Natural Killer Cells from Allergic Donors Are Defective in Their Response to CCL18 Chemokine
Abstract
1. Introduction
2. Results
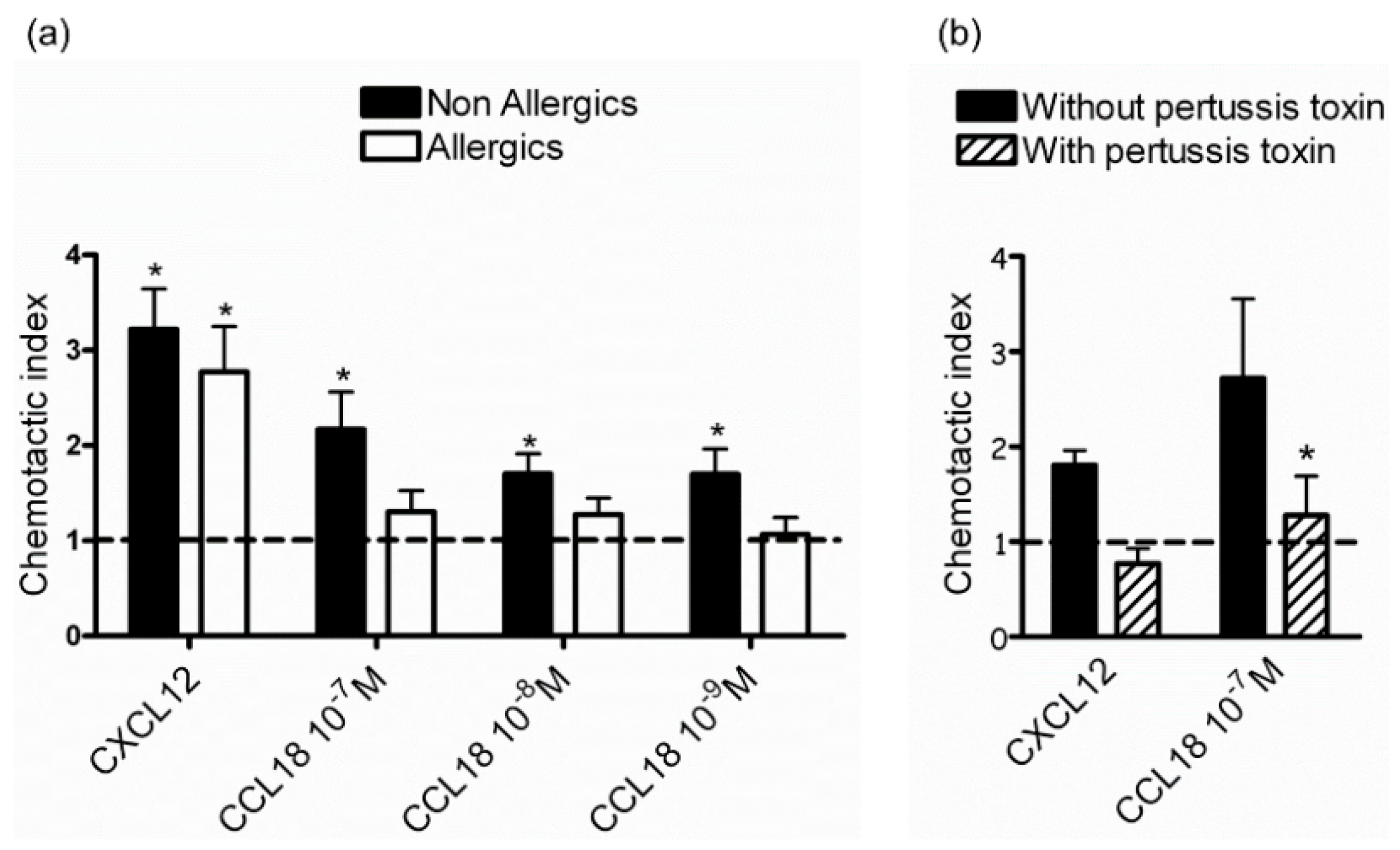
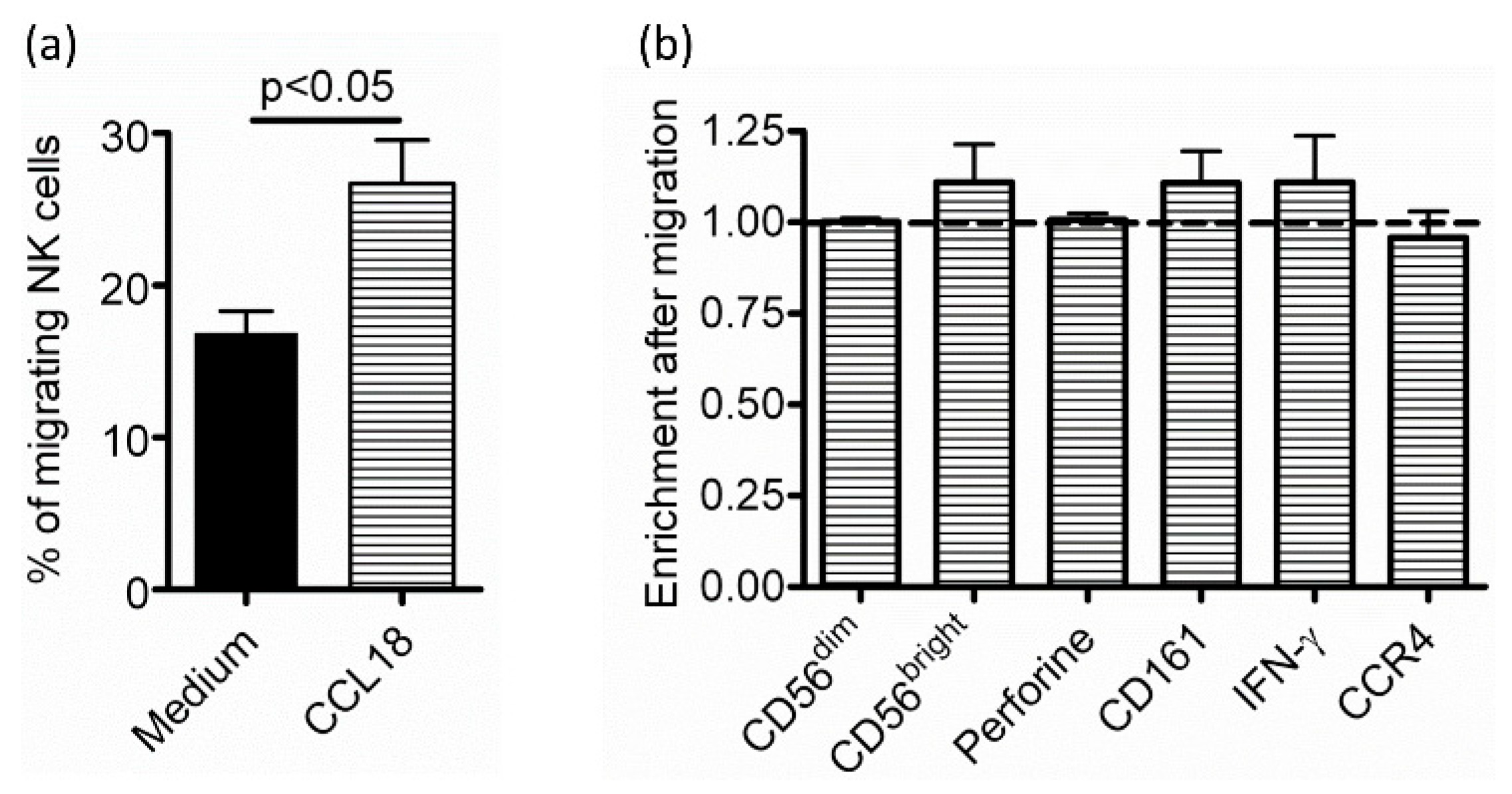
2.1. CCL18 Attracts NK Cells from Nonallergic Donors in a G-Protein-Dependent Manner
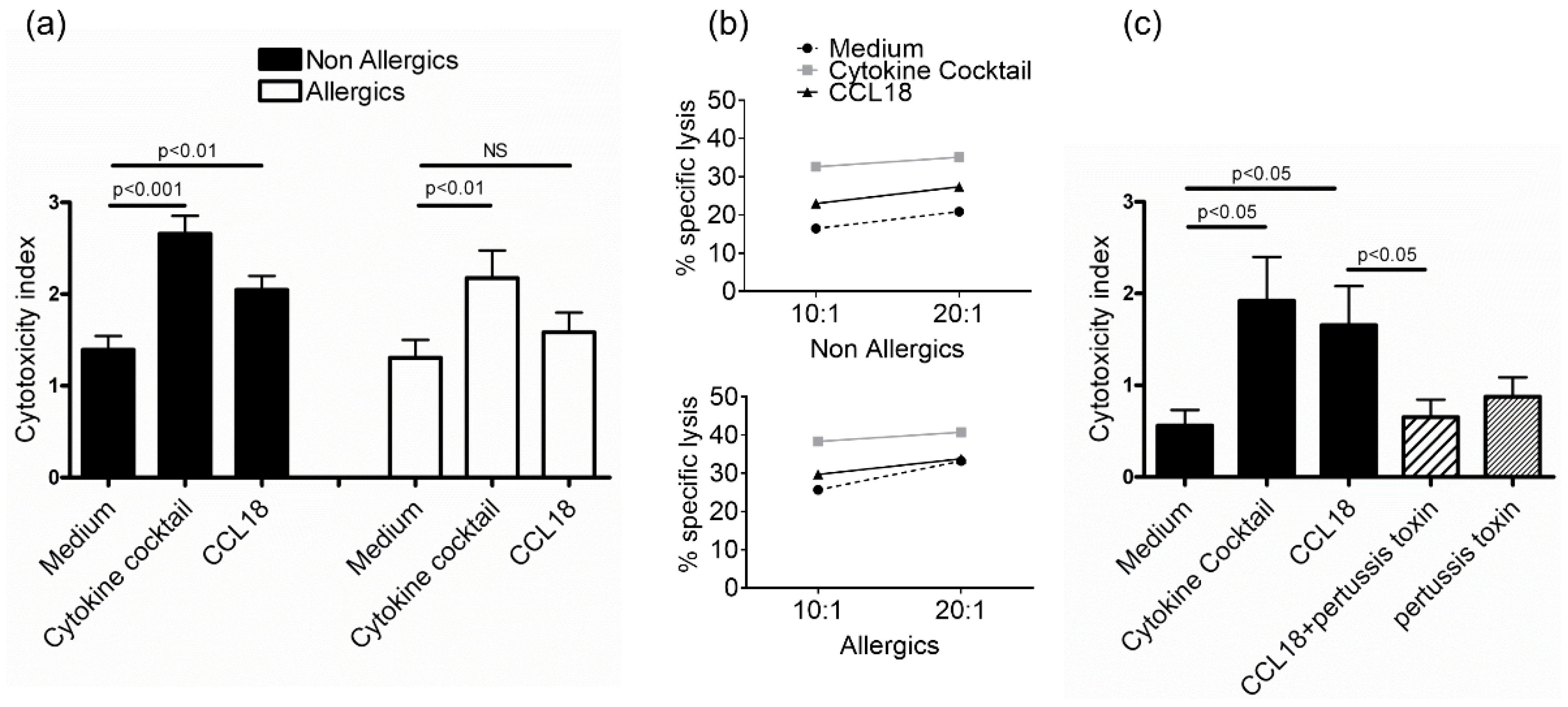
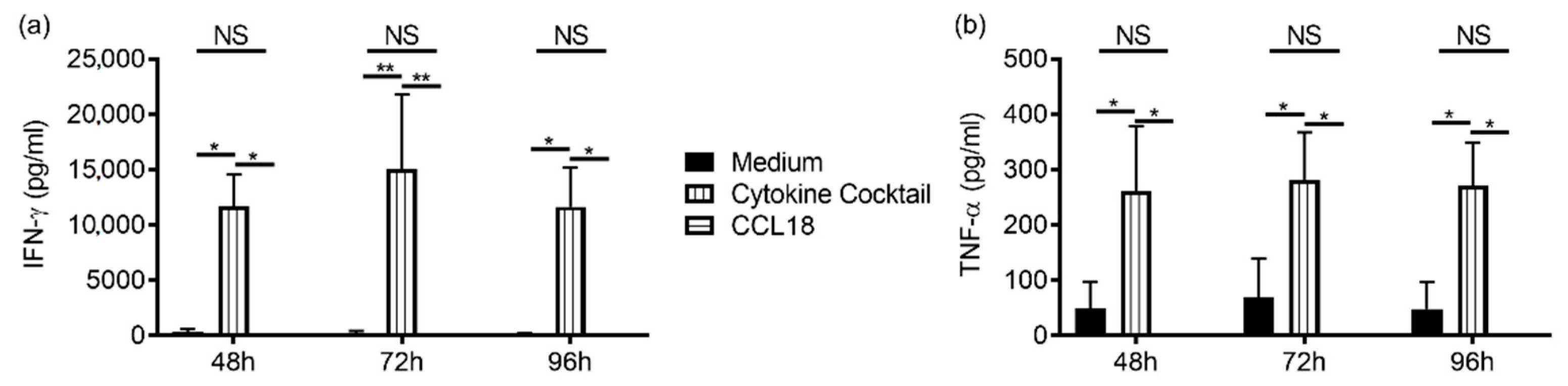
2.2. CCL18 Induces Cytotoxic Function of NK Cells from Nonallergic Donors in a G-Protein-Dependent Manner But Does Not Stimulate Cytokine Production
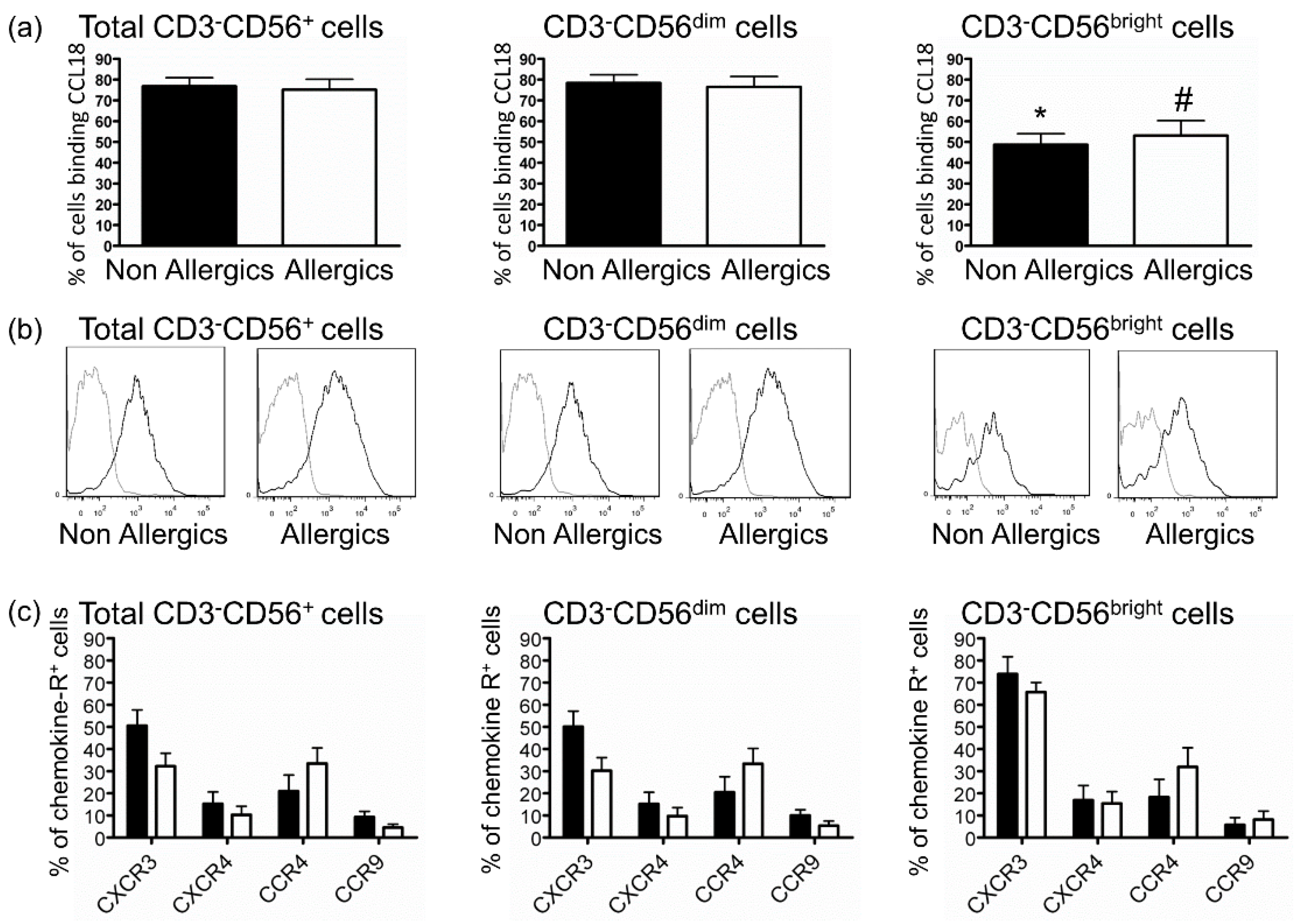
2.3. Defective Response of NK Cells from Allergic Patients to CCL18 Is Not Related to Decreased CCL18-Binding
3. Discussion
4. Materials and Methods
4.1. NK Cell Preparation
4.2. Staining of NK Cells
4.3. NK Cell Migration Assays
4.4. Cytotoxicity Assays
4.5. IFN-γ and TNF-α Production Measurement
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of Natural Killer Cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Moretta, A.; Marcenaro, E.; Sivori, S.; Della Chiesa, M.; Vitale, M.; Moretta, L. Early Liaisons between Cells of the Innate Immune System in Inflamed Peripheral Tissues. Trends Immunol. 2005, 26, 668–675. [Google Scholar] [CrossRef]
- Thorén, F.B.; Riise, R.E.; Ousbäck, J.; Della Chiesa, M.; Alsterholm, M.; Marcenaro, E.; Pesce, S.; Prato, C.; Cantoni, C.; Bylund, J.; et al. Human NK Cells Induce Neutrophil Apoptosis via an NKp46- and Fas-Dependent Mechanism. J. Immunol. 2012, 188, 1668–1674. [Google Scholar] [CrossRef]
- Awad, A.; Yassine, H.; Barrier, M.; Vorng, H.; Marquillies, P.; Tsicopoulos, A.; Duez, C. Natural Killer Cells Induce Eosinophil Activation and Apoptosis. PLoS ONE 2014, 9, e94492. [Google Scholar] [CrossRef]
- Barnig, C.; Cernadas, M.; Dutile, S.; Liu, X.; Perrella, M.A.; Kazani, S.; Wechsler, M.E.; Israel, E.; Levy, B.D. Lipoxin A4 Regulates Natural Killer Cell and Type 2 Innate Lymphoid Cell Activation in Asthma. Sci. Transl. Med. 2013, 5, 174ra26. [Google Scholar] [CrossRef]
