Hydroxyapatite-Coated SPIONs and Their Influence on Cytokine Release
Abstract
1. Introduction
2. Results and Discussion
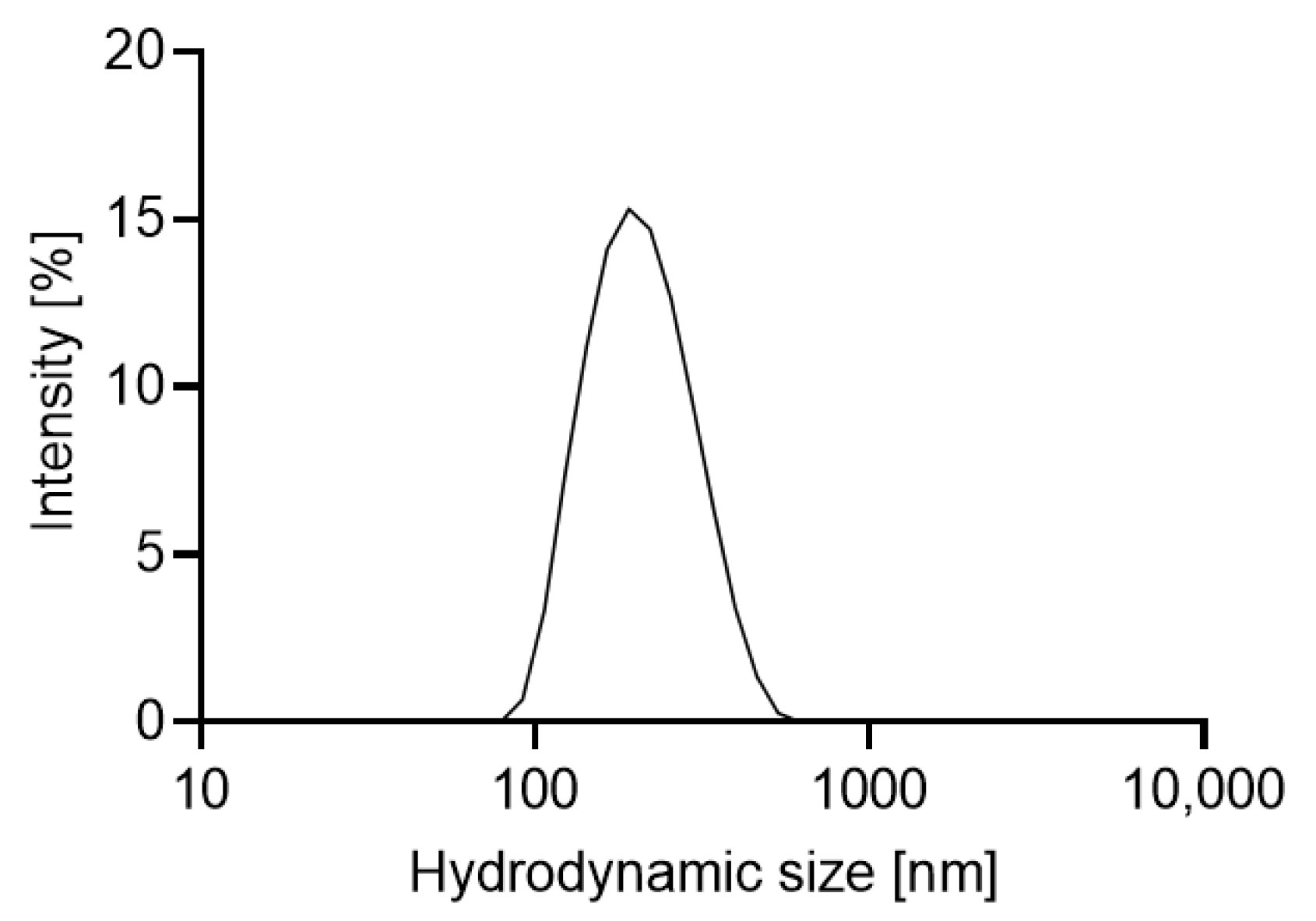
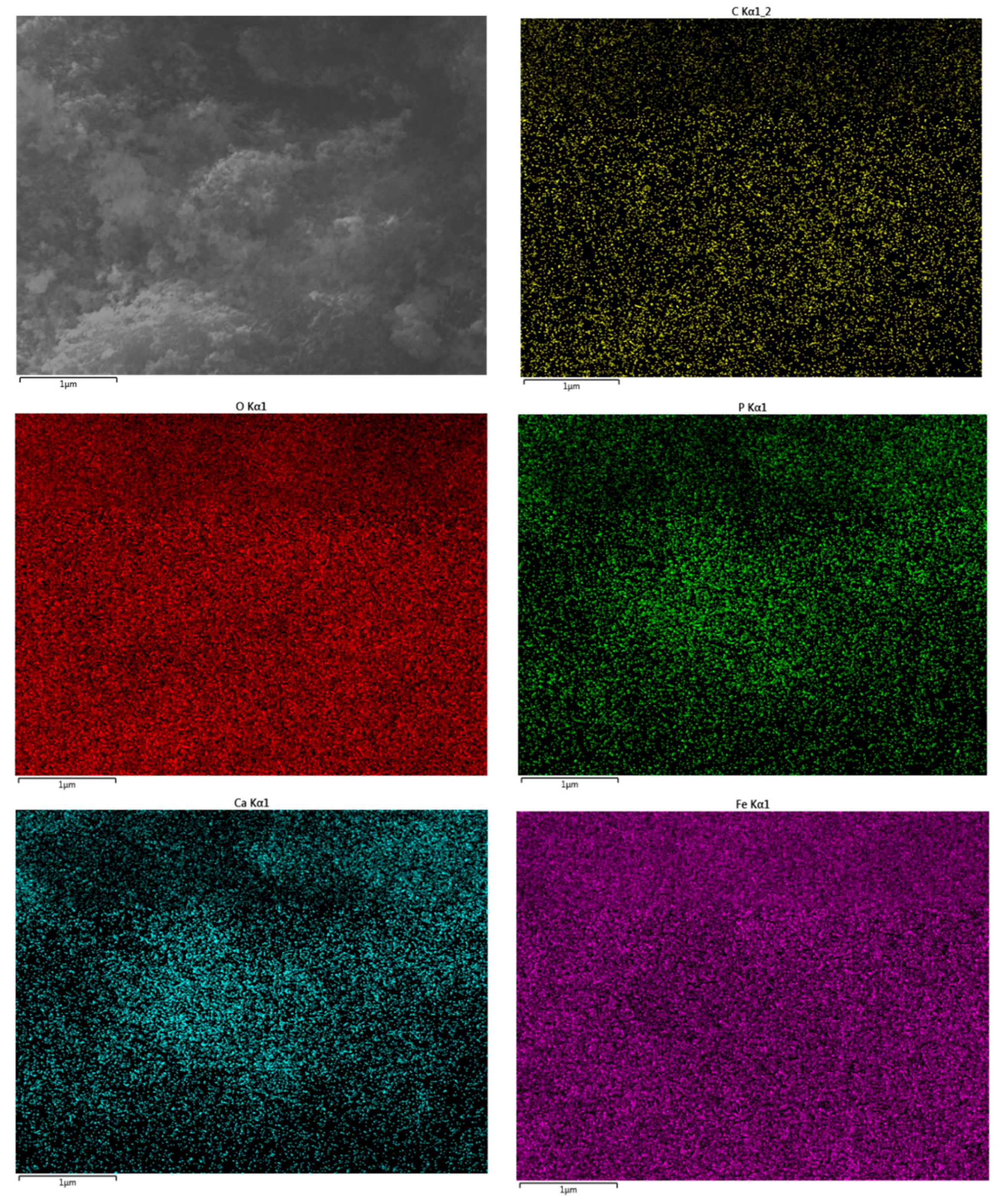
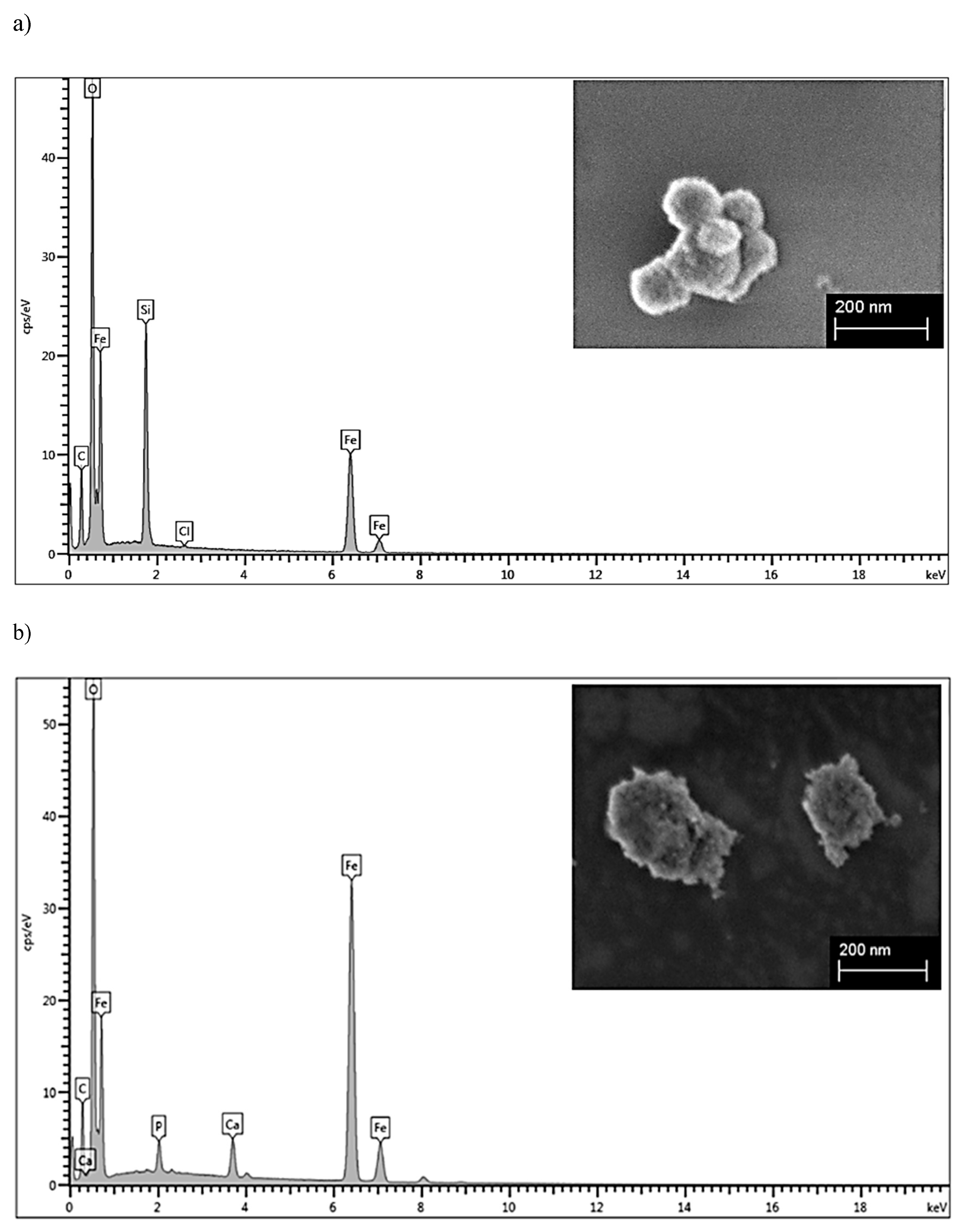
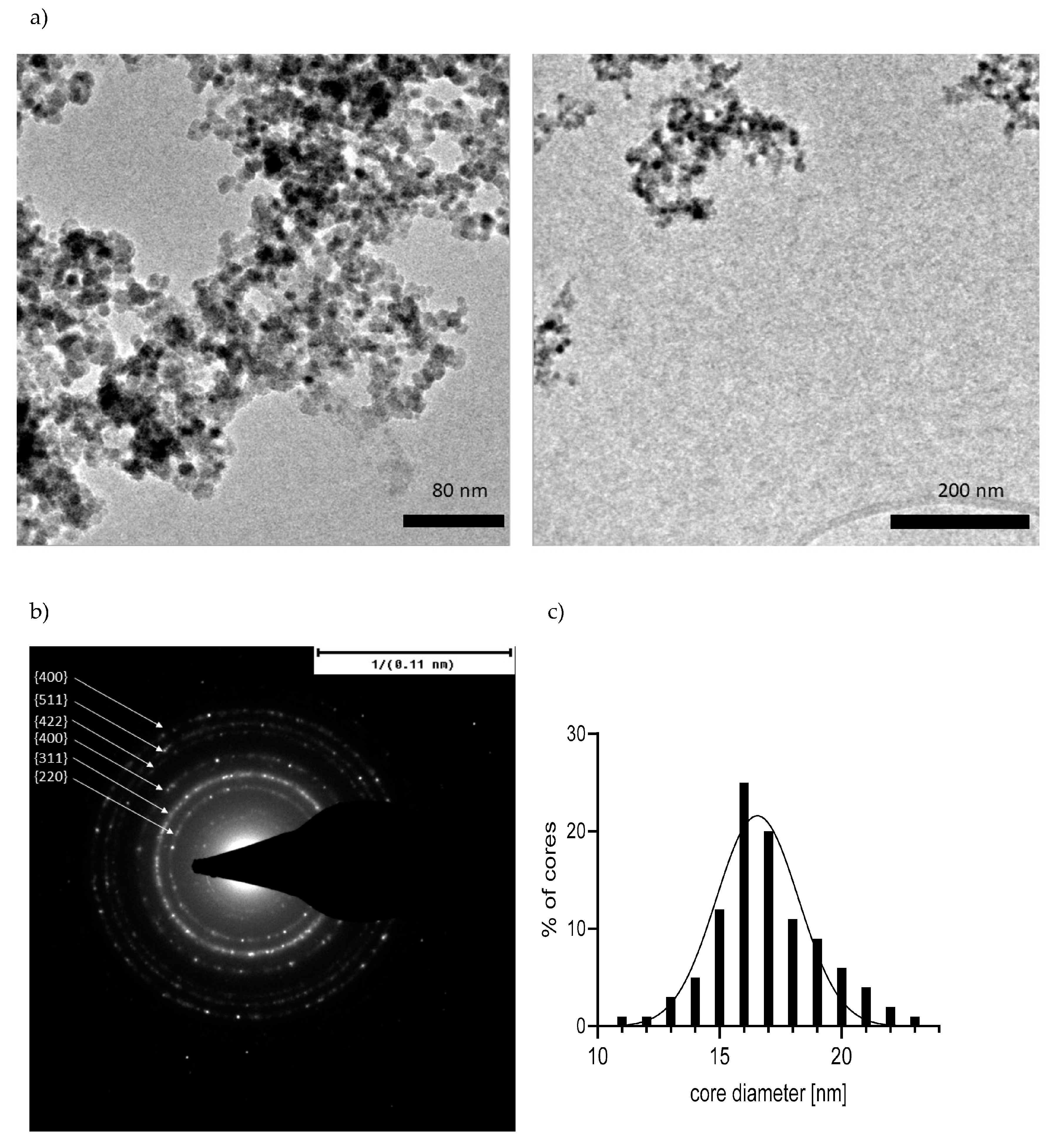
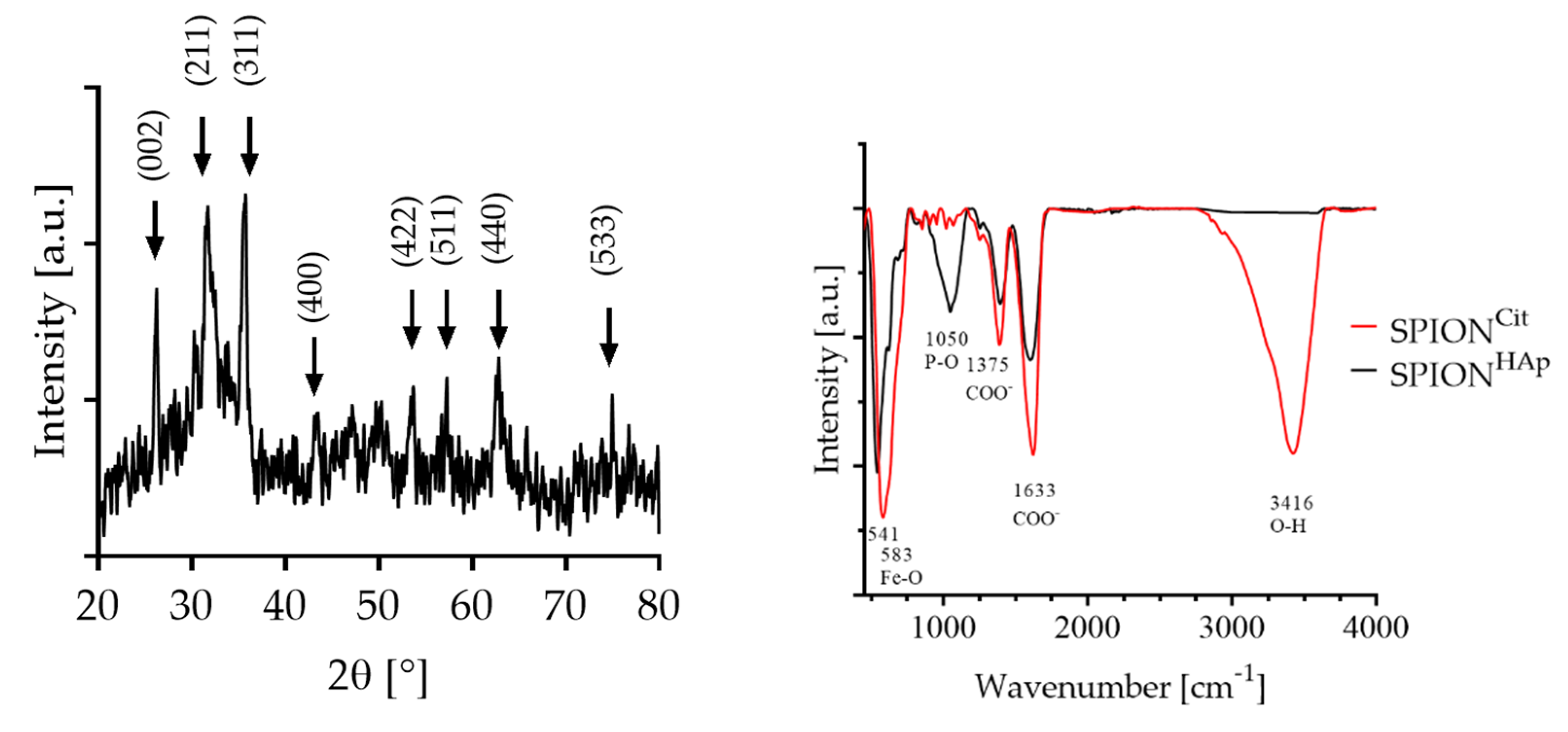
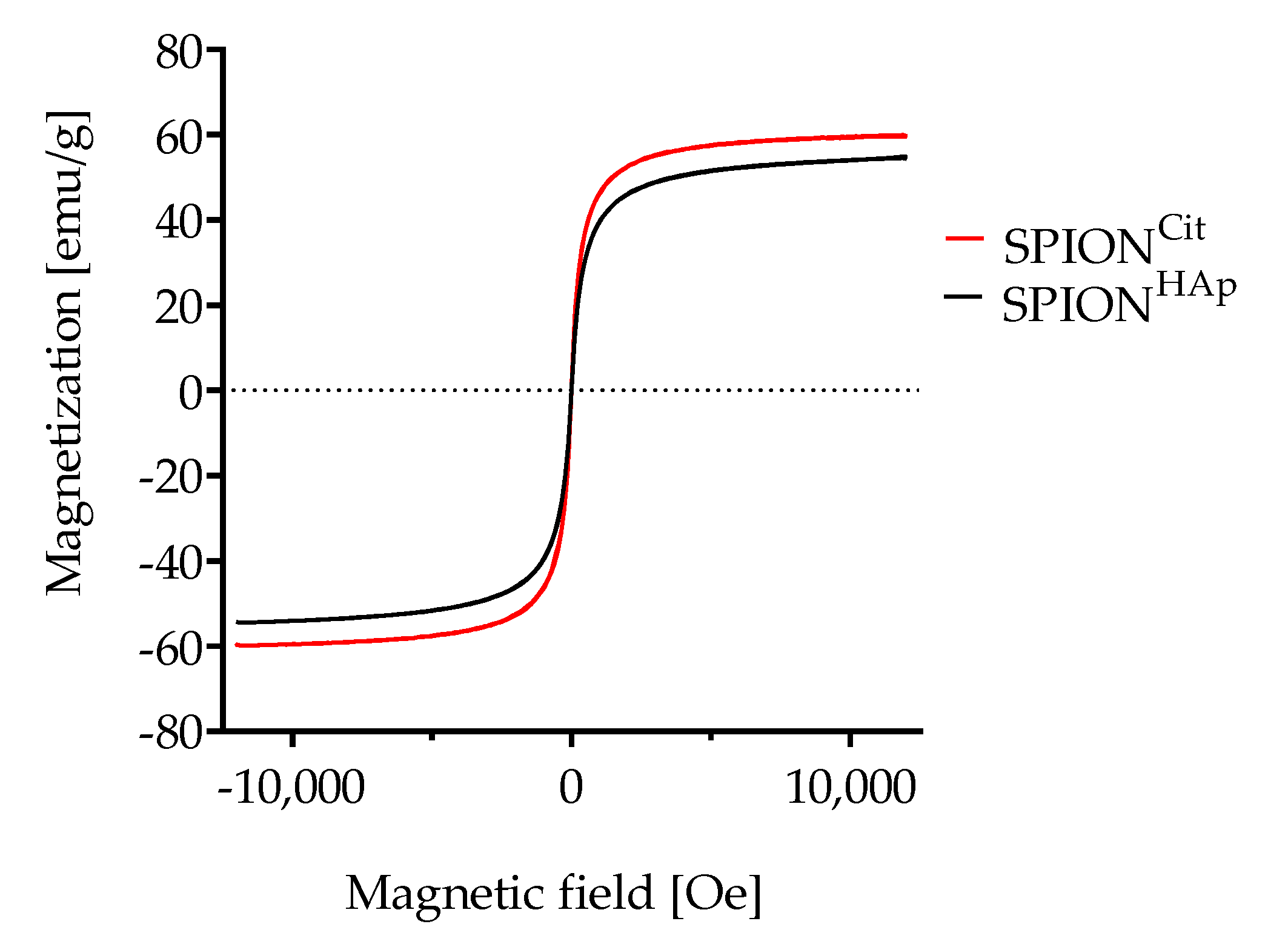
2.1. Characterizations of the Materials
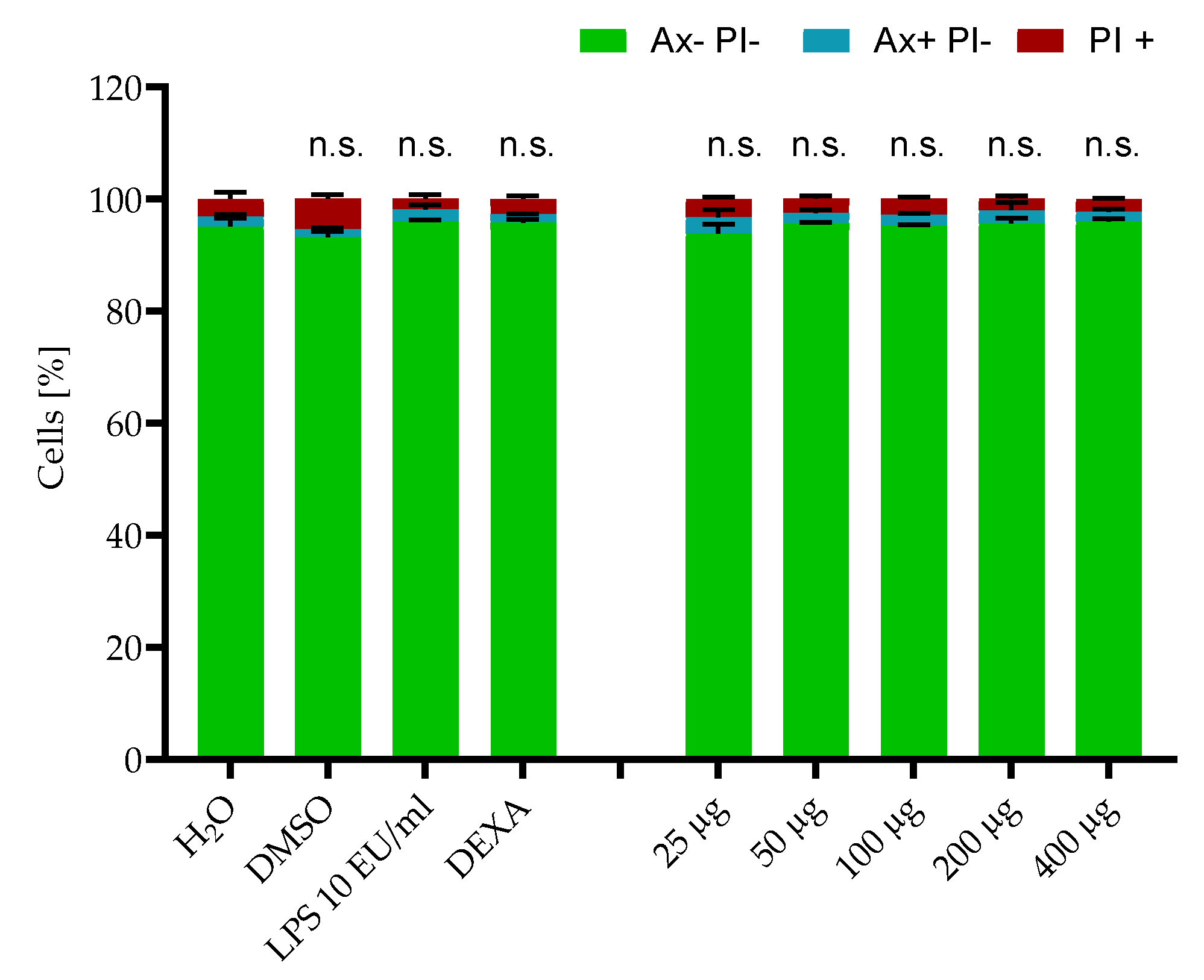
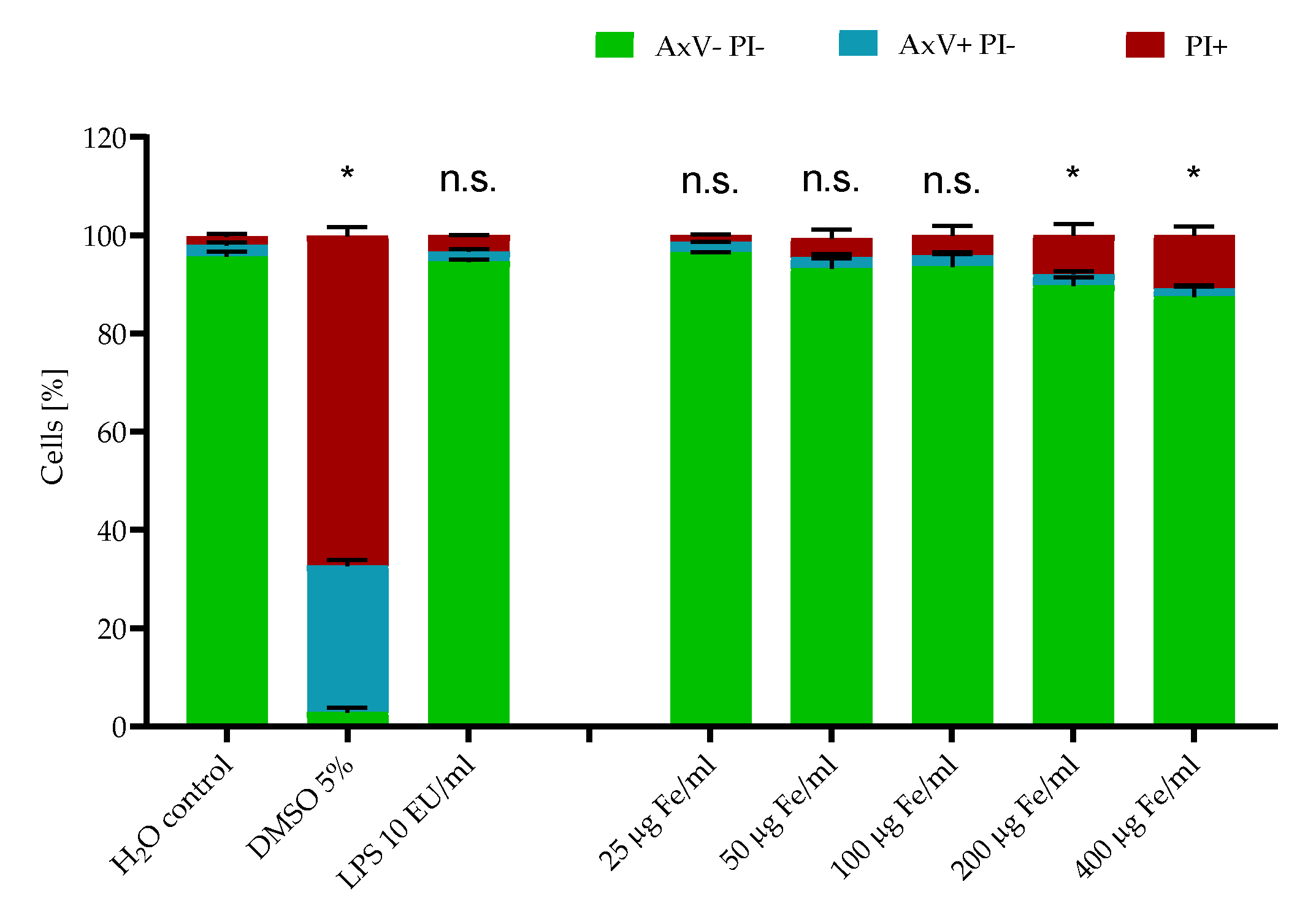
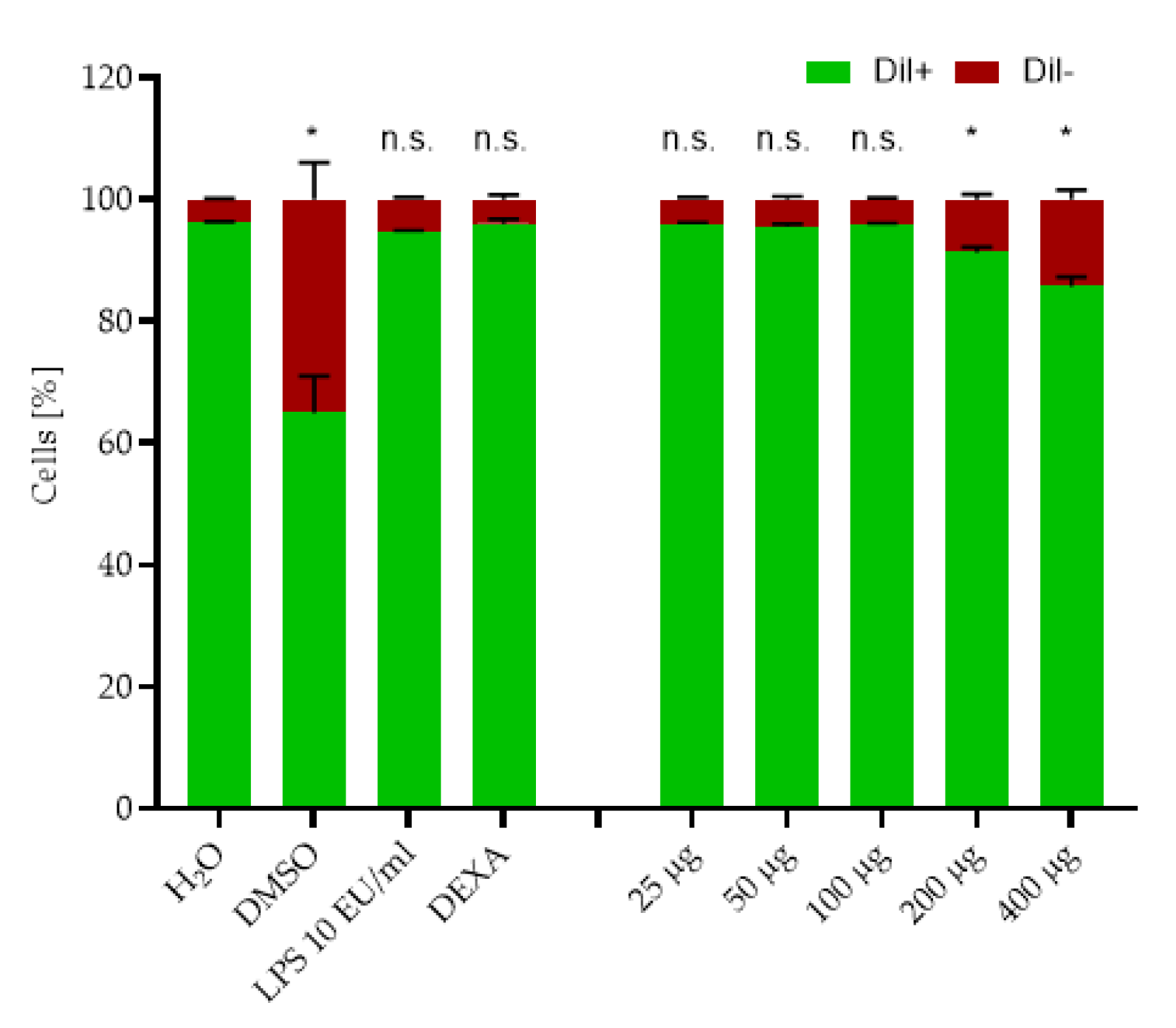
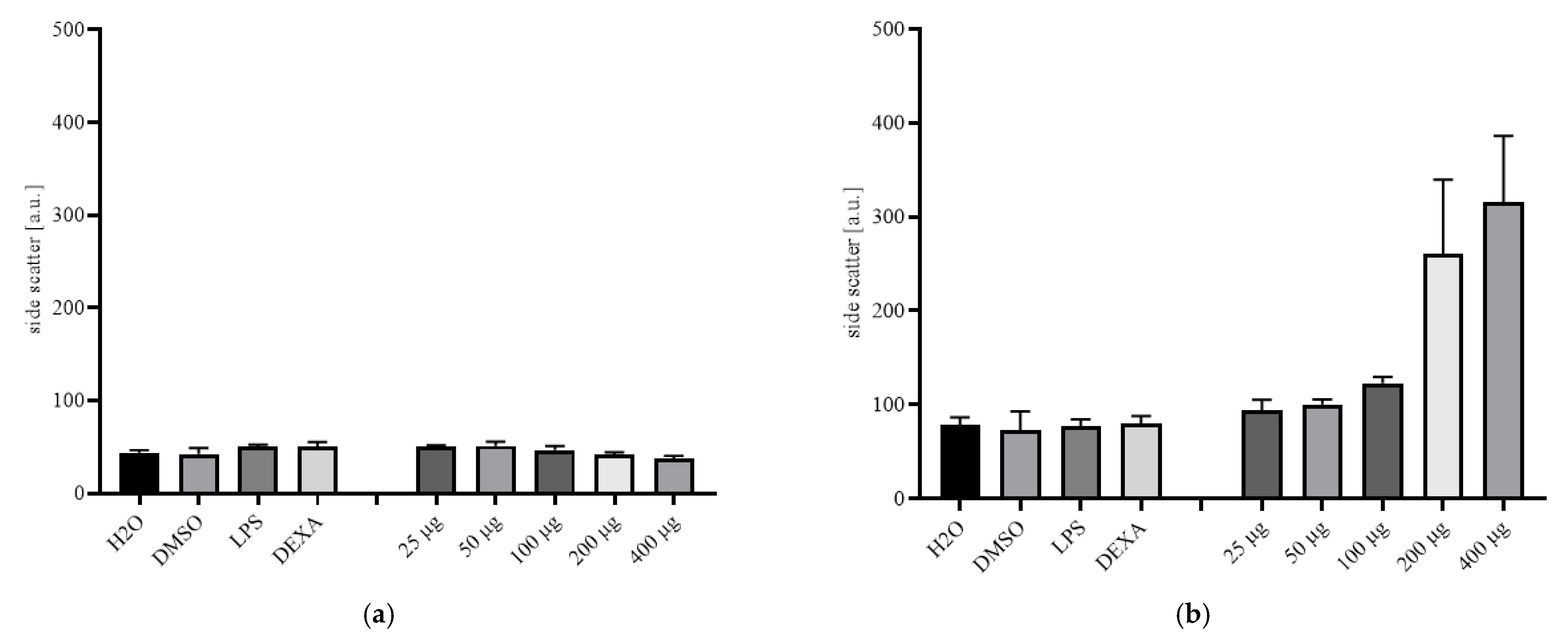
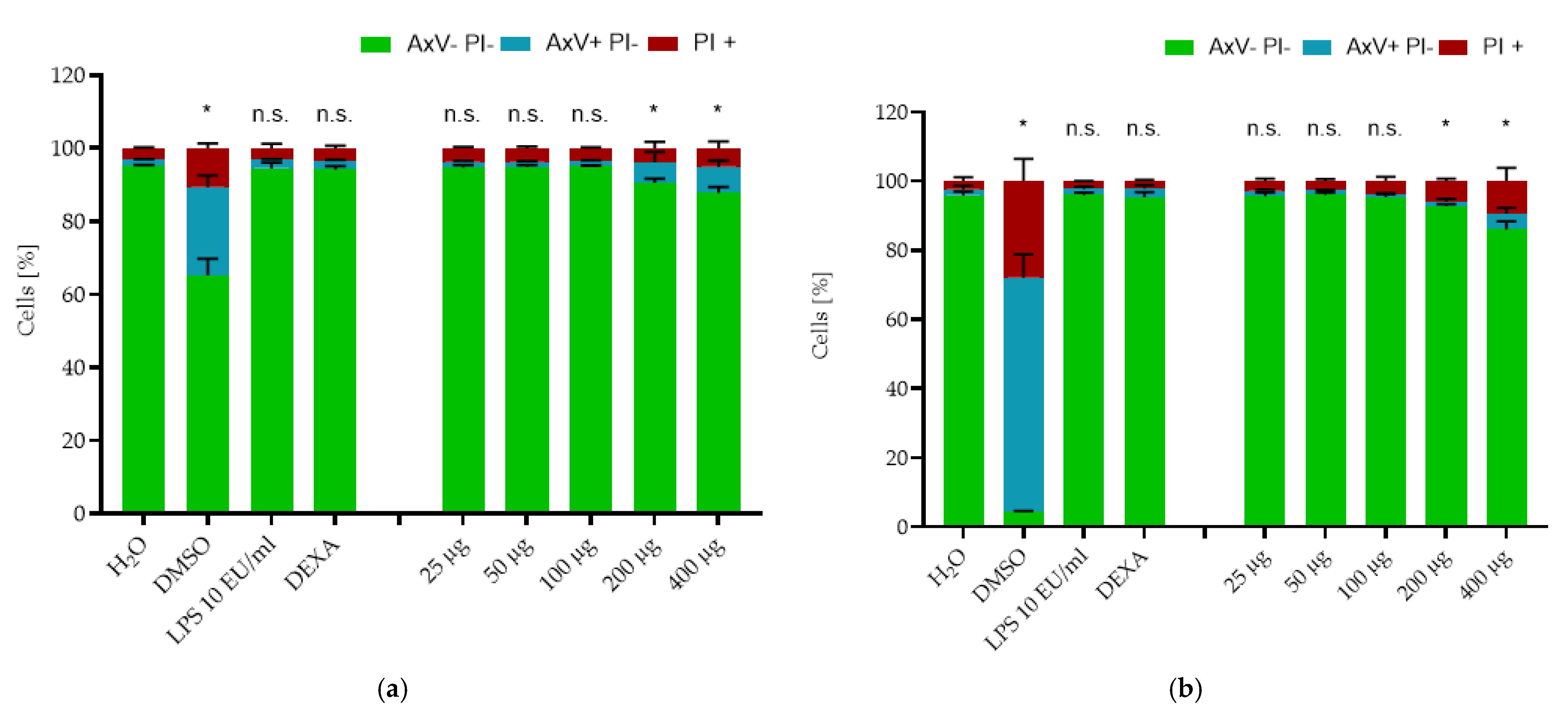
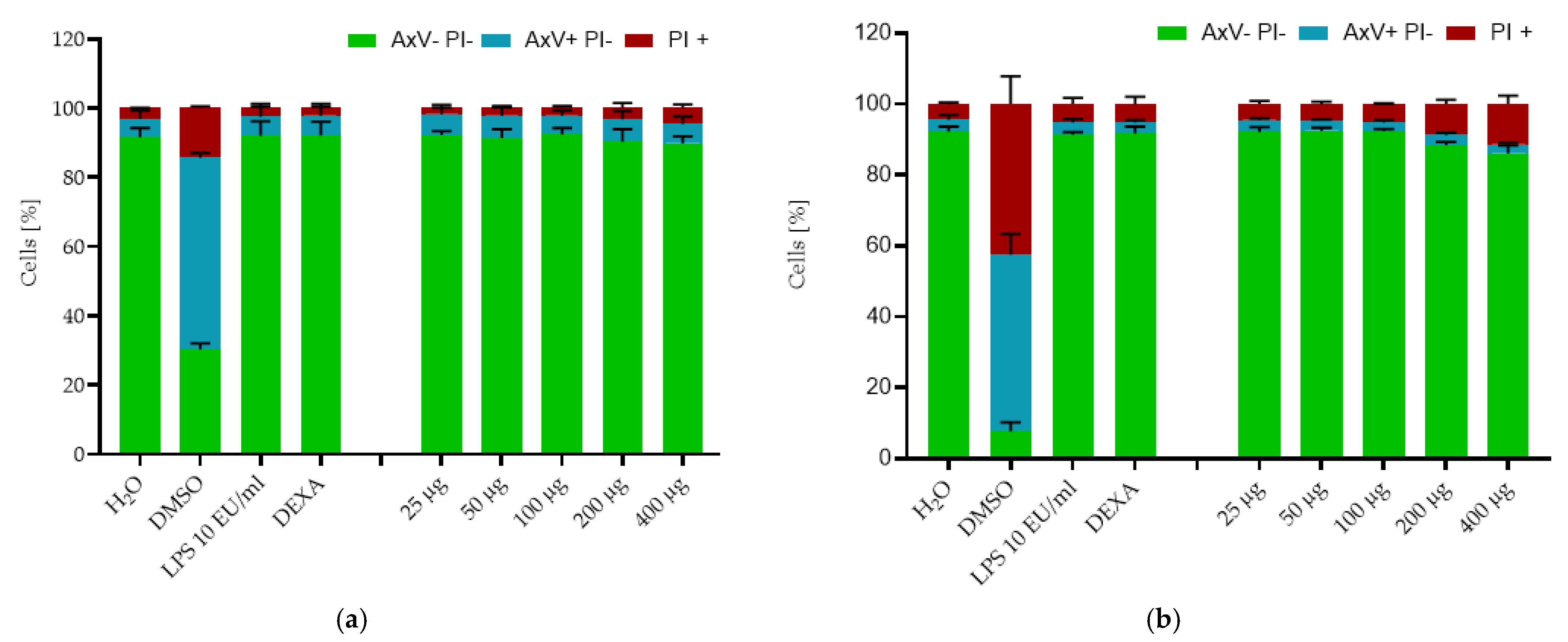
2.2. Cytotoxicity Testing
2.3. Cytokine Release Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Hydroxyapatite-Coated Iron Oxide Nanoparticles (SPIONHAp)
3.3. Iron Quantification
3.4. Hydrodynamic Particle Size and Zeta Potential
3.5. SEM Imaging
3.6. Energy-Dispersive X-ray Analysis (EDX)
3.7. Magnetic Susceptibility
3.8. X-ray Diffraction Analysis
3.9. Vibrating Sample Magnetometry (VSM)
3.10. TEM Imaging and Selected Area Diffraction
3.11. FTIR Measurements
3.12. Test for Endotoxin Contamination
3.13. Toxicological Tests
3.14. Macrophage Culture and Cytokine Analysis
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Kadouche, S.; Zemmouri, H.; Benaoumeur, K.; Drouiche, N.; Sharrock, P.; Lounici, H. Metal Ion Binding on Hydroxyapatite (Hap) and Study of the Velocity of Sedimentation. Procedia Eng. 2012, 33, 377–384. [Google Scholar] [CrossRef]
