DNA Methylation Patterns Correlate with the Expression of SCNN1A, SCNN1B, and SCNN1G (Epithelial Sodium Channel, ENaC) Genes
Abstract
1. Introduction
2. Results
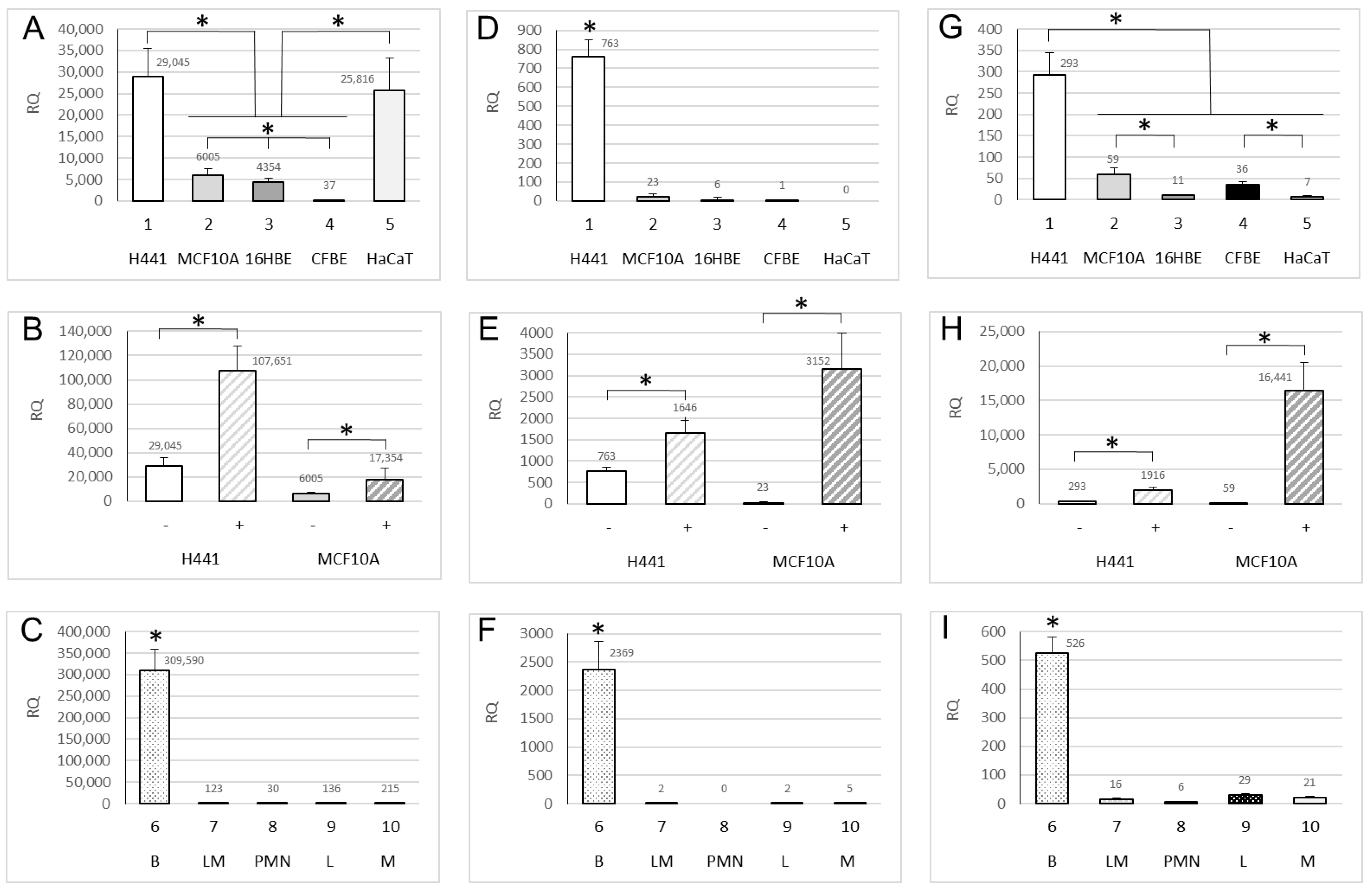
2.1. Expression of ENaC Genes
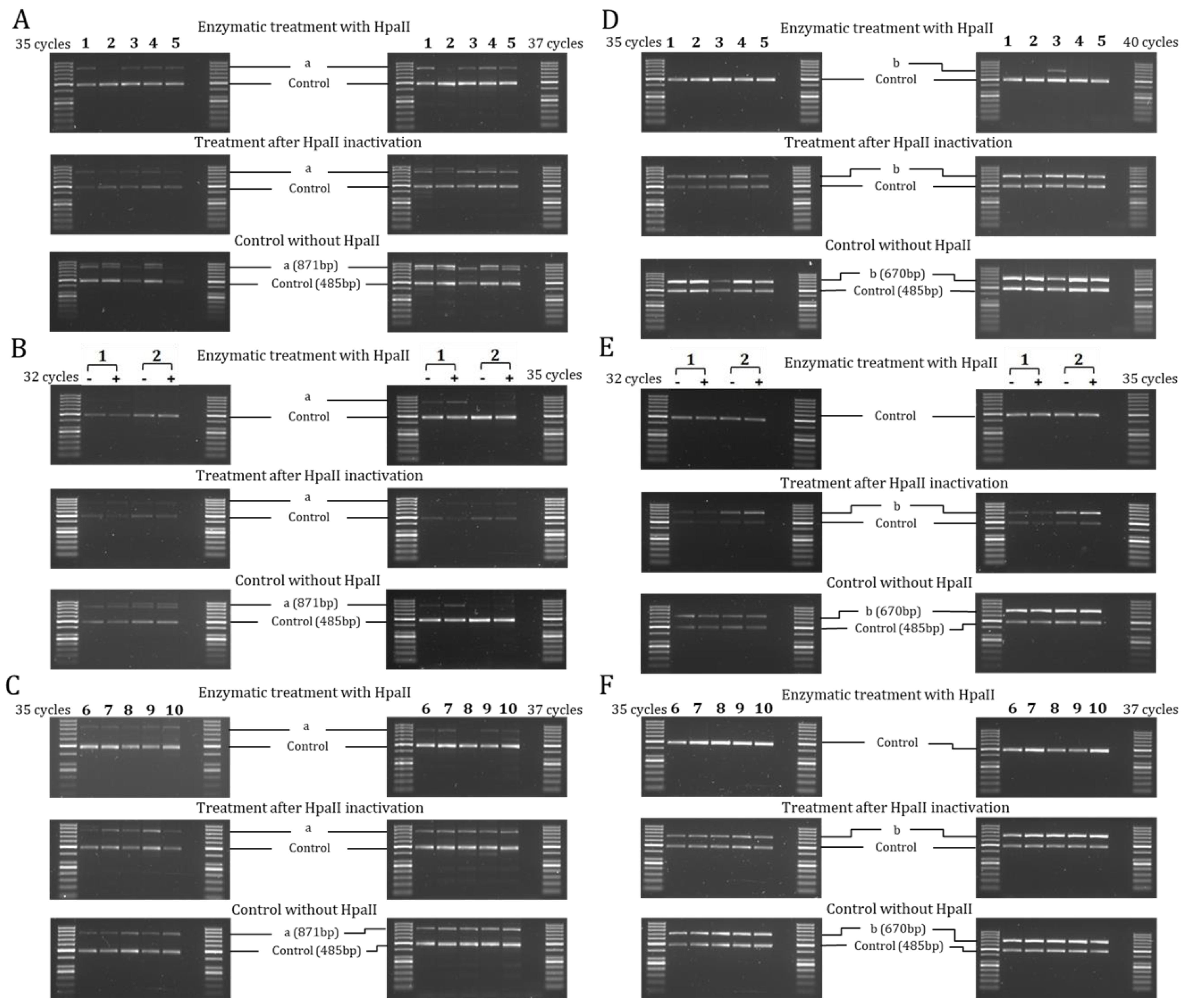
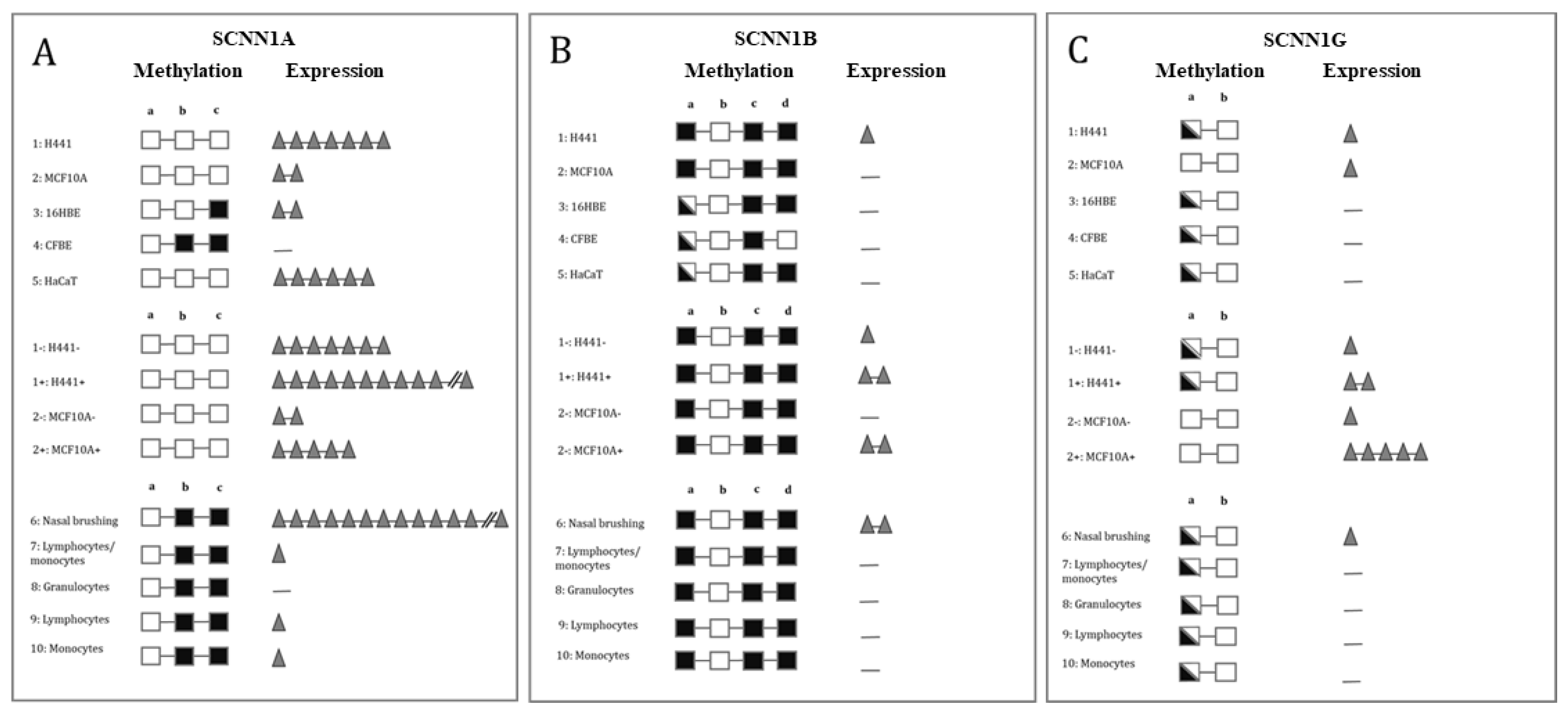
2.2. Methylation of ENaC Genes
3. Discussion
4. Materials and Methods
4.1. Cells and Culture Conditions
4.2. RNA Extraction and Expression Analysis
4.3. DNA Extraction and Methylation Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFTR | cystic fibrosis transmembrane conductance regulator |
| ENaC | epithelial sodium channel |
| CF | cystic fibrosis |
| SCNN1A | sodium channel epithelial 1 subunit alpha |
| SCNN1B | sodium channel epithelial 1 subunit beta |
| SCNN1G | sodium channel epithelial 1 subunit gamma |
| CFTR-RD | CFTR related disorders |
References
- Lucarelli, M.; Bruno, S.M.; Pierandrei, S.; Ferraguti, G.; Stamato, A.; Narzi, F.; Amato, A.; Cimino, G.; Bertasi, S.; Quattrucci, S.; et al. A Genotypic-Oriented View of CFTR Genetics Highlights Specific Mutational Patterns Underlying Clinical Macrocategories of Cystic Fibrosis. Mol. Med. 2015, 21, 257–275. [Google Scholar] [CrossRef]
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Krueger, B.; Diakov, A.; Korbmacher, J.; Haerteis, S.; Einsiedel, J.; Gmeiner, P.; Azad, A.K.; Cuppens, H.; Cassiman, J.-J.; et al. Functional Characterization of a Partial Loss-of-Function Mutation of the Epithelial Sodium Channel (ENaC) Associated with Atypical Cystic Fibrosis. Cell. Physiol. Biochem. 2010, 25, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.B.; Fong, P.; Groman, J.D.; Conrad, C.; Flume, P.; Diaz, R.; Harris, C.; Knowles, M.; Cutting, G.R. Mutations in the beta-subunit of the epithelial Na+ channel in patients with a cystic fibrosis-like syndrome. Hum. Mol. Genet. 2005, 14, 3493–3498. [Google Scholar] [CrossRef] [PubMed]
