Germinated Rhynchosia nulubilis Fermented with Lactobacillus pentosus SC65 Reduces Particulate Matter Induced Type II Alveolar Epithelial Apoptotic Cell Death
Abstract
1. Introduction
2. Results
2.1. LAB Fermentation Increases Total Polyphenolic Content and DPPH Radical Scavenging Activity in GR
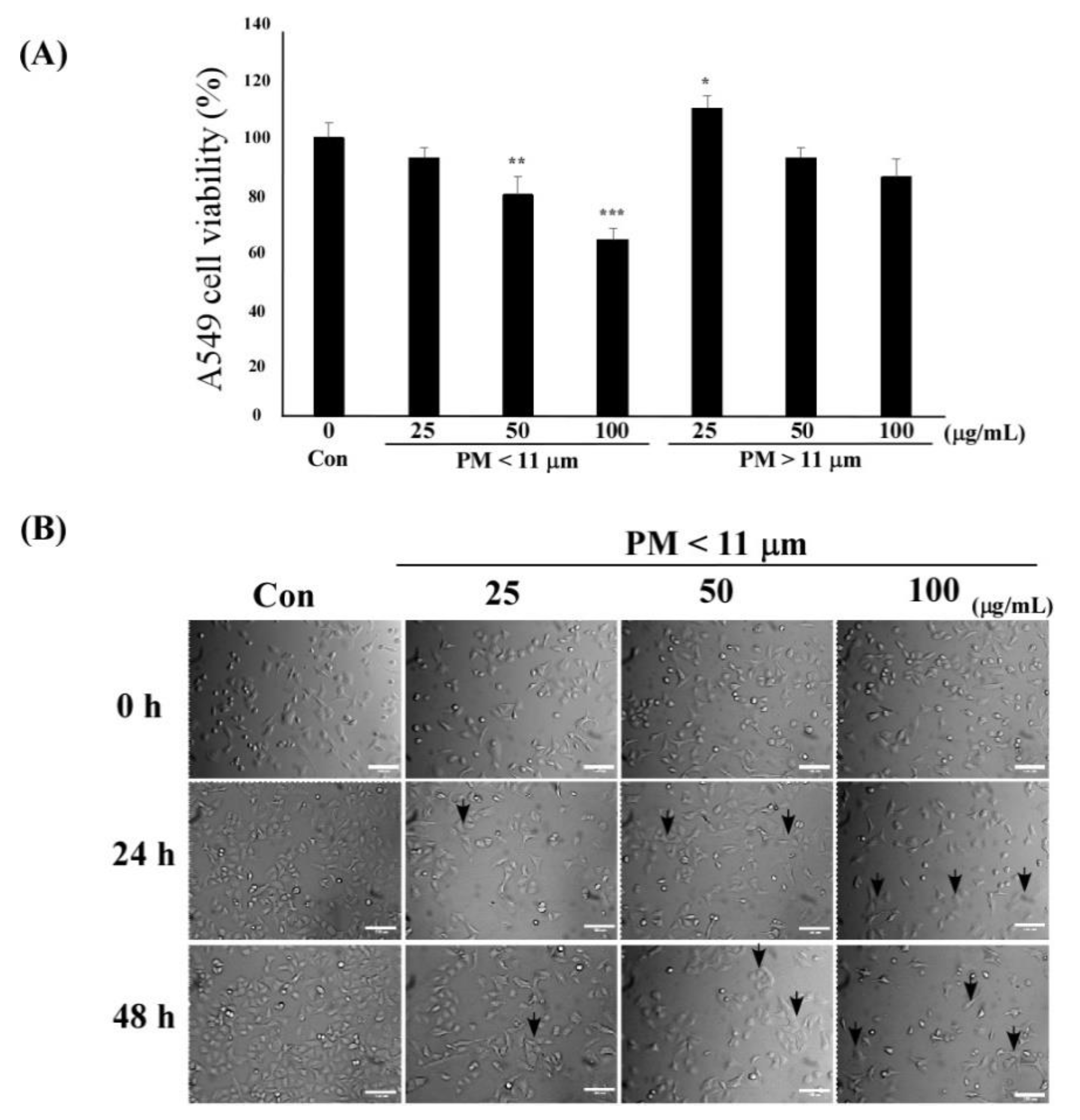
2.2. PM Decreases the Viability of A549 Cells
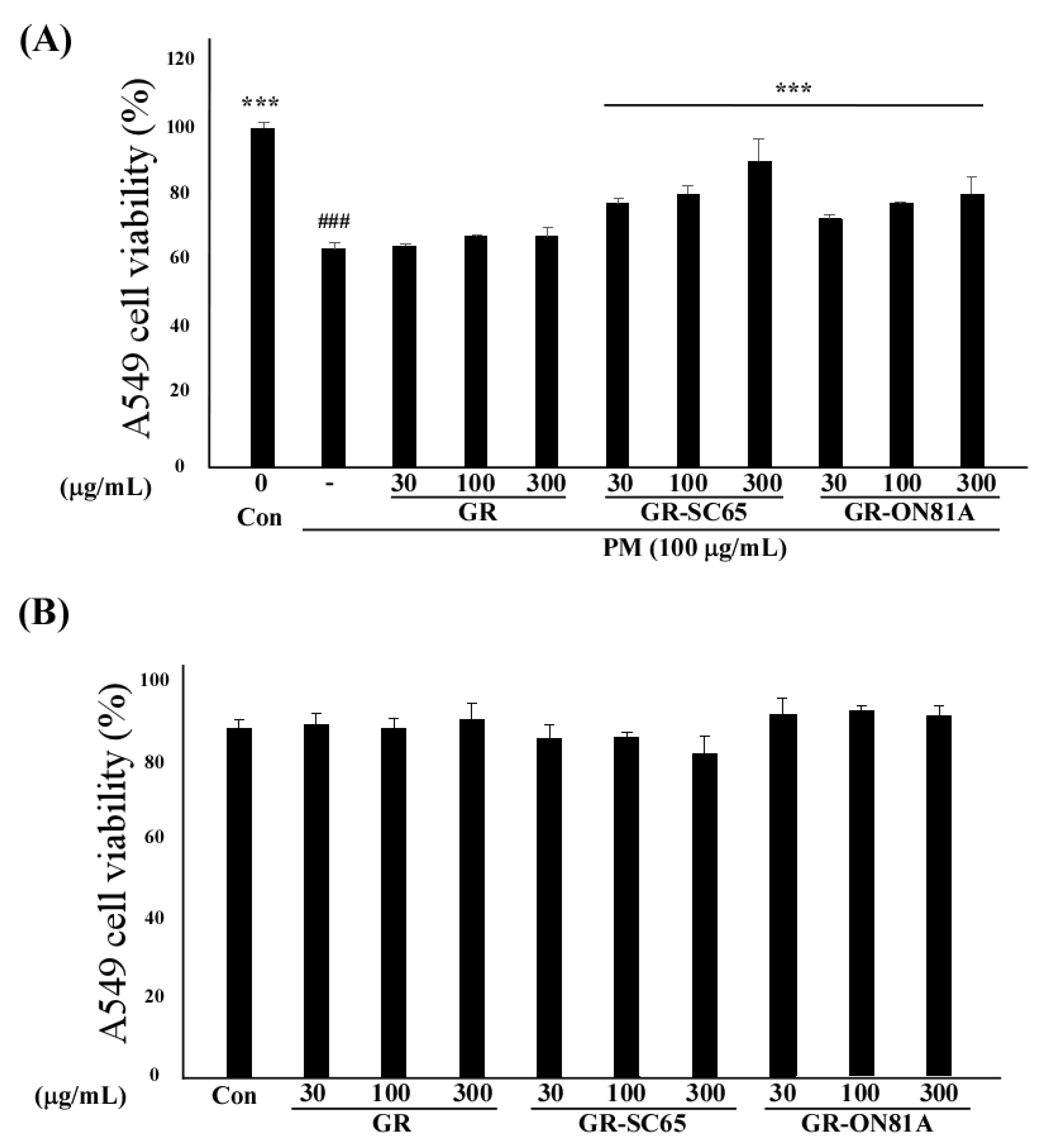
2.3. GR-SC65 Suppresses PM-Induced A549 Cell Death
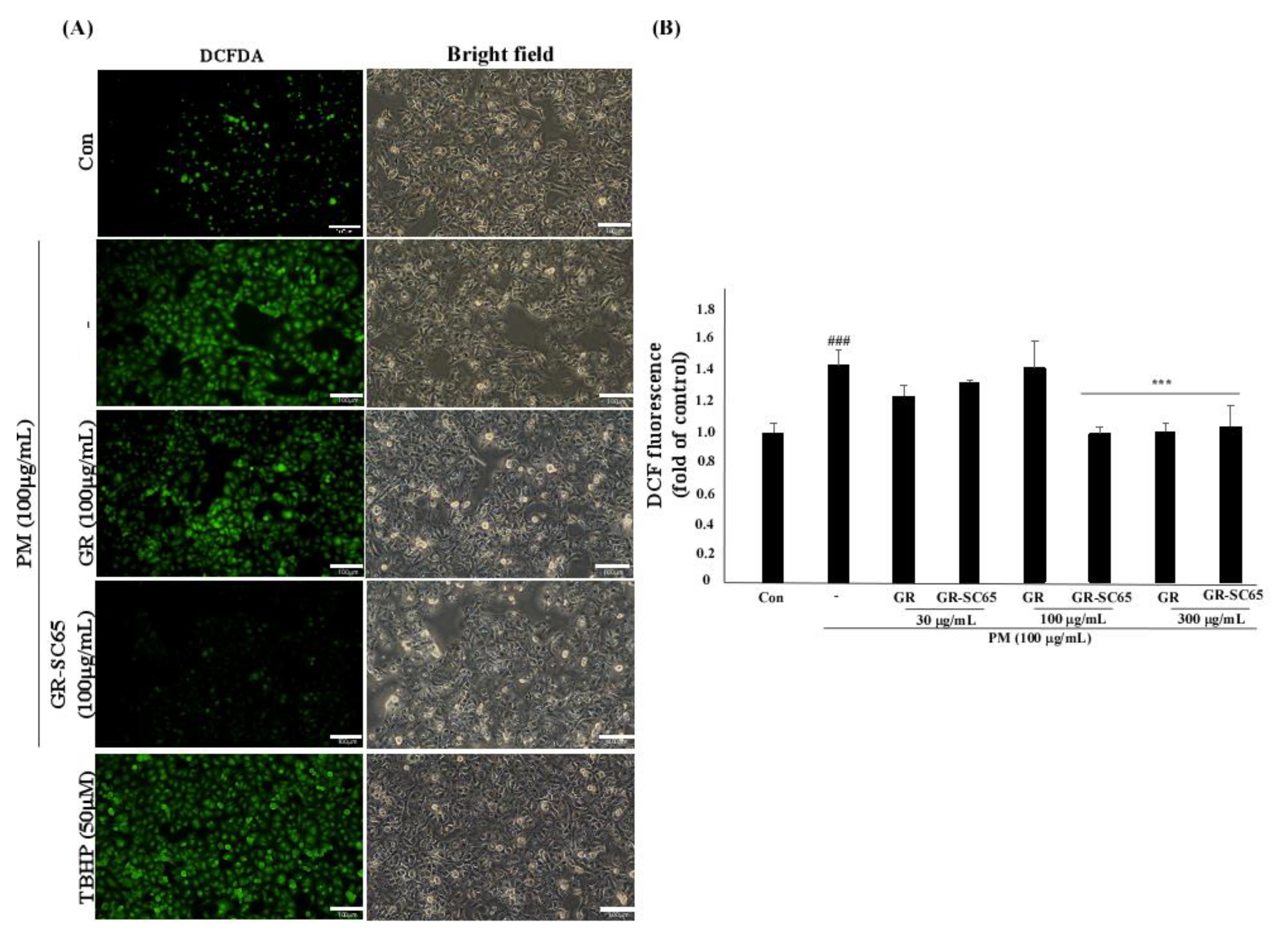
2.4. GR-SC65 Reduces PM-Induced ROS Generation in A549 Cells
2.5. GR-SC65 Protects A549 Cells from PM-Induced Apoptotic Cell Death
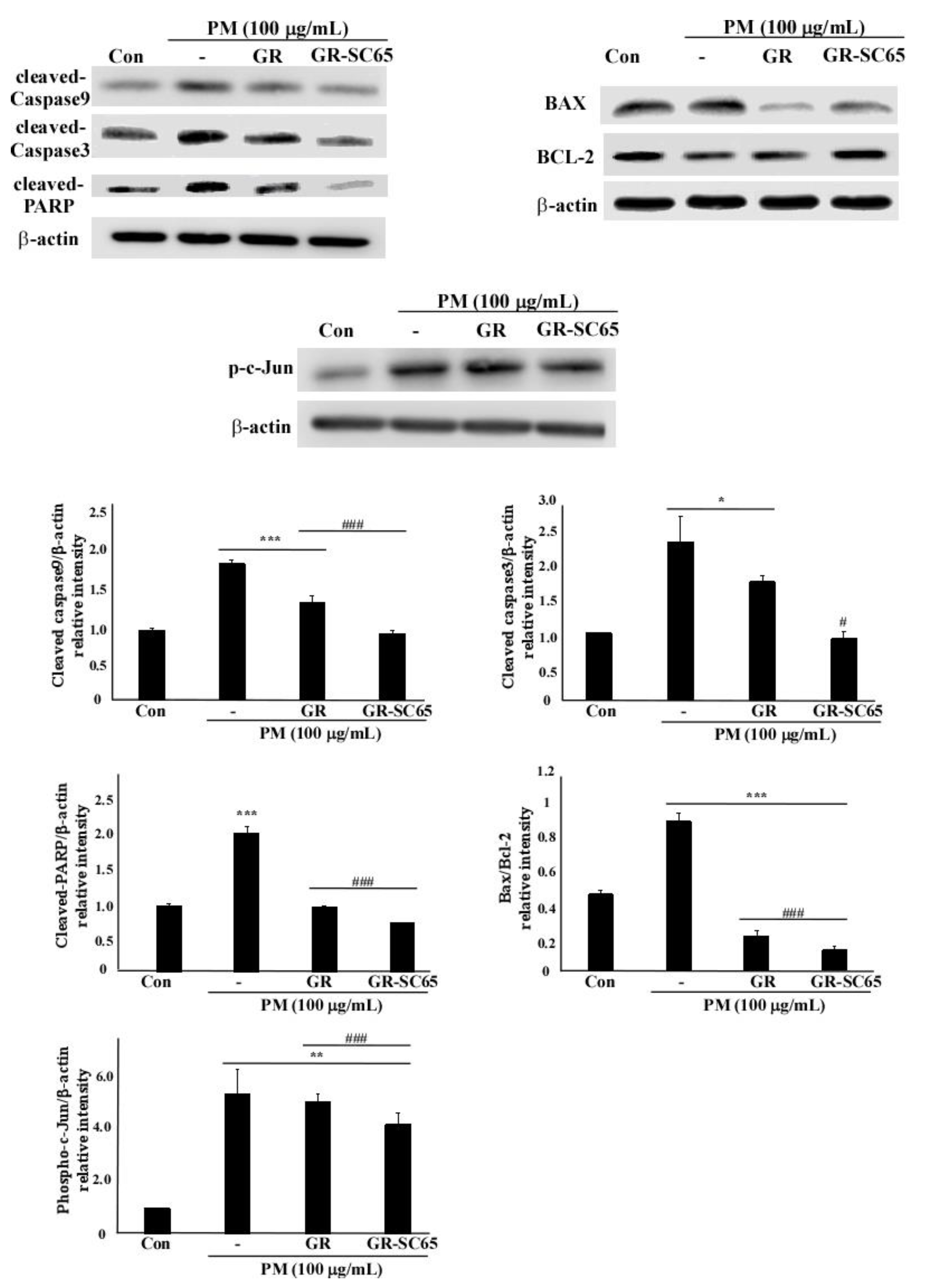
2.6. GR-SC65 Inhibits PM-Induced ROS-Dependent Apoptosis by Downregulating Active Caspase Protein Expression
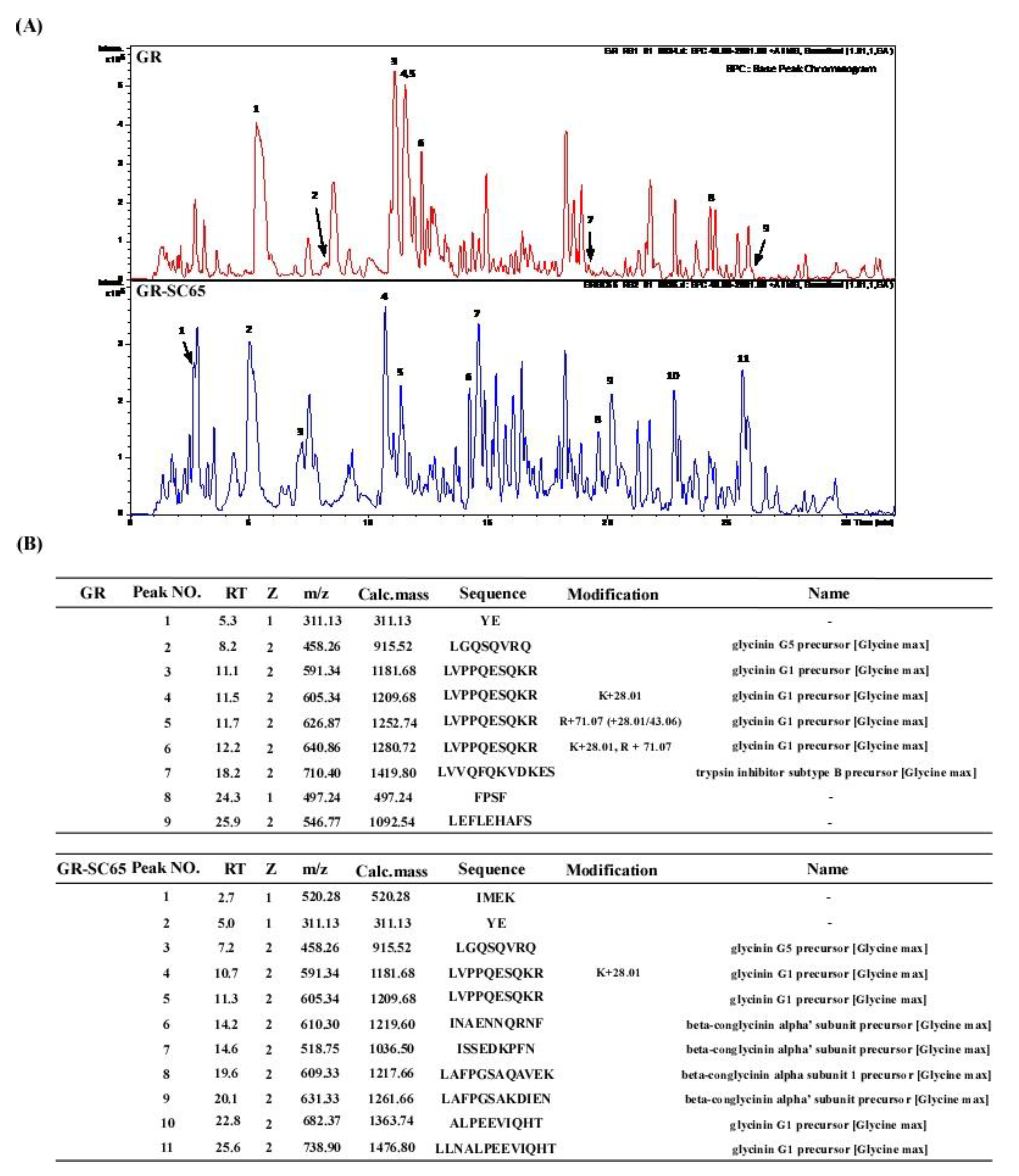
2.7. Comparison of Peptide Sequence of GR and GR-SC65
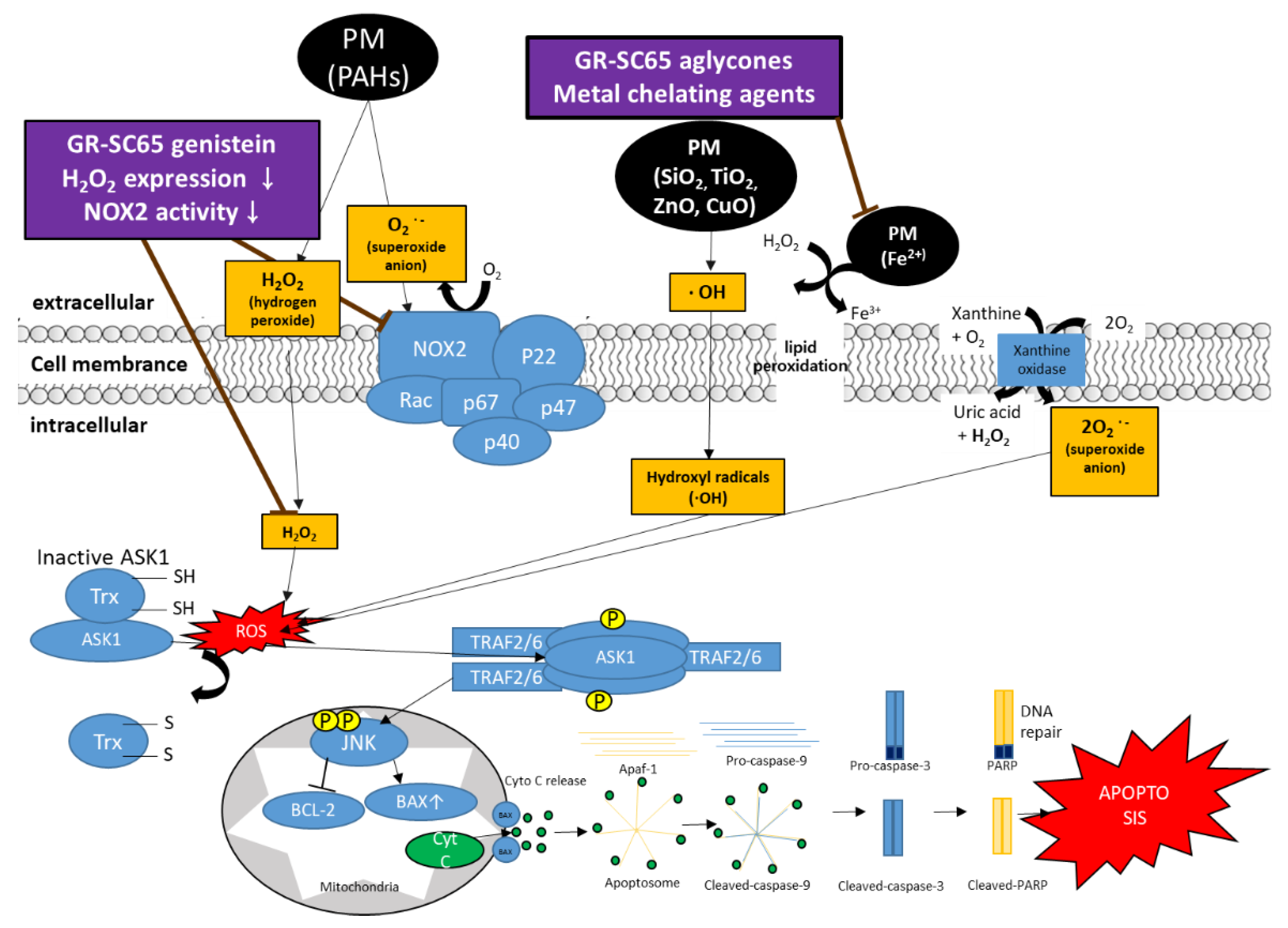
3. Discussion
4. Materials and Methods
4.1. Preparation of the Extract of Germinated Rhynchosia nulubilis (GR) Fermented with LAB
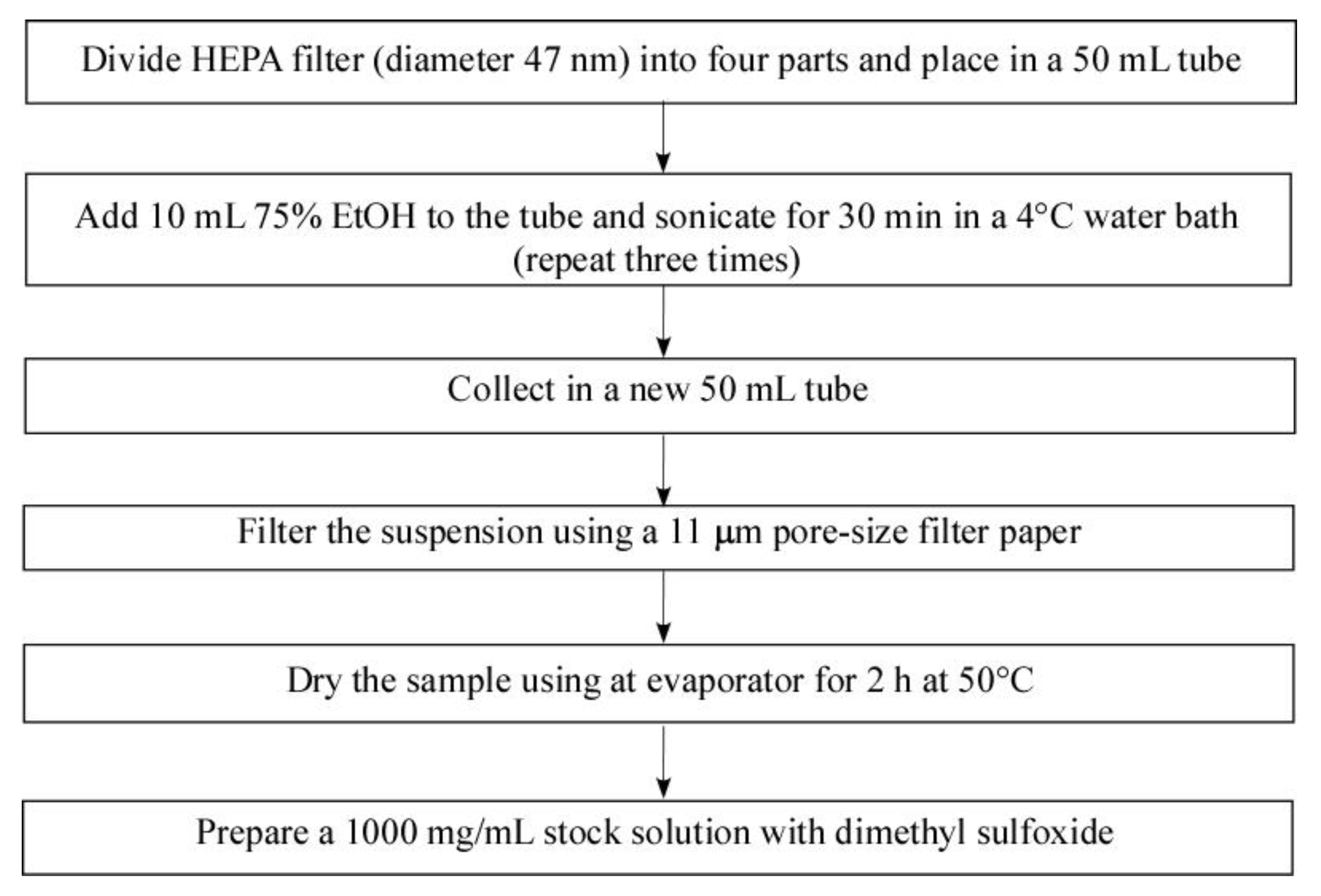
4.2. PM Collection and Extraction
4.3. Determination of Total Polyphenol Contents
4.4. DPPH Free Radical Scavenging Activity
4.5. Cell Culture
4.6. Morphology
4.7. Cell Viability
4.8. Reactive Oxygen Species (ROS) Assay
4.9. Hoechst 33342 and PI Double Staining
4.10. Western Blot Assay
4.11. Peptide Analysis by LC-MS
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Y.; Xie, X.; Jia, F.; He, J.; Li, Z.; Fu, M.; Hao, H.; Liu, Y.; Liu, J.Z.; Cowan, P.J.; et al. Ambient Fine Particulate Matter Induces Apoptosis of Endothelial Progenitor Cells Through Reactive Oxygen Species Formation. Cell. Physiol. Biochem. 2015, 35, 353–363. [Google Scholar] [CrossRef]
- Fernando, I.S.; Jayawardena, T.U.; Kim, H.-S.; Lee, W.W.; Vaas, A.; De Silva, H.; Abayaweera, G.; Nanayakkara, C.; Abeytunga, D.; Lee, D.-S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef]
