The Role of Non-Coding RNAs in the Neuroprotective Effects of Glutathione
Abstract
1. Introduction
2. Antioxidants against Oxidative Stress
2.1. Glutathione
2.2. Antioxidant Enzymes
2.3. Glutaredoxin, Thioredoxin and Peroxiredoxin
2.4. The Nrf2-Keap1 System
3. Uptake System for the Sources of GSH
3.1. Cysteine Uptake System
3.2. Cystine Uptake System
4. Neuroprotective Function of Non-Coding RNA
4.1. MicroRNAs
4.2. Long Non-Coding RNAs
4.3. Circular RNAs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| GSH | Glutathione |
| CNS | Central nervous system |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| ND | Neurodegenerative disease |
| H2O2 | Hydrogen peroxide |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| GCL | Glutamate-cysteine ligase |
| GSS | Glutathione synthetase |
| γ-GluCys | γ-glutamylcysteine |
| GCLc | Catalytic subunit of glutamate-cysteine ligase |
| GCLm | Modulatory subunit of glutamate-cysteine ligase |
| GSSG | Oxidized glutathione |
| GSR | Glutathione reductase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| GST | Glutathione-S-transferase |
| MRP | Multidrug-resistance-associated protein |
| γ-GT | γ-glutamyltranspeptidase |
| CysGly | Cysteinylglycine |
| Glycys | Glycylcysteine |
| ALS | Amyotrophic lateral scleorosis |
| Grx | Glutaredoxin |
| Trx | Thioredoxin |
| Prx | Peroxiredoxin |
| TrxR | Thioredoxin reductase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| sMAF | Small Musculo-aponeurotic fibrosarcoma |
| ARE | Antioxidant response element |
| KLF2 | Krüppel-like factor 2 |
| EAAC1 | Excitatory amino acid carrier 1 |
| EAAT | Excitatory amino acid transporter |
| SLC1 | Solute carrier family 1 |
| GLAST | Glutamate aspartate transporter |
| GLT-1 | Glutamate transporter-1 |
| mRNA | Messenger RNA |
| tRNA | Transfer RNA |
| rRNA | Ribosomal RNA |
| snRNA | Small nuclear RNA |
| snoRNA | Small nucleolar RNA |
| miRNA | MicroRNA |
| 3′-UTR | 3′-untranslated region |
| Pri-miRNA | Primary microRNA |
| Pre-miRNA | MicroRNA precursor |
| RISC | RNA -induced silencing complex |
| RECK | Reversion-inducing-cysteine-rich protein with kazal motifs |
| MDA | Malondialdehyde |
| BDNF | Brain-derived neurotrophic factor |
| TrkB | Tyrosine kinase receptor type21 |
| GSK3β | Glycogen synthase kinase 3β |
| PTEN | Phosphate and tensin homolog deleted from chromosome 10 |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal protein kinase |
| TIMP2 | Tissue inhibitors of metalloproteinase 2 |
| iNOS | Inducible nitric oxide synthase |
| KLK7 | Kallikrein-related peptidase 7 |
| TRAF6 | Tumor necrosis factor receptor associated factor 6 |
| NF-κB | Nuclear factor kappa B |
| HEY2 | Hairy/enhancer-of-split related with YRPW motif protein 2 |
| MCU | Mitochondrial calcium uniporter |
| Nox2 | Nicotinamide adenine dinucleotide phosphate oxiodase 2 |
| lncRNA | Long non-coding RNA |
| NEAT1 | Nuclear paraspeckle assembly transcript 1 |
| MEN | Multiple endocrine neoplasia |
| ARHGAP26 | Rho GTPase activating protein 26 |
| Parp8 | Poly (ADP-ribose) polymerase family, member 8 |
| IGF2 | Insulin like growth factor 2 |
| ROCK2 | Rho associated coiled-coil containing protein kinase 2 |
| BACE1-AS | β-secretase 1 antisense RNA |
| WT1-AS | Wilms’ tumor 1 antisense RNA |
| SOX21 | SRY-Box transcription factor 21 |
| FZD3/5 | Frizzled3/5 |
| circRNA | Circular RNA |
| ceRNA | Competitive endogenous RNA |
| CDR1 | Cerebellar degeneration-associated antigen 1 |
| TGFA | Transforming growth factor alpha |
| ARPE-19 | Adult retinal pigment epithelial cell line 19 |
| circHIPK3 | Circular RNA homeodomain-interacting protein kinase 3 |
| HCM | Human cardiac myocyte |
| HUVEC | Human umbilical vein endothelial cell |
| circIL4R | Circular RNA interleukin-4 receptor |
| circEPSTI1 | Circular RNA epithelial stroma linteraction 1 |
References
