Magnetic Core–Shell Molecularly Imprinted Nano-Conjugates for Extraction of Antazoline and Hydroxyantazoline from Human Plasma—Material Characterization, Theoretical Analysis and Pharmacokinetics
Abstract
1. Introduction
2. Results and Discussion
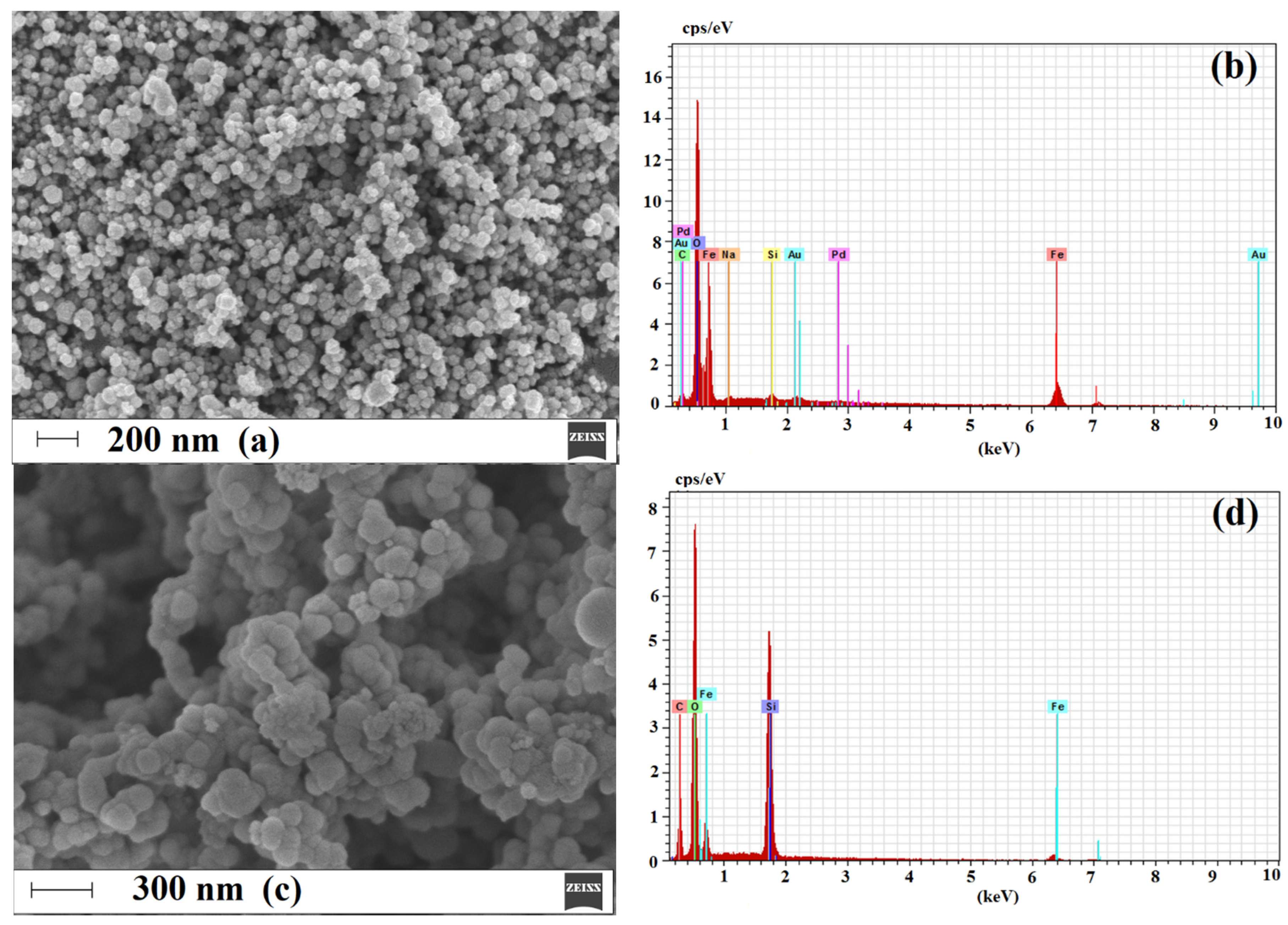
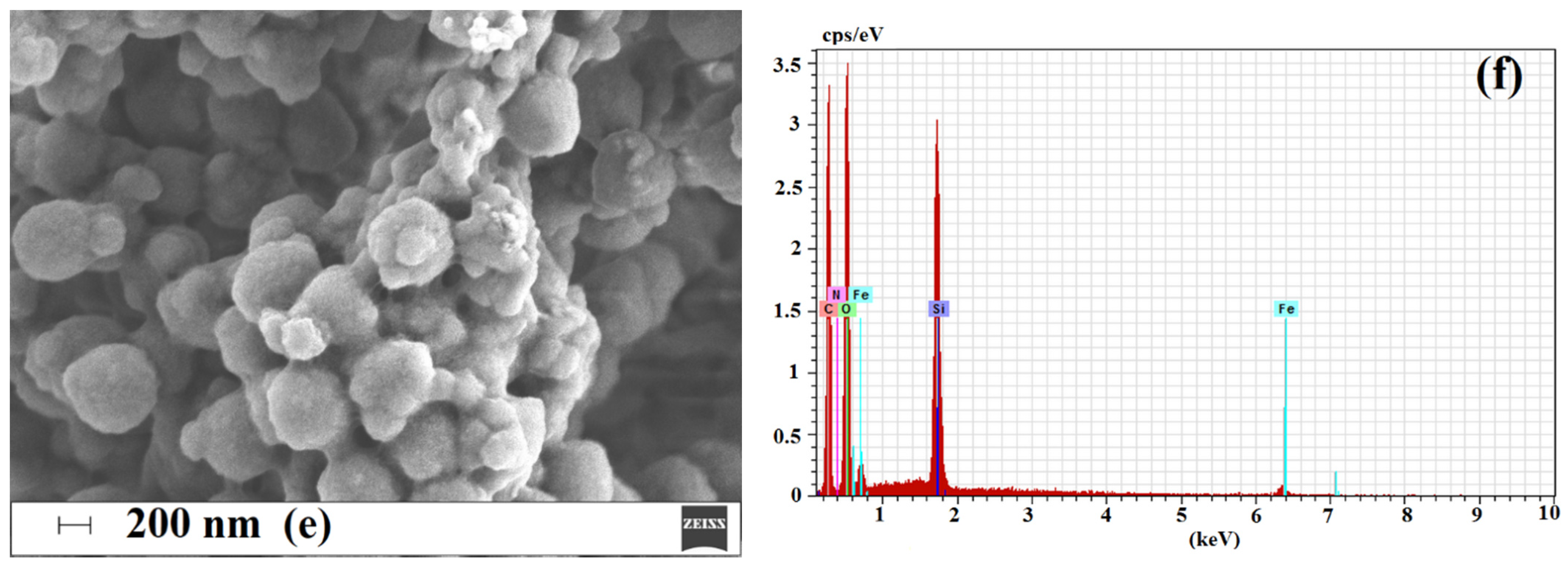
2.1. Characterization of Magnetic Sorbent
2.1.1. Adsorption Properties
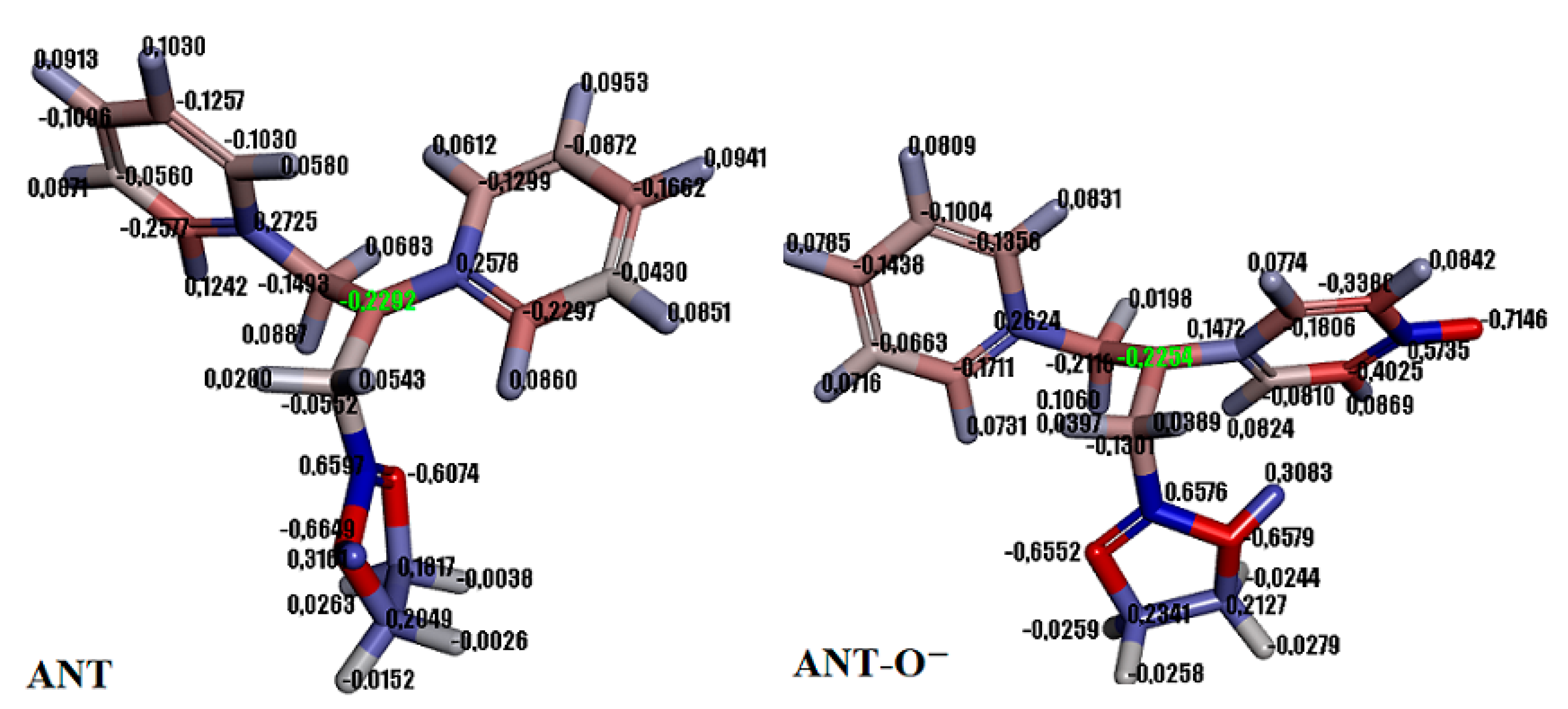
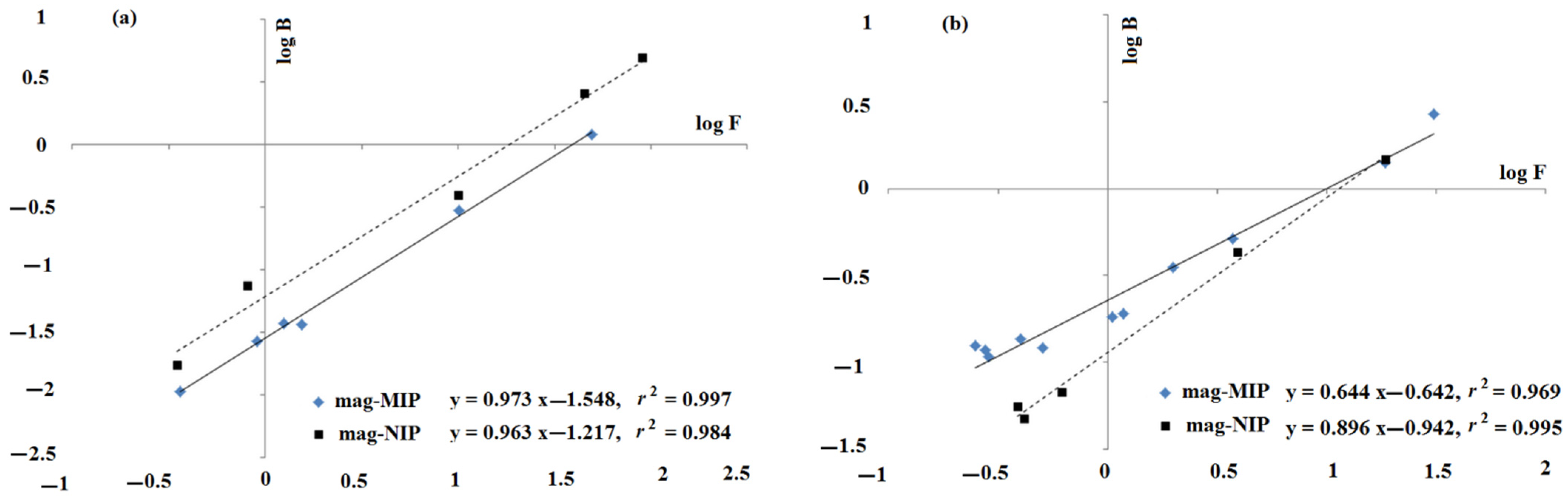
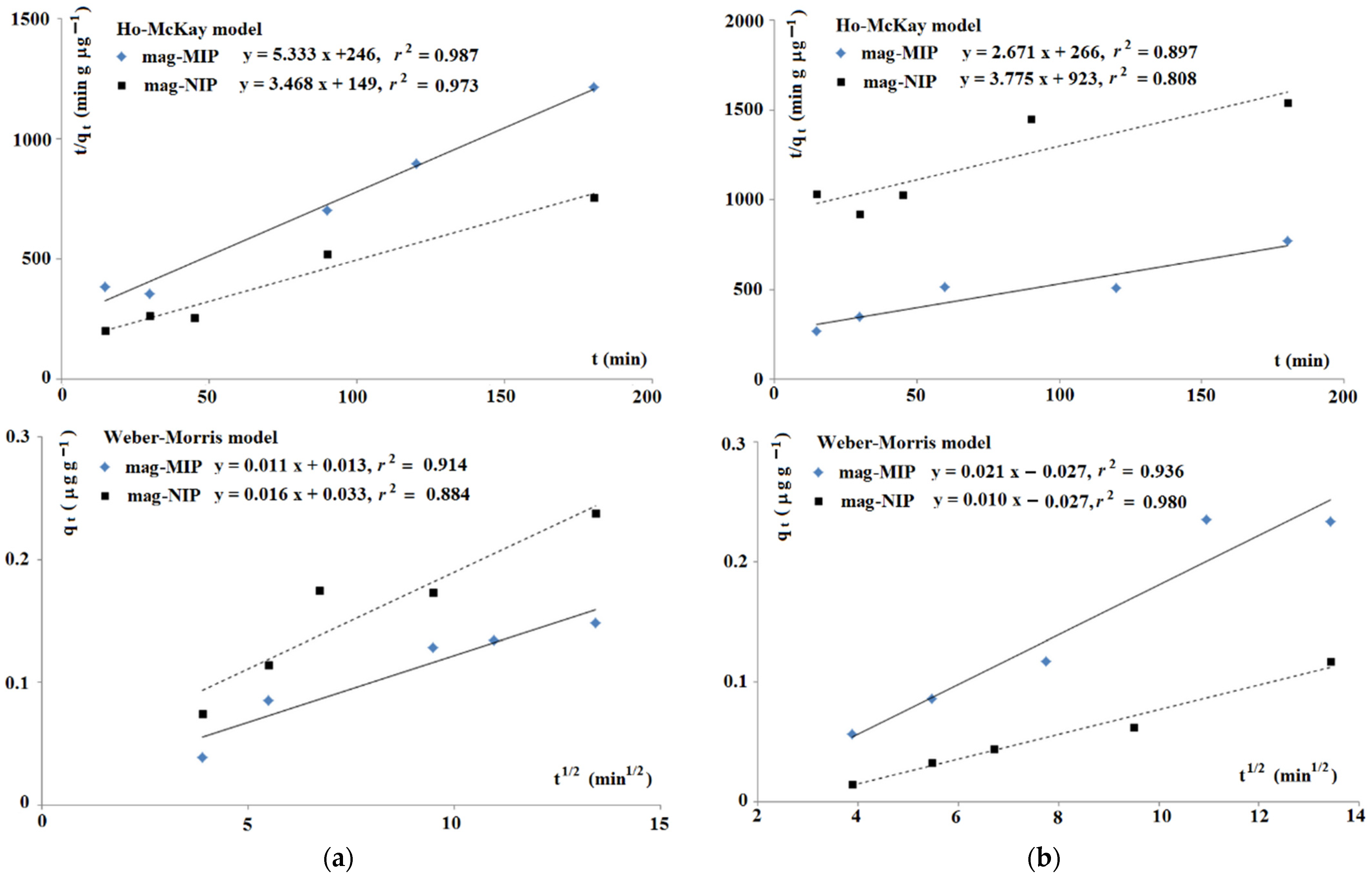
2.1.2. Theoretical Insight into Adsorption Process
2.1.3. Morphology and Composition of Imprinted Sorbent
2.2. Optimization of Dispersive Solid Phase Extraction
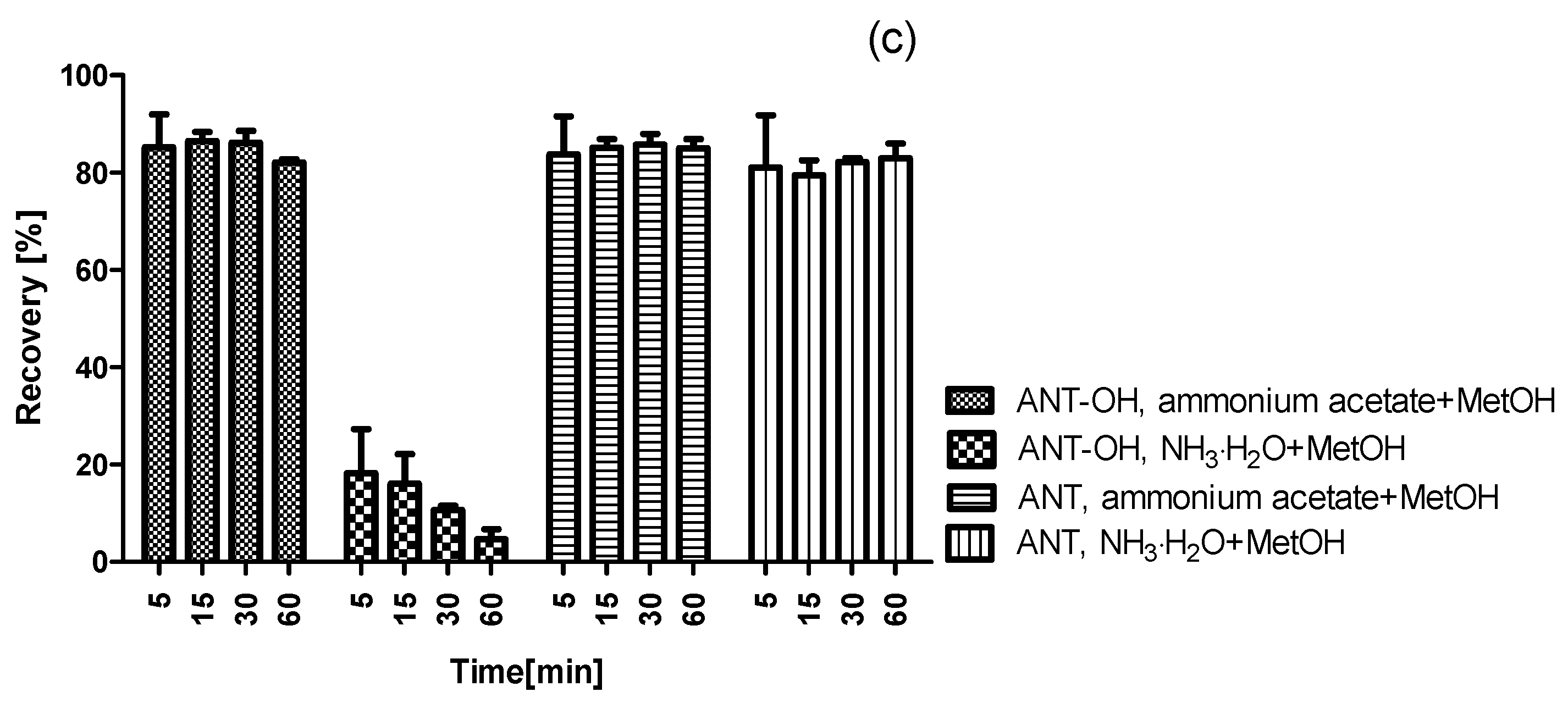
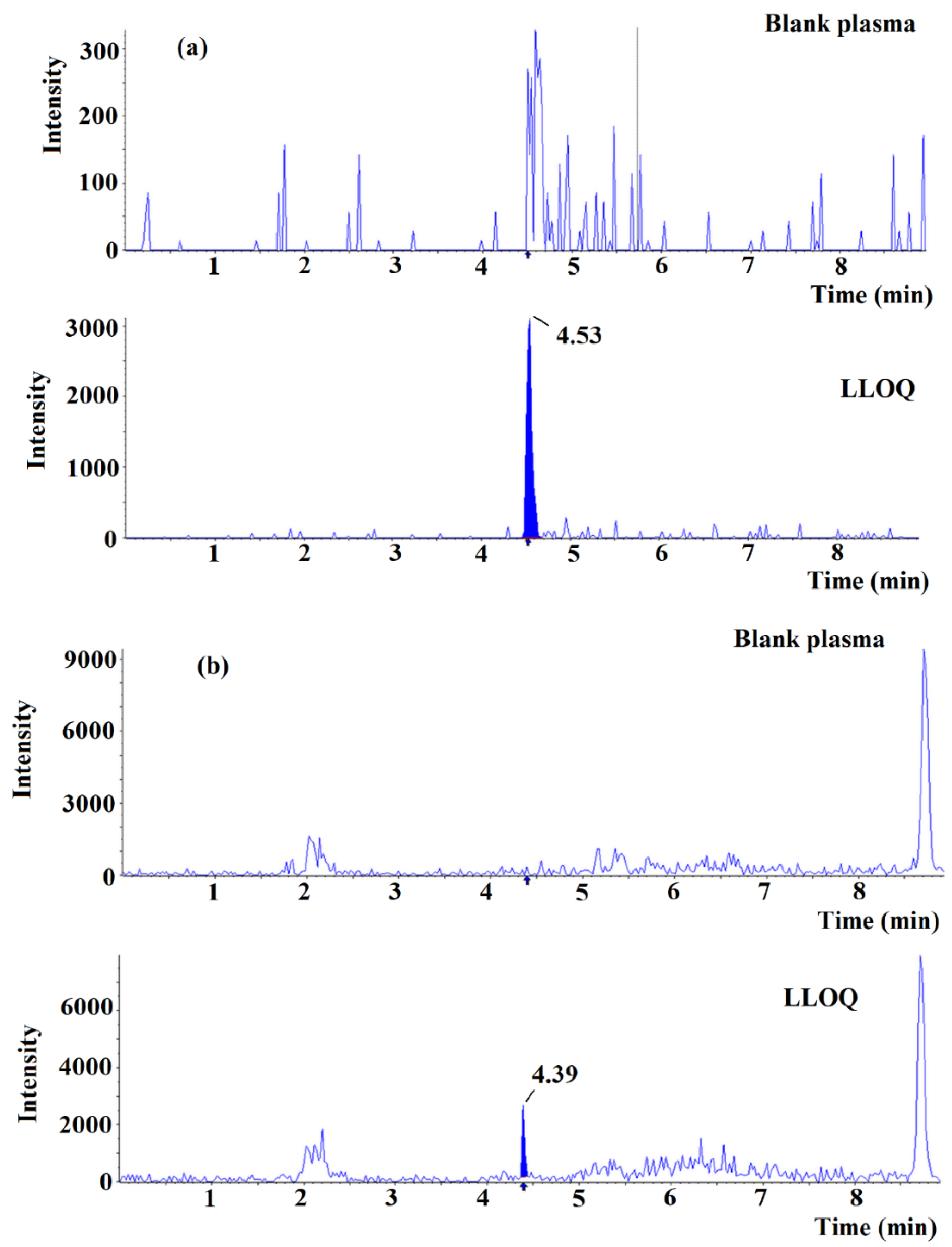
2.3. Validation of Method
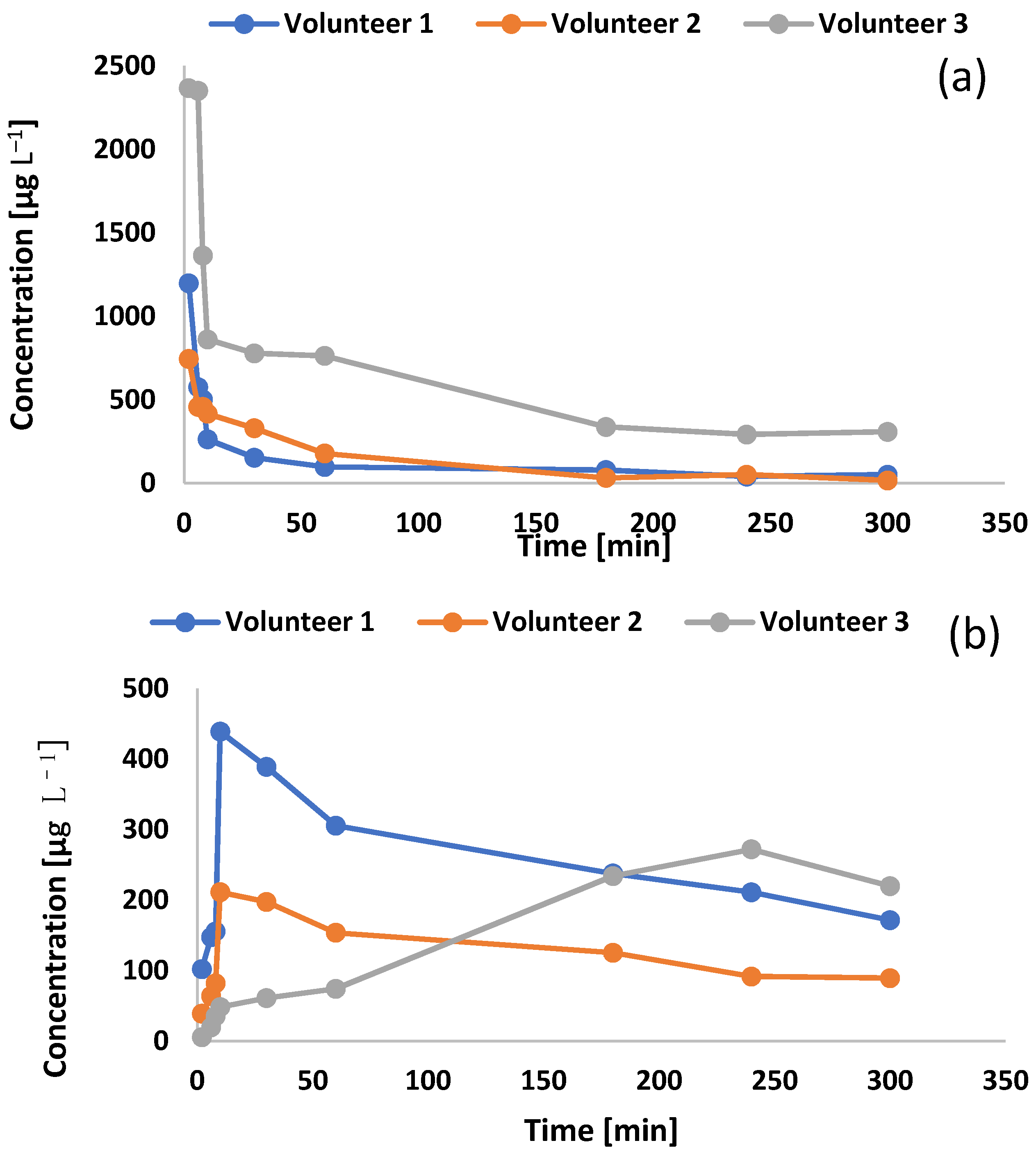
2.4. Pharmacokinetics
3. Materials and Methods
3.1. Chemicals
3.2. Sorbent
3.3. Instruments
3.4. Binding Study and Sample Preparation
3.5. Method Validation
3.6. Pharmacokinetic Study
3.7. Theory
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Synthesis of Magnetic Core and Functionalization

References