- Hiura, T.S.; Kaszubowski, M.P.; Li, N.; Nel, A.E. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J. Immunol. 1999, 163, 5582–5591. [Google Scholar]
- Wei, H.; Liang, F.; Cheng, W.; Zhou, R.; Wu, X.; Feng, Y.; Wang, Y. The mechanisms for lung cancer risk of PM2.5: Induction of epithelial-mesenchymal transition and cancer stem cell properties in human non-small cell lung cancer cells. Environ. Toxicol. 2017, 32, 2341–2351. [Google Scholar] [CrossRef]
- Billet, S.; Garçon, G.; Dagher, Z.; Verdin, A.; LeDoux, F.; Cazier, F.; Courcot, D.; Aboukais, A.; Shirali, P. Ambient particulate matter (PM2.5): Physicochemical characterization and metabolic activation of the organic fraction in human lung epithelial cells (A549). Environ. Res. 2007, 105, 212–223. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Niu, X.-Y.; He, X.-J.; Shu, J. Ginsenoside Rg1 reduces toxicity of fine particulate matter on human alveolar epithelial cells: A preliminary observation. Mol. Med. Rep. 2013, 9, 989–992. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Kim, J.-E.; Cho, S.-H.; Song, G.-Y.; Bae, J.-S. Inhibitory effects of protopanaxatriol type ginsenoside fraction (Rgx365) on particulate matter-induced pulmonary injury. J. Toxicol. Environ. Health Part A 2019, 82, 338–350. [Google Scholar] [CrossRef]
- Kim, H.; Song, M.-J. Traditional Plant-Based Therapies for Respiratory Diseases Found in North Jeolla Province, Korea. J. Altern. Complement. Med. 2012, 18, 287–293. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Shi, H.; Slavin, M.; Huang, H.; Whent, M.; Sheng, Y.; Yu, L. Chemical Composition of 13 Commercial Soybean Samples and Their Antioxidant and Anti-inflammatory Properties. J. Agric. Food Chem. 2012, 60, 10027–10034. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Humfrey, C.D. Phytoestrogens and human health effects: Weighing up the current evidence. Nat. Toxins 1998, 6, 51–59. [Google Scholar] [CrossRef]