- Yang, H.; Masse, S.; Zhang, H.; Helary, C.; Li, L.; Coradin, T. Surface reactivity of hydroxyapatite nanocoatings deposited on iron oxide magnetic spheres toward toxic metals. J. Colloid Interface Sci. 2014, 417, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.V.; Radtke, I.; Heumann, R.; Epple, M. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials 2006, 27, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xi, P.; Xie, G.; Chen, F.; Li, Z.; Bai, D.; Zeng, Z. Biocompatible hydroxyapatite nanoparticles as a redox luminescence switch. J. Biol. Inorg. Chem. 2011, 16, 1135–1140. [Google Scholar] [CrossRef]
- Syamchand, S.S.; Sony, G. Multifunctional hydroxyapatite nanoparticles for drug delivery and multimodal molecular imaging. Microchim. Acta 2015, 182, 1567–1589. [Google Scholar] [CrossRef]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Nguyen, V.T.; Kim, H.H.; Nam, S.Y.; Lee, K.D.; Oh, J. Hydroxyapatite Coated Iron Oxide Nanoparticles: A Promising Nanomaterial for Magnetic Hyperthermia Cancer Treatment. Nanomaterials 2017, 7, 426. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, W.; Wen, X.; He, B.; Zeng, X.; Wang, G.; Gu, Z. A novel calcium phosphate ceramic-magnetic nanoparticle composite as a potential bone substitute. Biomed. Mater. 2010, 5, 15001. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Wang, T.-W.; Sun, J.-S.; Wang, W.-H.; Lin, F.-H. A novel biomagnetic nanoparticle based on hydroxyapatite. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Cicha, I.; Alexiou, C. Cardiovascular applications of magnetic particles. J. Magn. Magn. Mater. 2021, 518. [Google Scholar] [CrossRef]
- Bell, C.S.; Mejias, R.; Miller, S.E.; Greer, J.M.; McClain, M.S.; Cover, T.L.; Giorgio, T.D. Magnetic Extraction of Acinetobacter baumannii Using Colistin-Functionalized gamma-Fe2O3/Au Core/Shell Composite Nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 26719–26730. [Google Scholar] [CrossRef]
