The Characterization of a Subependymal Giant Astrocytoma-Like Cell Line from Murine Astrocyte with mTORC1 Hyperactivation
Abstract
1. Introduction
2. Results
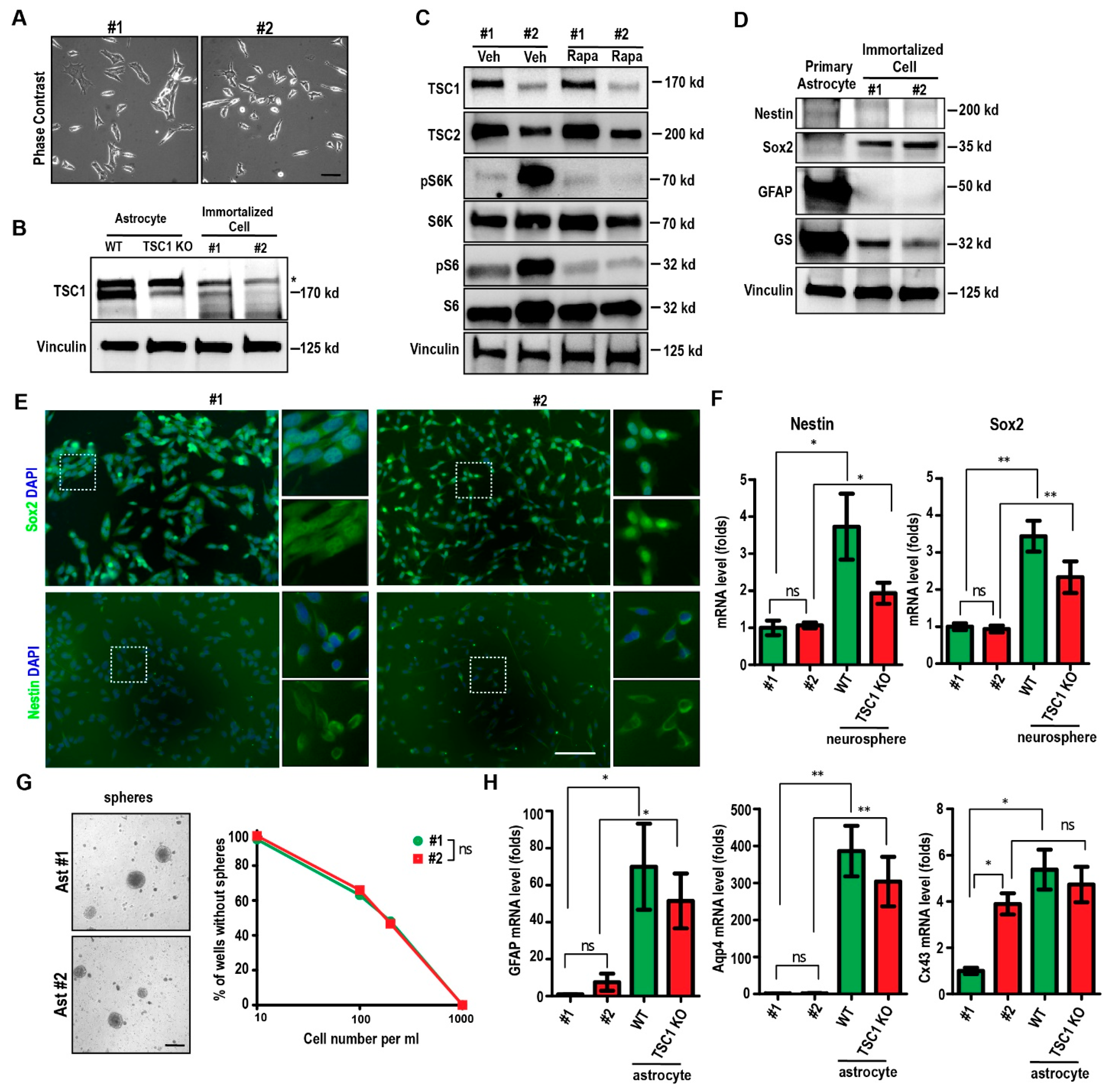
2.1. Spontaneous Immortalization of Isolated Astrocytes from Tsc1GFAP cKO Mouse
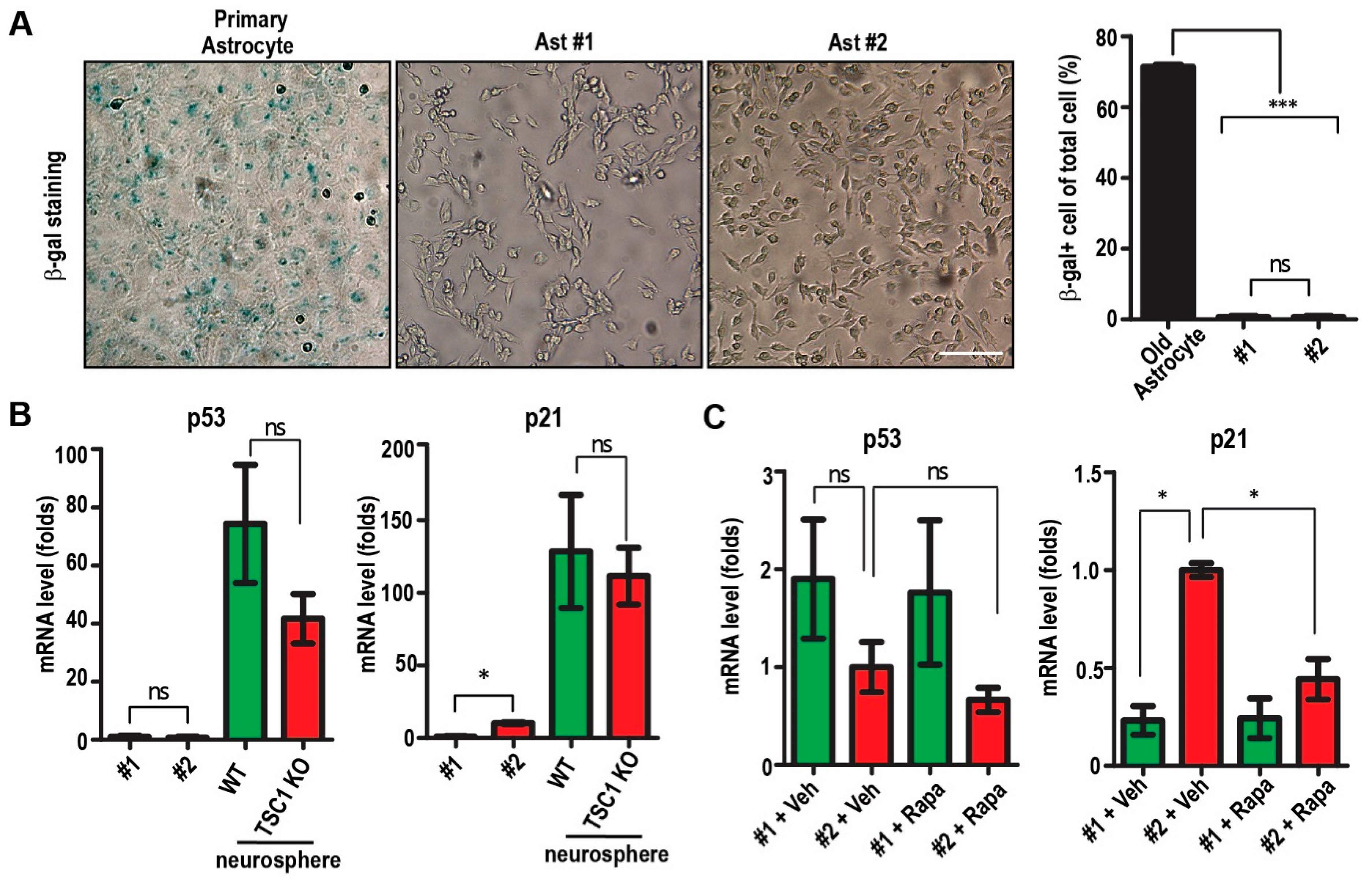
2.2. Passaging of Immortalized TSC1-Deficient Astrocytes
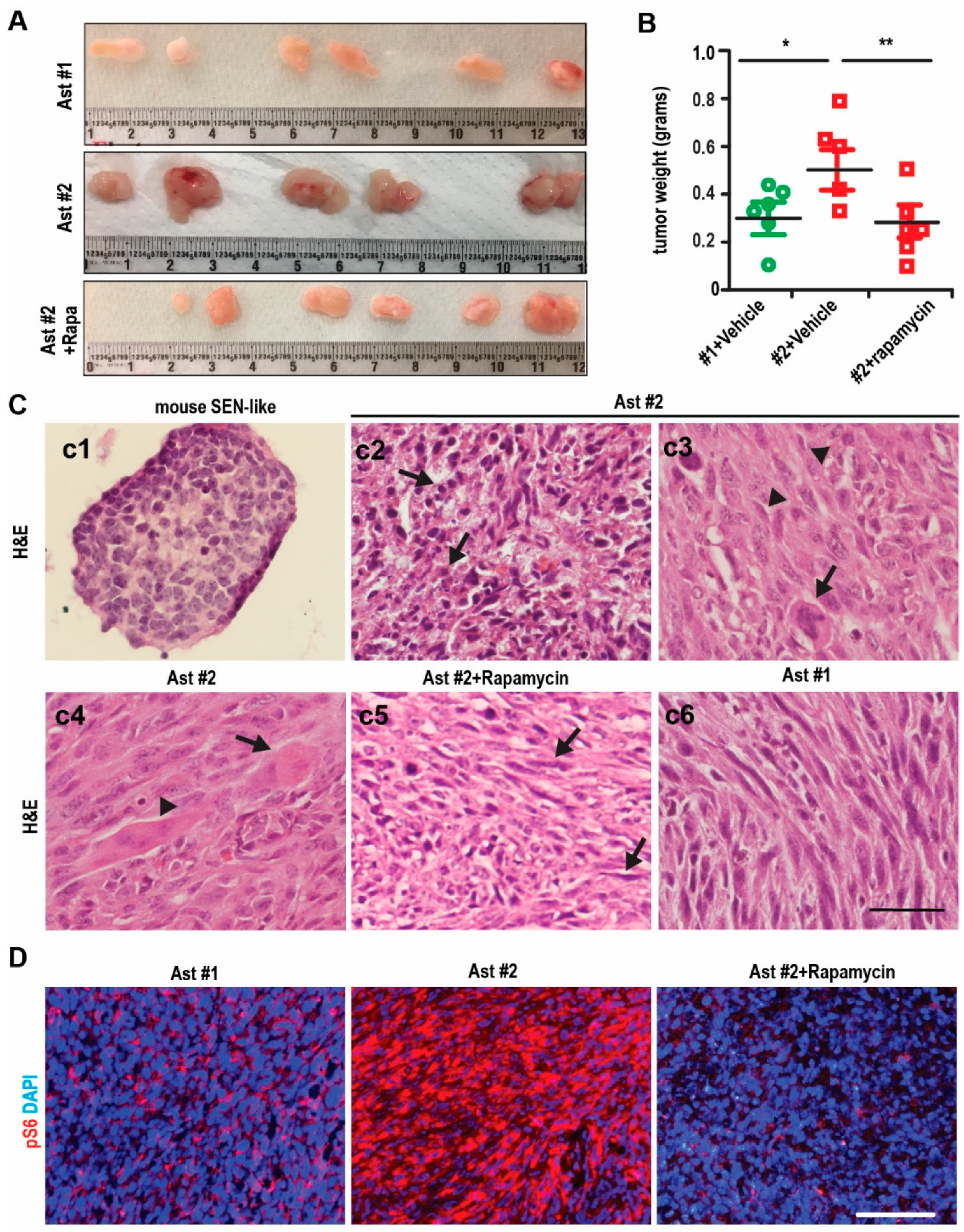
2.3. Tumorigenesis of Immortalized TSC1-Deficient Astrocyte to form SEGA-Like Tumors
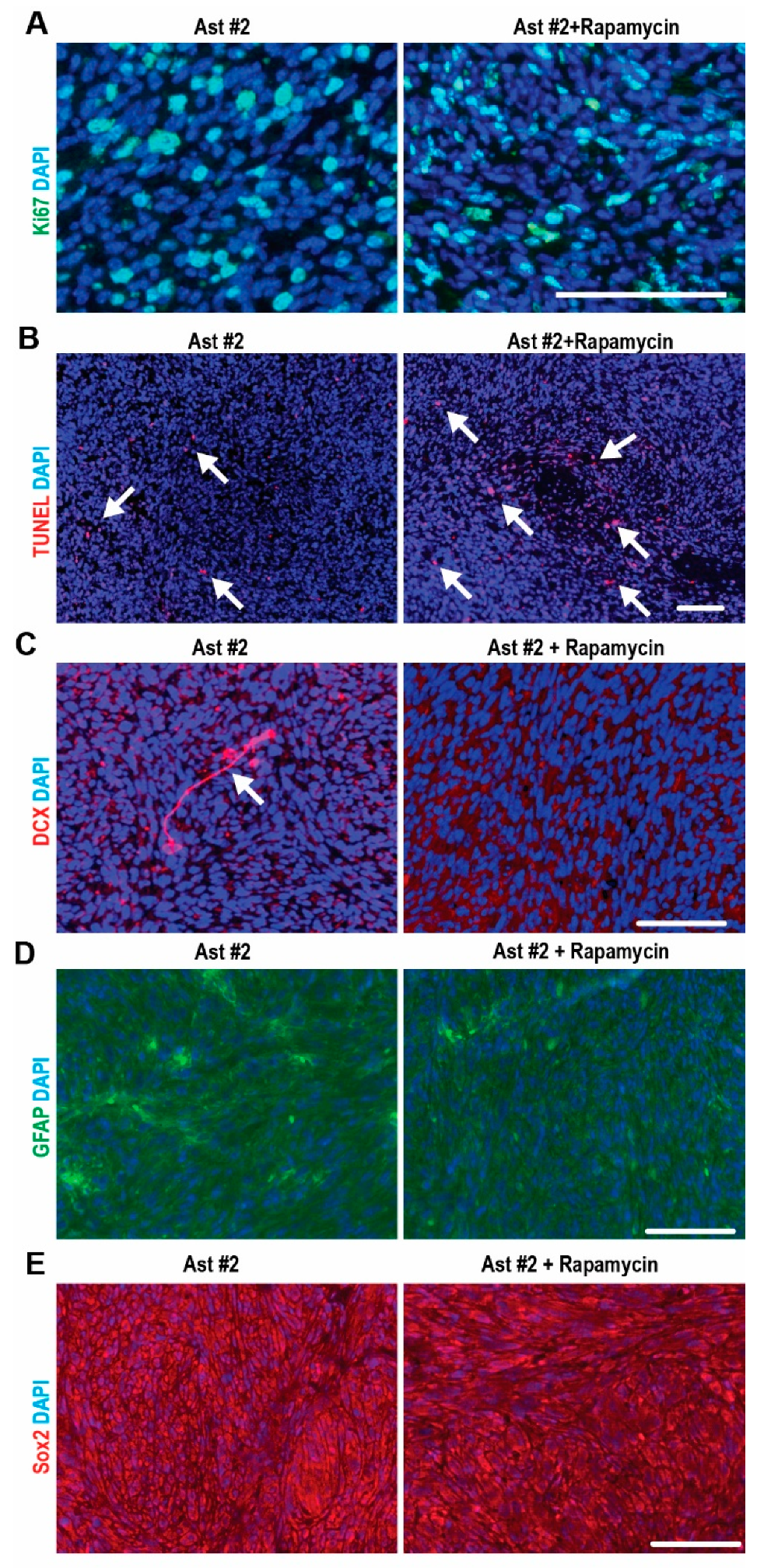
2.4. mTORC1-Dependent Survival and Differentiation of SEGA-Like Ast #2 Tumors
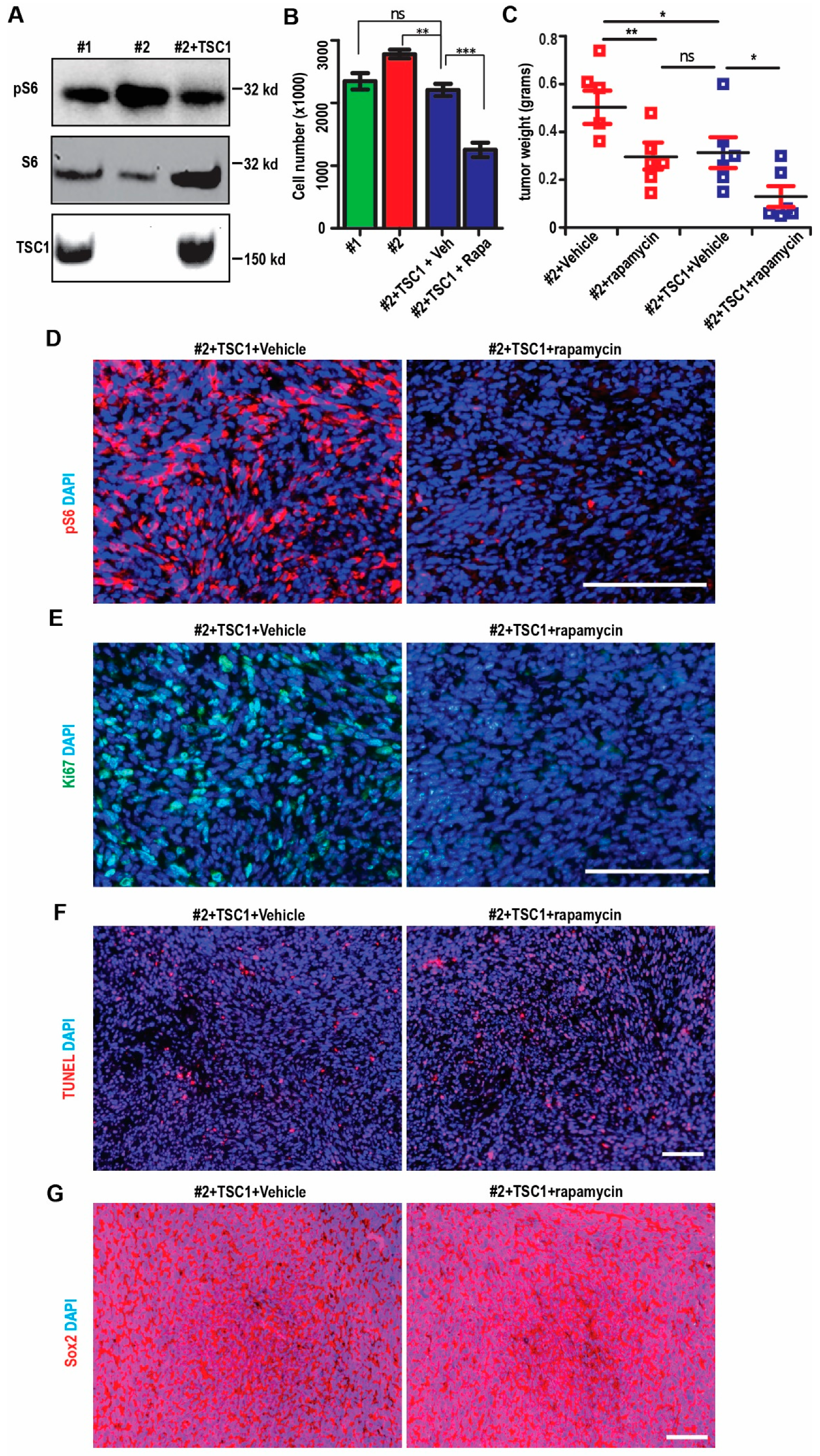
2.5. Restoration of mTORC1 Activation in SEGA-Like Tumors by Replenish Human TSC1
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Cultures
4.3. Virus Production and Infection
4.4. Cell Proliferation Assay
4.5. Subcutaneous Xenograft Models
4.6. Immunofluorescence
4.7. Histology and Immunostaining
4.8. Protein Extraction, SDS-PAGE and Western Blotting
4.9. Real-Time PCR
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, A.K.; Robson, W.L.M. Tuberous Sclerosis Complex: A Review. J. Pediatr. Health Care 2007, 21, 108–114. [Google Scholar] [CrossRef]