- Lee, A.-L.; Yu, Y.-P.; Hsieh, J.-F.; Kuo, M.-I.; Ma, Y.-S.; Lu, C.-P. Effect of germination on composition profiling and antioxidant activity of the polysaccharide-protein conjugate in black soybean [Glycine max (L.) Merr.]. Int. J. Biol. Macromol. 2018, 113, 601–606. [Google Scholar] [CrossRef]
- Cho, K.M.; Ha, T.J.; Lee, Y.B.; Seo, W.D.; Kim, J.Y.; Ryu, H.W.; Jeong, S.H.; Kang, Y.M.; Lee, J.H. Soluble phenolics and antioxidant properties of soybean (Glycine max L.) cultivars with varying seed coat colours. J. Funct. Foods 2013, 5, 1065–1076. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Peng, H.; Li, W.; Li, H.; Deng, Z.; Zhang, B. Extractable and non-extractable bound phenolic compositions and their antioxidant properties in seed coat and cotyledon of black soybean (Glycine max (L.) merr). J. Funct. Foods 2017, 32, 296–312. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Takahashi, R.; Ohmori, R.; Kiyose, C.; Momiyama, Y.; Ohsuzu, A.F.; Kondo, K. Antioxidant Activities of Black and Yellow Soybeans against Low Density Lipoprotein Oxidation. J. Agric. Food Chem. 2005, 53, 4578–4582. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.; Pardo, I.; Ferrer, S. Regulation of hdc expression and HDC activity by enological factors in lactic acid bacteria. J. Appl. Microbiol. 2008, 105, 1544–1551. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, J.Y.; Jeon, C.O. Source Tracking and Succession of Kimchi Lactic Acid Bacteria during Fermentation. J. Food Sci. 2015, 80, M1871–M1877. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, M. Antioxidant and Anti-Inflammatory Activity and Cytotoxicity of Ethanol Extracts from Rhynchosia nulubilis Cultivated with Ganoderma lucidum Mycelium. Prev. Nutr. Food Sci. 2018, 23, 326–334. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Halliwell, B.; Aeschbach, R.; Löliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- Imai, J.; Ide, N.; Nagae, S.; Moriguchi, T.; Matsuura, H.; Itakura, Y. Antioxidant and Radical Scavenging Effects of Aged Garlic Extract and its Constituents. Planta Medica 1994, 60, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Garro, M.S.; De Giori, G.S. Enzymatic hydrolysis of soybean protein using lactic acid bacteria. Food Chem. 2008, 111, 976–982. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F. Structural Analysis of Antioxidative Peptides from Soybean. beta.-Conglycinin. J. Agric. Food Chem. 1995, 43, 574–578. [Google Scholar] [CrossRef]
- Puchalska, P.; Marina, M.L.; García, M.C. Isolation and identification of antioxidant peptides from commercial soybean-based infant formulas. Food Chem. 2014, 148, 147–154. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Shah, A.S.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Newby, D.E.; Mills, N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 2013, 382, 1039–1048. [Google Scholar] [CrossRef]
- Hassanvand, M.S.; Naddafi, K.; Faridi, S.; Nabizadeh, R.; Sowlat, M.H.; Momeniha, F.; Gholampour, A.; Arhami, M.; Kashani, H.; Zare, A.; et al. Characterization of PAHs and metals in indoor/outdoor PM10/PM2.5/PM1 in a retirement home and a school dormitory. Sci. Total. Environ. 2015, 527–528, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Cho, H.-E.; Park, H.-J. Germinated black soybean fermented with Lactobacillus pentosus SC65 alleviates DNFB-induced delayed-type hypersensitivity in C57BL/6N mice. J. Ethnopharmacol. 2021, 265, 113236. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Shin, S.-R.; Kong, H.-J.; Choi, E.-M.; Woo, S.-C.; Lee, M.-H.; Yang, K.-M. Antioxidant activity of extracts from soybean and small black bean. Korean J. Food Preserv. 2014, 21, 404–411. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Crosby, L.M.; Waters, C.M.; Physiology, M. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L715–L731. [Google Scholar] [CrossRef]