- Oparin, A.I. The origin of life and the origin of enzymes. Adv. Enzymol. Relat. Areas Mol. Biol. 1965, 27, 347–380. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, D.; Seyboldt, R.; Weber, C.A.; Hyman, A.A.; Jülicher, F. Growth and division of active droplets provides a model for protocells. Nat. Phys. 2017, 13, 408–413. [Google Scholar] [CrossRef]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Higgs, P.G.; Lehman, N. The RNA World: Molecular cooperation at the origins of life. Nat. Rev. Genet. 2015, 16, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sullivan, F.X.; Cech, T.R. Intermolecular exon ligation of the rRNA precursor of Tetrahymena: Oligonucleotides can function as 5′ exons. Cell 1985, 43, 431–437. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, S.; Zaleta-Rivera, K. RNA: Interactions drive functionalities. Mol. Biol. Rep. 2020, 47, 1413–1434. [Google Scholar] [CrossRef]
- Richard Boland, C. Non-coding RNA: It’s not junk. Dig. Dis. Sci. 2017, 62, 1107–1109. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef]
- Demoulin, C.F.; Lara, Y.J.; Cornet, L.; François, C.; Baurain, D.; Wilmotte, A.; Javaux, E.J. Cyanobacteria evolution: Insight from the fossil record. Free Radic. Biol. Med. 2019, 140, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Hodgskiss, M.S.W.; Crockford, P.W.; Peng, Y.; Wing, B.A.; Horner, T.J. A productivity collapse to end Earth’s Great Oxidation. Proc. Natl. Acad. Sci. USA 2019, 116, 17207–17212. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, C.; Aoyama, K.; Nakaki, T. microRNA as a new agent for regulating neuronal glutathione synthesis and metabolism. AIMS Mol. Sci. 2015, 1, 124–143. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Freire, M.; de Cabo, R.; Bernier, M.; Sollott, S.J.; Fabbri, E.; Navas, P.; Ferrucci, L. Reconsidering the role of mitochondria in aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Ademowo, O.S.; Dias, H.K.I.; Burton, D.G.A.; Griffiths, H.R. Lipid (per) oxidation in mitochondria: An emerging target in the ageing process? Biogerontology 2017. [Google Scholar] [CrossRef]
- Aoyama, K.; Watabe, M.; Nakaki, T. Regulation of neuronal glutathione synthesis. J. Pharm. Sci. 2008, 108, 227–238. [Google Scholar] [CrossRef]
- Temiz, M.A.; Temur, A.; Elik, S. Antioxidant role and hepatoprotective effects of carob (Ceratonia siliqua L.) seeds against ethanol-induced oxidative stress in rats. J. Food Nutr. Res. 2015, 3, 57–61. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Kuo, H.C. Functional roles and networks of non-coding RNAs in the pathogenesis of neurodegenerative diseases. J. Biomed. Sci. 2020, 27, 49. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Ghezzi, P.; Jaquet, V.; Marcucci, F.; Schmidt, H. The oxidative stress theory of disease: Levels of evidence and epistemological aspects. Br. J. Pharm. 2017, 174, 1784–1796. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 2013, 14, 21021–21044. [Google Scholar] [CrossRef]
- Janáky, R.; Varga, V.; Hermann, A.; Saransaari, P.; Oja, S.S. Mechanisms of L-cysteine neurotoxicity. Neurochem. Res. 2000, 25, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine metabolism in neuronal redox homeostasis. Trends Pharm. Sci. 2018, 39, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Coody, T.K.; Jeong, M.Y.; Berg, J.A.; Winge, D.R.; Hughes, A.L. Cysteine toxicity drives age-related mitochondrial decline by altering iron homeostasis. Cell 2020, 180, 296–310.e18. [Google Scholar] [CrossRef]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Backos, D.S.; Fritz, K.S.; Roede, J.R.; Petersen, D.R.; Franklin, C.C. Posttranslational modification and regulation of glutamate-cysteine ligase by the α,β-unsaturated aldehyde 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2011, 50, 14–26. [Google Scholar] [CrossRef]
- Liu, Y.; Hyde, A.S.; Simpson, M.A.; Barycki, J.J. Emerging regulatory paradigms in glutathione metabolism. Adv. Cancer Res. 2014, 122, 69–101. [Google Scholar] [CrossRef] [PubMed]
