Abstract
Biocompatible nanoparticles (NPs) containing polymers, lipids (liposomes and micelles), dendrimers, ferritin, carbon nanotubes, quantum dots, ceramic, magnetic materials, and gold/silver have contributed to imaging diagnosis and targeted cancer therapy. However, only some NP drugs, including Doxil® (liposome-encapsulated doxorubicin), Abraxane® (albumin-bound paclitaxel), and Oncaspar® (PEG-Asparaginase), have emerged on the pharmaceutical market to date. By contrast, several phytochemicals that were found to be effective in cultured cancer cells and animal studies have not shown significant efficacy in humans due to poor bioavailability and absorption, rapid clearance, resistance, and toxicity. Research to overcome these drawbacks by using phytochemical NPs remains in the early stages of clinical translation. Thus, in the current review, we discuss the progress in nanotechnology, research milestones, the molecular mechanisms of phytochemicals encapsulated in NPs, and clinical implications. Several challenges that must be overcome and future research perspectives are also described.
1. Introduction
Cancer progression is the result of tumor development and subsequent metastasis, with features including increases in the growth rate and invasiveness of tumor cells [1]. Though cancer therapies such as chemotherapy, radiotherapy, immunotherapy, and surgery have been used to suppress cancer progression for years, cancer treatment is still frequently unsuccessful due to poor solubility, low stability, limited biodistribution, metabolism, chemoresistance, and toxicity [2].
Hence, to overcome the poor bioavailability of anticancer agents, including natural compounds, nanobiotechnologies such as nanoparticles (NPs) have been employed for efficient drug delivery to mitigate poor solubility and stability, prevent degradation by proteases, improve drug distribution, and reduce drug resistance [3].
Accumulating evidence reveals that organic NPs (such as polymeric conjugates, polymeric NPs, lipid-based carriers (liposomes and micelles), dendrimers, and ferritin) and inorganic NPs (such as carbon nanotubes, quantum dots (QDs), ceramic NPs, magnetic NPs, and gold/silver NPs) are useful nanomaterials for achieving the enhanced permeability and retention (EPR) effect [4,5]. Furthermore, emerging evidence demonstrates that NPs that include a hydrophilic central core, a target-oriented biocompatible outer layer, and a middle hydrophobic core containing the target site can improve drug/gene delivery in cancer cells and tissues for ligand- or antigen-targeted therapy [6].
Though anticancer nanodrugs such as Doxil and Abraxane are on the pharmaceutical market, several nano-phytochemicals, defined as nanomaterials and phytochemicals, including curcumin [7] and EGCG [8], are attractive cancer therapy candidates, as experimental data suggest that they result in improved drug delivery and have low toxicity in several cancers. Thus, in this review, we discuss the recent progress of NP biotechnology, the molecular mechanisms of nano-phytochemicals, and their implications for the possible clinical application of potent anticancer nanodrugs on the basis of experimental studies in several cancers. Future research perspectives are also suggested.
2. Research Milestones in Nanotechnology and Anticancer Nanodrugs
Over the past decade, nanotechnology has greatly contributed to biomedical sciences, including oncology, with the development of efficient delivery systems. Colloidal gold particles were first synthesized as a typical hydrophobic colloid by Turkevich’s group in 1951 [9], and in 1965, Bangham’s group introduced liposomes as nanocarriers with composite structures made of phospholipids for transporting proteins and drugs [10]. Since then, polymeric NPs have included biodegradable and biocompatible polymers of synthetic (polylactide, polylactide–polyglycolide copolymers, polycaprolactones, and polyacrylates) or natural (alginate, albumin, or chitosan) origin. Wichterle et al. discovered hydrophilic gels in 1960, and Langer and Folkman were the first to