- Mutesa, L.; Azad, A.K.; Verhaeghe, C.; Segers, K.; Vanbellinghen, J.-F.; Ngendahayo, L.; Rusingiza, E.K.; Mutwa, P.R.; Rulisa, S.; Koulischer, L.; et al. Genetic Analysis of Rwandan Patients with Cystic Fibrosis-Like Symptoms. Chest 2009, 135, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Rauh, R.; Vermeulen, F.; Jaspers, M.; Korbmacher, J.; Boissier, B.; Bassinet, L.; Fichou, Y.; Georges, M.D.; Stanke, F.; et al. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum. Mutat. 2009, 30, 1093–1103. [Google Scholar] [CrossRef]
- Agrawal, P.B.; Wang, R.; Li, H.L.; Schmitz-Abe, K.; Simone-Roach, C.; Chen, J.; Shi, J.; Louie, T.; Sheng, S.; Towne, M.C.; et al. The Epithelial Sodium Channel Is a Modifier of the Long-Term Nonprogressive Phenotype Associated with F508del CFTR Mutations. Am. J. Respir. Cell Mol. Biol. 2017, 57, 711–720. [Google Scholar] [CrossRef]
- Zhou, Z.; Duerr, J.; Johannesson, B.; Schubert, S.C.; Treis, D.; Harm, M.; Graeber, S.Y.; Dalpke, A.; Schultz, C.; Mall, M.A. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J. Cyst. Fibros. 2011, 10, S172–S182. [Google Scholar] [CrossRef]
- Rauh, R.; Hoerner, C. Korbmacher, deltabetagamma-ENaC is inhibited by CFTR but stimulated by cAMP in Xenopus laevis oocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Gentzsch, M.; Dang, H.; Dang, Y.; Garcia-Caballero, A.; Suchindran, H.; Boucher, R.C.; Stutts, M.J. The Cystic Fibrosis Transmembrane Conductance Regulator Impedes Proteolytic Stimulation of the Epithelial Na+ Channel. J. Biol. Chem. 2010, 285, 32227–32232. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.M.; Light, M.J.; Quinton, P.M. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl- channel function. Nat. Cell Biol. 1999, 402, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, B.-J.; Zhao, R.-Z.; Ji, H.-L. Epithelial Sodium Channels in Pulmonary Epithelial Progenitor and Stem Cells. Int. J. Biol. Sci. 2016, 12, 1150–1154. [Google Scholar] [CrossRef]
- Hanukoglu, I.; Hanukoglu, A. Epithelial sodium channel (ENaC) family: Phylogeny, structure–function, tissue distribution, and associated inherited diseases. Gene 2016, 579, 95–132. [Google Scholar] [CrossRef] [PubMed]