- Steullet, P.; Cabungcal, J.H.; Kulak, A.; Kraftsik, R.; Chen, Y.; Dalton, T.P.; Cuenod, M.; Do, K.Q. Redox dysregulation affects the ventral but not dorsal hippocampus: Impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J. Neurosci. 2010, 30, 2547–2558. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Rosca, M.; Fan, Y.; Hu, Y.; Feng, P.; Lee, H.G.; Monnier, V.M.; Fan, X. Gclc deficiency in mouse CNS causes mitochondrial damage and neurodegeneration. Hum. Mol. Genet. 2017, 26, 1376–1390. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Winkler, A.; Njålsson, R.; Carlsson, K.; Elgadi, A.; Rozell, B.; Abraham, L.; Ercal, N.; Shi, Z.Z.; Lieberman, M.W.; Larsson, A.; et al. Glutathione is essential for early embryogenesis—Analysis of a glutathione synthetase knockout mouse. Biochem. Biophys. Res. Commun. 2011, 412, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ristoff, E.; Larsson, A. Inborn errors in the metabolism of glutathione. Orphanet J. Rare Dis. 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Flores, A.; Martínez-González, J.J.; Rendón, J.L.; Del Arenal, I.P. The architecture of thiol antioxidant systems among invertebrate parasites. Molecules 2017, 22, 259. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, E.V.; Chernov, N.N.; Novichkova, M.D. Role of glutathione, glutathione transferase, and glutaredoxin in regulation of redox-dependent processes. Biochemistry 2014, 79, 1562–1583. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Marchan, R.; Hammond, C.L. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Asp. Med. 2009, 30, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Brandmann, M.; Hohnholt, M.C.; Blumrich, E.M. Glutathione-dependent detoxification processes in astrocytes. Neurochem. Res. 2015, 40, 2570–2582. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef]
- Greenwald, R.A. Superoxide dismutase and catalase as therapeutic agents for human diseases. A critical review. Free Radic. Biol. Med. 1990, 8, 201–209. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete identity. Protein Cell 2010, 1, 888–897. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef]
- McAlary, L.; Aquilina, J.A.; Yerbury, J.J. Susceptibility of mutant SOD1 to form a destabilized monomer predicts cellular aggregation and toxicity but not in vitro aggregation propensity. Front. Neurosci. 2016, 10, 499. [Google Scholar] [CrossRef]
- Góth, L.; Rass, P.; Páy, A. Catalase enzyme mutations and their association with diseases. Mol. Diagn. 2004, 8, 141–149. [Google Scholar] [CrossRef]
- Ho, Y.S.; Xiong, Y.; Ma, W.; Spector, A.; Ho, D.S. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J. Biol. Chem. 2004, 279, 32804–32812. [Google Scholar] [CrossRef]
- Hirono, A.; Sasaya-Hamada, F.; Kanno, H.; Fujii, H.; Yoshida, T.; Miwa, S. A novel human catalase mutation (358 T-->del) causing Japanese-type acatalasemia. Blood Cells Mol. Dis. 1995, 21, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Takahara, S. Progressive oral gangrene probably due to lack of catalase in the blood (acatalasaemia): Report of nine cases. Lancet 1952, 2, 1101–1104. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- Girotti, A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [CrossRef]
- Lei, X.G. Glutathione peroxidase-1 gene knockout on body antioxidant defense in mice. BioFactors 2001, 14, 93–99. [Google Scholar] [CrossRef]
- De Haan, J.B.; Crack, P.J.; Flentjar, N.; Iannello, R.C.; Hertzog, P.J.; Kola, I. An imbalance in antioxidant defense affects cellular function: The pathophysiological consequences of a reduction in antioxidant defense in the glutathione peroxidase-1 (Gpx1) knockout mouse. Redox Rep. 2003, 8, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Crack, P.J.; Cimdins, K.; Ali, U.; Hertzog, P.J.; Iannello, R.C. Lack of glutathione peroxidase-1 exacerbates Abeta-mediated neurotoxicity in cortical neurons. J. Neural Transm. 2006, 113, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Van Remmen, H.; Gu, M.; Qi, W.; Roberts, L.J., 2nd; Prolla, T.; Richardson, A. Embryonic fibroblasts from Gpx4+/- mice: A novel model for studying the role of membrane peroxidation in biological processes. Free Radic. Biol. Med. 2003, 35, 1101–1109. [Google Scholar] [CrossRef]