- Silva, L.H.; Cruz, F.F.; Morales, M.M.; Weiss, D.J.; Rocco, P.R. Magnetic targeting as a strategy to enhance therapeutic effects of mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 58. [Google Scholar] [CrossRef]
- Khalil, M.I. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron(III) salts as precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [CrossRef]
- Ansar, E.B.; Ajeesh, M.; Yokogawa, Y.; Wunderlich, W.; Varma, H.; Bandyopadhyay, A. Synthesis and Characterization of Iron Oxide Embedded Hydroxyapatite Bioceramics. J. Am. Ceram. Soc. 2012, 95, 2695–2699. [Google Scholar] [CrossRef]
- Pierri, E.; Tsamouras, D.; Dalas, E. Ferric phosphate precipitation in aqueous media. J. Cryst. Growth 2000, 213, 93–98. [Google Scholar] [CrossRef]
- Muhlberger, M.; Janko, C.; Unterweger, H.; Friedrich, R.P.; Friedrich, B.; Band, J.; Cebulla, N.; Alexiou, C.; Dudziak, D.; Lee, G.; et al. Functionalization of T Lymphocytes with Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles for Magnetically Controlled Immune Therapy. Int. J. Nanomed. 2019, 14, 8421–8432. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Friedrich, B.; Muhlberger, M.; Cebulla, N.; Schreiber, E.; Tietze, R.; Cicha, I.; Alexiou, C.; Dutz, S.; Boccaccini, A.R.; et al. Synthesis and Characterization of Citrate-Stabilized Gold-Coated Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2020, 25, 4425. [Google Scholar] [CrossRef] [PubMed]