- Bangel, N.; Dahlhoff, C.; Sobczak, K.; Weber, W.-M.; Kusche-Vihrog, K. Upregulated expression of ENaC in human CF nasal epithelium. J. Cyst. Fibros. 2008, 7, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, M.B. Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, R.C.; Lockwood, S.R.; Lide, E.; Bauer, R.; Suaud, L.; Grumbach, Y. Regulation of endogenous ENaC functional expression by CFTR and DeltaF508-CFTR in airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, 88–101. [Google Scholar] [CrossRef]
- Terlizzi, V.; Castaldo, G.; Salvatore, D.; Lucarelli, M.; Raia, V.; Angioni, A.; Carnovale, V.; Cirilli, N.; Casciaro, R.; Colombo, C.; et al. Genotype–phenotype correlation and functional studies in patients with cystic fibrosis bearing CFTR complex alleles. J. Med. Genet. 2016, 54, 224–235. [Google Scholar] [CrossRef]
- Lucarelli, M.; Narzi, L.; Pierandrei, S.; Bruno, S.M.; Stamato, A.; D’Avanzo, M.; Strom, R.; Quattrucci, S. A new complex allele of the CFTR gene partially explains the variable phenotype of the L997F mutation. Genet. Med. 2010, 12, 548–555. [Google Scholar] [CrossRef]
- Cutting, G.R. Modifier genes in Mendelian disorders: The example of cystic fibrosis. Ann. N. Y. Acad. Sci. 2010, 1214, 57–69. [Google Scholar] [CrossRef]
- Corvol, H.; Blackman, S.M.; Boëlle, P.-Y.; Gallins, P.J.; Pace, R.G.; Stonebraker, J.R.; Accurso, F.J.; Clement, A.; Collaco, J.M.; Dang, H.; et al. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat. Commun. 2015, 6, 8382. [Google Scholar] [CrossRef]
- Lucarelli, M.; Porcaro, L.; Biffignandi, A.; Costantino, L.; Giannone, V.; Alberti, L.; Bruno, S.M.; Corbetta, C.; Torresani, E.; Colombo, C.; et al. A New Targeted CFTR Mutation Panel Based on Next-Generation Sequencing Technology. J. Mol. Diagn. 2017, 19, 788–800. [Google Scholar] [CrossRef]
- Lucarelli, M.; Bruno, S.M.; Pierandrei, S.; Ferraguti, G.; Testino, G.; Truglio, G.; Strom, R.; Quattrucci, S. The Impact on Genetic Testing of Mutational Patterns of CFTR Gene in Different Clinical Macrocategories of Cystic Fibrosis. J. Mol. Diagn. 2016, 18, 554–565. [Google Scholar] [CrossRef][Green Version]