- Ran, Q.; Liang, H.; Ikeno, Y.; Qi, W.; Prolla, T.A.; Roberts, L.J., 2nd; Wolf, N.; Van Remmen, H.; Richardson, A. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Na, R.; Gu, M.; Richardson, A.; Ran, Q. Lipid peroxidation up-regulates BACE1 expression in vivo: A possible early event of amyloidogenesis in Alzheimer’s disease. J. Neurochem. 2008, 107, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 2008, 1780, 1304–1317. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Hanschmann, E.M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, glutaredoxins, and peroxiredoxins—Molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 2013, 19, 1539–1605. [Google Scholar] [CrossRef]
- Rhee, S.G. Overview on Peroxiredoxin. Mol. Cells 2016, 39, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Fledderus, J.O.; Boon, R.A.; Volger, O.L.; Hurttila, H.; Ylä-Herttuala, S.; Pannekoek, H.; Levonen, A.L.; Horrevoets, A.J. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1339–1346. [Google Scholar] [CrossRef]
- Abdalkader, M.; Lampinen, R.; Kanninen, K.M.; Malm, T.M.; Liddell, J.R. Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front. Neurosci. 2018, 12, 466. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Zhang, X.J.; Zhang, C.; Yang, Y.; He, J.N.; Chen, Y.X. Protective effects of leonurine against ischemic stroke in mice by activating nuclear factor erythroid 2-related factor 2 pathway. CNS Neurosci. Ther. 2019, 25, 1006–1017. [Google Scholar] [CrossRef]
- Shih, A.Y.; Li, P.; Murphy, T.H. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Suh, S.W.; Hamby, A.M.; Liu, J.; Chan, W.Y.; Chen, Y.; Swanson, R.A. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006, 9, 119–126. [Google Scholar] [CrossRef]
- Escartin, C.; Won, S.J.; Malgorn, C.; Auregan, G.; Berman, A.E.; Chen, P.C.; Déglon, N.; Johnson, J.A.; Suh, S.W.; Swanson, R.A. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J. Neurosci. 2011, 31, 7392–7401. [Google Scholar] [CrossRef]
- Kanai, Y.; Hediger, M.A. The glutamate and neutral amino acid transporter family: Physiological and pharmacological implications. Eur. J. Pharmacol. 2003, 479, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Zerangue, N.; Kavanaugh, M.P. Flux coupling in a neuronal glutamate transporter. Nature 1996, 383, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Zerangue, N.; Kavanaugh, M.P. Interaction of L-cysteine with a human excitatory amino acid transporter. J. Physiol. 1996, 493, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.D.; Torres-Salazar, D.; Divito, C.B.; Amara, S.G. Cysteine transport through excitatory amino acid transporter 3 (EAAT3). PLoS ONE 2014, 9, e109245. [Google Scholar] [CrossRef]
- Malik, A.R.; Willnow, T.E. Excitatory amino acid transporters in physiology and disorders of the central nervous system. Int. J. Mol. Sci. 2019, 20, 5671. [Google Scholar] [CrossRef] [PubMed]
- Bannai, S. Transport of cystine and cysteine in mammalian cells. Biochim. Biophys. Acta 1984, 779, 289–306. [Google Scholar] [CrossRef]
- Massie, A.; Boillée, S.; Hewett, S.; Knackstedt, L.; Lewerenz, J. Main path and byways: Non-vesicular glutamate release by system xc(-) as an important modifier of glutamatergic neurotransmission. J. Neurochem. 2015, 135, 1062–1079. [Google Scholar] [CrossRef]
- Pow, D.V. Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia 2001, 34, 27–38. [Google Scholar] [CrossRef]
- Liu, X.; Resch, J.; Rush, T.; Lobner, D. Functional upregulation of system xc- by fibroblast growth factor-2. Neuropharmacology 2012, 62, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Fogal, B.; Trettel, J.; Uliasz, T.F.; Levine, E.S.; Hewett, S.J. Changes in secondary glutamate release underlie the developmental regulation of excitotoxic neuronal cell death. Neuroscience 2005, 132, 929–942. [Google Scholar] [CrossRef]
- Jackman, N.A.; Uliasz, T.F.; Hewett, J.A.; Hewett, S.J. Regulation of system x(c)(-)activity and expression in astrocytes by interleukin-1β: Implications for hypoxic neuronal injury. Glia 2010, 58, 1806–1815. [Google Scholar] [CrossRef]