- Mühlberger, M.; Janko, C.; Unterweger, H.; Schreiber, E.; Band, J.; Lehmann, C.; Dudziak, D.; Lee, G.; Alexiou, C.; Tietze, R. Functionalization of T lymphocytes for magnetically controlled immune therapy: Selection of suitable superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2019, 473, 61–67. [Google Scholar] [CrossRef]
- Yewle, J.N.; Wei, Y.; Puleo, D.A.; Daunert, S.; Bachas, L.G. Oriented immobilization of proteins on hydroxyapatite surface using bifunctional bisphosphonates as linkers. Biomacromolecules 2012, 13, 1742–1749. [Google Scholar] [CrossRef]
- Yewle, J.N.; Puleo, D.A.; Bachas, L.G. Enhanced affinity bifunctional bisphosphonates for targeted delivery of therapeutic agents to bone. Bioconjug Chem. 2011, 22, 2496–2506. [Google Scholar] [CrossRef][Green Version]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Butoescu, N.; Jordan, O.; Burdet, P.; Stadelmann, P.; Petri-Fink, A.; Hofmann, H.; Doelker, E. Dexamethasone-containing biodegradable superparamagnetic microparticles for intra-articular administration: Physicochemical and magnetic properties, in vitro and in vivo drug release. Eur. J. Pharm. Biopharm. 2009, 72, 529–538. [Google Scholar] [CrossRef]
- Grosse, S.; Stenvik, J.; Nilsen, A.M. Iron oxide nanoparticles modulate lipopolysaccharide-induced inflammatory responses in primary human monocytes. Int. J. Nanomed. 2016, 11, 4625–4642. [Google Scholar] [CrossRef] [PubMed]
- Kutikhin, A.G.; Velikanova, E.A.; Mukhamadiyarov, R.A.; Glushkova, T.V.; Borisov, V.V.; Matveeva, V.G.; Antonova, L.V.; Filip’ev, D.E.; Golovkin, A.S.; Shishkova, D.K.; et al. Apoptosis-mediated endothelial toxicity but not direct calcification or functional changes in anti-calcification proteins defines pathogenic effects of calcium phosphate bions. Sci. Rep. 2016, 6, 27255. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Z.; Radauer-Preiml, I.; Andosch, A.; Casals, E.; Luetz-Meindl, U.; Cobaleda, M.; Lin, Z.; Jaberi-Douraki, M.; Italiani, P.; et al. Bacterial endotoxin (lipopolysaccharide) binds to the surface of gold nanoparticles, interferes with biocorona formation and induces human monocyte inflammatory activation. Nanotoxicology 2017, 11, 1157–1175. [Google Scholar] [CrossRef]
- Vasanthan, T.; Rochow, N.; Mian, F.; Codini, T.; DeFrance, B.; Fusch, G.; Samiee-Zafarghandy, S.; Fusch, C. LPS from bovine serum albumin drives TNF-alpha release during ex-vivo placenta perfusion experiments, contaminates the perfusion system but can be effectively removed by oxidative cleaning. Placenta 2014, 35, 1095–1098. [Google Scholar] [CrossRef]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis 1999, 180, 1584–1589. [Google Scholar] [CrossRef]
- Domingos Dias Cicarelli, J.E.V. Fábio Ely Martins Benseñor Early dexamethasone treatment for septic shock patients: A prospective randomized clinical trial. Sao Paulo Med. J. 2007. [Google Scholar] [CrossRef]
- Giles, A.J.; Hutchinson, M.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Vishwas, S.; Corrie, L.; Kumar, R.; Khursheed, R.; Kaur, J.; Kumar, R.; Arya, K.R.; Gulati, M.; Kumar, B.; et al. OUTBREAK of novel corona virus disease (COVID-19): Antecedence and aftermath. Eur. J. Pharmacol. 2020, 884, 173381. [Google Scholar] [CrossRef]
- Xiang, W.; Xue, H.; Wang, B.; Li, Y.; Zhang, J.; Jiang, C.; Liang, F.; Pang, J.; Yu, L. Combined application of dexamethasone and hyperbaric oxygen therapy yields better efficacy for patients with delayed encephalopathy after acute carbon monoxide poisoning. Drug Des. Dev. Ther. 2017, 11, 513–519. [Google Scholar] [CrossRef]