- Sánchez-Pérez, Y.; Chirino, Y.I.; Osornio-Vargas, Á.R.; Morales-Bárcenas, R.; Gutiérrez-Ruíz, C.; Vázquez-López, I.; García-Cuellar, C.M. DNA damage response of A549 cells treated with particulate matter (PM 10) of urban air pollutants. Cancer Lett. 2009, 278, 192–200. [Google Scholar] [CrossRef]
- Roig, N.; Sierra, J.; Rovira, J.; Schuhmacher, M.; Domingo, J.L.; Nadal, M. In vitro tests to assess toxic effects of airborne PM10 samples. Correlation with metals and chlorinated dioxins and furans. Sci. Total Environ. 2013, 443, 791–797. [Google Scholar] [CrossRef]
- Oeder, S.; Dietrich, S.; Weichenmeier, I.; Schober, W.; Pusch, G.; Jörres, R.A.; Schierl, R.; Nowak, D.; Fromme, H.; Behrendt, H.; et al. Toxicity and elemental composition of particulate matter from outdoor and indoor air of elementary schools in Munich, Germany. Indoor Air 2011, 22, 148–158. [Google Scholar] [CrossRef]
- Vuong, N.Q.; Breznan, D.; Goegan, P.; O’Brien, J.S.; Williams, A.; Karthikeyan, S.; Kumarathasan, P.; Vincent, R. In vitro toxicoproteomic analysis of A549 human lung epithelial cells exposed to urban air particulate matter and its water-soluble and insoluble fractions. Part. Fibre Toxicol. 2017, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Holme, J.A.; Schwarze, P.E. Activation of Proinflammatory Responses in Cells of the Airway Mucosa by Particulate Matter: Oxidant- and Non-Oxidant-Mediated Triggering Mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Seriani, R.; Junqueira, M.D.S.; De Toledo, A.C.; Martins, M.A.; Seckler, M.; Alencar, A.M.; Negri, E.M.; Silva, L.F.F.; Mauad, T.; Saldiva, P.H.N.; et al. Diesel exhaust particulates affect cell signaling, mucin profiles, and apoptosis in trachea explants of Balb/C mice. Environ. Toxicol. 