- Kojro, G.; Wroczyński, P. Cloud Point Extraction in the Determination of Drugs in Biological Matrices. J. Chromatogr. Sci. 2019, 58, 151–162. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Dempsey-Hibbert, N.C.; Peeters, M.; Tridente, A.; Banks, C.E. Molecularly imprinted polymer based electrochemical biosensors: Overcoming the challenges of detecting vital biomarkers and speeding up diagnosis. Talanta Open 2020, 2, 100018. [Google Scholar] [CrossRef]
- Sobiech, M.; Bujak, P.; Luliński, P.; Pron, A. Semiconductor nanocrystal–polymer hybrid nanomaterials and their application in molecular imprinting. Nanoscale 2019, 11, 12030–12074. [Google Scholar] [CrossRef] [PubMed]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing—Synthesis, characterisation and application. Sens. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Scigalski, P.; Kosobucki, P. Recent Materials Developed for Dispersive Solid Phase Extraction. Molecules 2020, 25, 4869. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Serpa, A.; González-Martín, R.; Sajid, M.; Pino, V. Greenness of magnetic nanomaterials in miniaturized extraction techniques: A review. Talanta 2021, 225, 122053. [Google Scholar] [CrossRef] [PubMed]
- Maciag, A.; Farkowski, M.M.; Chwyczko, T.; Beckowski, M.; Syska, P.; Kowalik, I.; Pytkowski, M.; Wozniak, J.; Dabrowski, R.; Szwed, H. Efficacy and safety of antazoline in the rapid cardioversion of paroxysmal atrial fibrillation (the AnPAF Study). Europace 2017, 19, 1637–1642. [Google Scholar] [CrossRef]
