Functionalization of Synthetic Bone Substitutes
Abstract
1. Introduction
2. Overview of Bone Fracture Healing
3. First Generation of Growth Factor Delivering Biomaterials
4. Alternative Carriers for Growth Factor Delivery
5. Examples of Synthetic Biomaterials for Growth Factor Delivery
6. Antibiotic Eluting Bone Substituents
7. Surgical-Site Surface Coating
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Busch, A.; Wegner, A.; Haversath, M.; Jäger, M. Bone Substitutes in Orthopaedic Surgery: Current Status and Future Perspectives. Z. Orthopädie Unf. 2020. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.J.; Park, J.Y.; Jung, J.W.; Jang, J.; Girdhari, R.; Kim, S.W.; Cho, D.W. Improvement of bone regeneration capability of ceramic scaffolds by accelerated release of their calcium ions. Tissue Eng. Part A 2014, 20, 2840–2849. [Google Scholar] [CrossRef]
- Jäger, M.; Westhoff, B.; Wild, A.; Krauspe, R. Bone harvesting from the iliac crest [Article in German]. Orthopade 2005, 34, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, A.G.; Raafat, A.; Yates, P.; Hutchison, J.D. Infection associated with the use of allograft bone from the north east Scotland Bone Bank. J. Hosp. Infect. 1997, 35, 215–222. [Google Scholar] [CrossRef]
- Behrend, C.; Carmouche, J.; Millhouse, P.W.; Ritter, L.; Moskal, J.; Rubery, P.; Puzas, E. Allogeneic and Autogenous Bone Grafts Are Affected by Historical Donor Environmental Exposure. Clin. Orthop. Relat Res. 2016, 474, 1405–1409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elsalanty, M.E.; Genecov, D.G. Bone grafts in craniofacial surgery. Craniomaxillofac. Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. 2), S96–S101. [Google Scholar] [PubMed]
- Güven, O.; Tekin, U.S. Healing of bone defects by an osteopromotion technique using solvent-dehydrated cortical bone plate: A clinical and radiological study. J. Craniofac. Surg. 2006, 17, 1105–1110. [Google Scholar] [CrossRef][Green Version]
- Lerner, T.; Bullmann, V.; Schulte, T.L.; Schneider, M.; Liljenqvist, U. A level-1 pilot study to evaluate of ultraporous beta-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur. Spine J. 2009, 18, 170–179. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.; Ferguson, J.Y.; Lau, A.C.K.; Diefenbeck, M.; Scarborough, M.; Ramsden, A.J.; Atkins, B.L. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: A prospective series of 100 cases. Bone Jt. J. 2016, 98, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Keramaris, N.C.; Calori, G.M.; Nikolaou, V.S.; Schemitsch, E.H.; Giannoudis, P.V. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury 2008, 39, S45–S57. [Google Scholar] [CrossRef]
- Kolar, P.; Gaber, T.; Perka, C.; Duda, G.N.; Buttgereit, F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin. Orthop. Relat. Res. 2011, 469, 3118–3126. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P. Molecular aspects of fracture healing: Which are the important molecules? Injury 2007, 38 (Suppl. 1), S11–S25. [Google Scholar] [CrossRef]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef]