introduce polymer systems appropriate for the controlled release of ionic molecules and macromolecules [11]. In 1978, Fritz Vögtle’s group became the first to synthesize dendrimers with structural stability [12]. Thereafter, Fraley et al. [13] reported the liposome-mediated delivery of DNA in 1980, and Gabizon et al. suggested that liposomal delivery improved the therapeutic index of encapsulated doxorubicin in 1982 [14]. In addition, QDs were synthesized by Ekimov’s group in 1982 [15], and in 1986, Matsumura and Maeda proposed the concept of the EPR effect, whereby nano-sized molecules accumulate more in tumor tissues than in normal tissues [16]. Among anticancer nanodrugs, the US FDA approved DOXIL (doxorubicin HCL liposome injection) for AIDS-related Kaposi’s sarcoma treatment in 1995 [17], DaunoXome (liposomal encapsulated daunorubicin) for HIV-related Kaposi’s sarcoma treatment in 1996 [18], Eligard (leuprolide acetate and polymer) for prostate cancer treatment in 2004 [19], Abraxane (albumin-bound paclitaxel injection) for metastatic pancreatic cancer treatment in 2005 [20], Oncaspar (Pegaspargase conjugated to mPEG) for acute lymphoblastic leukemia treatment in 2006 [21], Marqibo (vincristine sulfate liposome injection) for Philadelphia chromosome-negative lymphoblastic leukemia treatment in 2012 [22], and Onivyde (liposomal irinotecan) for pancreatic cancer treatment in 2015 [23]. Additionally, Myocet (non-PEGylated liposomal doxorubicin) was approved for metastatic breast cancer treatment in combination with cyclophosphamide in 2000 by the regulatory agencies of Europe and Canada [24]. Recently, anticancer nano-phytochemicals have been receiving increasing interest for their improved drug delivery and lower toxicity compared to synthetic anticancer agents. Among these compounds, gelatin/sugar-coated lycopene NPs were first characterized by Wegmann et al. in 2002 [25], and solid lipid NPs (SLNs) containing ferulic acid were characterized and developed as a sunscreen by Souto et al. in 2005 [26]. Additionally, Takahashi et al. prepared curcumin-loaded liposomes for encapsulation in 2006 [27], and in 2006, Hung et al. showed the NP potential of resveratrol coated with an emulsion-liposome blend, and no liver or kidney toxicity was detected [28]. Along the same line, systems with reported NP potential include gambogic acid-loaded micelles based on a chitosan derivative [29], ginsomes (ginsenoside-based NPs) for reinforcing the immune response of T and B lymphocyte in mice in 2009 [30], and EGCG encapsulated in PLA-PEG NPs [31] (Figure 1). Despite the advanced progress of nanotechnology over the past decade and a variety of potent nanodrugs or nano-phytochemicals, numerous questions and challenges remain for the future development of commercial anticancer nanodrugs. With this aim, this review focuses on a variety of NPs associated with phytochemicals, their drug delivery efficiency, and their anticancer efficacy. Then, future research directions are suggested.
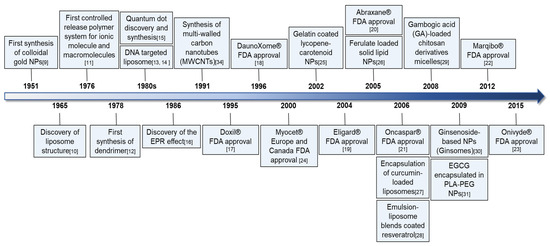
Figure 1.
Research milestones of nanotechnology, anticancer nanodrugs, and nano-phytochemicals.
3. Recent Advances in Nanotechnology Targeting Cancer Progression
A variety of NPs (1~100 nm) have been applied as efficient drug delivery systems, with advantages such as a high efficiency of drug loading and good bioavailability, drug delivery, and pharmacokinetics [32]. Generally, organic NPs include polymeric conjugates, polymeric nanoparticles, lipid-based carriers (liposomes and micelles), dendrimers, and ferritin, while inorganic NPs include carbon nanotubes, ceramic NPs, quantum dots, magnetic NPs, and gold/silver NPs [33] with specific nanostructure morphologies and sizes. Over the past decade, these NPs have been applied in the biomedical sciences for diagnosis and targeted drug delivery in cancer treatment (Figure 2).
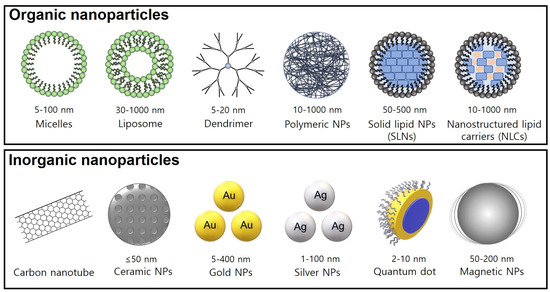
Figure 2.
Morphology and size of nanostructured NPs. The physicochemical properties of these NPs include biocompatibility, biodegradability, and controlled/targeted drug release.