- Burdo, J.; Dargusch, R.; Schubert, D. Distribution of the cystine/glutamate antiporter system xc- in the brain, kidney, and duodenum. J. Histochem. Cytochem. 2006, 54, 549–557. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Gregolin, C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta 1985, 839, 62–70. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Non-coding RNA genes and the modern RNA world. Nat. Rev. Genet. 2001, 2, 919–929. [Google Scholar] [CrossRef]
- Gerbi, S.A. Small nucleolar RNA. Biochem. Cell Biol. 1995, 73, 845–858. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, R. Regulatory non-coding RNAs: Revolutionizing the RNA world. Mol. Biol. Rep. 2014, 41, 3915–3923. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, S.; Zhang, M.; Xu, W.; Chen, Q.; Zheng, L.; Liu, P.; Zou, W. Acupuncture ameliorates neuronal cell death, inflammation, and ferroptosis and downregulated miR-23a-3p after intracerebral hemorrhage in rats. J. Mol. Neurosci. 2021. [Google Scholar] [CrossRef]
- Narasimhan, M.; Riar, A.K.; Rathinam, M.L.; Vedpathak, D.; Henderson, G.; Mahimainathan, L. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol. Lett. 2014, 228, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Jain, M.R.; Li, H.; Junn, E. MicroRNA-7 activates Nrf2 pathway by targeting Keap1 expression. Free Radic. Biol. Med. 2015, 89, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Si, E. MicroRNA-25 aggravates Aβ1-42-induced hippocampal neuron injury in Alzheimer’s disease by downregulating KLF2 via the Nrf2 signaling pathway in a mouse model. J. Cell. Biochem. 2019, 120, 15891–15905. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Li, Z.H.; Li, X.; Zheng, T.; Zhang, D.K. microRNA-592 blockade inhibits oxidative stress injury in Alzheimer’s disease astrocytes via the KIAA0319-mediated Keap1/Nrf2/ARE signaling pathway. Exp. Neurol. 2020, 324, 113128. [Google Scholar] [CrossRef]
- Francks, C.; Paracchini, S.; Smith, S.D.; Richardson, A.J.; Scerri, T.S.; Cardon, L.R.; Marlow, A.J.; MacPhie, I.L.; Walter, J.; Pennington, B.F.; et al. A 77-kilobase region of chromosome 6p22.2 is associated with dyslexia in families from the United Kingdom and from the United States. Am. J. Hum. Genet. 2004, 75, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Y.; Cui, H.F.; Qin, B.J. Monomethyl fumarate protects cerebral hemorrhage injury in rats via activating microRNA-139/Nrf2 axis. Eur. Rev. Med. Pharm. Sci. 2019, 23, 5012–5019. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, L.; Zheng, J.; Wang, K.; Deng, H.; Liu, P.; Chen, L.; Mu, H. MicroRNA-144 modulates oxidative stress tolerance in SH-SY5Y cells by regulating nuclear factor erythroid 2-related factor 2-glutathione axis. Neurosci. Lett. 2017, 655, 21–27. [Google Scholar] [CrossRef]
- Du, Y.; Chi, X.; An, W. Downregulation of microRNA-200c-3p reduces damage of hippocampal neurons in epileptic rats by upregulating expression of RECK and inactivating the AKT signaling pathway. Chem. Biol. Interact. 2019, 307, 223–233. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Yan, R.; Jin, S.; Wan, Z.; Cheng, J.; Li, N.; Chen, L.; Le, C. MicroRNA-204-5p mediates sevoflurane-induced cytotoxicity in HT22 cells by targeting brain-derived neurotrophic factor. Histol. Histopathol. 2020, 35, 1353–1361. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, L.; Shan, S.; Nie, Y.; Wang, Y. Vitexin improves neuron apoptosis and memory impairment induced by isoflurane via regulation of miR-409 expression. Adv. Clin. Exp. Med. 2020, 29, 135–145. [Google Scholar] [CrossRef]
- Wu, Q.; Shang, Y.; Shen, T.; Liu, F.; Xu, Y.; Wang, H. Neuroprotection of miR-214 against isoflurane-induced neurotoxicity involves the PTEN/PI3K/Akt pathway in human neuroblastoma cell line SH-SY5Y. Arch. Biochem. Biophys. 2019, 678, 108181. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.N.; Dong, Z.L.; Han, L.L. MicroRNA-410 inhibition of the TIMP2-dependent MAPK pathway confers neuroprotection against oxidative stress-induced apoptosis after ischemic stroke in mice. Brain Res. Bull. 2018, 143, 45–57. [Google Scholar] [CrossRef]