- Ketenjian, A.Y.; Arsenis, C. Morphological and biochemical studies during differentiation and calcification of fracture callus cartilage. Clin. Orthop. Relat. Res. 1975, 107, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Carano, A.D.; Filvaroff, E.H. Angiogenesis and bone repair. Drug Discov. Today 2003, 8, 980–989. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part Breviews 2008, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. substitutes in orthopaedic surgery: From basic science to clinical practice. Journal of materials science. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Lissenberg-Thunnissen, S.N.; David, J.J.; Sier, C.F.M.; Schipper, I.B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int. Orthop. 2011, 35, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Arrabal, P.M.; Visser, R.; Santos-Ruiz, L.; Becerra, J.; Cifuentes, M. Osteogenic molecules for clinical applications: Improving the BMP collagen System. Biol. Res. 2013, 46, 421–429. [Google Scholar] [CrossRef]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef]
- Lungu, A.; Titorencu, I.; Albu, M.G.; Florea, N.M.; Vasile, E.; Iovu, H.; Jinga, V.; Simonescu, M. The effect of BMP-4 loaded in 3D collagen-hyaluronic acid scaffolds on biocompatibility assessed with MG 63 osteoblast-like cells. Dig. J. Nanomater. Biostruct. 2011, 6, 1897–1908. [Google Scholar]
- Schmidmaier, G.; Schwabe, P.; Wildemann, B.; Haas, N.P. Use of bone morphogenetic proteins for treatment of non-unions and future perspectives. Injury 2007, 38, S35–S41. [Google Scholar] [CrossRef]
- Friess, W.; Uludag, H.; Foskett, S.; Biron, R.; Sargeant, C. Characterization of absorbable collagen sponges as rhBMP-2 carriers. Int. J. Pharm. 1999, 187, 91–99. [Google Scholar] [CrossRef]
- Brown, K.V.; Li, B.; Guda, T.; Perrien, D.S.; Guelcher, S.A.; Wenke, J.C. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue Eng. Part A 2011, 17, 1735–1746. [Google Scholar] [CrossRef]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone Morphogenetic Protein-2 Converts the Differentiation Pathway of C2C12 Myoblasts into the Osteoblast Lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef]
- Suliman, S.; Xing, Z.; Wu, X.; Xue, Y.; Pedersen, T.O.; Sun, Y.; Døskeland, A.P.; Nickel, J.; Waag, T.; Lygre, H.; et al. Release and bioactivity of bone morphogenetic protein-2 are affected by scaffold binding techniques in vitro and in vivo. J. Control Release 2015, 197, 148–157. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Bigham-Sadegh, A. Bone morphogenetic proteins: A powerful osteoinductive compound with non-negligible side effects and limitations. BioFactors 2014, 40, 459–481. [Google Scholar] [CrossRef]
- Sharma, A.; Meyer, F.; Hyvonen, M.; Best, S.M.; Cameron, R.E.; Rushton, N. Osteoinduction by combining bone morphogenetic protein (BMP)-2 with a bioactive novel nanocomposite. Bone Jt. Res. 2012, 1, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef]
- Martina, M.; Hutmacher, D.W. Biodegradable polymers applied in tissue engineering research: A review. Polym. Int. 2007, 56, 145–157. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2018, 64, 91–126. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef]
- Czech, T.; Oyewumi, M.O. Overcoming barriers confronting application of protein therapeutics in bone fracture healing. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lopac, S.K.; Torres, M.P.; Wilson-Welder, J.H.; Wannemuehler, M.J.; Narasimhan, B. Effect of polymer chemistry and fabrication method on protein release and stability from polyanhydride microspheres. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Madurantakam, P.A.; Rodriguez, I.A.; Beckman, M.J.; Simpson, D.G.; Bowlin, G.L. Evaluation of biological activity of bone morphogenetic proteins on exposure to commonly used electrospinning solvents. J. Bioact. Compat. Polym. 2011, 26, 578–589. [Google Scholar] [CrossRef]
