In Vitro Characterization of Human CD24hiCD38hi Regulatory B Cells Shows CD9 Is Not a Stable Breg Cell Marker
Abstract
1. Introduction
2. Results
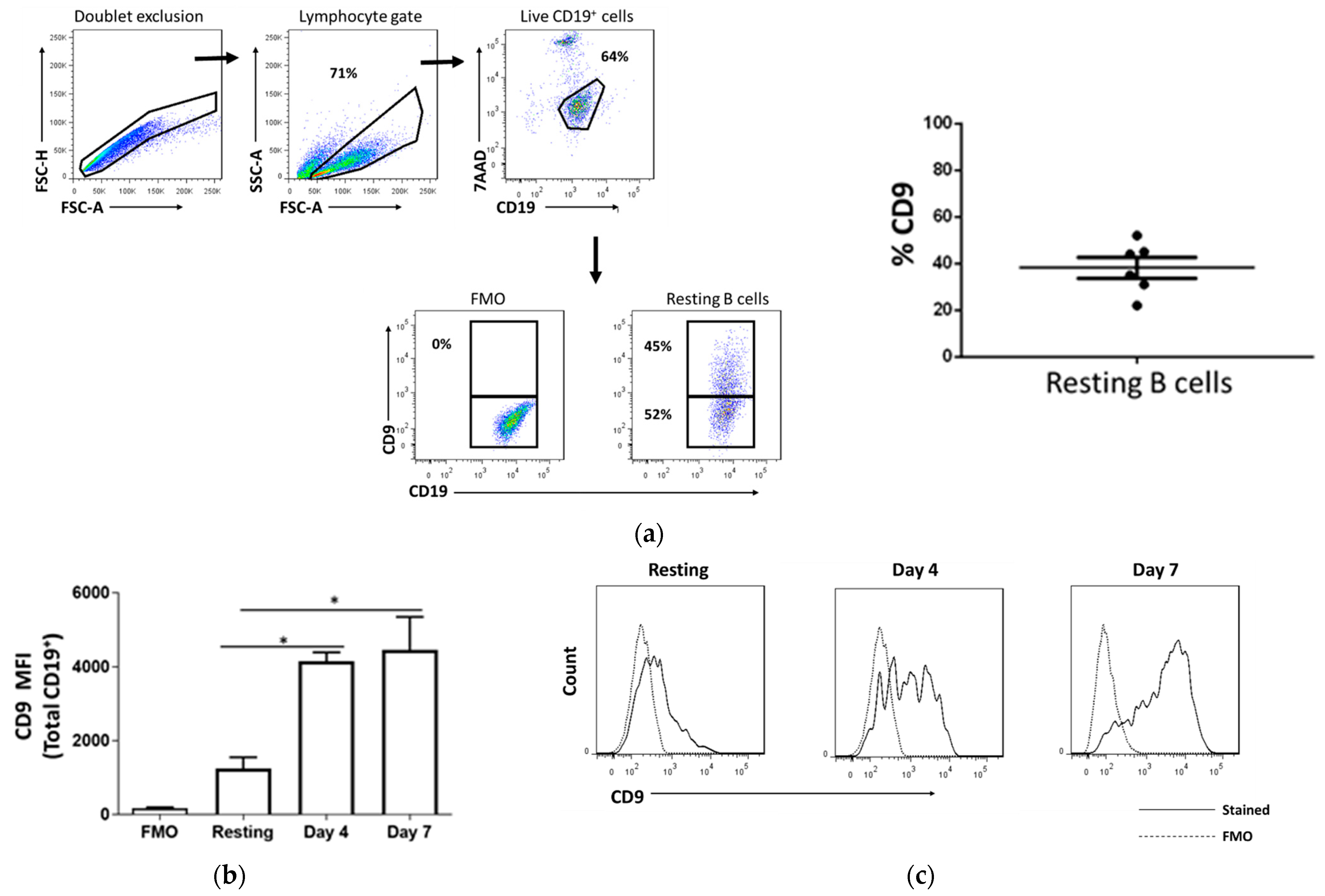
2.1. CD9 Is Expressed in Resting B Cells and Upregulated Upon Stimulation
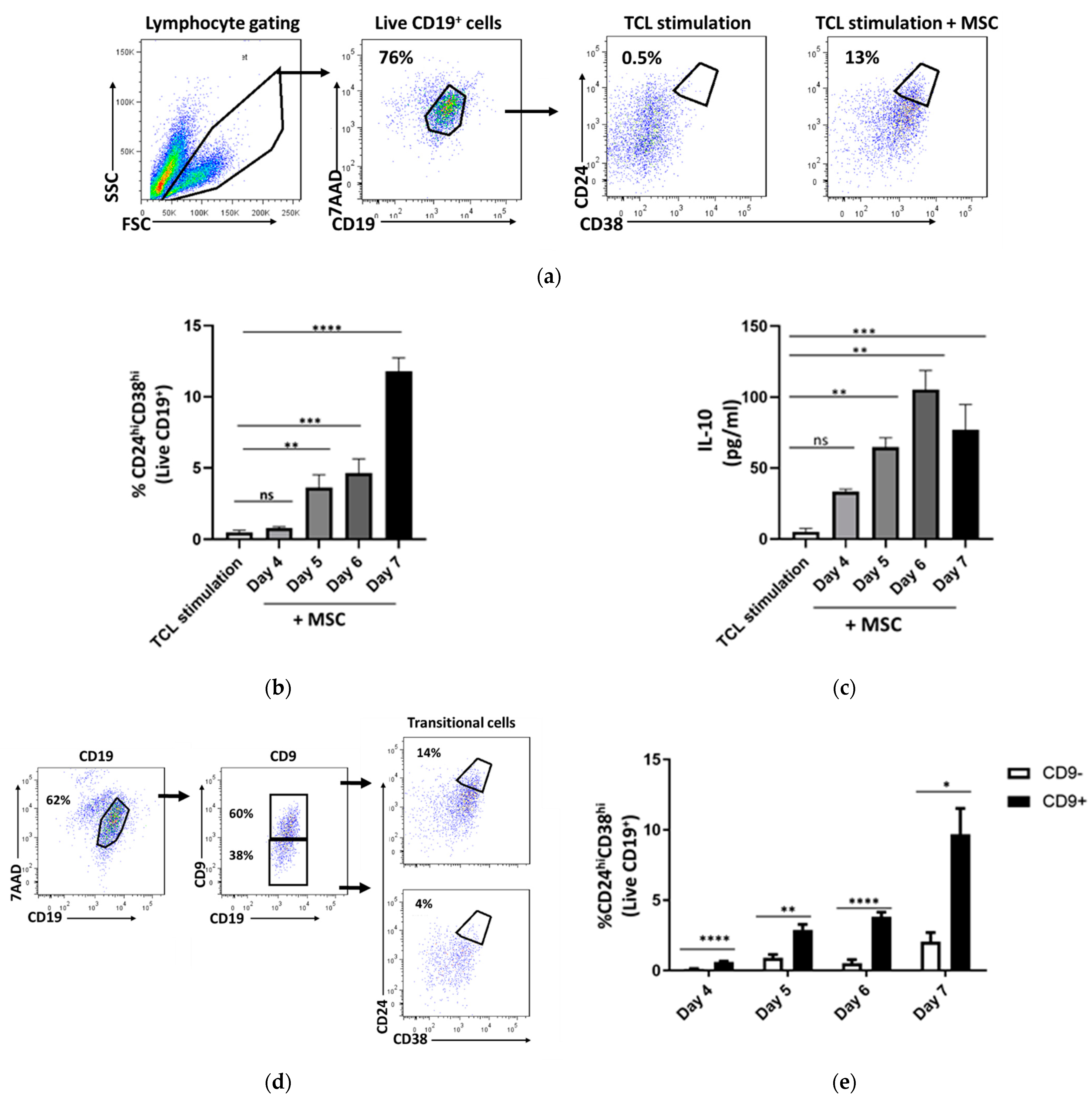
2.2. CD24hiCD38hi B Cells Are Enriched within Activated Cd9+ B Cells
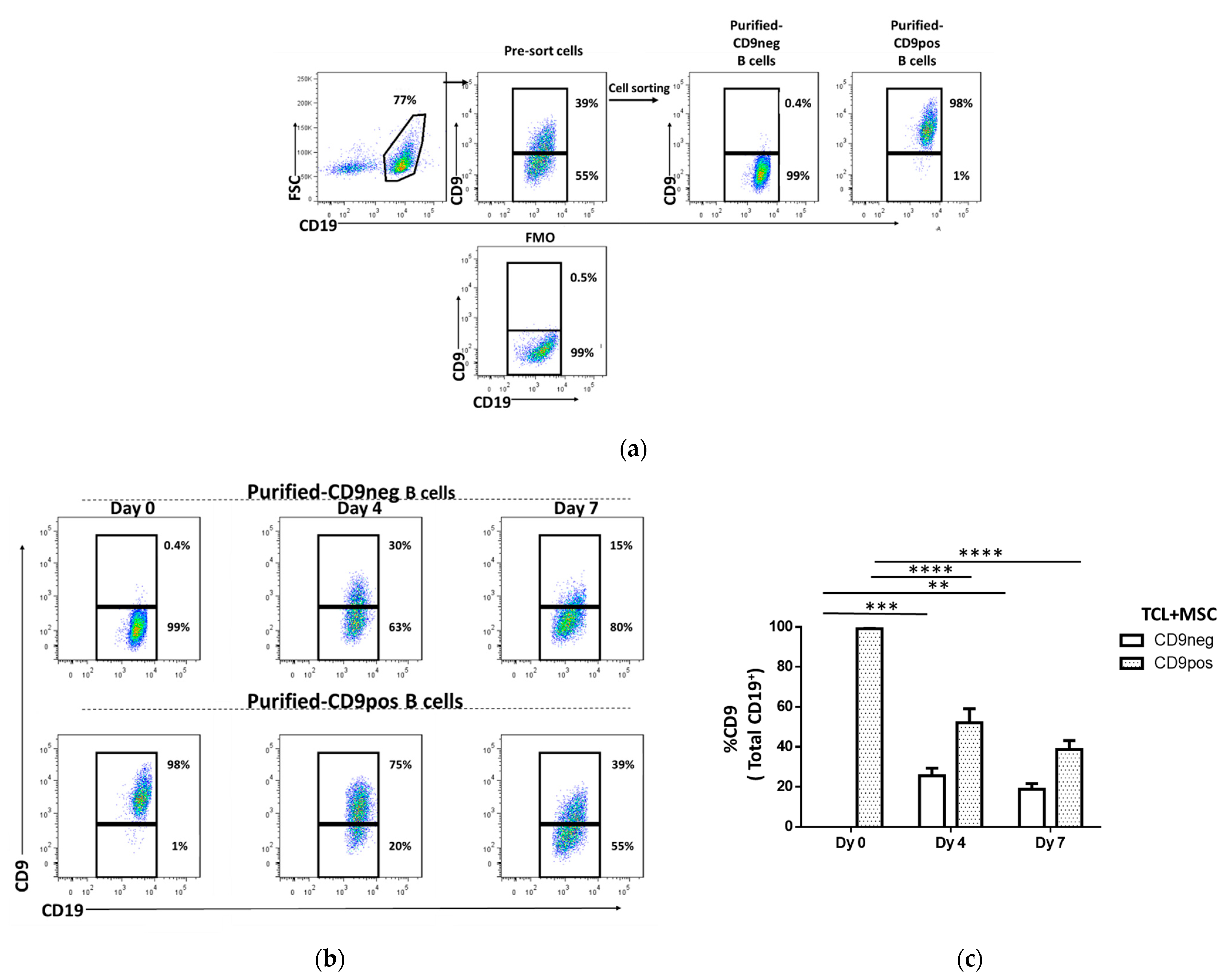
2.3. The Stability of CD9 as a Phenotypic Surface Marker
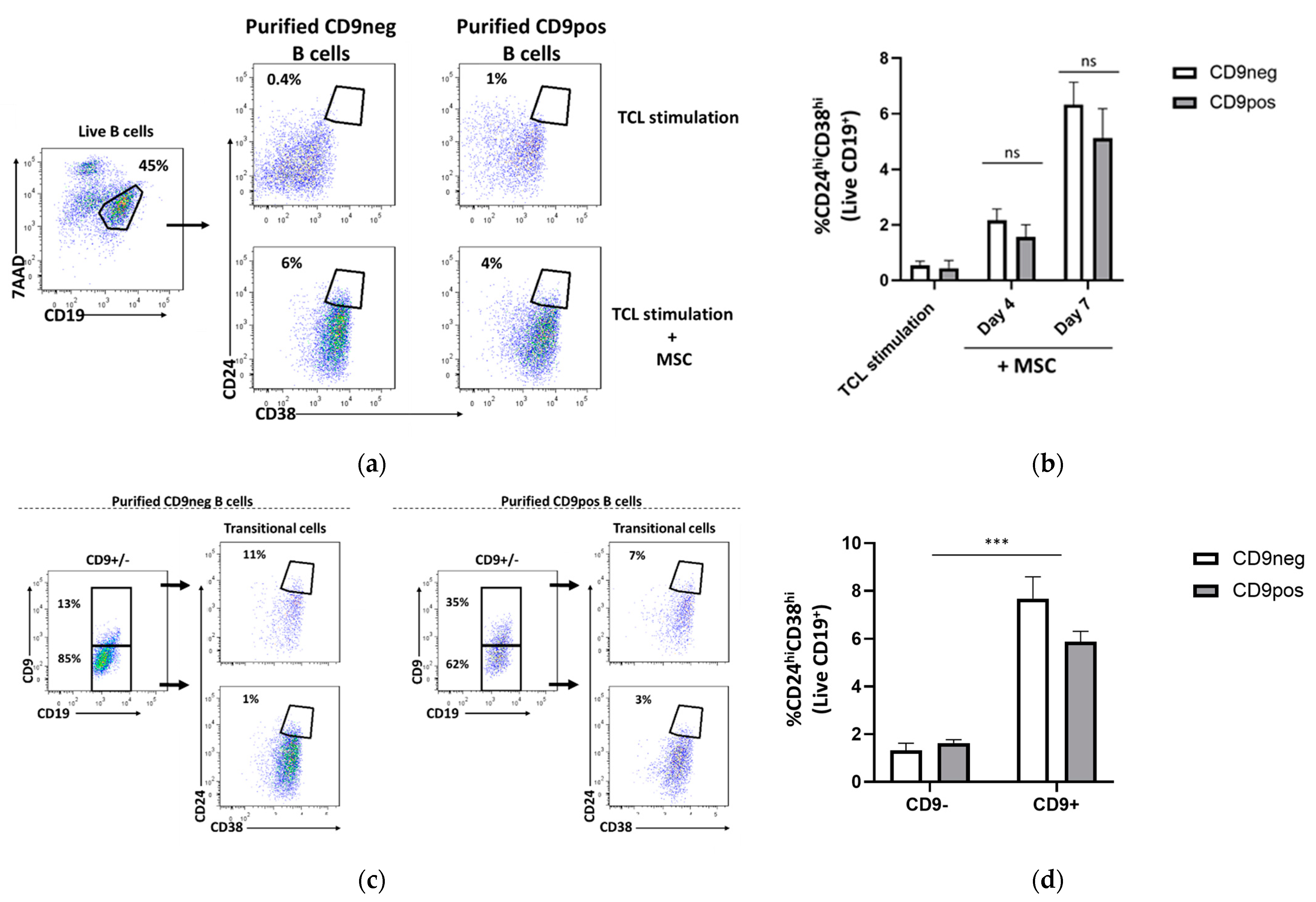
2.4. Transitional B Cell Induction Capacity on Purified CD9-Positive and CD9-Negative B Cells
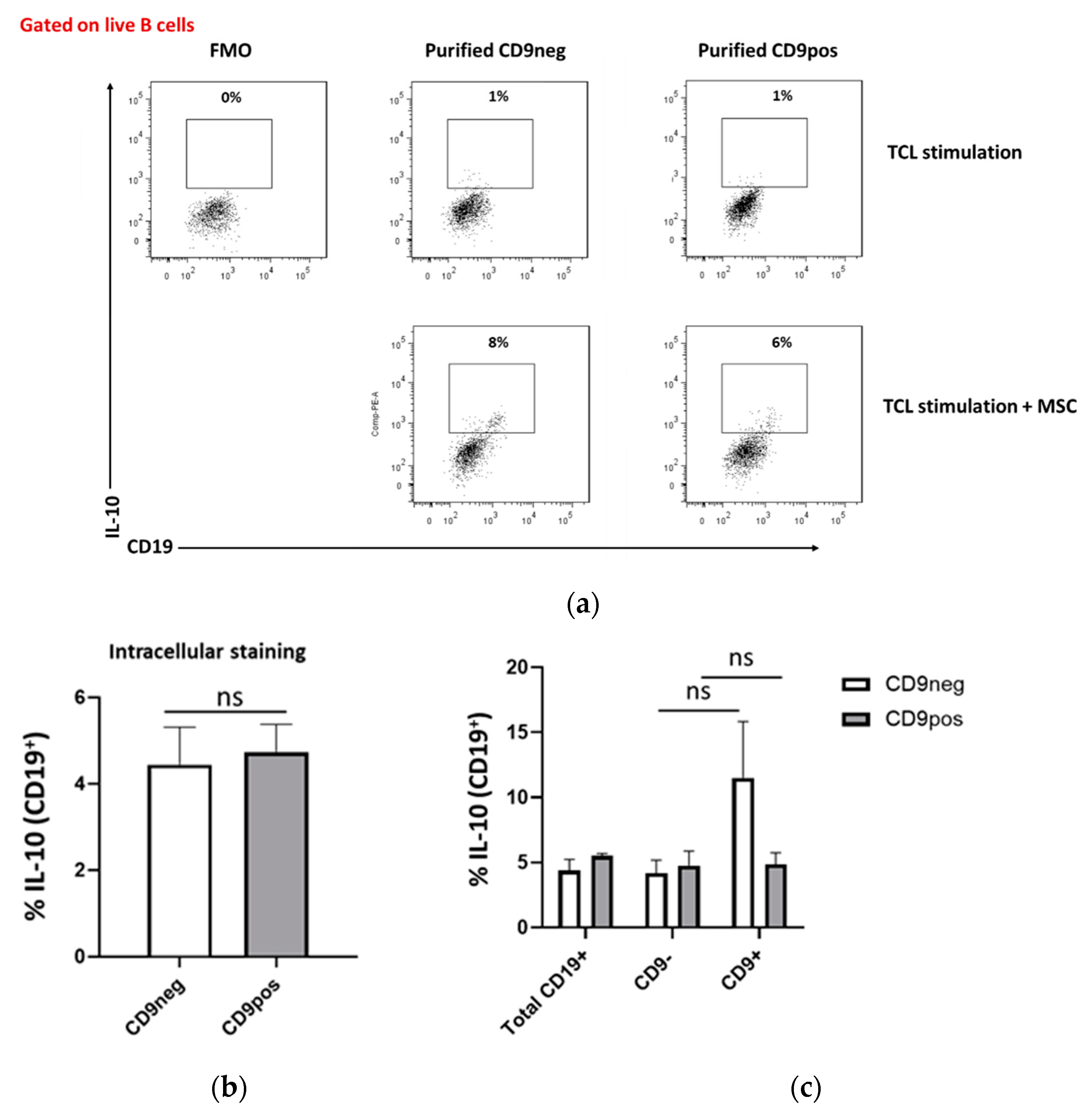
2.5. IL-10 Production Capacity of Purified CD9-Positive and CD9-Negative B Cells
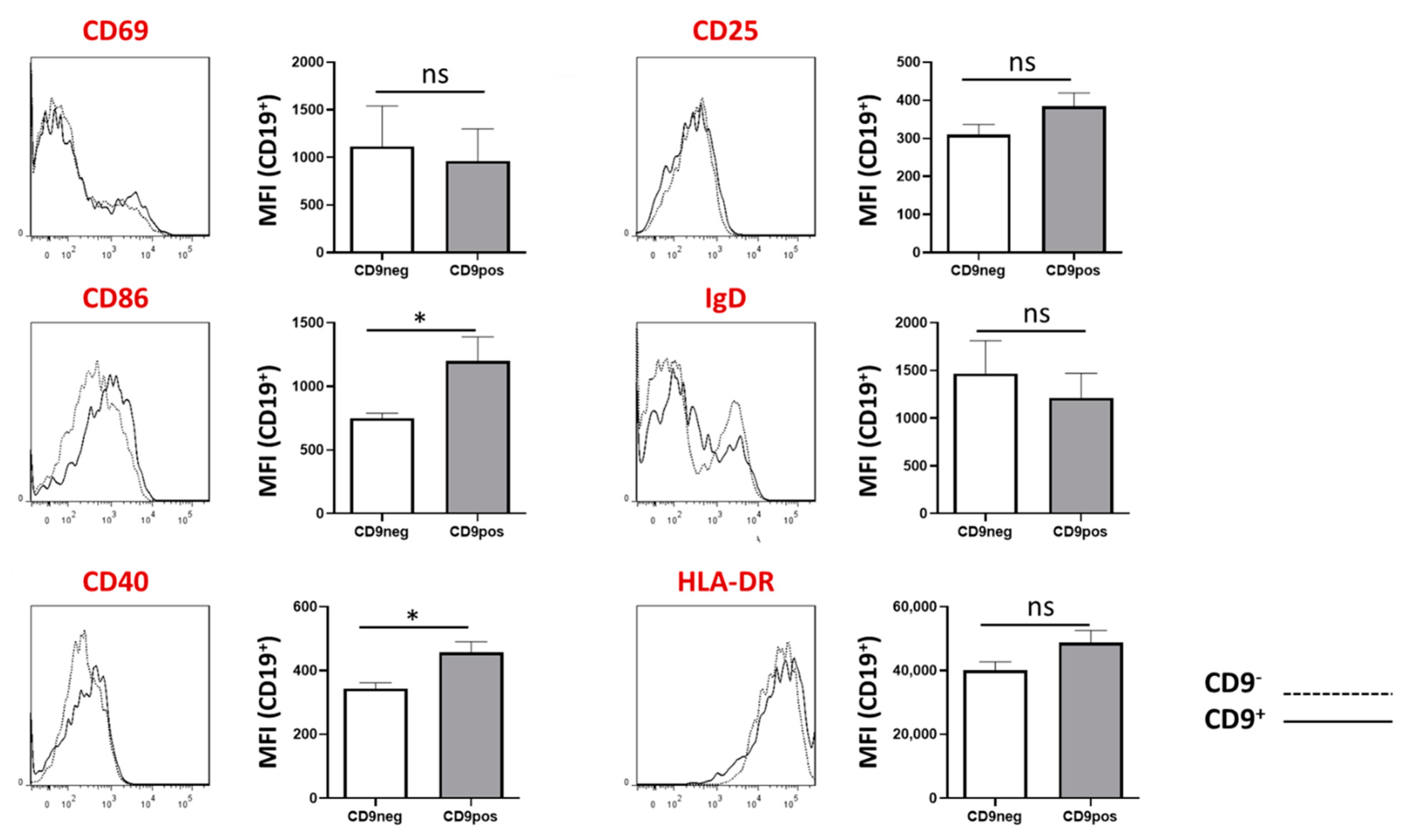
2.6. Expression of Other B Cell-Associated Markers in CD9+ and CD9− B Cells
3. Discussion
4. Materials and Methods
4.1. Mesenchymal Stem Cells
4.2. B Cells Isolation
4.3. Cell Sorting
4.4. B Cell Stimulation and Culture