- Chan, J.A.; Zhang, H.; Roberts, P.S.; Jozwiak, S.; Wieslawa, G.; Lewin-Kowalik, J.; Kotulska, K.; Kwiatkowski, D.J. Pathogenesis of Tuberous Sclerosis Subependymal Giant Cell Astrocytomas: Biallelic Inactivation ofTSC1orTSC2Leads to mTOR Activation. J. Neuropathol. Exp. Neurol. 2004, 63, 1236–1242. [Google Scholar] [CrossRef]
- Campen, C.J.; Porter, B.E. Subependymal Giant Cell Astrocytoma (SEGA) Treatment Update. Curr. Treat. Options Neurol. 2011, 13, 380–385. [Google Scholar] [CrossRef]
- Zhou, J.; Shrikhande, G.; Xu, J.; McKay, R.M.; Burns, D.K.; Johnson, J.E.; Parada, L.F. Tsc1 mutant neural stem/progenitor cells exhibit migration deficits and give rise to subependymal lesions in the lateral ventricle. Genes Dev. 2011, 25, 1595–1600. [Google Scholar] [CrossRef]
- Wang, C.; Haas, M.A.; Yang, F.; Yeo, S.; Okamoto, T.; Chen, S.; Wen, J.; Sarma, P.; Plas, D.R.; Guan, J.-L. Autophagic lipid metabolism sustains mTORC1 activity in TSC-deficient neural stem cells. Nat. Metab. 2019, 1, 1127–1140. [Google Scholar] [CrossRef]
- Zordan, P.; Cominelli, M.; Cascino, F.; Tratta, E.; Poliani, P.L.; Galli, R. Tuberous sclerosis complex–associated CNS abnormalities depend on hyperactivation of mTORC1 and Akt. J. Clin. Investig. 2018, 128, 1688–1706. [Google Scholar] [CrossRef]
- Blair, J.D.; Hockemeyer, D.; Bateup, H.S. Genetically engineered human cortical spheroid models of tuberous sclerosis. Nat. Med. 2018, 24, 1568–1578. [Google Scholar] [CrossRef]