- Jee, M.K.; Jung, J.S.; Choi, J.I.; Jang, J.A.; Kang, K.S.; Im, Y.B.; Kang, S.K. MicroRNA 486 is a potentially novel target for the treatment of spinal cord injury. Brain 2012, 135, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, W.; Nan, S.; Chang, M.; Fan, J. MicroRNA-326 inhibits apoptosis and promotes proliferation of dopaminergic neurons in Parkinson’s disease through suppression of KLK7-mediated MAPK signaling pathway. J. Mol. Neurosci. 2019, 69, 197–214. [Google Scholar] [CrossRef]
- Qu, X.; Wang, N.; Cheng, W.; Xue, Y.; Chen, W.; Qi, M. MicroRNA-146a protects against intracerebral hemorrhage by inhibiting inflammation and oxidative stress. Exp. Ther. Med. 2019, 18, 3920–3928. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Z.; Zhao, Y.; Chen, H.Z. MicroRNA-98 reduces amyloid β-protein production and improves oxidative stress and mitochondrial dysfunction through the Notch signaling pathway via HEY2 in Alzheimer’s disease mice. Int. J. Mol. Med. 2019, 43, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; You, C. miR-129-3p targeting of MCU protects against glucose fluctuation-mediated neuronal damage via a mitochondrial-dependent intrinsic apoptotic pathway. Diab. Metab. Synd. Obesity 2021, 14, 153–163. [Google Scholar] [CrossRef]
- Wu, D.M.; Wang, S.; Wen, X.; Han, X.R.; Wang, Y.J.; Shen, M.; Fan, S.H.; Zhuang, J.; Zhang, Z.F.; Shan, Q.; et al. Inhibition of microRNA-200a upregulates the expression of striatal dopamine receptor D2 to repress apoptosis of striatum via the cAMP/PKA signaling pathway in rats with Parkinson’s disease. Cell. Physiol. Biochem. 2018, 51, 1600–1615. [Google Scholar] [CrossRef]
- Shen, W.; Lu, Y.; Hu, J.; Le, H.; Yu, W.; Xu, W.; Yu, W.; Zheng, J. Mechanism of miR-320 in regulating biological characteristics of ischemic cerebral neuron by mediating Nox2/ROS pathway. J. Mol. Neurosci. 2020, 70, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, C.; Aoyama, K.; Matsumura, N.; Kikuchi-Utsumi, K.; Watabe, M.; Nakaki, T. Rhythmic oscillations of the microRNA miR-96-5p play a neuroprotective role by indirectly regulating glutathione levels. Nat. Commun. 2014, 5, 3823. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, C.; Kikuchi-Utsumi, K.; Aoyama, K.; Suzuki, R.; Okamoto, Y.; Matsumura, N.; Omata, D.; Maruyama, K.; Nakaki, T. Inhibition of miR-96-5p in the mouse brain increases glutathione levels by altering NOVA1 expression. Commun. Biol. 2021, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Kubicki, N.; Gnyawali, S.; Chan, Y.C.; Roy, S.; Khanna, S.; Sen, C.K. Natural vitamin E α-tocotrienol protects against ischemic stroke by induction of multidrug resistance-associated protein 1. Stroke 2011, 42, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, D.; Guo, J.; Chen, Z.; Chen, Y.; Zhang, J. Deficiency of NEAT1 prevented MPP(+)-induced inflammatory response, oxidative stress and apoptosis in dopaminergic SK-N-SH neuroblastoma cells via miR-1277-5p/ARHGAP26 axis. Brain Res. 2021, 1750, 147156. [Google Scholar] [CrossRef]
- Lu, J.; Xu, F.; Lu, H. LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci. 2020, 260, 118305. [Google Scholar] [CrossRef]
- Wang, C.; Wan, H.; Wang, Q.; Sun, H.; Sun, Y.; Wang, K.; Zhang, C. Safflor Yellow B attenuates ischemic brain injury via downregulation of long noncoding AK046177 and inhibition of MICRORNA-134 expression in rats. Oxid. Med. Cell. Longev. 2020, 2020, 4586839. [Google Scholar] [CrossRef]
- Nordin, M.; Bergman, D.; Halje, M.; Engström, W.; Ward, A. Epigenetic regulation of the Igf2/H19 gene cluster. Cell Proliferat. 2014, 47, 189–199. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, L.; Liu, J.; Zhu, T.; Xie, Z.; Sun, X.; Zhang, H. Metformin Protects against Oxidative Stress Injury Induced by Ischemia/Reperfusion via Regulation of the lncRNA-H19/miR-148a-3p/Rock2 Axis. Oxid. Med. Cell. Longev. 2019, 2019, 8768327. [Google Scholar] [CrossRef]