4.5. Detection of IL-10
4.6. Characterization of B Cell Surface Markers
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Y.; Xu, J.; Su, J.; Yang, Q.; Deng, W.; Zou, B.; Tan, Y.; Ding, Z.; Li, X. IL-10-producing regulatory B cells exhibit functional defects and play a protective role in severe endotoxic shock. Pharmacol. Res. 2019, 148, 104457. [Google Scholar] [CrossRef]
- Luo, J.; Guo, H.; Liu, Z.; Peng, T.; Hu, X.; Han, M.; Yang, X.; Zhou, X.; Li, H. Analysis of peripheral B cell subsets in patients with allergic rhinitis. Allergy Asthma Immunol. Res. 2018, 10, 236–243. [Google Scholar] [CrossRef]
- Mauri, C.; Gray, D.; Mushtaq, N.; Londei, M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 2003, 197, 489–501. [Google Scholar] [CrossRef]
- Sattler, S.; Ling, G.S.; Xu, D.; Hussaarts, L.; Romaine, A.; Zhao, H.; Fossati-Jimack, L.; Malik, T.; Cook, H.T.; Botto, M.; et al. IL-10-producing regulatory B cells induced by IL-33 (BregIL-33) effectively attenuate mucosal inflammatory responses in the gut. J. Autoimmun. 