- Pintacuda, G.; Martín, J.M.; Eggan, K.C. Mind the translational gap: Using iPS cell models to bridge from genetic discoveries to perturbed pathways and therapeutic targets. Mol. Autism 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Kotulska, K.; Borkowska, J.; Roszkowski, M.; Mandera, M.; Daszkiewicz, P.; Drabik, K.; Jurkiewicz, E.; Larysz-Brysz, M.; Nowak, K.; Grajkowska, W.; et al. Surgical Treatment of Subependymal Giant Cell Astrocytoma in Tuberous Sclerosis Complex Patients. Pediatr. Neurol. 2014, 50, 307–312. [Google Scholar] [CrossRef]
- Franz, D.N.; Leonard, J.; Tudor, C.; Chuck, G.; Care, M.; Sethuraman, G.; Dinopoulos, A.; Thomas, G.; Crone, K.R. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann. Neurol. 2006, 59, 490–498. [Google Scholar] [CrossRef]
- Krueger, D.A.; Care, M.M.; Holland, K.; Agricola, K.; Tudor, C.; Mangeshkar, P.; Wilson, K.A.; Byars, A.; Sahmoud, T.; Franz, D.N. Everolimus for Subependymal Giant-Cell Astrocytomas in Tuberous Sclerosis. N. Engl. J. Med. 2010, 363, 1801–1811. [Google Scholar] [CrossRef]
- Tomoto, K.; Fujimoto, A.; Inenaga, C.; Okanishi, T.; Imai, S.; Ogai, M.; Fukunaga, A.; Nakamura, H.; Sato, K.; Obana, A.; et al. Experience using mTOR inhibitors for subependymal giant cell astrocytoma in tuberous sclerosis complex at a single facility. BMC Neurol. 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013, 381, 125–132. [Google Scholar] [CrossRef]