- Yu, J.L.; Li, C.; Che, L.H.; Zhao, Y.H.; Guo, Y.B. Downregulation of long noncoding RNA H19 rescues hippocampal neurons from apoptosis and oxidative stress by inhibiting IGF2 methylation in mice with streptozotocin-induced diabetes mellitus. J. Cell. Physiol. 2019, 234, 10655–10670. [Google Scholar] [CrossRef]
- Li, Y.; Fang, J.; Zhou, Z.; Zhou, Q.; Sun, S.; Jin, Z.; Xi, Z.; Wei, J. Downregulation of lncRNA BACE1-AS improves dopamine-dependent oxidative stress in rats with Parkinson’s disease by upregulating microRNA-34b-5p and downregulating BACE1. Cell Cycle 2020, 19, 1158–1171. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, X.; Zhang, J.; Chen, L. Effect of lncRNA WT1-AS regulating WT1 on oxidative stress injury and apoptosis of neurons in Alzheimer’s disease via inhibition of the miR-375/SIX4 axis. Aging 2020, 12, 23974–23995. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Cheng, X.; Lian, Y.J.; Xu, H.L. Silencing of long noncoding RNA SOX21-AS1 relieves neuronal oxidative stress injury in mice with Alzheimer’s disease by upregulating FZD3/5 via the Wnt signaling pathway. Mol. Neurobiol. 2019, 56, 3522–3537. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Kjems, J.; Damgaard, C.K. Circular RNA and miR-7 in cancer. Cancer Res. 2013, 73, 5609–5612. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, T.; Wan, C.; Cang, Y. circRNA_0084043 contributes to the progression of diabetic retinopathy via sponging miR-140-3p and inducing TGFA gene expression in retinal pigment epithelial cells. Gene 2020, 747, 144653. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wang, L.; Huang, W. Circular RNA HIPK3 regulates human lens epithelial cell dysfunction by targeting the miR-221-3p/PI3K/AKT pathway in age-related cataract. Exp. Eye Res. 2020, 198, 108128. [Google Scholar] [CrossRef]
- Bai, M.; Pan, C.L.; Jiang, G.X.; Zhang, Y.M.; Zhang, Z. CircHIPK3 aggravates myocardial ischemia-reperfusion injury by binding to miRNA-124-3p. Eur. Rev. Med. Pharm. Sci. 2019, 23, 10107–10114. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, L.; Xu, C.; Wang, Y.; Fan, C. Hsa_circ_0001445 inhibits ox-LDL-induced HUVECs inflammation, oxidative stress and apoptosis by regulating miRNA-640. Perfusion 2020. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, L.; Yang, G.; Meng, F.; Wan, Y.; Wang, L.; Zhang, L. CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR-541-3p/GPX4 axis. Cell Biol. Int. 2020, 44, 2344–2356. [Google Scholar] [CrossRef]
- Wu, P.; Li, C.; Ye, D.M.; Yu, K.; Li, Y.; Tang, H.; Xu, G.; Yi, S.; Zhang, Z. Circular RNA circEPSTI1 accelerates cervical cancer progression via miR-375/409-3P/515-5p-SLC7A11 axis. Aging 2021, 13. [Google Scholar] [CrossRef] [PubMed]
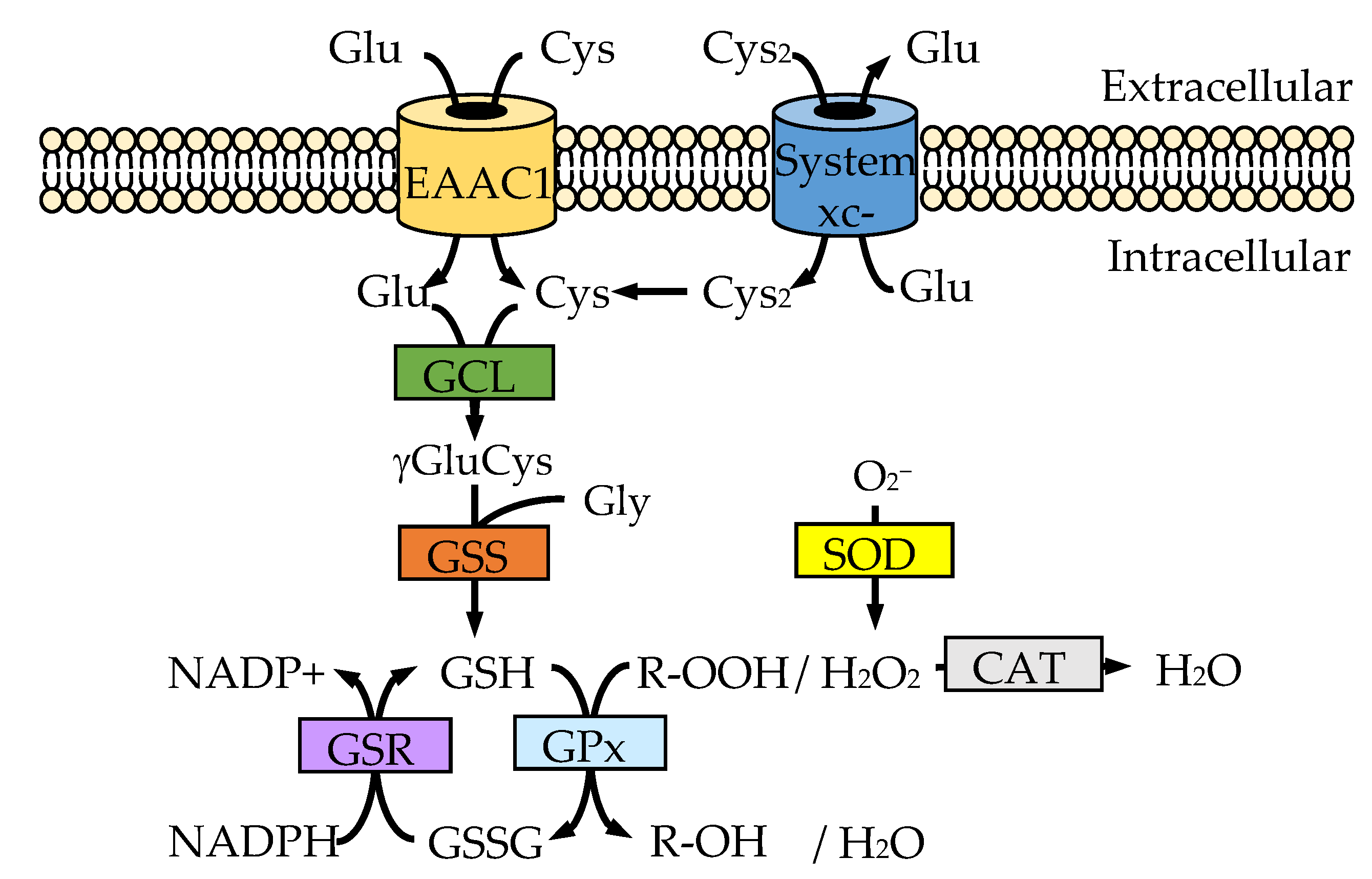
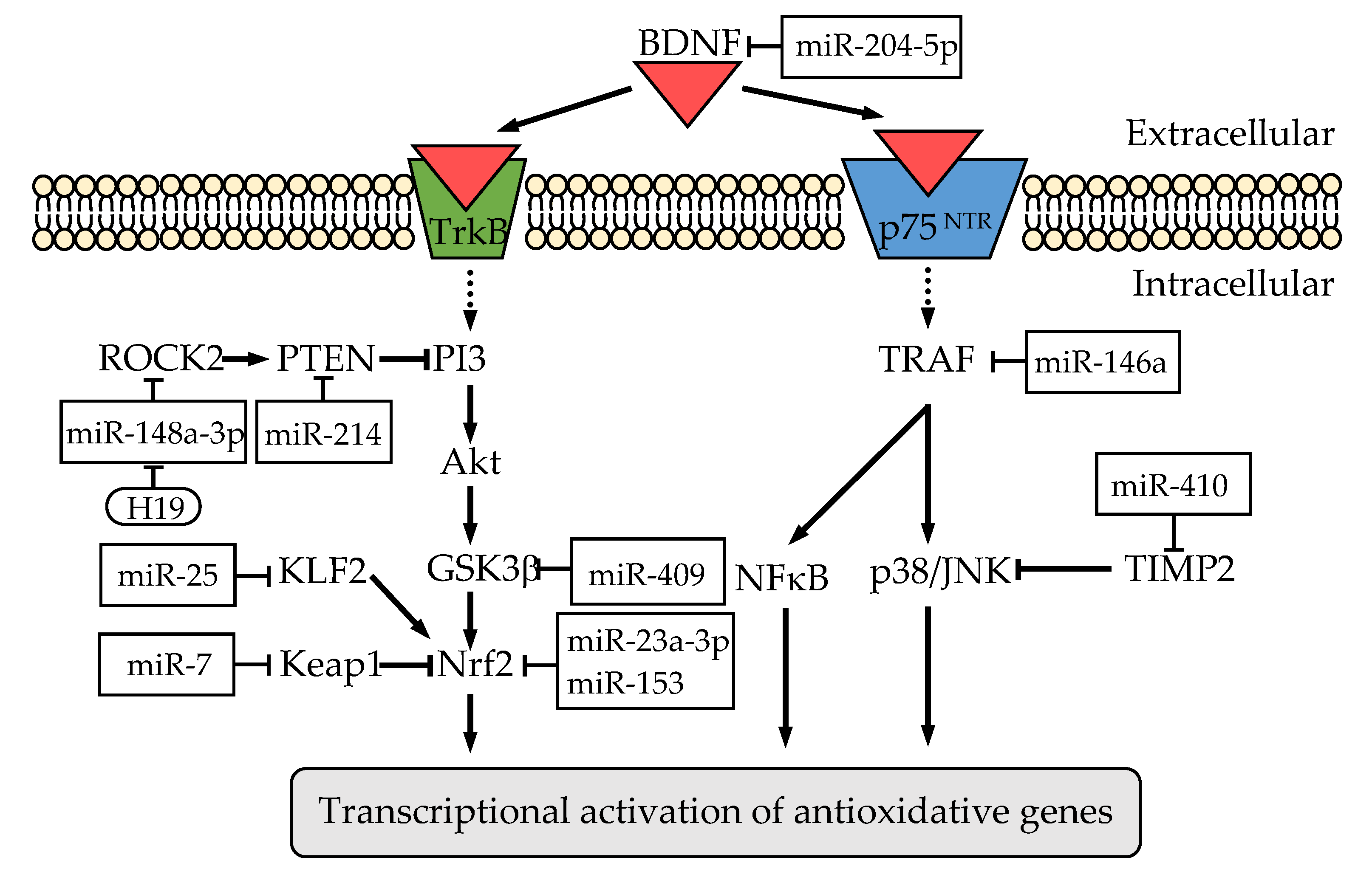
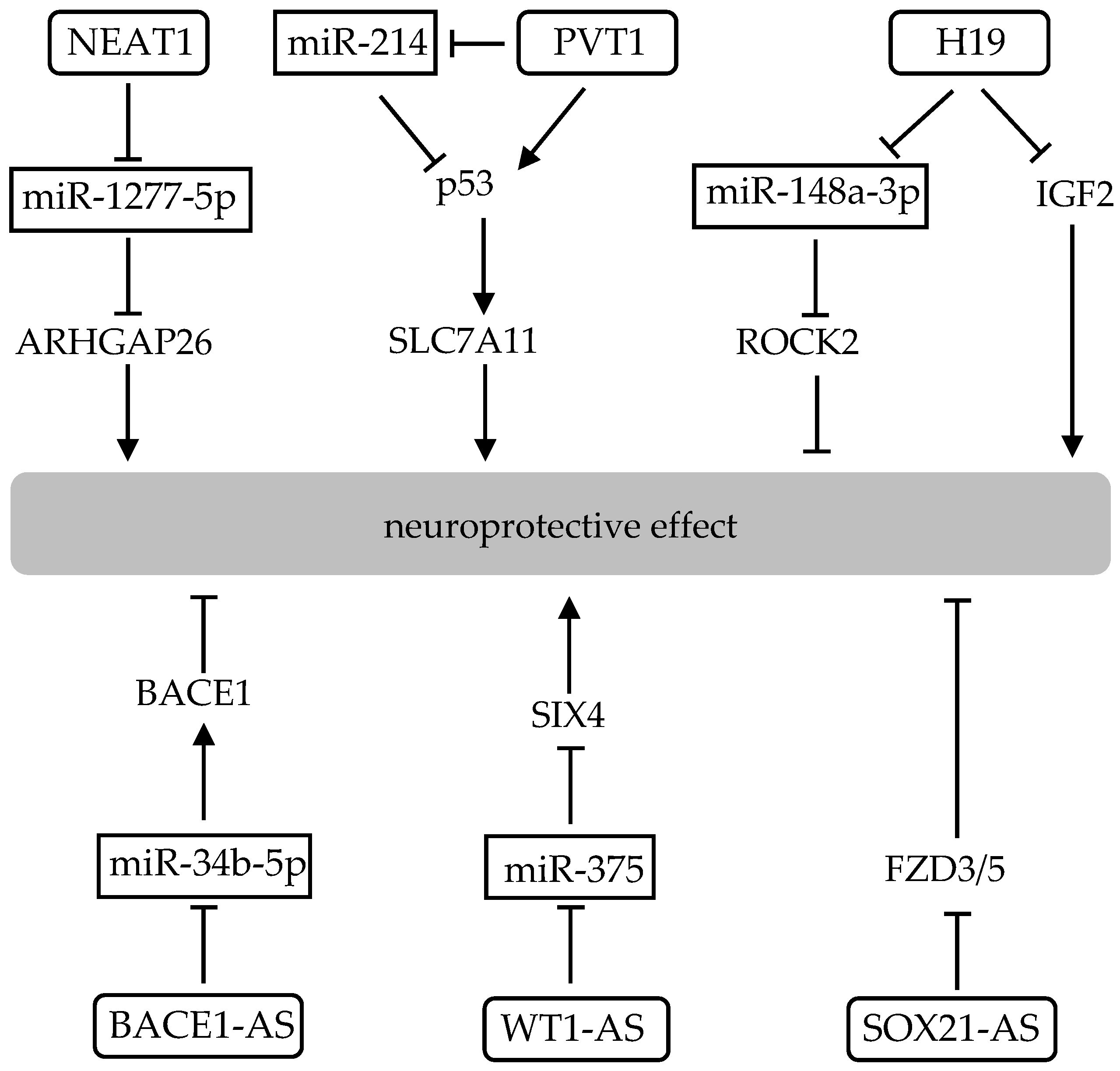

| MiRNA | Direct Target | Related Pathway | Effect on Redox States | Brain Tissue or Neuronal Cell | Ref | ||
|---|---|---|---|---|---|---|---|
| Glutathione | Antioxidative Enzymes | Oxidative Stress | |||||
| miR-7 | Keap1 | Nrf2 pathway | GSH ↑ | GCLm ↑ | CBA *2 ↓ | SH-SY5Y cell | [103] |
| miR-23a-3p | Nrf2 | Nrf2 pathway | n.d. | n.d. | ROS ↑ MDA ↑ | brain *1 | [101] |
| miR-25 | KLF2 | Nrf2 pathway | GSH ↓ | GST ↓ Trx ↓ | n.d. | hippocampus | [104] |
| miR-96-5p | EAAC1 | Cys transport | GSH ↓ | n.d. | ROS ↑ | substantia nigra | [121] |
| miR-96-5p | NOVA1 | Cys transport | GSH ↓ | n.d. | ROS ↑ | dentate gyrus of hippocampus | [122] |
| miR-98 | HEY2 | Notch signaling | GSH ↑ | GPx ↑ SOD ↓ | MDA ↓ | hippocampus | [117] |
| miR-129-3p | MCU | MMP2 pathway | GSH/GSSG ↓ | SOD ↓ | ROS ↑ | primary hippocampal neurons | [118] |
| miR-139 | n.d. | Nrf2 pathway | GSH ↑ | CAT ↑ SOD ↑ | MDA ↓ | SH-SY5Y cell | [107] |
| miR-144 | n.d. | Nrf2 pathway | GSH ↓ | GPx ↓ | ROS ↑ | SH-SY5Y cell | [108] |
| miR-146a | TRAF6 | NF-κB pathway | n.d. | GPx ↑ SOD ↑ | MDA ↓ | brain *1 | [116] |
| miR-153 | Nrf2 | Nrf2 pathway | n.d. | GCLc ↓ | ROS ↑ | SH-SY5Y cell | [102] |
| miR-199a-5p | MRP1 | GSSG clearlance | GSSG ↑ | n.d. | n.d. | primary cortical neurons | [123] |