2014, 50, 107–122. [Google Scholar] [CrossRef]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19+CD24hiCD38hi B Cells Exhibit Regulatory Capacity in Healthy Individuals but Are Functionally Impaired in Systemic Lupus Erythematosus Patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Mohd Jaya, F.N.; Liu, Z.; Chan, G.C.F. Early Treatment of Interleukin-33 can Attenuate Lupus Development in Young NZB/W F1 Mice. Cells 2020, 9, 2448. [Google Scholar] [CrossRef]
- Mauri, C.; Menon, M. The expanding family of regulatory B cells. Int. Immunol. 2015, 27, 479–486. [Google Scholar] [CrossRef]
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity 2016, 44, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Flores-Borja, F.; Bosma, A.; Ng, D.; Reddy, V.; Ehrenstein, M.R.; Isenberg, D.A.; Mauri, C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med. 2013, 5, 173ra23. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, J.; Pefanis, E.; Chao, J.; Rothschild, G.; Tachibana, I.; Chen, J.K.; Ivanov, I.I.; Rabadan, R.; Takeda, Y.; et al. Transcriptomics Identify CD9 as a Marker of Murine IL-10-Competent Regulatory B Cells. Cell Rep. 2015, 13, 1110–1117. [Google Scholar] [CrossRef]
- Brosseau, C.; Colas, L.; Magnan, A.; Brouard, S. CD9 tetraspanin: A new pathway for the regulation of inflammation? Front. Immunol. 2018, 9, 2316. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.; Durand, M.; Colas, L.; Durand, E.; Foureau, A.; Cheminant, M.A.; Bouchaud, G.; Castan, L.; Klein, M.; Magnan, A.; et al. CD9+ Regulatory B Cells Induce T Cell Apoptosis via IL-10 and Are Reduced in Severe Asthmatic Patients. Front. Immunol. 2018, 9, 3034. [Google Scholar] [CrossRef]
- Braza, F.; Chesne, J.; Durand, M.; Dirou, S.; Brosseau, C.; Mahay, G.; Cheminant, M.A.; Magnan, A.; Brouard, S. A regulatory CD9+ B-cell subset inhibits HDM-induced allergic airway inflammation. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 1421–1431. [Google Scholar] [CrossRef]