- Determan, A.S.; Wilson, J.H.; Kipper, M.J.; Wannemuehler, M.J.; Narasimhan, B. Protein stability in the presence of polymer degradation products: Consequences for controlled release formulations. Biomaterials 2006, 27, 3312–3320. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, E.; Holmes, C.; Witham, T.; Grayson, W.L. Growth factor-eluting technologies for bone tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 184–194. [Google Scholar] [CrossRef]
- Qasim, M.; Chae, D.S.; Lee, N.Y. Bioengineering strategies for bone and cartilage tissue regeneration using growth factors and stem cells. J. Biomed. Mater. Res. 2020, 108, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Sinha, M. A review on carrier systems for bone morphogenetic protein-2. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 904–925. [Google Scholar] [CrossRef] [PubMed]
- Jennissen, H.P. Aspects of Multimodal Hybrid Biomaterials. Curr. Dir. Biomed. Eng. 2019, 5, 303–306. [Google Scholar] [CrossRef]
- Bayer, E.A.; Gottardi, R.; Fedorchak, M.V. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J. Control Release 2015, 219, 129–140. [Google Scholar] [CrossRef]
- Kanczler, J.M.; Ginty, P.J.; White, L.; Clarke, N.M.P.; Howdle, S.M.; Shakesheff, K.M.; Oreffo, R.O.C. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials 2010, 31, 1242–1250. [Google Scholar] [CrossRef]
- Sänger, T.; Laub, M.; Jennissen, H.P. Release of 125I-rhBMP-2 from Foamed Poly-(D.,L)-Lactide. Biomed. Tech. 2013, 58 (Suppl. 1), 989–990. [Google Scholar] [CrossRef] [PubMed]
- Sänger, T.; Asran, A.S.; Jennissen, H.P. Immobilization and release of rhVEGF and rhBMP-2 from PDLLA nanofiber-scaffolds. J. Tissue Eng. Regen. Med. 2014, 8 (Suppl. 1), 458. [Google Scholar] [CrossRef]
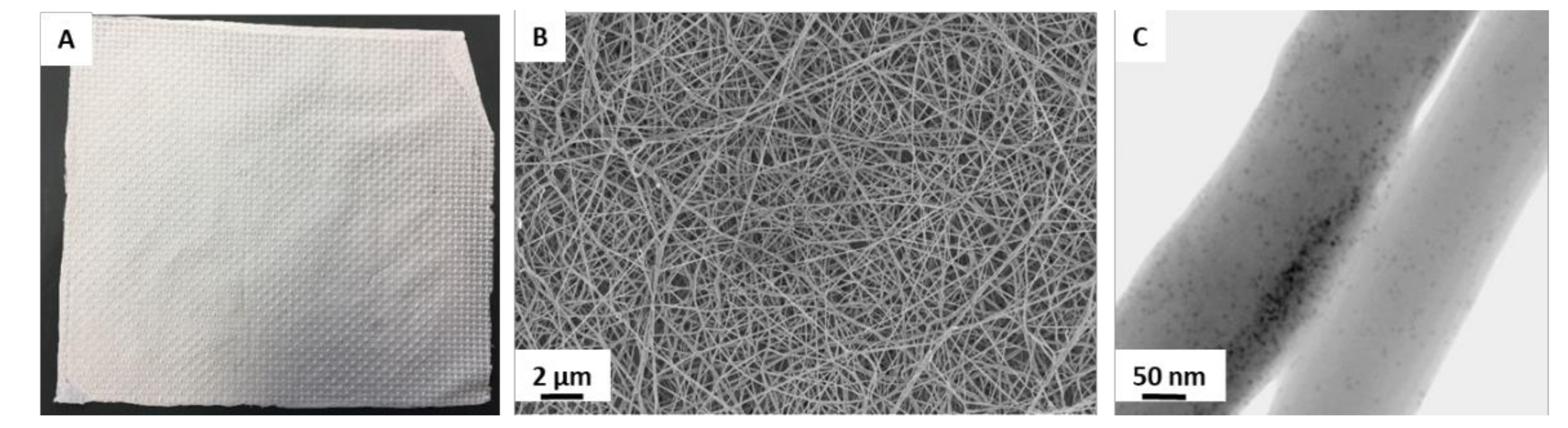
- Sowislok, A.; Jennissen, H.P. Immobilization of rhBMP-2 and rhVEGF on Electrospun PDLLA-Scaffolds for Sequential Release. Biomed. Eng. Biomed. Tech. 2019, 64, S48. [Google Scholar] [CrossRef]
- Sowislok, A.; Dohle, E.; Jennissen, H.P. Preparation of electrospun nanofiber tubular scaffolds as carriers for rhBMP-2 and rhVEGF165 to enhance bone induction. Biomed. Eng. Biomed. Tech. 2019, 64, S213. [Google Scholar] [CrossRef]
- Zurlinden, K.; Laub, M.; Jennissen, H.P. Immobilization and Controlled Release of Vascular (VEGF) and Bone Growth Factors (BMP-2) on Bone Replacement Materials. Biomed. Tech. 2012, 57 (Suppl. 1), 989–991. [Google Scholar] [CrossRef]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol–collagen–hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Ammann, C.G.; Nogler, M.; Fille, M.; Frommelt, l.; Kühn, K.-D.; Fölsch, C. Lyophilized allogeneic bone tissue as an antibiotic carrier. Cell Tissue Bank. 2016, 17, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Bidossi, A.; Bottagisio, M.; Logoluso, N.; De Vecchi, E. In Vitro Evaluation of Gentamicin or Vancomycin Containing Bone Graft Substitute in the Prevention of Orthopedic Implant-Related Infections. Int. J. Mol. Sci. 2020, 21, 9250. [Google Scholar] [CrossRef]
