Brain Region-Dependent Effects of Neuropeptide Y on Conditioned Social Fear and Anxiety-Like Behavior in Male Mice
Abstract
1. Introduction
2. Results
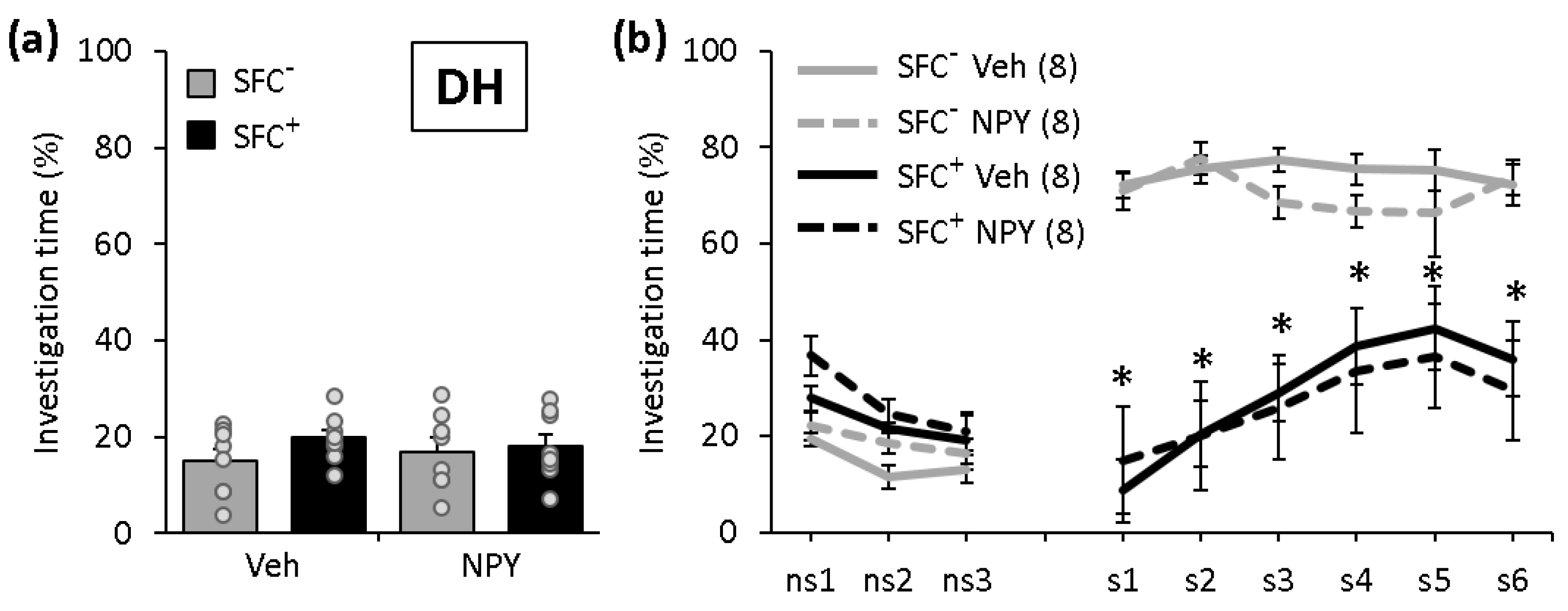
2.1. NPY Does Not Affect the Expression of Social Fear When Administered into the DH
2.2. NPY Reduces the Expression of Social Fear When Administered into the DLS
2.3. NPY Reduces the Expression of Social Fear When Administered into the CeA, But Not When Administered into the MeA or BLA
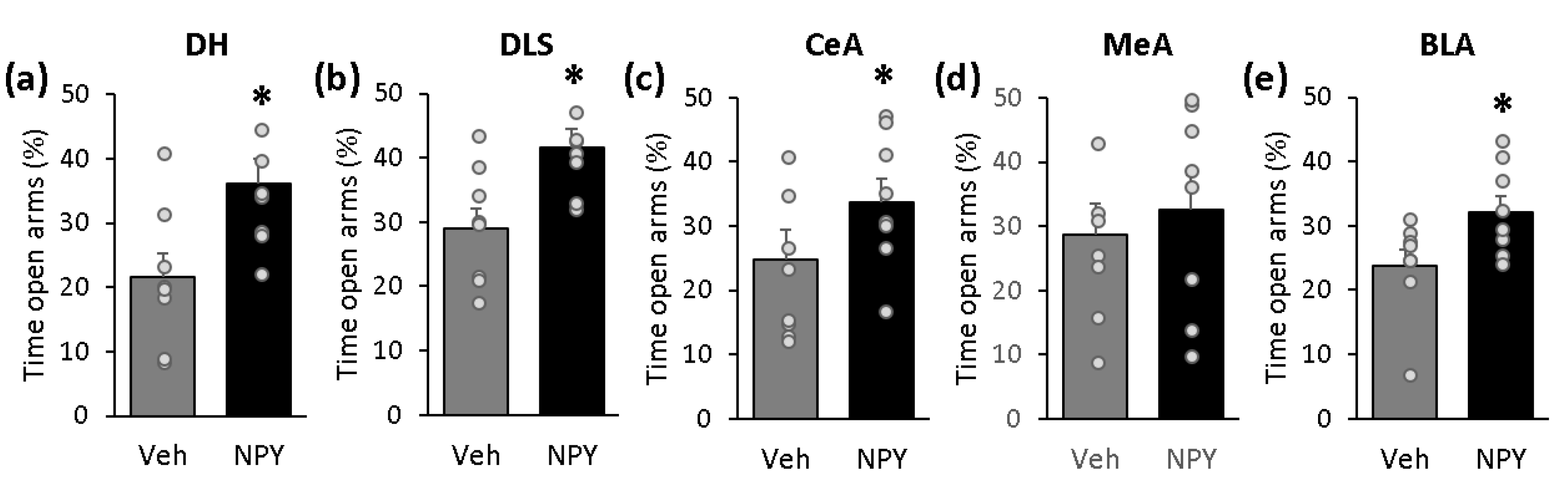
2.4. NPY Exerts Anxiolytic-Like Effects When Administered into the DH, DLS, CeA and BLA, But Not When Administered into the MeA
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Stereotaxic Cannula Implantation
4.3. Intracerebral Infusions
4.4. Social Fear Conditioning (SFC) Paradigm
4.5. Elevated Plus-Maze (EPM) Test
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Stanley, B.G.; Leibowitz, S.F. Neuropeptide Y: Stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984, 35, 2635–2642. [Google Scholar] [CrossRef]
- Colmers, W.F.; Bleakman, D. Effects of neuropeptide Y on the electrical properties of neurons. Trends Neurosci. 1994, 17, 373–379. [Google Scholar] [CrossRef]
- Vezzani, A.; Sperk, G.; Colmers, W.F. Neuropeptide Y: Emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999, 22, 25–30. [Google Scholar] [CrossRef]
- Michalkiewicz, M.; Michalkiewicz, T.; Kreulen, D.L.; McDougall, S.J. Increased blood pressure responses in neuropeptide Y transgenic rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Magni, P. Hormonal control of the neuropeptide Y system. Curr. Protein Pept. Sci. 2003, 4, 45–57. [Google Scholar] [CrossRef]
- Hökfelt, T.; Stanic, D.; Sanford, S.D.; Gatlin, J.C.; Nilsson, I.; Paratcha, G.; Ledda, F.; Fetissov, S.; Lindfors, C.; Herzog, H.; et al. NPY and its involvement in axon guidance, neurogenesis, and feeding. Nutrition 2008, 24, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Dumont, Y.; Fournier, A.; St-Pierre, S.; Quirion, R. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J. Neurosci. 1993, 13, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Dumont, Y.; Fournier, A.; St-Pierre, S.; Quirion, R. Autoradiographic distribution of [125I]Leu31,Pro34]PYY and [125I]PYY3-36 binding sites in the rat brain evaluated with two newly developed Y1 and Y2 receptor radioligands. Synapse 1996, 22, 139–158. [Google Scholar] [CrossRef]
- Parker, R.M.; Herzog, H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 1999, 11, 1431–1448. [Google Scholar] [CrossRef]
- Chang, R.S.; Lotti, V.J.; Chen, T.B.; Cerino, D.J.; Kling, P.J. Neuropeptide Y (NPY) binding sites in rat brain labeled with 125I-Bolton-Hunter NPY: Comparative potencies of various polypeptides on brain NPY binding and biological responses in the rat vas deferens. Life Sci. 1985, 37, 2111–2122. [Google Scholar] [CrossRef]
- De Quidt, M.E.; Emson, P.C. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system-II. Immunohistochemical analysis. Neuroscience 1986, 18, 545–618. [Google Scholar] [CrossRef]