3.1. Carbon-Based NPs
Carbon-based NPs are generally classified as single-walled carbon nanotubes (SWCNTs) and multiple-walled carbon nanotubes (MWCNTs) [34], which were first reported by Sumio Iijima [35,36] in 1991. These CNTs have attracted interest for their unique physicochemical properties that allow them to cross the cell membrane [37], as well as their highly versatile materials and enormous potential for biomedical applications [38]. However, CNTs are known to be cytotoxic in MC4L2 cells and mice. The antitumor effect of CNTs was confirmed in an animal model of breast cancer [39], and their increased cytotoxicity was attributed to the induction of oxidative stress in murine breast cancer [40]. Srivastava et al. [41] also demonstrated that MWCNTs induced oxidative stress and apoptosis in A549 cells. Similarly, Wang et al. [42] suggested that SWCNTs induced oxidative damage and apoptosis in PC-12 cells along with autophagy through the AKT–TSC2–mTOR pathway in non-small cell lung cancer cells (NSCLCs) [43]. Recently, Rh2 ginsenoside-hyaluronic acid-functionalized zinc oxide (Rh2HAZnO) was shown to have an enhanced antitumor effect compared to Rh2 alone in A549, HT29, and MCF7 cancer cells [44], and ZnO/carbon nanotubes were FDA-approved as safe in the body [45].
3.2. Ceramic NPs
Ceramic NPs (CNs) have emerged as drug delivery vehicles [46]. Silica nanoparticles (SNPs), discovered in 1992 by Kresge et al. [47], are known to have superior textual properties, such as a high surface area, narrow pore size distribution, large pore volume, and tunable pore diameter, for disease diagnosis and cancer imaging [48]. Recently, Yuan et al. [49] reported that SNPs encapsulating doxorubicin exerted antitumor effects through a burst release at an early stage and sustained release at a later stage. However, ceramic NPs have been reported to induce oxidative stress and inflammation in the lungs, liver, heart, and brain, leading to prethrombosis, genotoxicity, carcinogenicity, and teratogenicity, or brain toxicity [50]. Similarly, cerium oxide (CeO2) NPs induced apoptosis via ROS generation and p53-dependent mitochondrial signaling in HCT116 colorectal cancer cells [51]. Recently, Cinnamomum cassia extract was reported to protect against liver and kidney damage induced by nickel nanoparticles (Ni-NPs), which are used in applications such as ceramics and nanomedicine, in male Sprague Dawley rats [52]. Moreover, licorice isoliquiritigenin-encapsulated mesoporous silica nanoparticles (MSNs-ISL NPs) significantly suppressed receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast generation, and the effect was greater than that achieved using ISL or MSNs alone [53].
3.3. Metal NPs
NPs containing metals such as gold, silver, copper, zinc, and palladium have unique physicochemical properties that are suitable for drug delivery and ligand targeting [54]. Among these complexes, gold NPs are regarded as bio-inert and non-cytotoxic and are used for medical applications such as MRI for imaging diagnosis and the photothermal treatment of cancer, in which NPs generate heat when exposed to near-infrared (NIR) laser light [55], with no acute cytotoxicity [56]. Indeed, Tsai et al. [57] reported that gold NPs inhibited proinflammatory cytokine production and TLR9 translocation via CpG oligodeoxynucleotides (CpG-ODNs), and Farooq et al. [58] demonstrated the anticancer and cytotoxic effects and intracellular localization of AuNPs in HeLa cells. Similarly, silver NPs have been used for biomedical applications such as infertility, antibacterial effects, skin damage, burns, and cancer treatment despite their harmful nanotoxicity [59,60]. Gurunathan et al. [61] reported that AgNPs, which are characterized by high yield, solubility, and stability, exerted dose-dependent cytotoxicity in MDA-MB-231 cells through the activation of lactate dehydrogenase (LDH), caspase-3, and reactive oxygen species (ROS) in breast cancer [62]. Similarly, B-AgNPs and F-AgNPs derived from Bacillus tequilensis and Calocybe indica (milky mushroom) extract induced apoptosis via the activation of p53, p-Erk1/2, and caspase-3 signaling and the downregulation of Bcl-2 in MDA-MB-231 breast cancer cells [62]. Interestingly, the release of Zn2+ from zinc peroxide nanoparticles (ZnO2 NPs) exerted an anticancer effect in a synergistic fashion with ROS production [63]. Ruenraroengsak et al. [64] demonstrated that mesoporous silica nanolayer (MSN)-ZnO-AuNSs reduced the viability of CAL51/CALDOX cells and MCF7/MCF-7-TX cells, while MSN-ZnO-AuNSs conjugated with Frizzled-7 (FZD-7) enhanced the toxicity by three-fold in resistant MCF-7TX cells. Further study on the efficacy and toxicity of metal NPs in animals and humans is required for their potential use in cancer diagnosis and therapy.