- Hwang, S.-K.; Lee, J.-H.; Yang, J.-E.; Lim, C.-S.; Lee, J.-A.; Lee, Y.-S.; Lee, K.; Kaang, B.-K. Everolimus improves neuropsychiatric symptoms in a patient with tuberous sclerosis carrying a novel TSC2 mutation. Mol. Brain 2016, 9, 56. [Google Scholar] [CrossRef]
- Overwater, I.E.; Rietman, A.B.; Van Eeghen, A.M.; De Wit, M.C.Y. Everolimus for the treatment of refractory seizures associated with tuberous sclerosis complex (TSC): Current perspectives. Ther. Clin. Risk Manag. 2019, 15, 951–955. [Google Scholar] [CrossRef]
- Rufini, A.; Tucci, P.J.F.; Celardo, I.; Melino, G. Senescence and aging: The critical roles of p53. Oncogene 2013, 32, 5129–5143. [Google Scholar] [CrossRef] [PubMed]
- Aramburu, J.; Ortells, M.C.; Tejedor, S.; Buxadé, M.; López-Rodríguez, C. Transcriptional regulation of the stress response by mTOR. Sci. Signal. 2014, 7, re2. [Google Scholar] [CrossRef] [PubMed]
- Scheidenhelm, D.K.; Gutmann, D.H. Mouse models of tuberous sclerosis complex. J. Child Neurol. 2004, 19, 726–733. [Google Scholar] [CrossRef]