2014, 30, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Velali, E.; Papachristou, E.; Pantazaki, A.; Choli-Papadopoulou, T.; Planou, S.; Kouras, A.; Manoli, E.; Besis, A.; Voutsa, D.; Samara, C. Redox activity and in vitro bioactivity of the water-soluble fraction of urban particulate matter in relation to particle size and chemical composition. Environ. Pollut. 2016, 208, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Q.; Yang, X.; Li, G.; Zhang, J.; Zhou, X.; Jiang, W. Cytotoxicity of the soluble and insoluble fractions of atmospheric fine particulate matter. J. Environ. Sci. 2020, 91, 105–116. [Google Scholar] [CrossRef]
- Dos Santos, M.; Gómez, D.; Dawidowski, L.; Gautier, E.; Smichowski, P. Determination of water-soluble and insoluble compounds in size classified airborne particulate matter. Microchem. J. 2009, 91, 133–139. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Hsu, L.; Lee, C.; Chiang, Y.; Lee, M.; How, J.; Wu, C.; Huang, C.; Lee, I.-T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef]
- Vogel, C.F.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- Goodale, B.C.; La Du, J.; Tilton, S.C.; Sullivan, C.M.; Bisson, W.H.; Waters, K.M.; Tanguay, R.L. Ligand-Specific Transcriptional Mechanisms Underlie Aryl Hydrocarbon Receptor-Mediated Developmental Toxicity of Oxygenated PAHs. Toxicol. Sci. 2015, 147, 397–411. [Google Scholar] [CrossRef]
- Jheng, H.-F.; Hayashi, K.; Matsumura, Y.; Kawada, T.; Seno, S.; Matsuda, H.; Inoue, K.; Nomura, W.; Takahashi, H.; Goto, T. Anti-Inflammatory and Antioxidative Properties of Isoflavones Provide Renal Protective Effects Distinct from Those of Dietary Soy Proteins against Diabetic Nephropathy. Mol. Nutr. Food Res. 2020, 64, e2000015. [Google Scholar] [CrossRef] [PubMed]
- Vernaza, M.G.; Dia, V.P.; De Mejia, E.G.; Kil Chang, Y. Antioxidant and antiinflammatory properties of germinated and hydrolysed Brazilian soybean flours. Food Chem. 2012, 134, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Salika, A.; Theodoropoulou, A. Generation of hydroxyl radicals by urban suspended particulate air matter. The role of iron ions. Atmos. Environ. 2000, 34, 2379–2386. [Google Scholar] [CrossRef]