- Bjerke-Kroll, B.T.; Christ, A.B.; McLawhorn, A.S.; Sculco, P.K.; Jules-Elysée, K.M.; Sculco, T.P. Periprosthetic joint infections treated with two-stage revision over 14 years: An evolving microbiology profile. J. Arthroplast. 2014, 29, 877–882. [Google Scholar] [CrossRef]
- Jamei, O.; Gjoni, S.; Zenelaj, B.; Kressmann, B.; Belaieff, W.; Hannouche, D.; Uçkay, I. Which Orthopaedic Patients Are Infected with Gram-negative Non-fermenting Rods? J. Bone Jt. Infect. 2017, 2, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Piper, K.E.; Jacobson, M.J.; Cofield, R.H.; Sperling, J.W.; Sanchez-Sotelo, J.; Osmon, D.R.; McDowell, A.; Patrick, S.; Steckelberg, J.M.; Mandrekar, J.N.; et al. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 2009, 47, 1878–1884. [Google Scholar] [CrossRef]
- Hill, C.; Flamant, R.; Mazas, F.; Evrard, J. Prophylactic cefazolin versus placebo in total hip replacement. Report of a multicentre double-blind randomised trial. Lancet 1981, 1, 795–796. [Google Scholar] [CrossRef]
- Peel, T.; Astbury, S.; Cheng, A.C.; Paterson, D.; Buising, K.; Spelman, T.; Tran-Duy, A.; de Steiger, R.S. Multicentre randomised double-blind placebo controlled trial of combination vancomycin and cefazolin surgical antibiotic prophylaxis: The Australian surgical antibiotic prophylaxis (ASAP) trial. BMJ Open 2019, 9, e033718. [Google Scholar] [CrossRef] [PubMed]
- Coraça-Hubér, D.C.; Fille, M.; Hausdorfer, J.; Pfaller, K.; Nogler, M. Evaluation of MBEC™-HTP biofilm model for studies of implant associated infections. J. Orthop. Res. 2012, 30, 1176–1180. [Google Scholar] [CrossRef]
- Zimmerli, W.; Moser, C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol. Med. Microbiol. 2012, 65, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Isefuku, S.; Joyner, C.J.; Simpson, A.H. Gentamicin may have an adverse effect on osteogenesis. J. Orthop. Trauma. 2003, 17, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Joukhadar, C.; Dehghanyar, P.; Traunmüller, F.; Sauermann, R.; Mayer-Helm, B.; Georgopoulos, A.; Müller, M. Increase of microcirculatory blood flow enhances penetration of ciprofloxacin into soft tissue. Antimicrob. Agents Chemother. 2005, 49, 4149–4153. [Google Scholar] [CrossRef] [PubMed]
- So, W.; Kuti, J.L.; Nicolau, D.P. Population Pharmacokinetics of Cefazolin in Serum and Tissue for Patients with Complicated Skin and Soft Tissue Infections (cSSTI). Infect. Dis Ther. 2014, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Lipsky, B.A. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar] [CrossRef]
- Wittmann, D.H.; Kuipers, T.H.; Fock, R.; Höll, M.; Bauernfeind, A. Bone concentrations of imipenem after a dose of imipenem/cilastatin. Infection 1986, 14 (Suppl. 2), S130–S137. [Google Scholar] [CrossRef]
- Graziani, A.L.; Lawson, L.A.; Gibson, G.A.; Steinberg, M.A.; MacGregor, R.R. Vancomycin concentrations in infected and noninfected human bone. Antimicrob. Agents Chemother. 1988, 32, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Massias, L.; Dubois, C.; de Lentdecker, P.; Brodaty, O.; Fischler, M.; Farinotti, R. Penetration of vancomycin in uninfected sternal bone. Antimicrob. Agents Chemother. 1992, 36, 2539–2541. [Google Scholar] [CrossRef]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Wininger, D.A.; Fass, R.J. Antibiotic-impregnated cement and beads for orthopedic infections. Antimicrob. Agents Chemother. 