- Cornell, R.M.A.U.S. The Iron Oxide; Wiley-VCH Verlag: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Hofmeister, H.; Huisken, F.; Kohn, B.; Alexandrescu, R.; Cojocaru, S.; Crunteanu, A.; Morjan, I.; Diamandescu, L. Filamentary iron nanostructures from laser-induced pyrolysis of iron pentacarbonyl and ethylene mixtures. Appl. Phys. A Mater. Sci. Process. 2001, 72, 7–11. [Google Scholar] [CrossRef]
- Lari, L.; Steinhauer, S.; Lazarov, V.K. In situ TEM oxidation study of Fe thin-film transformation to single-crystal magnetite nanoparticles. J. Mater. Sci. 2020, 55, 12897–12905. [Google Scholar] [CrossRef]
- Swanson, H.E. Standard X-ray Diffraction Powder Patterns: Section 5; US Department of Commerce, National Bureau of Standards: Gaithersburg, MA, USA, 1967; Volume 25.
- Yang, Z.-P.; Gong, X.-Y.; Zhang, C.-J. Recyclable Fe3O4/hydroxyapatite composite nanoparticles for photocatalytic applications. Chem. Eng. J. 2010, 165, 117–121. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Affolter, C.; Pretsch, E.; Bhuhlmann, P.; Affolter, C. Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Rao, K.M.; Swamy, C.K. Infrared spectra of potassium citrate monohydrate single crystals. Indian J. Phys. 1974, 48, 225–235. [Google Scholar]
- Li, Y.S.; Church, J.S.; Woodhead, A.L. Woodhead. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- Laskin, D.L. Macrophages and inflammatory mediators in chemical toxicity: A battle of forces. Chem. Res. Toxicol. 2009, 22, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Duerr, S.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. Riccardi A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 271–279. [Google Scholar] [CrossRef]
