]
- Limat, A.; Hunziker, T. Cultivation of keratinocytes from the outer root sheath of human hair follicles. Methods Mol. Med. 1996, 2, 21–31. [Google Scholar] [CrossRef]
- Feldmeyer, L.; Keller, M.; Niklaus, G.; Hohl, D.; Werner, S.; Beer, H.D. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr. Biol. 2007, 17, 1140–1145. [Google Scholar] [CrossRef]
- Lee, P.; Lee, D.J.; Chan, C.; Chen, S.W.; Ch’en, I.; Jamora, C. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature 2009, 458, 519–523. [Google Scholar] [CrossRef]
- Deng, Y.; Chang, C.; Lu, Q. The Inflammatory Response in Psoriasis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y. Role of keratinocytes in the development of vitiligo. Ann. Dermatol. 2012, 24, 115–125. [Google Scholar] [CrossRef]
- Tobin, D.J.; Fenton, D.A.; Kendall, M.D. Cell degeneration in alopecia areata. An ultrastructural study. Am. J. Dermatol. 1991, 13, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Choi, D.K.; Sohn, K.C.; Kim, S.Y.; Min Ha, J.; Ho Lee, Y.; Im, M.; Seo, Y.J.; Deok Kim, C.; Lee, J.H.; et al. Double-stranded RNA induces inflammation via the NF-κB pathway and inflammasome activation in the outer root sheath cells of hair follicles. Sci. Rep. 2017, 7, 44127. [Google Scholar] [CrossRef]
- Gu, S.; Xing, C.; Han, J.; Tso, M.O.; Hong, J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol. Vis. 2009, 15, 99–107. [Google Scholar]
- Guo, T.; Wang, W.; Zhang, J.; Chen, X.; Li, B.Z.; Li, L.S. Experimental study on repairing damage of corneal surface by mesenchymal stem cells transplantation. Zhonghua Yan Ke Za Zhi 2006, 42, 246–250. [Google Scholar] [PubMed]
- Ma, Y.; Xu, Y.; Xiao, Z.; Yang, W.; Zhang, C.; Song, E.; Du, Y.; Li, L. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells 2006, 24, 315–321. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, M.K.; Shin, M.S.; Lee, H.J.; Ko, J.H.; Wee, W.R.; Lee, J.H. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells 2008, 26, 1047–1055. [Google Scholar] [CrossRef]
- Regmi, S.; Pathak, S.; Kim, J.O.; Yong, C.S.; Jeong, J.H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 2019, 98, 151041. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J. Natural Killer Cells: At the Forefront of Modern Immunology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–428. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Chu, T.W.; Santos, L.; McElwee, K.J. Biology of the hair follicle and mechanisms of nonscarring and scarring alopecia. Semin. Cutan Med. Surg. 2015, 34, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Woo, Y.J.; Sohn, K.M.; Jeong, K.H.; Kang, H. Wnt/β-catenin and ERK pathway activation: A possible mechanism of photobiomodulation therapy with light-emitting diodes that regulate the proliferation of human outer root sheath cells. Lasers Surg. Med. 2017, 49, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Shtutman, M.; Zhurinsky, J.; Simcha, I.; Albanese, C.; D’Amico, M.; Pestell, R.; Ben-Ze’ev, A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5522–5527. [Google Scholar] [CrossRef]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Andl, T.; Bagasra, A.; Lu, M.M.; Epstein, D.J.; Morrisey, E.E.; Millar, S.E. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 2001, 107, 69–82. [Google Scholar] [CrossRef]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. Bioessays 2005, 27, 247–261. [Google Scholar] [CrossRef]
- Purba, T.S.; Haslam, I.S.; Shahmalak, A.; Bhogal, R.K.; Paus, R. Mapping the expression of epithelial hair follicle stem cell-related transcription factors LHX2 and SOX9 in the human hair follicle. Exp. Dermatol. 2015, 24, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Kadaja, M.; Keyes, B.E.; Lin, M.; Pasolli, H.A.; Genander, M.; Polak, L.; Stokes, N.; Zheng, D.; Fuchs, E. SOX9: A stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014, 28, 328–341. [Google Scholar] [CrossRef]
- Vidal, V.P.; Chaboissier, M.C.; Lützkendorf, S.; Cotsarelis, G.; Mill, P.; Hui, C.C.; Ortonne, N.; Ortonne, J.P.; Schedl, A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 2005, 15, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Teh, M.-T.; Mackenzie, I.C.; Waseem, A. Keratin K15 as a Biomarker of Epidermal Stem Cells. Int. J. Mol. Sci. 2013, 14, 19385–19398. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, Y.J.; Park, H.R.; Lee, D.G.; Jeong, K.H.; Kang, H. The Effect of JAK Inhibitor on the Survival, Anagen Re-Entry, and Hair Follicle Immune Privilege Restoration in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2020, 21, 5137. [Google Scholar] [CrossRef]
- Zhang, H.; Nan, W.; Wang, S.; Zhang, T.; Si, H.; Yang, F.; Li, G. Epidermal Growth Factor Promotes Proliferation and Migration of Follicular Outer Root Sheath Cells via Wnt/β-Catenin Signaling. Cell Physiol. Biochem. 2016, 39, 360–370. [Google Scholar] [CrossRef]