3.4. Quantum Dots
Quantum dots (QDs) are among the emerging engineering nanomaterials that have shown promise as a platform for cancer detection and diagnosis; examples include CdSe, ZnS, CdSe, ZnS, and CdS, which have unique optical and chemical properties [65]. Generally, colloidal QDs have been synthesized for diagnostic and therapeutic purposes in living systems through the processes of core development, shell growth, solubilization, and biological binding [66]. Lee et al. [67] reported that MNP-QD conjugates enhanced cellular uptake in HeLa cells without non-specific binding to the cell membrane, making them a promising new cell imaging technique. However, one drawback of QDs is their toxicity, which is caused by the release of factors that induce oxidative stress, including ROS, inflammatory cytokines, and metal ions [68]. Recently, curcumin quantum dots (CurQDs) were found to enhance the degradation of bacterial biofilms compared to curcumin alone [69], and folic acid [70] and chlorophyllin [71] have also emerged as promising QD NP candidates for imaging diagnosis.
3.5. Magnetic NPs
Magnetic NPs (MNPs) have been widely used for diverse applications, including magnetic biosensing (diagnostics), magnetic imaging, magnetic separation, drug and gene delivery, and hyperthermia therapy [72]. Interestingly, among MNPs, magnetic iron oxide NPs conjugated with integrin αvβ6 antibodies are known to have antitumor effects in oral squamous cell carcinoma [73]. Recently, galbanic acid-coated Fe3O4 MNPs were found to exert cytotoxic effects in PC3, LNCaP, and DU145 prostate cancer cells via the downregulation of AR, while galbanic acid was cytotoxic only in LNCaP cells [74].
3.6. Polymeric NPs
Polymeric NPs consisting of a reservoir system (nanocapsule) and matrix system (nanosphere) can incorporate hydrophilic/hydrophobic drug particles by coating inert materials in imaging, targeted drug delivery, and biomedical applications [75]. Known natural polymeric NPs include gelatin, chitosan, collagen, gum arabic, starch [76], dextran [77], alginate [78], and polylactic acid, while synthetic polymeric NPs in the form of dendrimers include N-(2-hydroxypropyl) methacrylamide (HPMA), polyglycolide or polyglycolic acid (PGA), PGA polyethylene glycol (PEG), polypropylenimine, polyamidoamine, and hydrogels [79,80]. Among the listed compounds, chitosan, a cationic polysaccharide, has been effectively used for the treatment of several cancers; its beneficial properties are biodegradability, mucoadhesiveness, enhanced absorption, and biocompatibility [81,82]. For instance, Amjadi et al. [83] suggested that DOX@BET-loaded PGNPs—betanin (BET) and doxorubicin (DOX) encapsulated by gelatin nanoparticles (GNPs)—showed better anticancer activity in MCF-7 cells compared to DOX or BET alone. Similarly, pretreatment with collagozomes followed by paclitaxel micelles facilitated drug penetration into pancreatic ductal adenocarcinoma, resulting in better antitumor activity [84], while gum arabic-encapsulated gold NPs (GA-AuNPs) combined with the application of lasers induced cell death in lung tumor tissues via the inhibition of inflammation, angiogenesis, and lipid peroxidation [85]. Furthermore, Babu et al. [86] suggested that the combinatorial delivery of cisplatin and p62siRNA/β5 plasmid DNA-mediated chitosan-coated polylactic acid NPs enhanced the antitumor effect of cisplatin in resistant ovarian cancer cells. Additionally, Strong et al. [87] reported that polyhydrogels with silica–gold nanoshells loaded with either doxorubicin or a DNA duplex enhanced drug release by 2–5-fold after exposure to NIR light in CT 26-WT colon cancer cells.