- Munoz, L.E.; Maueroder, C.; Chaurio, R.; Berens, C.; Herrmann, M.; Janko, C. Colourful death: Six-parameter classification of cell death by flow cytometry--dead cells tell tales. Autoimmunity 2013, 46, 336–341. [Google Scholar] [CrossRef]
- Muhlberger, M.; Unterweger, H.; Band, J.; Lehmann, C.; Heger, L.; Dudziak, D.; Alexiou, C.; Lee, G.; Janko, C. Loading of Primary Human T Lymphocytes with Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles Does Not Impair Their Activation after Polyclonal Stimulation. Cells 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Hochdorfer, T.; Tiedje, C.; Stumpo, D.J.; Blackshear, P.J.; Gaestel, M.; Huber, M. LPS-induced production of TNF-alpha and IL-6 in mast cells is dependent on p38 but independent of TTP. Cell Signal. 2013, 25, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Jan Bertil Eggesbø, I.H. Arne Torbjørn Høstmark and Peter Kierulf. LPS induced release of IL-1β, IL-6, IL-8 and TNF-α in EDTA or heparin anticoagulated whole blood from persons with high or low levels of serum HDL. Cytokine 1996, 8, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liew, L.N.; Kuo, I.C.; Huang, C.H.; Goh, D.L.; Chua, K.Y. The modulatory effects of lipopolysaccharide-stimulated B cells on differential T-cell polarization. Immunology 2008, 125, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, S.; Hassannia, B.; Delrue, I.; Goossens, V.; Wiernicki, B.; Dondelinger, Y.; Bertrand, M.J.; Krysko, D.V.; Vuylsteke, M.; Vandenabeele, P.; et al. A real-time fluorometric method for the simultaneous detection of cell death type and rate. Nat. Protoc. 2016, 11, 1444–1454. [Google Scholar] [CrossRef] [PubMed]