- Farkowski, M.M.; Maciag, A.; Zurawska, M.; Pytkowski, M.; Kowalik, I.; Wozniak, J.; Sterlinski, M.; Szwed, H. Comparative effectiveness and safety of antazolinebased and propafenonebased strategies for pharmacological cardioversion of shortduration atrial fibrillation in the emergency department. Pol. Arch. Med. Wewn 2016, 126, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, M.T.; Wrobel, W.; Wilkosz, K.; Wrona, K.; Bula, K.; Mizia-Stec, K. Pharmacological Cardioversion With Antazoline in Atrial Fibrillation: The Results of the CANT Study. J. Am. Heart Assoc. 2018, 7, e010153. [Google Scholar] [CrossRef]
- Piotrowski, R.; Giebultowicz, J.; Baran, J.; Sikorska, A.; Gralak-Lachowska, D.; Soszynska, M.; Wroczynski, P.; Kulakowski, P. Antazoline-insights into drug-induced electrocardiographic and hemodynamic effects: Results of the ELEPHANT II substudy. Ann. Noninvasive. Electrocardiol. 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Binkowski, B.J.; Makowski, M.; Kubinski, P.; Lubinski, A. Effect of Antazoline on Electrophysiological Properties of Atrial Muscle and Conduction System of the Heart. Cardiovasc. Drugs Ther. 2018, 32, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Giebultowicz, J.; Piotrowski, R.; Baran, J.; Kulakowski, P.; Wroczynski, P. Application of a novel liquid chromatography/tandem mass spectrometry method for the determination of antazoline in human plasma: Result of ELEPHANT-I [ELEctrophysiological, pharmacokinetic and hemodynamic effects of PHenazolinum (ANTazoline mesylate)] human pharmacokinetic study. J. Pharm Biomed. Anal. 2016, 123, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Giebultowicz, J.; Korytowska, N.; Piotrowski, R.; Kulakowski, P.; Latacz, G.; Szymanska, E.; Wisniowska, B.; Polak, S. Characterization of In Vitro and In Vivo Metabolism of Antazoline Using Liquid Chromatography-Tandem Mass Spectrometry. Int. J. Mol. Sci 2020, 21, 9693. [Google Scholar] [CrossRef]
- Kang, M.J.; Song, W.H.; Shim, B.H.; OH, S.Y.; Lee, H.Y.; Chung, E.Y.; Sohn, Y.; Lee, J. Pharmacologically Active Metabolites of Currently Marketed Drugs: Potential Resources for New Drug Discovery and Development. Pharm. Soc. Jpn. 2010, 130, 1325–1337. [Google Scholar] [CrossRef]
- Ali, H.M.; Beckett, A.H. Identification of two in vitro metabolic products after liver microsomal incubation of antazoline. J. Chromatogr. 1981, 210, 350–355. [Google Scholar] [CrossRef]