- Deniz, G.; van de Veen, W.; Akdis, M. Natural Killer Cells in Patients with Allergic Diseases. J. Allergy Clin. Immunol. 2013, 132, 527–535. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J. Role of Natural Killer Cells in Airway Inflammation. Allergy Asthma Immunol. Res. 2018, 10, 448–456. [Google Scholar] [CrossRef]
- Lin, S.-J.; Chang, L.-Y.; Yan, D.-C.; Huang, Y.-J.; Lin, T.-J.; Lin, T.-Y. Decreased Intercellular Adhesion Molecule-1 (CD54) and L-Selectin (CD62L) Expression on Peripheral Blood Natural Killer Cells in Asthmatic Children with Acute Exacerbation. Allergy 2003, 58, 67–71. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, J.; Xiao, W.; Feng, J.; Sun, R.; Tian, Z. Involvement of Human Natural Killer Cells in Asthma Pathogenesis: Natural Killer 2 Cells in Type 2 Cytokine Predominance. J. Allergy Clin. Immunol. 2005, 115, 841–847. [Google Scholar] [CrossRef]
- Scordamaglia, F.; Balsamo, M.; Scordamaglia, A.; Moretta, A.; Mingari, M.C.; Canonica, G.W.; Moretta, L.; Vitale, M. Perturbations of Natural Killer Cell Regulatory Functions in Respiratory Allergic Diseases. J. Allergy Clin. Immunol. 2008, 121, 479–485. [Google Scholar] [CrossRef]
- Duvall, M.G.; Barnig, C.; Cernadas, M.; Ricklefs, I.; Krishnamoorthy, N.; Grossman, N.L.; Bhakta, N.R.; Fahy, J.V.; Bleecker, E.R.; Castro, M.; et al. Natural Killer Cell-Mediated Inflammation Resolution Is Disabled in Severe Asthma. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Devulder, J.; Chenivesse, C.; Ledroit, V.; Fry, S.; Lobert, P.-E.; Hober, D.; Tsicopoulos, A.; Duez, C. Aberrant Anti-Viral Response of Natural Killer Cells in Severe Asthma. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Korsgren, M.; Persson, C.G.; Sundler, F.; Bjerke, T.; Hansson, T.; Chambers, B.J.; Hong, S.; Van Kaer, L.; Ljunggren, H.G.; Korsgren, O. Natural Killer Cells Determine Development of Allergen-Induced Eosinophilic Airway Inflammation in Mice. J. Exp. Med. 1999, 189, 553–562. [Google Scholar] [CrossRef]