3.7. Lipid-Based NPs
Lipid-based NPs with low toxicity include liposomes, micelles, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs) [88,89]. However, a major drawback of SLNs is their low drug loading capacity due to the release of the loaded drug solution [90]. NLCs have a less defined lattice defect with an incomplete crystal or amorphous structure. Consequently, drug loading occurs at the defect site, leading to greater accumulation and less excretion of the drug, since the compartment is surrounded by solid lipids [91,92]. Liposomes have a bilayer structure that is mainly composed of phospholipids, and they can encapsulate both hydrophobic and hydrophilic drugs because of the amphiphilic properties of phospholipids. Furthermore, liposomes can improve stability and efficiency by preventing the drug from being metabolized [93,94]. Notably, lipid NPs with aqueous cores can be used as carriers of hydrophilic drugs, mainly for oral delivery, while polymeric NPs, including carbon nanotubes, dendrimers, and quantum dots, can encapsulate water-insoluble drugs in their hydrophobic cores for injectable drug delivery systems [95]. Wang et al. [96] suggested that myricetin nanoliposomes (MYR-NLs) enhanced apoptosis in DBTRG-05MG glioblastoma multiforme (GBM) cells by reducing glycolysis. Similarly, Maroufi et al. [97] reported that myricetin-loaded nanostructured lipid carriers (NLCs) enhanced the anticancer effect of DXT via the downregulation of Mcl-1, survivin, and cyclin B1 and the upregulation of Bid and Bax in MDA-MB231 breast cancer cells.
3.8. Dendrimers
Dendrimers are manufactured using several macromolecules, including polypropyleneimine (PPI), polyamidoamine (PAMAM), poly-L-lycine (PLL), melamine, triazine, and poly(ethylene glycol) (PEG) [98]. Dendrimers are suitable for loading hydrophilic and hydrophobic drugs and are characterized by branches, distinct molecular weights, and a globular assembly with a meticulous surface, and they have been studied since Dr. Donald Tomalia first published his work on poly(amidoamine) (PAMAM) dendrimers in 1985 [99]. Indeed, Dickwalkar et al. [100] reported that polyamidoamine dendrimers (PAMAMG4.0-NH2) conjugated with the omega-3 fatty acid docosahexaenoic acid (DHA) and paclitaxel (PTX) enhanced the anticancer activity of PTX compared to PTX or PAX (PAMAMG4.0 -NH2 -PTX) alone in upper gastrointestinal cancer cells. Recently, Mignani et al. [101] suggested that several anticancer phytochemicals, including epirubicin (4′-epi-isomer of DOX), methotrexate, daidzein, genistein, 7-ethyl-10-hydroxycamtothecin (SN-38), colchicine, 7-butyl-10-aminocamptothecin, lamellarin D, digoxin, biotin, a tubulysin D analog, and 10-hydroxycamptothecin, could be conjugated only with dendrimers for enhanced anticancer activity. Accumulating evidence also suggests that dendrimers combined with phytochemicals can serve as drug and gene carriers that impart good solubility and bioavailability to hydrophobic drugs in cancer therapy [101,102]. Aas et al. [103] reported that dendrosomal farnesiferol C (DFC) significantly enhanced the anti-proliferative effect in a time- and dose-dependent manner compared to FC alone in AGS cells.
3.9. The Enhanced Permeability and Retention Effect
The enhanced permeability and retention (EPR) effect first coined by Matsumura and Maeda [16] is defined the phenomenon of macromolecules or high molecular weight drug and nanomedicine accumulation inside solid tumor models compared to healthy tissue counterparts [4]. EPR effect is usually induced by a leaky tumor vasculature by the accelerated angiogenesis and impaired lymphatic drainage by the disorganized growth of tumors [104,105]. In details, NPs with appropriate sizes can evade the tumor capillaries and be retained in the tumor tissues for days due to the lack of lymphatic drainage. Additionally, particles with high positive charges can bind non-specifically to the negatively charged luminal surface due to the presence of sulfate and carboxylate sugar moieties [106]. It is noteworthy that nitric oxide (NO), prostaglandins, and bradykinin, which act as vasodilators, can enhance the EPR effect in tumors by increasing their vascular permeability [107]. Furthermore, some nanomedicine formulations are effective in the treatment of multidrug resistance (MDR) [108]. In contrast, Jain et al. [109] claimed that elevated interstitial fluid pressure and heterogeneous blood supply limit macromolecular delivery to tumors. Furthermore, a key challenge is the promotion of the EPR effect in patients with EPR-insensitive tumor phenotypes since the EPR-insensitive phenotype is known to have smaller endothelial fenestrations, heterogeneously high or low pericyte coverage, more developed and branched vasculatures, a relatively dense ECR, and more developed immune profiles compared to EPR sensitive phenotypes [105]. Thus, the role of EPR is still in question in terms of clinical translation and different human tumor types due to their heterogeneity [105,110]. Among the three major targeted drug delivery methods, namely, passive targeting, active targeting, and physical targeting; passive targeting acts via the EPR effect, by which tumor cells preferentially absorb NPs [111]. In active targeting, NPs that are functionalized with ligands such as proteins, antibodies, and peptides effectively interact with overexpressed receptors at the target site [112]. Physical targeting utilizes external sources or fields, such as radiation, ultrasound, photothermal and magnetic hyperthermia therapies, to guide NPs to the target site and control drug release through changes in pH and/or temperature especially in EPR-insensitive tumor phenotypes [103,106]. Thus, Theek et al. [113] revealed that increased accumulation of liposomes was shown in two EPR-insensitive phenotypes such as highly stromal BxPC-3 pancreatic carcinoma xenograft and highly cellular A431 epidermal xenograft after ultrasound irradiation compared to untreated controls.