- Costa, V.; Aigner, S.; Vukcevic, M.; Sauter, E.; Behr, K.; Ebeling, M.; Dunkley, T.; Friedlein, A.; Zoffmann, S.; Meyer, C.A.; et al. mTORC1 Inhibition Corrects Neurodevelopmental and Synaptic Alterations in a Human Stem Cell Model of Tuberous Sclerosis. Cell Rep. 2016, 15, 86–95. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J.; Chen, M.; Li, J.; Sun, Y.; Zhang, Y.; Zhu, Y.; Wang, L.; Zhang, C. Abnormal Neural Progenitor Cells Differentiated from Induced Pluripotent Stem Cells Partially Mimicked Development of TSC2 Neurological Abnormalities. Stem Cell Rep. 2017, 8, 883–893. [Google Scholar] [CrossRef]
- Kwiatkowski, D.J.; Manning, B.D. Molecular Basis of Giant Cells in Tuberous Sclerosis Complex. N. Engl. J. Med. 2014, 371, 778–780. [Google Scholar] [CrossRef]
- Lopes, M.B.S.; Altermatt, H.J.; Scheithauer, B.W.; Shepherd, C.W.; Vandenberg, S.R. Immunohistochemical characterization of subependymal giant cell astrocytomas. Acta Neuropathol. 1996, 91, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Magri, L.; Cambiaghi, M.; Cominelli, M.; Alfaro-Cervello, C.; Cursi, M.; Pala, M.; Bulfone, A.; Garcìa-Verdugo, J.M.; Leocani, L.; Minicucci, F.; et al. Sustained Activation of mTOR Pathway in Embryonic Neural Stem Cells Leads to Development of Tuberous Sclerosis Complex-Associated Lesions. Cell Stem Cell 2011, 9, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Price, T.N.C.; Burke, J.F.; Mayne, L.V. A novel human astrocyte cell line (A735) with astrocyte-specific neurotransmitter function. In Vitro Cell. Dev. Biol. Anim. 1999, 35, 279–288. [Google Scholar] [CrossRef]