1996, 40, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Lovallo, J.; Helming, J.; Jafari, S.M.; Owusu-Forfie, A.; Donovan, S.; Minnock, C.; Adib, F. Intraoperative intra-articular injection of gentamicin: Will it decrease the risk of infection in total shoulder arthroplasty? J. Shoulder Elb. Surg. 2014, 23, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Cancienne, J.M.; Burrus, M.T.; Weiss, D.B.; Yarboro, S.R. Applications of Local Antibiotics in Orthopedic Trauma. Orthop. Clin. N. Am. 2015, 46, 495–510. [Google Scholar] [CrossRef]
- Soundrapandian, C.; Basu, D.; Sa, B.; Datta, S. Local drug delivery system for the treatment of osteomyelitis: In vitro evaluation. Drug Dev. Ind. Pharm. 2011, 37, 538–546. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A.C. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin. Orthop. Relat. Res. 2004, 427, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kallala, R.; Harris, W.E.; Ibrahim, M.; Dipane, M.; McPherson, E. Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty: Safety profile and complication rates. Bone Jt. Res. 2018, 7, 570–579. [Google Scholar] [CrossRef]
- Qin, C.H.; Zhou, C.H.; Song, H.J.; Cheng, G.-Y.; Zhang, H.-A.; Fang, J.; Tao, R. Infected bone resection plus adjuvant antibiotic-impregnated calcium sulfate versus infected bone resection alone in the treatment of diabetic forefoot osteomyelitis. BMC Musculoskelet. Disord. 2019, 20, 246. [Google Scholar] [CrossRef]
- Ferguson, J.; Diefenbeck, M.; McNally, M. Ceramic Biocomposites as Biodegradable Antibiotic Carriers in the Treatment of Bone Infections. J. Bone Jt. Infect. 2017, 2, 38–51. [Google Scholar] [CrossRef]
- Thabit, A.K.; Fatani, D.F.; Bamakhrama, M.S.; Barnawi, O.A.; Basudan, L.O.; Alhejaili, S.F. Antibiotic penetration into bone and joints: An updated review. Int. J. Infect. Dis. 2019, 81, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Chan, E.K.; Gupta, S.; Diwan, A.D. Polymethylmethacrylate bone cements and additives: A review of the literature. World J. Orthop. 2013, 4, 67–74. [Google Scholar] [CrossRef]
- Lawrie, C.M.; Jo, S.; Barrack, T.; Roper, S.; Wright, R.W.; Nunley, R.M.; Barrack, R.L. Local delivery of tobramycin and vancomycin in primary total knee arthroplasty achieves minimum inhibitory concentrations for common bacteria causing acute prosthetic joint infection. Bone Jt. J. 2020, 102, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Hausner, M.; Wuertz, S. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl. Environ. Microbiol. 1999, 65, 3710–3713. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef]
- Boles, B.R.; Singh, P.K. Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc. Natl. Acad. Sci. USA 2008, 105, 12503–12508. [Google Scholar] [CrossRef] [PubMed]
- Faron, M.L.; Ledeboer, N.A.; Buchan, B.W. Resistance Mechanisms, Epidemiology, and Approaches to Screening for Vancomycin-Resistant Enterococcus in the Health Care Setting. J. Clin. Microbiol. 2016, 54, 2436–2447. [Google Scholar] [CrossRef]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- Thompson, J.M.; Miller, R.J.; Ashbaugh, A.G.; Dillen, C.A.; Pickett, J.E.; Wang, Y.; Ortines, R.V.; Sterling, R.S.; Francis, K.P.; Bernthal, N.M.; et al. Mouse model of Gram-negative prosthetic joint infection reveals therapeutic targets. JCI Insight. 2018, 3, e121737. [Google Scholar] [CrossRef] [PubMed]