- Hasan, M.M.; Thompson-Snipes, L.; Klintmalm, G.; Demetris, A.J.; O’Leary, J.; Oh, S.; Joo, H. CD24hiCD38hi and CD24hiCD27+ Human Regulatory B Cells Display Common and Distinct Functional Characteristics. J. Immunol. 2019, 203, 2110–2120. [Google Scholar] [CrossRef]
- Franquesa, M.; Mensah, F.K.; Huizinga, R.; Strini, T.; Boon, L.; Lombardo, E.; Delarosa, O.; Laman, J.D.; Grinyõ, J.M.; Weimar, W.; et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells 2015, 33, 880–891. [Google Scholar] [CrossRef]
- Luk, F.; Carreras-Planella, L.; Korevaar, S.S.; de Witte, S.F.H.; Borràs, F.E.; Betjes, M.G.H.; Baan, C.C.; Hoogduijn, M.J.; Franquesa, M. Inflammatory conditions dictate the effect of mesenchymal stem or stromal cells on B cell function. Front. Immunol. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Bankó, Z.; Pozsgay, J.; Szili, D.; Tóth, M.; Gáti, T.; Nagy, G.; Rojkovich, B.; Sármay, G. Induction and Differentiation of IL-10-Producing Regulatory B Cells from Healthy Blood Donors and Rheumatoid Arthritis Patients. J. Immunol. 2017, 198, 1512–1520. [Google Scholar] [CrossRef]