| SPIONCit | SPIONHAp | |
|---|---|---|
| Z-average in nm | 109 ± 1 | 190.7 ± 11.5 |
| polydispersity index | 0.189 ± 0.01 | 0.114 ± 0.02 |
| ζ in mV | −46.9 ± 0.9 | −28.1 ± 1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, B.; Auger, J.-P.; Dutz, S.; Cicha, I.; Schreiber, E.; Band, J.; Boccacccini, A.R.; Krönke, G.; Alexiou, C.; Tietze, R. Hydroxyapatite-Coated SPIONs and Their Influence on Cytokine Release. Int. J. Mol. Sci. 2021, 22, 4143. https://doi.org/10.3390/ijms22084143
Friedrich B, Auger J-P, Dutz S, Cicha I, Schreiber E, Band J, Boccacccini AR, Krönke G, Alexiou C, Tietze R. Hydroxyapatite-Coated SPIONs and Their Influence on Cytokine Release. International Journal of Molecular Sciences. 2021; 22(8):4143. https://doi.org/10.3390/ijms22084143
Chicago/Turabian StyleFriedrich, Bernhard, Jean-Philippe Auger, Silvio Dutz, Iwona Cicha, Eveline Schreiber, Julia Band, Aldo R. Boccacccini, Gerhard Krönke, Christoph Alexiou, and Rainer Tietze. 2021. "Hydroxyapatite-Coated SPIONs and Their Influence on Cytokine Release" International Journal of Molecular Sciences 22, no. 8: 4143. https://doi.org/10.3390/ijms22084143
APA StyleFriedrich, B., Auger, J.-P., Dutz, S., Cicha, I., Schreiber, E., Band, J., Boccacccini, A. R., Krönke, G., Alexiou, C., & Tietze, R. (2021). Hydroxyapatite-Coated SPIONs and Their Influence on Cytokine Release. International Journal of Molecular Sciences, 22(8), 4143. https://doi.org/10.3390/ijms22084143