- Ple, C.; Barrier, M.; Amniai, L.; Marquillies, P.; Bertout, J.; Tsicopoulos, A.; Walzer, T.; Lassalle, P.; Duez, C. Natural Killer Cells Accumulate in Lung-Draining Lymph Nodes and Regulate Airway Eosinophilia in a Murine Model of Asthma. Scand. J. Immunol. 2010, 72, 118–127. [Google Scholar] [CrossRef]
- Haworth, O.; Cernadas, M.; Levy, B.D. NK Cells Are Effectors for Resolvin E1 in the Timely Resolution of Allergic Airway Inflammation. J. Immunol. 2011, 186, 6129–6135. [Google Scholar] [CrossRef]
- Haspeslagh, E.; van Helden, M.J.; Deswarte, K.; De Prijck, S.; van Moorleghem, J.; Boon, L.; Hammad, H.; Vivier, E.; Lambrecht, B.N. Role of NKp46+ Natural Killer Cells in House Dust Mite-Driven Asthma. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The Biology of Human Natural Killer-Cell Subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- De Maria, A.; Bozzano, F.; Cantoni, C.; Moretta, L. Revisiting Human Natural Killer Cell Subset Function Revealed Cytolytic CD56(Dim)CD16+ NK Cells as Rapid Producers of Abundant IFN-Gamma on Activation. Proc. Natl. Acad. Sci. USA 2011, 108, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, E.; Yessaad, N.; Gregoire, E.; Hudspeth, K.; Luci, C.; Mavilio, D.; Hardwigsen, J.; Vivier, E. Mapping of NKp46(+) Cells in Healthy Human Lymphoid and Non-Lymphoid Tissues. Front. Immunol. 2012, 3, 344. [Google Scholar] [CrossRef] [PubMed]
- Soriani, A.; Stabile, H.; Gismondi, A.; Santoni, A.; Bernardini, G. Chemokine Regulation of Innate Lymphoid Cell Tissue Distribution and Function. Cytokine Growth Factor Rev. 2018, 42, 47–55. [Google Scholar] [CrossRef]
- Berahovich, R.D.; Lai, N.L.; Wei, Z.; Lanier, L.L.; Schall, T.J. Evidence for NK Cell Subsets Based on Chemokine Receptor Expression. J. Immunol. 2006, 177, 7833–7840. [Google Scholar] [CrossRef] [PubMed]