- Giebultowicz, J.; Kojro, G.; Piotrowski, R.; Kulakowski, P.; Wroczynski, P. Cloud-point extraction is compatible with liquid chromatography coupled to electrospray ionization mass spectrometry for the determination of antazoline in human plasma. J. Pharm. Biomed. Anal. 2016, 128, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chu, Y.; Li, X.; Wan, B.; Yu, T.; Wang, L.; Hao, L.; Guo, M. Determination of antazoline hydrochloride in rat plasma and excreta by reversed-phase ion-pair chromatography and its application to pharmacokinetics. Biomed. Chromatogr. 2013, 27, 1595–1602. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Sobiech, M.; Żołek, T.; Luliński, P.; Maciejewska, D. A computational exploration of imprinted polymer affinity based on voriconazole metabolites. Analyst 2014, 139, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Luliński, P.; Sobiech, M.; Żołek, T.; Maciejewska, D. A separation of tyramine on a 2-(4-methoxyphenyl)ethylamine imprinted polymer: An answer from theoretical and experimental studies. Talanta 2014, 129, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Żołek, T.; Luliński, P.; Maciejewska, D. A computational model for selectivity evaluation of 2-(3,4-dimethoxyphenyl)ethylamine (homoveratrylamine) imprinted polymers towards biogenic compounds. Anal. Chim. Acta 2011, 693, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, M.; Giebułtowicz, J.; Luliński, P. Theoretical and experimental proof for selective response of imprinted sorbent—analysis of hordenine in human urine. J. Chromatogr. A 2020, 1613, 460677. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Sobiech, M.; Rużycka, M.; Luliński, P. Theoretical and experimental approach to hydrophilic interaction dispersive solid-phase extraction of 2-aminothiazoline-4-carboxylic acid from human post-mortem blood. J. Chromatogr. A 2019, 1587, 61–72. [Google Scholar] [CrossRef]
- Petrovic, J.; Pesic, V.; Lauschke, V.M. Frequencies of clinically important CYP2C19 and CYP2D6 alleles are graded across Europe. Eur. J. Hum. Genet. 2020, 28, 88–94. [Google Scholar] [CrossRef]
- Hu, C.; Deng, J.; Zhao, Y.; Xia, L.; Huang, K.; Ju, S.; Xiao, N. A novel core–shell magnetic nano-sorbent with surface molecularly imprinted polymer coating for the selective solid phase extraction of dimetridazole. Food Chem. 2014, 158, 366–373. [Google Scholar] [CrossRef]
- Sobiech, M.; Giebułtowicz, J.; Luliński, P. Application of Magnetic Core–Shell Imprinted Nanoconjugates for the Analysis of Hordenine in Human Plasma-Preliminary Data on Pharmacokinetic Study after Oral Administration. J. Agric. Food Chem. 2020, 68, 14502–14512. [Google Scholar] [CrossRef]