- Abolfath, R.M.; Van Duin, A.C.T.; Brabec, T. Reactive Molecular Dynamics Study on the First Steps of DNA Damage by Free Hydroxyl Radicals. J. Phys. Chem. A 2011, 115, 11045–11049. [Google Scholar] [CrossRef]
- Toscano, M.; Russo, N. Soybean aglycones antioxidant activity. A theoretical investigation. Comput. Theor. Chem. 2016, 1077, 119–124. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, L.; Zhang, Y.; Hu, H.; Shi, Y.; Liang, S.; Zhao, T.; Fu, Y.; Duan, J.; Sun, Z. Cytotoxicity induced by fine particulate matter (PM2.5) via mitochondria-mediated apoptosis pathway in human cardiomyocytes. Ecotoxicol. Environ. Saf. 2018, 161, 198–207. [Google Scholar] [CrossRef]
- Nadeau, P.J.; Charette, S.J.; Toledano, M.B.; Landry, J. Disulfide Bond-mediated Multimerization of Ask1 and Its Reduction by Thioredoxin-1 Regulate H2O2-induced c-Jun NH2-terminal Kinase Activation and Apoptosis. Mol. Biol. Cell 2007, 18, 3903–3913. [Google Scholar] [CrossRef]
- Papadakis, E.S.; Finegan, K.G.; Wang, X.; Robinson, A.C.; Guo, C.; Kayahara, M.; Tournier, C. The regulation of Bax by c-Jun N-terminal protein kinase (JNK) is a prerequisite to the mitochondrial-induced apoptotic pathway. FEBS Lett. 2006, 580, 1320–1326. [Google Scholar] [CrossRef]
- Hongmei, Z. Extrinsic and Intrinsic Apoptosis Signal Pathway Review. In Apoptosis and Medicine; InTechOpen: London, UK, 2012. [Google Scholar]
- Zhou, Z.; Liu, Y.; Duan, F.; Qin, M.; Wu, F.; Sheng, W.; Yang, L.; Liu, J.; He, K. Transcriptomic Analyses of the Biological Effects of Airborne PM2.5 Exposure on Human Bronchial Epithelial Cells. PLoS ONE 2015, 10, e0138267. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Song, M.-J.; Lee, H.-J.; Park, T.-S.; Kim, M.I.; Park, H.-J. Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis. Int. J. Mol. Sci. 2018, 19, 3504. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kwon, H.-K.; Kim, H.S.; Kim, M.I.; Park, H.-J. Hair Growth Promoting Effect of 4HGF Encapsulated with PGA Nanoparticles (PGA-4HGF) by β-Catenin Activation and Its Related Cell Cycle Molecules. Int. J. Mol. Sci. 2019, 20, 3447. [Google Scholar] [CrossRef] [PubMed]
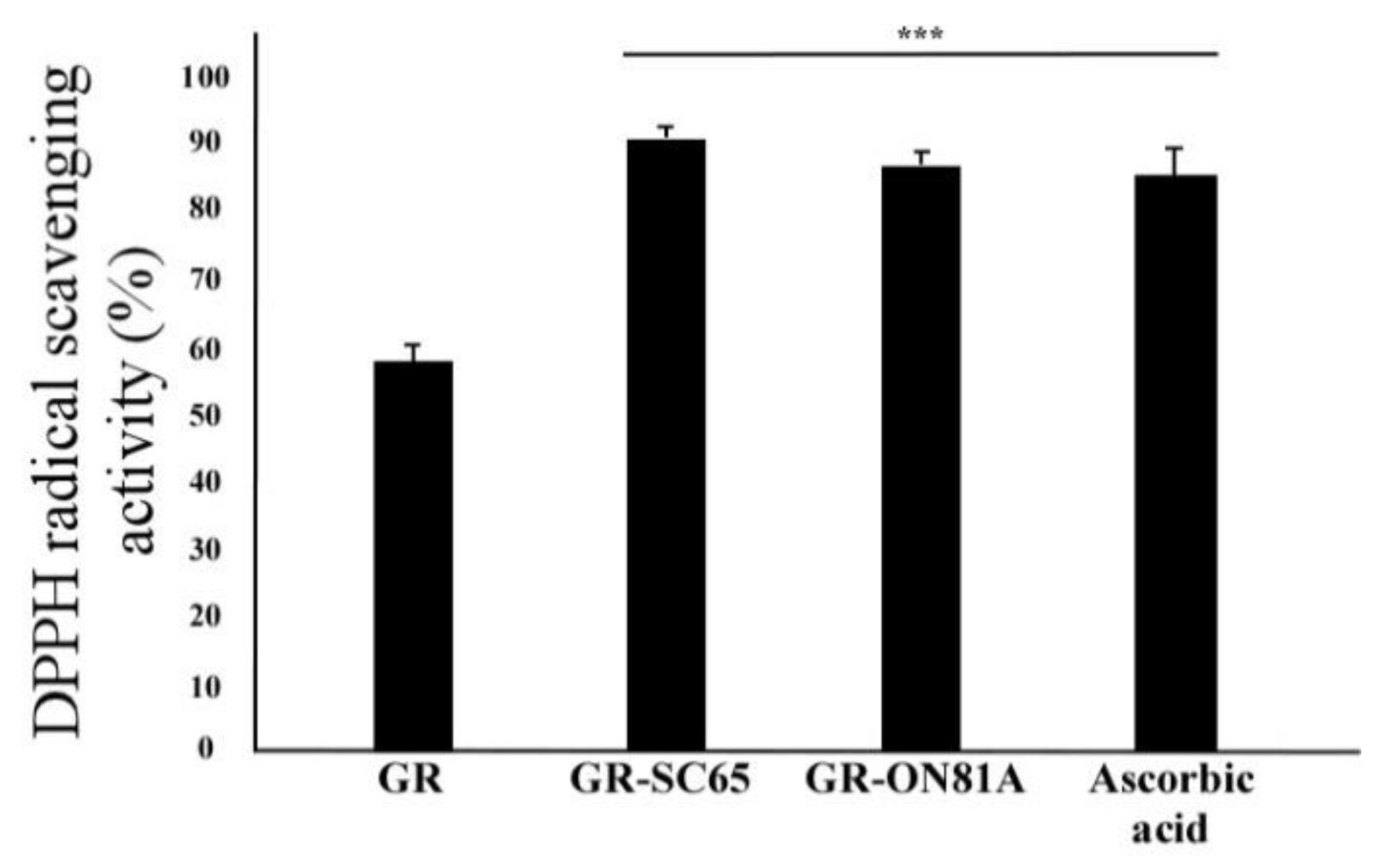

| Temperature [°C] | Sample | Total Phenolic Content (mg Gallic Acid Equivalents (GAE)/g Sample) |
|---|---|---|
| 27 | GR | 80.9 ± 0.6 |
| GR-SC65 | 113.2 ± 2.3 *** | |
| GR-ON81A | 89.8 ± 0.2 | |
| 50 | GR | 93.8 ± 1.3 |
| GR-SC65 | 161.6 ± 1.9 ### | |
| GR-ON81A | 158.8 ± 2.8 ### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Park, H.-J. Germinated Rhynchosia nulubilis Fermented with Lactobacillus pentosus SC65 Reduces Particulate Matter Induced Type II Alveolar Epithelial Apoptotic Cell Death. Int. J. Mol. Sci. 2021, 22, 3660. https://doi.org/10.3390/ijms22073660
Lee H-J, Park H-J. Germinated Rhynchosia nulubilis Fermented with Lactobacillus pentosus SC65 Reduces Particulate Matter Induced Type II Alveolar Epithelial Apoptotic Cell Death. International Journal of Molecular Sciences. 2021; 22(7):3660. https://doi.org/10.3390/ijms22073660
Chicago/Turabian StyleLee, Hye-Ji, and Hye-Jin Park. 2021. "Germinated Rhynchosia nulubilis Fermented with Lactobacillus pentosus SC65 Reduces Particulate Matter Induced Type II Alveolar Epithelial Apoptotic Cell Death" International Journal of Molecular Sciences 22, no. 7: 3660. https://doi.org/10.3390/ijms22073660
APA StyleLee, H.-J., & Park, H.-J. (2021). Germinated Rhynchosia nulubilis Fermented with Lactobacillus pentosus SC65 Reduces Particulate Matter Induced Type II Alveolar Epithelial Apoptotic Cell Death. International Journal of Molecular Sciences, 22(7), 3660. https://doi.org/10.3390/ijms22073660