- Rusnakova, V.; Honsa, P.; Dzamba, D.; Ståhlberg, A.; Kubista, M.; Anderova, M. Heterogeneity of Astrocytes: From Development to Injury—Single Cell Gene Expression. PLoS ONE 2013, 8, e69734. [Google Scholar] [CrossRef]
- Nakamura, Y.; Becker, L.E. Subependymal giant-cell tumor: Astrocytic or neuronal? Acta Neuropathol. 1983, 60, 271–277. [Google Scholar] [CrossRef]
- Dębiec-Rychter, M.; Jesionek-Kupnicka, R.; Zakrzewski, K.; Liberski, P.P. Cytogenetic changes in two cases of subependymal giant-cell astrocytoma. Cancer Genet. Cytogenet. 1999, 109, 29–33. [Google Scholar] [CrossRef]
- Lee, A.; Maldonado, M.; Baybis, M.; Walsh, C.A.; Scheithauer, B.; Yeung, R.; Parent, J.; Weiner, H.L.; Crino, P.B. Markers of cellular proliferation are expressed in cortical tubers. Ann. Neurol. 2003, 53, 668–673. [Google Scholar] [CrossRef]
- Grajkowska, W.; Kotulska, K.; Jurkiewicz, E.; Roszkowski, M.; Daszkiewicz, P.; Jóźwiak, S.; Matyja, E. Subependymal giant cell astrocytomas with atypical histological features mimicking malignant gliomas. Folia Neuropathol. 2011, 49, 39–46. [Google Scholar]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Cheah, P.