- Maghazachi, A.A. Role of Chemokines in the Biology of Natural Killer Cells. Curr. Top. Microbiol. Immunol. 2010, 341, 37–58. [Google Scholar] [CrossRef]
- Chenivesse, C.; Tsicopoulos, A. CCL18—Beyond Chemotaxis. Cytokine 2018, 109, 52–56. [Google Scholar] [CrossRef]
- de Nadaï, P.; Charbonnier, A.-S.; Chenivesse, C.; Sénéchal, S.; Fournier, C.; Gilet, J.; Vorng, H.; Chang, Y.; Gosset, P.; Wallaert, B.; et al. Involvement of CCL18 in Allergic Asthma. J. Immunol. 2006, 176, 6286–6293. [Google Scholar] [CrossRef]
- Günther, C.; Bello-Fernandez, C.; Kopp, T.; Kund, J.; Carballido-Perrig, N.; Hinteregger, S.; Fassl, S.; Schwärzler, C.; Lametschwandtner, G.; Stingl, G.; et al. CCL18 Is Expressed in Atopic Dermatitis and Mediates Skin Homing of Human Memory T Cells. J. Immunol. 2005, 174, 1723–1728. [Google Scholar] [CrossRef]
- Pirayesh, A.; Ferdosi, S.; Shirzad, H.; Amani, S.; Bahadivand Chegini, H.; Bagheri, N.; Sadeghian, L.; Torkamand, F. Differential Expression of CCL18 in Moderate/Severe and Mild Persistent Allergic Rhinitis and Its Correlation with Disease Parameters. J. Immunoass. Immunochem. 2018, 39, 485–495. [Google Scholar] [CrossRef]
- Tsicopoulos, A.; Chang, Y.; Ait Yahia, S.; de Nadai, P.; Chenivesse, C. Role of CCL18 in Asthma and Lung Immunity. Clin. Exp. Allergy 2013, 43, 716–722. [Google Scholar] [CrossRef]
- Adema, G.J.; Hartgers, F.; Verstraten, R.; de Vries, E.; Marland, G.; Menon, S.; Foster, J.; Xu, Y.; Nooyen, P.; McClanahan, T.; et al. A Dendritic-Cell-Derived C-C Chemokine That Preferentially Attracts Naive T Cells. Nature 1997, 387, 713–717. [Google Scholar] [CrossRef]
- Lindhout, E.; Vissers, J.L.; Hartgers, F.C.; Huijbens, R.J.; Scharenborg, N.M.; Figdor, C.G.; Adema, G.J. The Dendritic Cell-Specific CC-Chemokine DC-CK1 Is Expressed by Germinal Center Dendritic Cells and Attracts CD38-Negative Mantle Zone B Lymphocytes. J. Immunol. 2001, 166, 3284–3289. [Google Scholar] [CrossRef]