| miR-200a | n.d. | PKA pathway | n.d. | GPx ↓ SOD ↓ | MDA ↑ | striatum | [119] |
| miR-200c-3p | RECK | PI3K/AKT pathway | n.d. | GPx ↓ SOD ↓ | MDA ↑ | hippocampus | [109] |
| miR-204-5p | BDNF | TrkB pathway | GSH ↓ | SOD ↓ | ROS ↑ MDA ↑ | HT-22 cell | [110] |
| miR-214 | PTEN | PI3K/AKT pathway | GSH ↑ | SOD ↑ | MDA ↓ | SH-SY5Y cell | [112] |
| miR-320 | Nox2 | Nox2 pathway | n.d. | GPx ↑ CAT ↑ SOD ↑ | ROS ↓ MDA ↓ | primary neuron | [120] |
| miR-326 | KLK7 | p38/JNK pathway | n.d. | SOD ↓ GPx ↓ | MDA ↑ | striatum | [115] |
| miR-409 | n.d. | PI3K/AKT pathway | GSH ↑ | SOD ↑ | ROS ↓ | PC-12 cell | [111] |
| miR-410 | TIMP2 | p38/JNK pathway | n.d. | GPx ↑ SOD ↑ | MDA ↓(serum) | hippocampal neurons | [113] |
| miR-486 | NeuroD6 | p38/JNK pathway | n.d. | GPx ↓ | ROS ↑ | spinal cord | [114] |
| miR-592 | KIAA0319 | Nrf2 pathway | GSH ↓ | CAT ↓ SOD ↓ | ROS ↑ MDA ↑ | cortical astrocytes | [105] |
| LncRNA | Direct Target | Function | Effect on Redox States | Brain Tissue or Neuronal Cell | Ref | ||
|---|---|---|---|---|---|---|---|
| Glutathione | Antioxidative Enzymes | Oxidative Stress | |||||
| AK046177 | n.d. | Nrf2/CREB regulation acting with miR-134 | n.d. | GPx ↓ SOD ↓ | ROS ↑ MDA ↑ | primary cortical cell | [127] |
| BACE1-AS | miR-34b-5p | BACE1 upregulation acting as miR-34b-5p sponge | n.d. | GPx ↓ SOD ↓ | MDA ↑ | substantia nigra | [131] |
| H19 | miR-148a-3p | ROCK2 upregulation acting as miR-148a-3p sponge | n.d. | GPx ↓ SOD ↓ | MDA ↑ | Neuro2a cell | [129] |
| H19 | IGF2 | inhibition of antioxidative gene transcription | GSH ↓ | GPx ↓ CAT ↓ SOD ↓ | n.d. | hippocampal neuron | [130] |
| NEAT1 | miR-1277-5p | ARHGAP26 upregulation acting as miR-1277-5p sponge | n.d. | GPx ↓ SOD ↓ | MDA ↑ | SK-N-SH cell | [125] |
| PVT1 | miR-214-3p | TP53 and TFRC upregulation acting as miR-1277-5p sponge | GSH ↑ | GPx ↑ | MDA ↓ | SK-N-SH cell | [126] |
| SOX21-AS1 | FZD3/5 | inactivation of Wnt signalin pathway | n.d. | GPx ↓ CAT ↓ SOD ↓ | ROS ↑ MDA ↑ 4-HNE * ↑ | hippocampal neuron | [133] |
| WT1-AS | miR-375 | SIX4 upregulation acting as miR-375 sponge | n.d. | GPx ↑ SOD ↑ | ROS ↓ MDA ↓ | SH-SY5Y cell | [132] |
| CircRNA | Direct Target | Function | Effect on Redox States | Tissue or Cell | Ref | ||
|---|---|---|---|---|---|---|---|
| Glutathione | Antioxidative Enzymes | Oxidative Stress | |||||
| circRNA_0084043 | miR-140-3p | TGFA upregulation acting as miR-221-3p sponge | n.d. | GPx ↓ SOD ↓ | MDA ↑ | ARPE-19 cells | [136] |
| circHIPK | miR-221-3p | PI3K/AKT pathway activation acting as miR-140-3p sponge | n.d. | GPx ↑ | MDA ↓ | LECs | [137] |
| circHIPK | miR-124-3p | apoptosis induction acting as miR-124-3p sponge | n.d. | GPx ↓ SOD ↓ | MDA ↑ LDH *1 ↑ | HCM | [138] |
| circRNA_0001445 | miR-640 | protective function acting as miR-640 sponge | n.d. | GPx ↑ SOD ↑ | MDA ↓ | HUVECs | [139] |
| circIL4R | miR-541-3p | GPx4 upregulation acting as miR-541-3p sponge | n.d. | n.d. | ROS ↓ MDA ↓ | hepatocellular carcinoma | [140] |
| circEPSTI1 | miR-375 miR-409-3p miR-515-5p | SLC7A11 upregulation acting as sponge of miR-375, -409-3p and -515-5p | GSH/GSSG ↑ | n.d. | Liperfluo *2 ↓ | cervical cancer | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, C.; Aoyama, K. The Role of Non-Coding RNAs in the Neuroprotective Effects of Glutathione. Int. J. Mol. Sci. 2021, 22, 4245. https://doi.org/10.3390/ijms22084245
Kinoshita C, Aoyama K. The Role of Non-Coding RNAs in the Neuroprotective Effects of Glutathione. International Journal of Molecular Sciences. 2021; 22(8):4245. https://doi.org/10.3390/ijms22084245
Chicago/Turabian StyleKinoshita, Chisato, and Koji Aoyama. 2021. "The Role of Non-Coding RNAs in the Neuroprotective Effects of Glutathione" International Journal of Molecular Sciences 22, no. 8: 4245. https://doi.org/10.3390/ijms22084245
APA StyleKinoshita, C., & Aoyama, K. (2021). The Role of Non-Coding RNAs in the Neuroprotective Effects of Glutathione. International Journal of Molecular Sciences, 22(8), 4245. https://doi.org/10.3390/ijms22084245