- Voilley, N.; Lingueglia, E.; Champigny, G.; Mattei, M.G.; Waldmann, R.; Lazdunski, M.; Barbry, P. The lung amiloride-sensitive Na+ channel: Biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proc. Natl. Acad. Sci. USA 1994, 91, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Voilley, N.; Bassilana, F.; Mignon, C.; Merscher, S.; Mattei, M.G.; Carle, G.F.; Lazdunski, M.; Barbry, P. Cloning, chromosomal localization, and physical linkage of the beta and gamma subunits (SCNN1B and SCNN1G) of the human epithelial amiloride-sensitive sodium channel. Genomics 1995, 28, 560–565. [Google Scholar] [CrossRef]
- Ludwig, M.; Bolkenius, U.; Wickert, L.; Marynen, P.; Bidlingmaier, F. Structural organisation of the gene encoding the α-subunit of the human amiloride-sensitive epithelial sodium channel. Qual. Life Res. 1998, 102, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.P.; Loftus, R.W.; Liu, K.Z.; Itani, O.A. Genomic organization of the 5′ end of human beta-ENaC and preliminary characterization of its promoter. Am. J. Physiol. Renal Physiol. 2002, 282, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, S.D.; Loftus, R.W.; Itani, O.A.; Thomas, C.P. Human amiloride-sensitive epithelial Na+ channel gamma subunit promoter: Functional analysis and identification of a polypurine-polypyrimidine tract with the potential for triplex DNA formation. Biochem. J. 2000, 347, 105–114. [Google Scholar] [CrossRef]
- Zhang, L.N.; Karp, P.; Gerard, C.J.; Pastor, E.; Laux, D.; Munson, K.; Yan, Z.; Liu, X.; Godwin, S.; Thomas, C.P.; et al. Dual Therapeutic Utility of Proteasome Modulating Agents for Pharmaco-gene Therapy of the Cystic Fibrosis Airway. Mol. Ther. 2004, 10, 990–1002. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Q.; Wang, L.; Wang, H.; Yin, F. A DNA methylation signature to improve survival prediction of gastric cancer. Clin. Epigenetics 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Qian, Y.; Wong, C.C.; Xu, J.; Chen, H.; Zhang, Y.; Kang, W.; Wang, H.; Zhang, L.; Li, W.; Chu, E.S.; et al. Sodium Channel Subunit SCNN1B Suppresses Gastric Cancer Growth and Metastasis via GRP78 Degradation. Cancer Res. 2017, 77, 1968–1982. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Liu, C.; Fan, R.; Duan, S.; Xu, X.; Zhao, J.; Mao, S.; Zhu, W.; Hao, L.; Yin, F.; et al. Association of SCNN1B promoter methylation with essential hypertension. Mol. Med. Rep. 2016, 14, 5422–5428. [Google Scholar] [CrossRef]
- Edinger, R.S.; Yospin, J.; Perry, C.; Kleyman, T.R.; Johnson, J.P. Regulation of epithelial Na+ channels (ENaC) by methylation: A novel methyltransferase stimulates ENaC activity. J. Biol. Chem. 2006, 281, 9110–9117. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A.; Galietta, L.J. Targeting ion channels in cystic fibrosis. J. Cyst. Fibros. 2015, 14, 561–570. [Google Scholar] [CrossRef]
- Martin, S.L.; Saint-Criq, V.; Hwang, T.-C.; Csanády, L. Ion channels as targets to treat cystic fibrosis lung disease. J. Cyst. Fibros. 2018, 17, S22–S27. [Google Scholar] [CrossRef]
- Moore, P.J.; Tarran, R. The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis lung disease. Expert Opin. Ther. Targets 2018, 22, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Aarbiou, J.; Copreni, E.; Buijs-Offerman, R.M.; Van Der Wegen, P.; Castellani, S.; Carbone, A.; Tilesi, F.; Fradiani, P.; Hiemstra, P.S.; Yueksekdag, G.; et al. Lentiviral small hairpin RNA delivery reduces apical sodium channel activity in differentiated human airway epithelial cells. J. Gene Med. 2012, 14, 733–745. [Google Scholar] [CrossRef]
- Burrows, E.; Southern, K.W.; Noone, P. Sodium channel blockers for cystic fibrosis. Cochrane Database Syst. Rev. 2006, 3, CD005087. [Google Scholar]