- Vulcano, M.; Struyf, S.; Scapini, P.; Cassatella, M.; Bernasconi, S.; Bonecchi, R.; Calleri, A.; Penna, G.; Adorini, L.; Luini, W.; et al. Unique Regulation of CCL18 Production by Maturing Dendritic Cells. J. Immunol. 2003, 170, 3843–3849. [Google Scholar] [CrossRef]
- Chenivesse, C.; Chang, Y.; Azzaoui, I.; Ait Yahia, S.; Morales, O.; Plé, C.; Foussat, A.; Tonnel, A.-B.; Delhem, N.; Yssel, H.; et al. Pulmonary CCL18 Recruits Human Regulatory T Cells. J. Immunol. 2012, 189, 128–137. [Google Scholar] [CrossRef]
- Krohn, S.; Garin, A.; Gabay, C.; Proudfoot, A.E.I. The Activity of CCL18 Is Principally Mediated through Interaction with Glycosaminoglycans. Front. Immunol. 2013, 4, 193. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.A.; Ling, M.F.; Leung, J.; Shreffler, W.G.; Luster, A.D. Identification of Human CCR8 as a CCL18 Receptor. J. Exp. Med. 2013, 210, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, Y.; Gong, C.; Yu, F.; Su, S.; Chen, J.; Liu, B.; Deng, H.; Wang, F.; Lin, L.; et al. CCL18 from Tumor-Associated Macrophages Promotes Breast Cancer Metastasis via PITPNM3. Cancer Cell 2011, 19, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Catusse, J.; Wollner, S.; Leick, M.; Schröttner, P.; Schraufstätter, I.; Burger, M. Attenuation of CXCR4 Responses by CCL18 in Acute Lymphocytic Leukemia B Cells. J. Cell. Physiol. 2010, 225, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Krohn, S.C.; Bonvin, P.; Proudfoot, A.E.I. CCL18 Exhibits a Regulatory Role through Inhibition of Receptor and Glycosaminoglycan Binding. PLoS ONE 2013, 8, e72321. [Google Scholar] [CrossRef]
- Azzaoui, I.; Yahia, S.A.; Chang, Y.; Vorng, H.; Morales, O.; Fan, Y.; Delhem, N.; Ple, C.; Tonnel, A.-B.; Wallaert, B.; et al. CCL18 Differentiates Dendritic Cells in Tolerogenic Cells Able to Prime Regulatory T Cells in Healthy Subjects. Blood 2011, 118, 3549–3558. [Google Scholar] [CrossRef]