- Chang-Chin, W.; Yang-Kai, H.; Wei-Jen, C.; Yun-Ching, W.; Chen-Chie, W.; Kai-Chiang, Y. Limitation of the antibiotic-eluting bone graft substitute: An example of gentamycin-impregnated calcium sulfate. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 80–87. [Google Scholar] [CrossRef]
- Zhang, P.; Ding, L.; Kasugai, S. Effect of doxycycline doped bone substitute on vertical bone augmentation on rat calvaria. Dent. Mater. J. 2019, 38, 211–217. [Google Scholar] [CrossRef]
- Johnson, C.T.; Sok, M.C.P.; Martin, K.E.; Kalelkar, P.P.; Caplin, J.D.; Botchwey, E.A.; García, A.J. BMP-2 co-delivery reduces S. aureus infection and regenerates critical-sizedsegmental bone defects. Sci. Adv. 2019, 5, eaaw1228. [Google Scholar] [CrossRef]
- Jung, S.W.; Oh, S.H.; Lee, I.S.; Byun, J.-H.; Lee, J.H. In Situ Gelling Hydrogel with Anti-Bacterial Activity and Bone Healing Property for Treatment of Osteomyelitis. Tissue Eng. Regen. Med. 2019, 16, 479–490. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Ripamonti, U.; Duarte, R.; Ferretti, C. Re-evaluating the induction of bone formation in primates. Biomaterials 2014, 35, 9407–9422. [Google Scholar] [CrossRef]
- García-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised scaffolds: Just a framework? Current knowledge and future directions. J. Tissue Eng. 2020, 11, 2041731420942903. [Google Scholar] [CrossRef] [PubMed]
- Tosounidis, T.H.; Calori, G.M.; Giannoudis, P.V. The use of Reamer-irrigator-aspirator in the management of long bone osteomyelitis: An update. Eur. J. Trauma Emerg. Surg. 2016, 42, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Shonuga, O.; Ellis, S.; Fragomen, A.; Kennedy, J.; Kudryashov, V.; Lane, J.M. A prospective comparison of 3 approved systems for autologous bone marrow concentration demonstrated nonequivalency in progenitor cell number and concentration. J. Orthop. Trauma. 2014, 28, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Henze, K.; Herten, M.; Haversath, M.; Busch, A.; Brandau, S.; Hackel, A.; Flohé, S.B.; Jäger, M. Surgical vacuum filter-derived stromal cells are superior in proliferation to human bone marrow aspirate. Stem Cell Res. Ther. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Herten, M.; Haversath, M.; Kaiser, C.; Brandau, S.; Jäger, M. Ceramic Scaffolds in a Vacuum Suction Handle for Intraoperative Stromal Cell Enrichment. Int. J. Mol. Sci. 2020, 21, 6393. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busch, A.; Jäger, M.; Mayer, C.; Sowislok, A. Functionalization of Synthetic Bone Substitutes. Int. J. Mol. Sci. 2021, 22, 4412. https://doi.org/10.3390/ijms22094412
Busch A, Jäger M, Mayer C, Sowislok A. Functionalization of Synthetic Bone Substitutes. International Journal of Molecular Sciences. 2021; 22(9):4412. https://doi.org/10.3390/ijms22094412
Chicago/Turabian StyleBusch, André, Marcus Jäger, Constantin Mayer, and Andrea Sowislok. 2021. "Functionalization of Synthetic Bone Substitutes" International Journal of Molecular Sciences 22, no. 9: 4412. https://doi.org/10.3390/ijms22094412
APA StyleBusch, A., Jäger, M., Mayer, C., & Sowislok, A. (2021). Functionalization of Synthetic Bone Substitutes. International Journal of Molecular Sciences, 22(9), 4412. https://doi.org/10.3390/ijms22094412