- Zhou, Z.; Treis, D.; Schubert, S.C.; Harm, M.; Schatterny, J.; Hirtz, S.; Duerr, J.; Boucher, R.C.; Mall, M.A. Preventive but not late amiloride therapy reduces morbidity and mortality of lung disease in betaENaC-overexpressing mice. Am. J. Respir. Crit. Care Med. 2008, 178, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Manunta, M.D.I.; Tagalakis, A.D.; Attwood, M.; Aldossary, A.M.; Barnes, J.L.; Munye, M.M.; Weng, A.; McAnulty, R.J.; Hart, S.L. Delivery of ENaC siRNA to epithelial cells mediated by a targeted nanocomplex: A therapeutic strategy for cystic fibrosis. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Crosby, J.R.; Zhao, C.; Jiang, C.; Bai, D.; Katz, M.; Greenlee, S.; Kawabe, H.; McCaleb, M.; Rotin, D.; Guo, S.; et al. Inhaled ENaC antisense oligonucleotide ameliorates cystic fibrosis-like lung disease in mice. J. Cyst. Fibros. 2017, 16, 671–680. [Google Scholar] [CrossRef]
- Scott, D.W.; Walker, M.P.; Sesma, J.; Wu, B.; Stuhlmiller, T.J.; Sabater, J.R.; Abraham, W.M.; Crowder, T.M.; Christensen, D.J.; Tarran, R. SPX-101 Is a Novel Epithelial Sodium Channel–targeted Therapeutic for Cystic Fibrosis That Restores Mucus Transport. Am. J. Respir. Crit. Care Med. 2017, 196, 734–744. [Google Scholar] [CrossRef]
- Coote, K.; Atherton-Watson, H.C.; Sugar, R.; Young, A.; MacKenzie-Beevor, A.; Gosling, M.; Bhalay, G.; Bloomfield, G.; Dunstan, A.; Bridges, R.J.; et al. Camostat Attenuates Airway Epithelial Sodium Channel Function in Vivo through the Inhibition of a Channel-Activating Protease. J. Pharmacol. Exp. Ther. 2009, 329, 764–774. [Google Scholar] [CrossRef]
- Shei, R.-J.; Peabody, J.E.; Kaza, N.; Rowe, S.M. The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis. Curr. Opin. Pharmacol. 2018, 43, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Murabito, A.; Ghigo, A. Inhaled Biologicals for the Treatment of Cystic Fibrosis. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 19–26. [Google Scholar] [CrossRef]
- Mroz, M.S.; Harvey, B.J. Ursodeoxycholic acid inhibits ENaC and Na/K pump activity to restore airway surface liquid height in cystic fibrosis bronchial epithelial cells. Steroids 2019, 151, 108461. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, M.; Ferraguti, G.; Fuso, A. Active Demethylation of Non-CpG Moieties in Animals: A Neglected Research Area. Int. J. Mol. Sci. 2019, 20, 6272. [Google Scholar] [CrossRef]