- Chang, Y.; de Nadai, P.; Azzaoui, I.; Morales, O.; Delhem, N.; Vorng, H.; Tomavo, S.; Ait Yahia, S.; Zhang, G.; Wallaert, B.; et al. The Chemokine CCL18 Generates Adaptive Regulatory T Cells from Memory CD4+ T Cells of Healthy but Not Allergic Subjects. FASEB J. 2010, 24, 5063–5072. [Google Scholar] [CrossRef]
- Schraufstatter, I.U.; Zhao, M.; Khaldoyanidi, S.K.; DiScipio, R.G. The Chemokine CCL18 Causes Maturation of Cultured Monocytes to Macrophages in the M2 Spectrum. Immunology 2012, 135, 287–298. [Google Scholar] [CrossRef]
- Neel, N.F.; Schutyser, E.; Sai, J.; Fan, G.-H.; Richmond, A. Chemokine Receptor Internalization and Intracellular Trafficking. Cytokine Growth Factor Rev. 2005, 16, 637–658. [Google Scholar] [CrossRef]
- Inngjerdingen, M.; Damaj, B.; Maghazachi, A.A. Expression and Regulation of Chemokine Receptors in Human Natural Killer Cells. Blood 2001, 97, 367–375. [Google Scholar] [CrossRef]
- Walzer, T.; Vivier, E. G-Protein-Coupled Receptors in Control of Natural Killer Cell Migration. Trends Immunol. 2011, 32, 486–492. [Google Scholar] [CrossRef]
- Maghazachi, A.A.; Skalhegg, B.S.; Rolstad, B.; Al-Aoukaty, A. Interferon-Inducible Protein-10 and Lymphotactin Induce the Chemotaxis and Mobilization of Intracellular Calcium in Natural Killer Cells through Pertussis Toxin-Sensitive and -Insensitive Heterotrimeric G-Proteins. FASEB J. 1997, 11, 765–774. [Google Scholar] [CrossRef]
- Fadnes, B.; Husebekk, A.; Svineng, G.; Rekdal, Ø.; Yanagishita, M.; Kolset, S.O.; Uhlin-Hansen, L. The Proteoglycan Repertoire of Lymphoid Cells. Glycoconj. J. 2012, 29, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Brusilovsky, M.; Radinsky, O.; Cohen, L.; Yossef, R.; Shemesh, A.; Braiman, A.; Mandelboim, O.; Campbell, K.S.; Porgador, A. Regulation of Natural Cytotoxicity Receptors by Heparan Sulfate Proteoglycans in -Cis: A Lesson from NKp44. Eur. J. Immunol. 2015, 45, 1180–1191. [Google Scholar] [CrossRef]