- Lynch, D.R.; Walker, M.W.; Miller, R.J.; Snyder, S.H. Neuropeptide Y receptor binding sites in rat brain: Differential autoradiographic localizations with 125I-peptide YY and 125I-neuropeptide Y imply receptor heterogeneity. J. Neurosci. 1989, 9, 2607–2619. [Google Scholar] [CrossRef]
- Bowers, M.E.; Choi, D.C.; Ressler, K.J. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiol. Behav. 2012, 107, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M. The NPY system in stress, anxiety and depression. Neuropeptides 2004, 38, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Feder, A.; Wegener, G.; Bailey, C.; Saxena, S.; Charney, D.; Mathé, A.A. Central functions of neuropeptide Y in mood and anxiety disorders. Expert Opin. Ther. Targets 2011, 15, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M.; Soderpalm, B.; Engel, J.A.; Widerlov, E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology 1989, 98, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M.; McLeod, S.; Brot, M.; Heinrichs, S.C.; Menzaghi, F.; Koob, G.F.; Britton, K.T. Anxiolytic-like action of neuropeptide Y: Mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology 1993, 8, 357–363. [Google Scholar] [CrossRef]
- Kask, A.; Rägo, L.; Harro, J. Anxiolytic-like effect of neuropeptide Y (NPY) and NPY13-36 microinjected into vicinity of locus coeruleus in rats. Brain Res. 1998, 788, 345–348. [Google Scholar] [CrossRef]
- Trent, N.L.; Menard, J.L. Infusions of neuropeptide Y into the lateral septum reduce anxiety-related behaviors in the rat. Pharmacol. Biochem. Behav. 2011, 99, 580–590. [Google Scholar] [CrossRef]
- Smiałowska, M.; Wierońska, J.M.; Domin, H.; Zieba, B. The effect of intrahippocampal injection of group II and III metobotropic glutamate receptor agonists on anxiety; the role of neuropeptide Y. Neuropsychopharmacology 2007, 32, 1242–1250. [Google Scholar] [CrossRef]
- Sharko, A.C.; Kaigler, K.F.; Fadel, J.R.; Wilson, M.A. Ethanol-induced anxiolysis and neuronal activation in the amygdala and bed nucleus of the stria terminalis. Alcohol 2016, 50, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Thorsell, A.; Repunte-Canonigo, V.; O’Dell, L.E.; Chen, S.A.; King, A.R.; Lekic, D.; Koob, G.F.; Sanna, P.P. Viral vector-induced amygdala NPY overexpression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain 2007, 130, 1330–1337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kask, A.; Nguyen, H.P.; Pabst, R.; Von Hörsten, S. Neuropeptide Y Y1 receptor-mediated anxiolysis in the dorsocaudal lateral septum: Functional antagonism of corticotropin-releasing hormone-induced anxiety. Neuroscience 2001, 104, 799–806. [Google Scholar]
- Sajdyk, T.J.; Vandergriff, M.G.; Gehlert, D.R. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur. J. Pharmacol. 1999, 368, 143–147. [Google Scholar] [CrossRef]
- Broqua, P.; Wettstein, J.G.; Rocher, M.N.; Gauthier-Martin, B.; Junien, J.L. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav. Pharmacol. 1995, 6, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, R.M.; Holmes, A.; Heilig, M.; Crawley, J.N. Anxiolytic-like actions of centrally-administered neuropeptide Y, but not galanin, in C57BL/6J mice. Pharmacol. Biochem. Behav. 2005, 80, 427–436. [Google Scholar] [CrossRef]