- Yoon, S.O.; Lee, I.Y.; Zhang, X.; Zapata, M.C.; Choi, Y.S. CD9 may contribute to the survival of human germinal center B cells by facilitating the interaction with follicular dendritic cells. FEBS Open Bio 2014, 4, 370–376. [Google Scholar] [CrossRef]
- Barrena, S.; Almeida, J.; Yunta, M.; López, A.; Fernández-Mosteirín, N.; Giralt, M.; Romero, M.; Perdiguer, L.; Delgado, M.; Orfao, A.; et al. Aberrant expression of tetraspanin molecules in B-cell chronic lymphoproliferative disorders and its correlation with normal B-cell maturation. Leukemia 2005, 19, 1376–1383. [Google Scholar] [CrossRef]
- Mauri, C.; Menon, M. Human regulatory B cells in health and disease: Therapeutic potential. J. Clin. Investig. 2017, 127, 772–779. [Google Scholar] [CrossRef]
- Lighaam, L.C.; Unger, P.-P.A.; Vredevoogd, D.W.; Verhoeven, D.; Vermeulen, E.; Turksma, A.W.; ten Brinke, A.; Rispens, T.; van Ham, S.M. In vitro-Induced Human IL-10+ B Cells Do Not Show a Subset-Defining Marker Signature and Plastically Co-express IL-10 With Pro-Inflammatory Cytokines. Front. Immunol. 2018, 9, 1913. [Google Scholar] [CrossRef]
- Mohd Jaya, F.N.; Garcia, S.G.; Borràs, F.E.; Chan, G.C.F.; Franquesa, M. Paradoxical role of Breg-inducing cytokines in autoimmune diseases. J. Transl. Autoimmun. 2019, 2, 100011. [Google Scholar] [CrossRef]
- Mauri, C.; Bosma, A. Immune Regulatory Function of B Cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef]
- Carreras-Planella, L.; Monguió-Tortajada, M.; Borràs, F.E.; Franquesa, M. Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. Front. Immunol. 2019, 10, 1288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Jaya, F.N.; Garcia, S.G.; Borras, F.E.; Guerrero, D.; Chan, G.C.F.; Franquesa, M. In Vitro Characterization of Human CD24hiCD38hi Regulatory B Cells Shows CD9 Is Not a Stable Breg Cell Marker. Int. J. Mol. Sci. 2021, 22, 4583. https://doi.org/10.3390/ijms22094583
Mohd Jaya FN, Garcia SG, Borras FE, Guerrero D, Chan GCF, Franquesa M. In Vitro Characterization of Human CD24hiCD38hi Regulatory B Cells Shows CD9 Is Not a Stable Breg Cell Marker. International Journal of Molecular Sciences. 2021; 22(9):4583. https://doi.org/10.3390/ijms22094583
Chicago/Turabian StyleMohd Jaya, Fatin N., Sergio G. Garcia, Francesc E. Borras, Dolores Guerrero, Godfrey C. F. Chan, and Marcella Franquesa. 2021. "In Vitro Characterization of Human CD24hiCD38hi Regulatory B Cells Shows CD9 Is Not a Stable Breg Cell Marker" International Journal of Molecular Sciences 22, no. 9: 4583. https://doi.org/10.3390/ijms22094583
APA StyleMohd Jaya, F. N., Garcia, S. G., Borras, F. E., Guerrero, D., Chan, G. C. F., & Franquesa, M. (2021). In Vitro Characterization of Human CD24hiCD38hi Regulatory B Cells Shows CD9 Is Not a Stable Breg Cell Marker. International Journal of Molecular Sciences, 22(9), 4583. https://doi.org/10.3390/ijms22094583