- Panina-Bordignon, P.; Papi, A.; Mariani, M.; Di Lucia, P.; Casoni, G.; Bellettato, C.; Buonsanti, C.; Miotto, D.; Mapp, C.; Villa, A.; et al. The C-C Chemokine Receptors CCR4 and CCR8 Identify Airway T Cells of Allergen-Challenged Atopic Asthmatics. J. Clin. Investig. 2001, 107, 1357–1364. [Google Scholar] [CrossRef]
- Loetscher, P.; Seitz, M.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. Activation of NK Cells by CC Chemokines. Chemotaxis, Ca2+ Mobilization, and Enzyme Release. J. Immunol. 1996, 156, 322–327. [Google Scholar] [PubMed]
- Taub, D.D.; Sayers, T.J.; Carter, C.R.; Ortaldo, J.R. Alpha and Beta Chemokines Induce NK Cell Migration and Enhance NK-Mediated Cytolysis. J. Immunol. 1995, 155, 3877–3888. [Google Scholar]
- Yoneda, O.; Imai, T.; Goda, S.; Inoue, H.; Yamauchi, A.; Okazaki, T.; Imai, H.; Yoshie, O.; Bloom, E.T.; Domae, N.; et al. Fractalkine-Mediated Endothelial Cell Injury by NK Cells. J. Immunol. 2000, 164, 4055–4062. [Google Scholar] [CrossRef]
- Nieto, M.; Navarro, F.; Perez-Villar, J.J.; del Pozo, M.A.; González-Amaro, R.; Mellado, M.; Frade, J.M.; Martínez-A, C.; López-Botet, M.; Sánchez-Madrid, F. Roles of Chemokines and Receptor Polarization in NK-Target Cell Interactions. J. Immunol. 1998, 161, 3330–3339. [Google Scholar]
- Leung, S.Y.; Yuen, S.T.; Chu, K.-M.; Mathy, J.A.; Li, R.; Chan, A.S.Y.; Law, S.; Wong, J.; Chen, X.; So, S. Expression Profiling Identifies Chemokine (C-C Motif) Ligand 18 as an Independent Prognostic Indicator in Gastric Cancer. Gastroenterology 2004, 127, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, S.; Natsugoe, S.; Tokuda, K.; Nakajo, A.; Xiangming, C.; Iwashige, H.; Aridome, K.; Hokita, S.; Aikou, T. Clinical Impact of Intratumoral Natural Killer Cell and Dendritic Cell Infiltration in Gastric Cancer. Cancer Lett. 2000, 159, 103–108. [Google Scholar] [CrossRef]
- Tasaki, Y.; Fukuda, S.; Iio, M.; Miura, R.; Imai, T.; Sugano, S.; Yoshie, O.; Hughes, A.L.; Nomiyama, H. Chemokine PARC Gene (SCYA18) Generated by Fusion of Two MIP-1alpha/LD78alpha-like Genes. Genomics 1999, 55, 353–357. [Google Scholar] [CrossRef]