-S.; Prabhakar, S.; Yellen, D.; Beauchamp, R.L.; Zhang, X.; Kasamatsu, S.; Bronson, R.T.; Thiele, E.A.; Kwiatkowski, D.J.; Stemmer-Rachamimov, A.; et al. Gene therapy for tuberous sclerosis complex type 2 in a mouse model by delivery of AAV9 encoding a condensed form of tuberin. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Gao, K.; Wang, C.R.; Jiang, F.; Wong, A.Y.K.; Su, N.; Jiang, J.H.; Chai, R.C.; Vatcher, G.; Teng, J.; Chen, J.; et al. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia 2013, 61, 2063–2077. [Google Scholar] [CrossRef]
- Wang, C.; Yeo, S.; Haas, M.A.; Guan, J.-L. Autophagy gene FIP200 in neural progenitors non–cell autonomously controls differentiation by regulating microglia. J. Cell Biol. 2017, 216, 2581–2596. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, C.-C.; Bian, Z.C.; Zhu, Y.; Guan, J.-L. FIP200 is required for maintenance and differentiation of postnatal neural stem cells. Nat. Neurosci. 2013, 16, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Yeo, S.; Karsli-Uzunbas, G.; White, E.; Mizushima, N.; Virgin, H.W.; Guan, J.L. Elevated p62/SQSTM1 determines the fate of autophagy-deficient neural stem cells by increasing superoxide. J. Cell Biol. 2016, 212, 545–560. [Google Scholar] [CrossRef]