3.10. The Reticuloendothelial System Barrier in Nanoparticle Drug Delivery
One of the clinical translation issues with NP drugs has been the reticuloendothelial system (RES) barrier because hepatocytes and Kupffer cells in the RES in the liver usually take up NPs bound to serum proteins, depending on their size and surface properties [103,114]. Thus, for the purpose of clinical translation, a good strategy to block or deplete macrophages is to boost the efficiency of nanoparticle drugs. Recently, Tang et al. suggested that d-self-peptide-labeled liposomes (DSLs) could reduce interactions between phagocytes and NPs by forming a long-lasting mask [115] since the modification of NPs with polyethylene glycol (PEG) reduced their clearance by macrophages and their internalization [116]. Similarly, multicore iron oxide NPs coated with a poly(4-vinylpyridine) polyethylene glycol copolymer have low RES retention and high urinary excretion in the kidneys [117].
4. Molecular Mechanisms of Nano-Phytochemicals in Cancer Progression
Recently, phytochemicals, including functional food and anticancer supplements, have emerged as effective anticancer agents for cancer therapy with fewer side effects than conventional treatments [118]. Among phytochemicals, curcumin, epigallocatechin gallate (EGCG), ginsenosides, lycopene, and resveratrol are more attractive than others. In particular, curcumin, the Indian spice turmeric from Curcuma longa, has been shown to exert an anticancer effect in several cancers, including breast, colon, and pancreas, brain, and liver cancers [119]. Despite its beneficial effects, its therapeutic efficacy is limited due to poor bioavailability, poor absorption, rapid metabolism, and rapid systemic elimination [120,121,122]. Thus, to overcome the shortcomings of natural compounds such as curcumin, several types of NPs have been applied with phytochemicals in cancer therapy, as shown in Table 1.

Table 1.
Efficacy and molecular mechanism of nano-phytochemicals in several cancers.
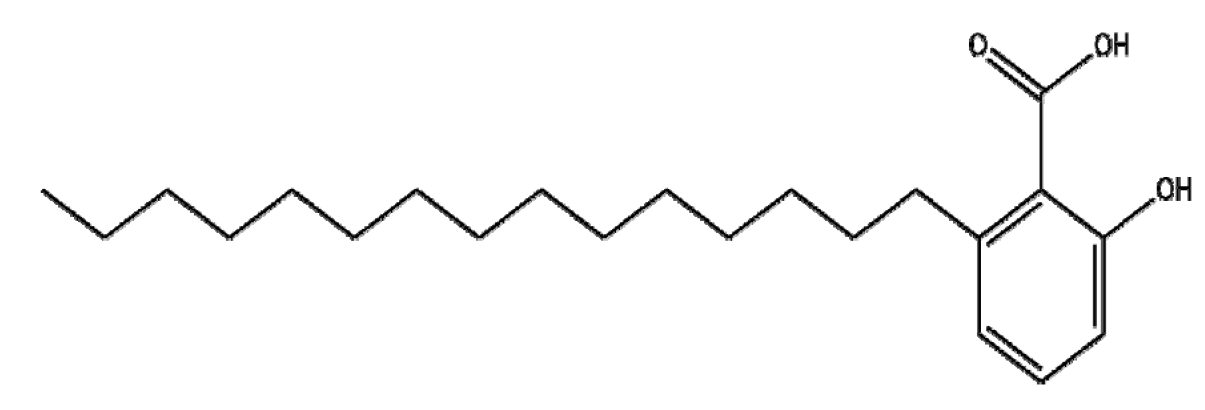
4.1. Anacardic Acid
Anacardic acid (AA) from Anacardium occidentale has antitumor effects in several cancers. AA inhibited proliferation, invasion, and migration and induced G0/G1-phase arrest and apoptosis in MDA-MB-231 cells via the inhibition of Hsp90-dependent endoplasmic reticulum stress (ERS)-related molecules, such as GRP78, Hsp70, CDK-4, MMP-9, Bcl-2, and Mcl-1 [123]. To increase the efficacy of this molecule, nanotechnology with AA has been adopted [124]. Kushwah et al. [125] reported that docetaxel (DTX)-loaded bovine serum albumin (BSA) covalently conjugated with AA and gemcitabine (GEM) nanoparticles (AA-GEM-BSA NPs) significantly improved the cellular uptake, apoptosis, and pharmacokinetic profile in MCF-7 and MDA-MB-231 breast cancer cells compared to DTX and GEM alone. Specifically, AA-GEM-BSA NPs were associated with significantly sustained release, enhanced stability against enzymatic degradation, delayed DTX release, and higher levels of the apoptosis index in MCF-7 and MDA-MB-231 cells as compared to a combination of GEM and DTX. Thus, the combination of AA and DTX or GEM encapsulated by BSA protein is a promising candidate for breast cancer treatment.