- Buentke, E.; Heffler, L.C.; Wilson, J.L.; Wallin, R.P.A.; Löfman, C.; Chambers, B.J.; Ljunggren, H.-G.; Scheynius, A. Natural Killer and Dendritic Cell Contact in Lesional Atopic Dermatitis Skin--Malassezia-Influenced Cell Interaction. J. Investig. Dermatol. 2002, 119, 850–857. [Google Scholar] [CrossRef]
- Sheehy, M.E.; McDermott, A.B.; Furlan, S.N.; Klenerman, P.; Nixon, D.F. A Novel Technique for the Fluorometric Assessment of T Lymphocyte Antigen Specific Lysis. J. Immunol. Methods 2001, 249, 99–110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amniai, L.; Ple, C.; Barrier, M.; de Nadai, P.; Marquillies, P.; Vorng, H.; Chenivesse, C.; Tsicopoulos, A.; Duez, C. Natural Killer Cells from Allergic Donors Are Defective in Their Response to CCL18 Chemokine. Int. J. Mol. Sci. 2021, 22, 3879. https://doi.org/10.3390/ijms22083879
Amniai L, Ple C, Barrier M, de Nadai P, Marquillies P, Vorng H, Chenivesse C, Tsicopoulos A, Duez C. Natural Killer Cells from Allergic Donors Are Defective in Their Response to CCL18 Chemokine. International Journal of Molecular Sciences. 2021; 22(8):3879. https://doi.org/10.3390/ijms22083879
Chicago/Turabian StyleAmniai, Latiffa, Coline Ple, Mathieu Barrier, Patricia de Nadai, Philippe Marquillies, Han Vorng, Cécile Chenivesse, Anne Tsicopoulos, and Catherine Duez. 2021. "Natural Killer Cells from Allergic Donors Are Defective in Their Response to CCL18 Chemokine" International Journal of Molecular Sciences 22, no. 8: 3879. https://doi.org/10.3390/ijms22083879
APA StyleAmniai, L., Ple, C., Barrier, M., de Nadai, P., Marquillies, P., Vorng, H., Chenivesse, C., Tsicopoulos, A., & Duez, C. (2021). Natural Killer Cells from Allergic Donors Are Defective in Their Response to CCL18 Chemokine. International Journal of Molecular Sciences, 22(8), 3879. https://doi.org/10.3390/ijms22083879