- Luliński, P.; Dana, M.; Maciejewska, D. Synthesis and characterization of 4-(2-aminoethyl)aniline imprinted polymer as a highly effective sorbent of dopamine. Talanta 2014, 119, 623–631. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Bioanalytical Method Validation; Committee for Medicinal Products for Human Use: London, UK, 2012; EMEA/CHMP/EWP/192217/2009. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision d. 01; Gaussian Inc.: Wallingford, CT, USA, 2009; Volume 201. [Google Scholar]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Systemes, D. BIOVIA, Discovery Studio Modeling Environment. Release 2019; Dassault Systemes: San Diego, CA, USA, 2018. [Google Scholar]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giebułtowicz, J.; Korytowska, N.; Sobiech, M.; Polak, S.; Wiśniowska, B.; Piotrowski, R.; Kułakowski, P.; Luliński, P. Magnetic Core–Shell Molecularly Imprinted Nano-Conjugates for Extraction of Antazoline and Hydroxyantazoline from Human Plasma—Material Characterization, Theoretical Analysis and Pharmacokinetics. Int. J. Mol. Sci. 2021, 22, 3665. https://doi.org/10.3390/ijms22073665
Giebułtowicz J, Korytowska N, Sobiech M, Polak S, Wiśniowska B, Piotrowski R, Kułakowski P, Luliński P. Magnetic Core–Shell Molecularly Imprinted Nano-Conjugates for Extraction of Antazoline and Hydroxyantazoline from Human Plasma—Material Characterization, Theoretical Analysis and Pharmacokinetics. International Journal of Molecular Sciences. 2021; 22(7):3665. https://doi.org/10.3390/ijms22073665
Chicago/Turabian StyleGiebułtowicz, Joanna, Natalia Korytowska, Monika Sobiech, Sebastian Polak, Barbara Wiśniowska, Roman Piotrowski, Piotr Kułakowski, and Piotr Luliński. 2021. "Magnetic Core–Shell Molecularly Imprinted Nano-Conjugates for Extraction of Antazoline and Hydroxyantazoline from Human Plasma—Material Characterization, Theoretical Analysis and Pharmacokinetics" International Journal of Molecular Sciences 22, no. 7: 3665. https://doi.org/10.3390/ijms22073665
APA StyleGiebułtowicz, J., Korytowska, N., Sobiech, M., Polak, S., Wiśniowska, B., Piotrowski, R., Kułakowski, P., & Luliński, P. (2021). Magnetic Core–Shell Molecularly Imprinted Nano-Conjugates for Extraction of Antazoline and Hydroxyantazoline from Human Plasma—Material Characterization, Theoretical Analysis and Pharmacokinetics. International Journal of Molecular Sciences, 22(7), 3665. https://doi.org/10.3390/ijms22073665