- Fuso, A.; Ferraguti, G.; Grandoni, F.; Ruggeri, R.; Scarpa, S.; Strom, R.; Lucarelli, M. Early demethylation of non-CpG, CpC-rich, elements in the myogenin 5′-flanking region: A priming effect on the spreading of active demethylation. Cell Cycle 2010, 9, 3965–3976. [Google Scholar] [CrossRef]
- Monti, N.; Cavallaro, R.A.; Stoccoro, A.; Nicolia, V.; Scarpa, S.; Kovacs, G.G.; Fiorenza, M.T.; Lucarelli, M.; Aronica, E.; Ferrer, I.; et al. CpG and non-CpG Presenilin1 methylation pattern in course of neurodevelopment and neurodegeneration is associated with gene expression in human and murine brain. Epigenetics 2020, 15, 781–799. [Google Scholar] [CrossRef]
- Gruenert, D.C.; Willems, M.; Cassiman, J.J.; Frizzell, R.A. Established cell lines used in cystic fibrosis re-search. J. Cyst. Fibros. 2004, 3, 191–196. [Google Scholar] [CrossRef]
- De Rocco, D.; Pompili, B.; Castellani, S.; Morini, E.; Cavinato, L.; Cimino, G.; Mariggiò, M.A.; Guarnieri, S.; Conese, M.; Del Porto, P.; et al. Assembly and Functional Analysis of an S/MAR Based Episome with the Cystic Fibrosis Transmembrane Conductance Regulator Gene. Int. J. Mol. Sci. 2018, 19, 1220. [Google Scholar] [CrossRef]
- Auriche, C.; Carpani, D.; Conese, M.; Caci, E.; Zegarra-Moran, O.; Donini, P.; Ascenzioni, F. Functional human CFTR produced by a stable minichromosome. EMBO Rep. 2002, 3, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Auriche, C.; Di Domenico, E.G.; Pierandrei, S.; Lucarelli, M.; Castellani, S.; Conese, M.; Melani, R.; Zegarra-Moran, O.; Ascenzioni, F. CFTR expression and activity from the human CFTR locus in BAC vectors, with regulatory regions, isolated by a single-step procedure. Gene Ther. 2010, 17, 1341–1354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luly, F.R.; Lévêque, M.; Licursi, V.; Cimino, G.; Martin-Chouly, C.; Théret, N.; Negri, R.; Cavinato, L.; Ascenzioni, F.; Del Porto, P. MiR-146a is over-expressed and controls IL-6 production in cystic fibrosis macrophages. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fuso, A.; Scarpa, S.; Grandoni, F.; Strom, R.; Lucarelli, M. A reassessment of semiquantitative analytical procedures for DNA methylation: Comparison of bisulfite- and HpaII polymerase-chain-reaction-based methods. Anal. Biochem. 2006, 350, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, M.; Fuso, A.; Strom, R.; Scarpa, S. The Dynamics of Myogenin Site-specific Demethylation Is Strongly Correlated with Its Expression and with Muscle Differentiation. J. Biol. Chem. 2001, 276, 7500–7506. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierandrei, S.; Truglio, G.; Ceci, F.; Del Porto, P.; Bruno, S.M.; Castellani, S.; Conese, M.; Ascenzioni, F.; Lucarelli, M. DNA Methylation Patterns Correlate with the Expression of SCNN1A, SCNN1B, and SCNN1G (Epithelial Sodium Channel, ENaC) Genes. Int. J. Mol. Sci. 2021, 22, 3754. https://doi.org/10.3390/ijms22073754
Pierandrei S, Truglio G, Ceci F, Del Porto P, Bruno SM, Castellani S, Conese M, Ascenzioni F, Lucarelli M. DNA Methylation Patterns Correlate with the Expression of SCNN1A, SCNN1B, and SCNN1G (Epithelial Sodium Channel, ENaC) Genes. International Journal of Molecular Sciences. 2021; 22(7):3754. https://doi.org/10.3390/ijms22073754
Chicago/Turabian StylePierandrei, Silvia, Gessica Truglio, Fabrizio Ceci, Paola Del Porto, Sabina Maria Bruno, Stefano Castellani, Massimo Conese, Fiorentina Ascenzioni, and Marco Lucarelli. 2021. "DNA Methylation Patterns Correlate with the Expression of SCNN1A, SCNN1B, and SCNN1G (Epithelial Sodium Channel, ENaC) Genes" International Journal of Molecular Sciences 22, no. 7: 3754. https://doi.org/10.3390/ijms22073754
APA StylePierandrei, S., Truglio, G., Ceci, F., Del Porto, P., Bruno, S. M., Castellani, S., Conese, M., Ascenzioni, F., & Lucarelli, M. (2021). DNA Methylation Patterns Correlate with the Expression of SCNN1A, SCNN1B, and SCNN1G (Epithelial Sodium Channel, ENaC) Genes. International Journal of Molecular Sciences, 22(7), 3754. https://doi.org/10.3390/ijms22073754