- Doles, J.; Storer, M.; Cozzuto, L.; Roma, G.; Keyes, W.M. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 2012, 26, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Welsch, K.; Holstein, J.; Laurence, A.; Ghoreschi, K. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur. J. Immunol. 2017, 47, 1096–1107. [Google Scholar] [CrossRef]
- Harel, S.; Higgins, C.A.; Cerise, J.E.; Dai, Z.; Chen, J.C.; Clynes, R.; Christiano, A.M. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci. Adv. 2015, 1, e1500973. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Bettermann, A.; Tokura, Y.; Takigawa, M.; Paus, R. Collapse and restoration of MHC class-I-dependent immune privilege: Exploiting the human hair follicle as a model. Am. J. Pathol. 2004, 164, 623–634. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef]
- Wu, X.J.; Zhu, J.W.; Jing, J.; Xue, D.; Liu, H.; Zheng, M.; Lu, Z.F. VEGF165 modulates proliferation, adhesion, migration and differentiation of cultured human outer root sheath cells from central hair follicle epithelium through VEGFR-2 activation in vitro. J. Dermatol. Sci. 2014, 73, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Jeong, K.H.; Kim, J.E.; Kang, H. Synthesized Ceramide Induces Growth of Dermal Papilla Cells with Potential Contribution to Hair Growth. Ann. Dermatol. Vol. 2019, 31, 164–174. [Google Scholar] [CrossRef]
- Veraitch, O.; Mabuchi, Y.; Matsuzaki, Y.; Sasaki, T.; Okuno, H.; Tsukashima, A.; Amagai, M.; Okano, H.; Ohyama, M. Induction of hair follicle dermal papilla cell properties in human induced pluripotent stem cell-derived multipotent LNGFR(+)THY-1(+) mesenchymal cells. Sci. Rep. 2017, 7, 42777. [Google Scholar] [CrossRef]
- Park, J.; Jun, E.K.; Son, D.; Hong, W.; Jang, J.; Yun, W.; Yoon, B.S.; Song, G.; Kim, I.Y.; You, S. Overexpression of Nanog in amniotic fluid–derived mesenchymal stem cells accelerates dermal papilla cell activity and promotes hair follicle regeneration. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Rahmani, W.; Abbasi, S.; Hagner, A.; Raharjo, E.; Kumar, R.; Hotta, A.; Magness, S.; Metzger, D.; Biernaskie, J. Hair Follicle Dermal Stem Cells Regenerate the Dermal Sheath, Repopulate the Dermal Papilla, and Modulate Hair Type. Dev. Cell 2014, 31, 543–558. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Lee, D.-H.; Shin, Y.-H.; Yoo, B.-Y.; Lee, K.-M.; Song, K.-Y.; Seo, S.-J.; Whang, S.-J.; Kim, Y.-J.; Yang, E.-K.; et al. Development of isolation and cultivation method for outer root sheath cells from human hair follicle and construction of bioartificial skin. Biotechnol. Bioprocess Eng. 2003, 8, 151–157. [Google Scholar] [CrossRef]
- Shafiee, A.; Khosrotehrani, K. In vitro Co-culture of Mesenchymal Stem Cells and Endothelial Colony Forming Cells. Bio-Protocol 2017, 7, e2587. [Google Scholar] [CrossRef]
- Wasserman, T.H.; Twentyman, P. Use of a colorimetric microtiter (MTT) assay in determining the radiosensitivity of cells from murine solid tumors. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 699–702. [Google Scholar] [CrossRef]
- Gong, J.; Meng, H.-B.; Hua, J.; Song, Z.-S.; He, Z.-G.; Zhou, B.; Qian, M.-P. The SDF-1/CXCR4 axis regulates migration of transplanted bone marrow mesenchymal stem cells towards the pancreas in rats with acute pancreatitis. Mol. Med. Rep. 2014, 9, 1575–1582. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Park, S.-H.; Park, H.-R.; Lee, Y.; Kang, H.; Kim, J.-E. Mesenchymal Stem Cells Antagonize IFN-Induced Proinflammatory Changes and Growth Inhibition Effects via Wnt/β-Catenin and JAK/STAT Pathway in Human Outer Root Sheath Cells and Hair Follicles. Int. J. Mol. Sci. 2021, 22, 4581. https://doi.org/10.3390/ijms22094581
Lee Y-J, Park S-H, Park H-R, Lee Y, Kang H, Kim J-E. Mesenchymal Stem Cells Antagonize IFN-Induced Proinflammatory Changes and Growth Inhibition Effects via Wnt/β-Catenin and JAK/STAT Pathway in Human Outer Root Sheath Cells and Hair Follicles. International Journal of Molecular Sciences. 2021; 22(9):4581. https://doi.org/10.3390/ijms22094581
Chicago/Turabian StyleLee, Yu-Jin, Song-Hee Park, Hye-Ree Park, Young Lee, Hoon Kang, and Jung-Eun Kim. 2021. "Mesenchymal Stem Cells Antagonize IFN-Induced Proinflammatory Changes and Growth Inhibition Effects via Wnt/β-Catenin and JAK/STAT Pathway in Human Outer Root Sheath Cells and Hair Follicles" International Journal of Molecular Sciences 22, no. 9: 4581. https://doi.org/10.3390/ijms22094581
APA StyleLee, Y.-J., Park, S.-H., Park, H.-R., Lee, Y., Kang, H., & Kim, J.-E. (2021). Mesenchymal Stem Cells Antagonize IFN-Induced Proinflammatory Changes and Growth Inhibition Effects via Wnt/β-Catenin and JAK/STAT Pathway in Human Outer Root Sheath Cells and Hair Follicles. International Journal of Molecular Sciences, 22(9), 4581. https://doi.org/10.3390/ijms22094581