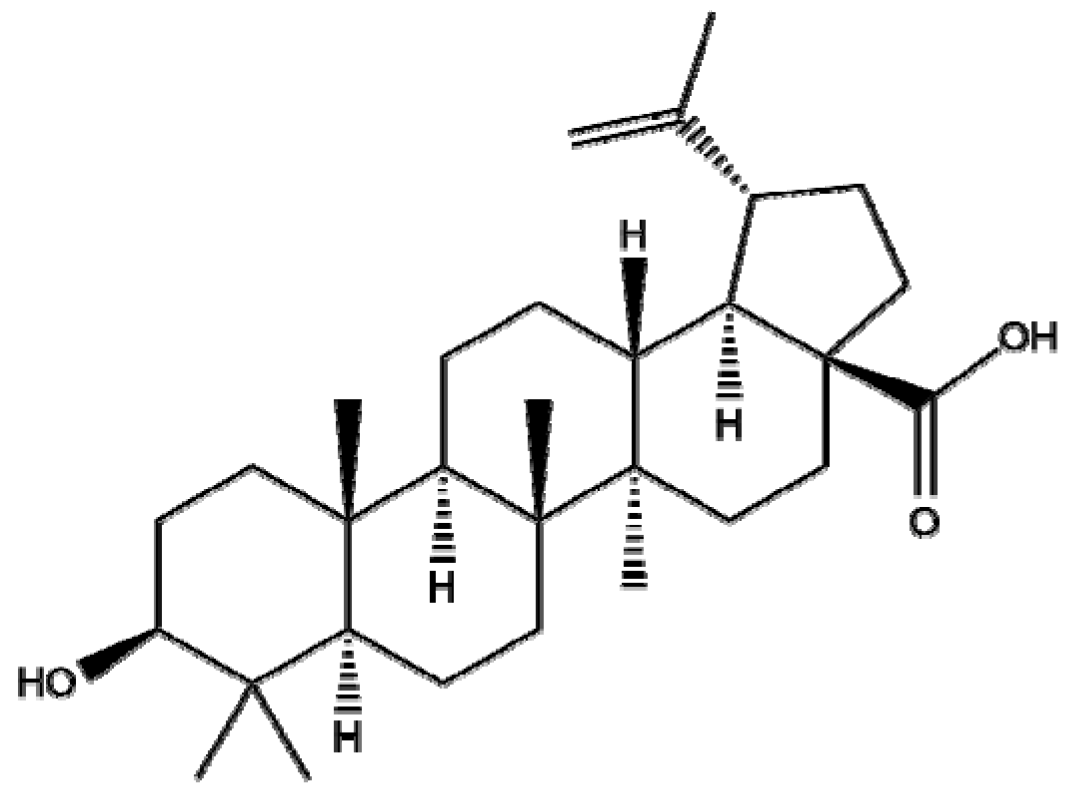
4.2. Betulinic Acid
Though betulinic acid (BA), a pentacyclic triterpenoid derived from birch tree, has a broad spectrum of biological and medicinal properties, it has some drawbacks, such as poor aqueous solubility and a short half-life in vivo [126]. Thus, several types of nanoscale delivery systems, such as polymeric NPs, magnetic NPs, liposomes, polymeric conjugates, nanoemulsions, cyclodextrin complexes, and carbon nanotubes, have been developed for the efficient delivery of BA [127]. Saneja et al. revealed that GEM -BA NPs significantly enhanced cytotoxicity and ROS generation in pancreatic cancer Panc-1 cells compared to GEM NPs or GEM and BA or GEM alone [128]. Farcas et al. [129] indicated that BA-loaded magnetoliposomes, as a potent anticancer agent, enhanced the antitumor effect in MDA-MB-231 breast cancer cells without significant cytotoxicity in normal breast epithelial MCF 10A cells. Similarly, Kumar et al. [130] reported that poly(lactic- co-glycolic acid)-loaded NPs of BA (PLGA-loaded NPs of BA) significantly decreased the expression of i-NOS, Bcl-2, and Bcl-xl and increased the expression of BAD, BAX, and caspase-9/3 compared to the untreated control in a diethylnitrosamine (DEN)-induced hepatocellular carcinoma (HCC) rat model. Overall, polymeric or lipid NPs with BA are promising candidates for treating breast, pancreas, and liver cancers and have outperformed BA controls, indicating that further study on BA-NPs is required in humans to determine their pharmacodynamic and pharmacokinetic profiles.