- Lach, G.; de Lima, T.C. Role of NPY Y1 receptor on acquisition, consolidation and extinction on contextual fear conditioning: Dissociation between anxiety, locomotion and non-emotional memory behavior. Neurobiol. Learn. Mem. 2013, 103, 26–33. [Google Scholar] [CrossRef]
- Gutman, A.R.; Yang, Y.; Ressler, K.J.; Davis, M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J. Neurosci. 2008, 28, 12682–12690. [Google Scholar] [CrossRef]
- Fendt, M.; Bürki, H.; Imobersteg, S.; Lingenhöhl, K.; McAllister, K.H.; Orain, D.; Uzunov, D.P.; Chaperon, F. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: An NPY Y1 receptor independent effect. Psychopharmacology 2009, 206, 291–301. [Google Scholar] [CrossRef]
- Pickens, C.L.; Adams-Deutsch, T.; Nair, S.G.; Navarre, B.M.; Heilig, M.; Shaham, Y. Effect of pharmacological manipulations of neuropeptide Y and corticotropin-releasing factor neurotransmission on incubation of conditioned fear. Neuroscience 2009, 164, 1398–1406. [Google Scholar] [CrossRef]
- Verma, D.; Tasan, R.O.; Herzog, H.; Sperk, G. NPY controls fear conditioning and fear extinction by combined action on Y1 and Y2 receptors. Br. J. Pharmacol. 2012, 166, 1461–1473. [Google Scholar] [CrossRef]
- Verma, D.; Wood, J.; Lach, G.; Mietzsch, M.; Weger, S.; Heilbronn, R.; Herzog, H.; Bonaventure, P.; Sperk, G.; Tasan, R.O. NPY Y2 receptors in the central amygdala reduce cued but not contextual fear. Neuropharmacology 2015, 99, 665–674. [Google Scholar] [CrossRef]
- Verma, D.; Hörmer, B.; Bellmann-Sickert, K.; Thieme, V.; Beck-Sickinger, A.G.; Herzog, H.; Sperk, G.; Tasan, R.O. Pancreatic polypeptide and its central Y4 receptors are essential for cued fear extinction and permanent suppression of fear. Br. J. Pharmacol. 2016, 173, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Zoicas, I. Neuropeptide Y reduces expression of social fear via simultaneous activation of Y1 and Y2 receptors. J. Psychopharmacol. 2019, 33, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Zoicas, I. Neuropeptide Y as alternative pharmacotherapy for antidepressant-resistant social fear. Int. J. Mol. Sci. 2020, 21, 8220. [Google Scholar] [CrossRef] [PubMed]
- Kask, A.; Harro, J.; von Hörsten, S.; Redrobe, J.P.; Dumont, Y.; Quirion, R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci. Biobehav. Rev. 2002, 26, 259–283. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Muller, J. Emotional memory and psychopathology. Philos. Trans. R Soc. Lond. B Biol. Sci. 1997, 352, 1719–1726. [Google Scholar]
- Reichmann, F.; Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109. [Google Scholar] [CrossRef]
- Tasan, R.O.; Verma, D.; Wood, J.; Lach, G.; Hörmer, B.; de Lima, T.C.; Herzog, H.; Sperk, G. The role of Neuropeptide Y in fear conditioning and extinction. Neuropeptides 2016, 55, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; LeDoux, J.E. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 1999, 23, 229–232. [Google Scholar] [CrossRef]