- Wang, C.; Yoo, Y.; Fan, H.; Kim, E.; Guan, K.-L.; Guan, J.-L. Regulation of Integrin beta 1 recycling to lipid rafts by Rab1a to promote cell migration. J. Biol. Chem. 2010, 285, 29398–29405. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Angst, G.; Haas, M.; Yang, F.; Wang, C. The Characterization of a Subependymal Giant Astrocytoma-Like Cell Line from Murine Astrocyte with mTORC1 Hyperactivation. Int. J. Mol. Sci. 2021, 22, 4116. https://doi.org/10.3390/ijms22084116
Tang X, Angst G, Haas M, Yang F, Wang C. The Characterization of a Subependymal Giant Astrocytoma-Like Cell Line from Murine Astrocyte with mTORC1 Hyperactivation. International Journal of Molecular Sciences. 2021; 22(8):4116. https://doi.org/10.3390/ijms22084116
Chicago/Turabian StyleTang, Xin, Gabrielle Angst, Michael Haas, Fuchun Yang, and Chenran Wang. 2021. "The Characterization of a Subependymal Giant Astrocytoma-Like Cell Line from Murine Astrocyte with mTORC1 Hyperactivation" International Journal of Molecular Sciences 22, no. 8: 4116. https://doi.org/10.3390/ijms22084116
APA StyleTang, X., Angst, G., Haas, M., Yang, F., & Wang, C. (2021). The Characterization of a Subependymal Giant Astrocytoma-Like Cell Line from Murine Astrocyte with mTORC1 Hyperactivation. International Journal of Molecular Sciences, 22(8), 4116. https://doi.org/10.3390/ijms22084116