- LeDoux, J.E. The amygdala. Curr. Biol. 2007, 17, R868–R874. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, K.A.; Maren, S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 2001, 21, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Maren, S.; Phan, K.L.; Liberzon, I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 2013, 14, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Slattery, D.A.; Neumann, I.D. Brain oxytocin in social fear conditioning and its extinction: Involvement of the lateral septum. Neuropsychopharmacology 2014, 39, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Menon, R.; Neumann, I.D. Neuropeptide S reduces fear and avoidance of con-specifics induced by social fear conditioning and social defeat, respectively. Neuropharmacology 2016, 108, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Sajdyk, T.J.; Schober, D.A.; Smiley, D.L.; Gehlert, D.R. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol. Biochem. Behav. 2002, 71, 419–423. [Google Scholar] [CrossRef]
- File, S.E.; Hyde, J.R. Can social interaction be used to measure anxiety? Br. J. Pharmacol. 1978, 62, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.; Neumann, I.D. Animal models of social avoidance and social fear. Cell Tissue Res. 2013, 354, 107–118. [Google Scholar] [CrossRef]
- Kornhuber, J.; Zoicas, I. Neuropeptide Y prolongs non-social memory in a brain region- and receptor-specific way in male mice. Neuropharmacology 2020, 175, 108199. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Zoicas, I. Social fear memory requires two stages of protein synthesis in mice. Int. J. Mol. Sci. 2020, 21, 5537. [Google Scholar] [CrossRef]
- Toth, I.; Neumann, I.D.; Slattery, D.A. Social fear conditioning: A novel and specific animal model to study social anxiety disorder. Neuropsychopharmacology 2012, 37, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.; Neumann, I.D.; Slattery, D.A. Social fear conditioning as an animal model of social anxiety disorder. Curr. Protoc. Neurosci. 2013, 63, 9–42. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornhuber, J.; Zoicas, I. Brain Region-Dependent Effects of Neuropeptide Y on Conditioned Social Fear and Anxiety-Like Behavior in Male Mice. Int. J. Mol. Sci. 2021, 22, 3695. https://doi.org/10.3390/ijms22073695
Kornhuber J, Zoicas I. Brain Region-Dependent Effects of Neuropeptide Y on Conditioned Social Fear and Anxiety-Like Behavior in Male Mice. International Journal of Molecular Sciences. 2021; 22(7):3695. https://doi.org/10.3390/ijms22073695
Chicago/Turabian StyleKornhuber, Johannes, and Iulia Zoicas. 2021. "Brain Region-Dependent Effects of Neuropeptide Y on Conditioned Social Fear and Anxiety-Like Behavior in Male Mice" International Journal of Molecular Sciences 22, no. 7: 3695. https://doi.org/10.3390/ijms22073695
APA StyleKornhuber, J., & Zoicas, I. (2021). Brain Region-Dependent Effects of Neuropeptide Y on Conditioned Social Fear and Anxiety-Like Behavior in Male Mice. International Journal of Molecular Sciences, 22(7), 3695. https://doi.org/10.3390/ijms22073695