4.3. Curcumin
It is well known that curcumin (diferuloylmethane:CU)—a hydrophobic polyphenol—modulates enzymes, transcription factors, kinases, inflammatory cytokines, growth factors, and proapoptotic and antiapoptotic proteins in several cancers [119]. Though CU has been shown to be a potent anticancer compound in numerous cancers in vitro and in vivo, several clinical trials have found that its efficacy is limited in humans because of characteristics that reduce its bioavailability, such as low water solubility, poor absorption, a high rate of metabolism, the inactivity of metabolic products, and/or rapid elimination and clearance from the body [120,131]. As one of the approaches to overcome the disadvantages of CU, NPs that include liposomal encapsulation and emulsions have been employed for many years [132]. Li et al. [133] indicated that liposomal CU enhanced cytotoxicity and apoptosis in LoVo and Colo205 colorectal cancer cells and exerted significant (p < 0.05) synergistic effects with oxaliplatin in a colorectal xenograft model via the inhibition of CD31, VEGF, and IL-8 and the activation of PARP cleavage. Similarly, Pandelidou et al. [134] demonstrated that egg phosphatidylcholine (EPC) liposomes conjugated with CU showed significantly better cytotoxicity compared to the CU control in HCT116 and HCT15 colorectal cancer cells. Notably, Arya et al. [135] demonstrated that CU-loaded chitosan/polyethylene glycol (PEG)-blended PLGA NPs exhibited significant cytotoxic, anti-invasive, and apoptotic effects compared to the CU control in Panc-1 and Mia Paca-2 pancreatic cancer cells via the activation of BAX, caspase-3, and PAPR cleavage and the inhibition of BCL-2. Moreover, Khan et al. [136] reported that CU-loaded chitosan nanoparticles (CLCsNPs) produced better antitumor effects in cervical cancers compared to chitosan NPs (CsNPs) alone. Furthermore, CU-resveratrol-gelucire 50/13 (CRG) SLNs had better cytotoxicity in HCT116 cells compared to CU-resveratrol-gelucire 50/13-HPβCD (CRG-CD) [137] due to their greater bioavailability, and the adverse effects were limited [131]. Overall, CU NPs have shown significant anticancer activity in several cancers and are promising as potential combinatorial agents with classical anticancer drugs.
4.4. EGCG
Accumulating evidence has confirmed that epigallocatechin gallate (EGCG), a flavone-3-ol polyphenol from green tea, is mainly absorbed in the intestine [138] and inhibits NF-κB, epithelial–mesenchymal transition (EMT), and cellular invasion in several cancers via its interaction with DNA methyltransferases (DNMTs), histone deacetylases (HDACs), Pin1, TGFR-II, MMP-2, and MMP-9. In an aim to improve the insolubility, instability, and low tissue distribution of EGCG, Chu et al. [139] investigated polymeric CU/EGCG-loaded NPs in an orthotopic prostate tumor model. The NPs exerted stronger antitumor effects compared to the CU/EGCG combination control by targeting CD34 and P-selectin since hyaluronic acid targets CD44 and fucoidan targets P-selection in tumor vasculature. Zhang et al. [140] demonstrated that, compared to EGCG alone, polymeric EGCG-loaded PLGA NPs had enhanced anticancer activity in A549 and H1299 lung cancer cells. The anticancer effects occurred via the inhibition of NF-κB and its related proteins, such as Bcl-2, Bcl-xL, COX-2, TNF-α, cyclin D1, c-Myc, TWIST1, and MMP-2. Similarly, Velavan et al. [141] demonstrated that BSA-encapsulated magnetite nanoparticles (MNPs) loaded with EGCG (nano-EGCG) showed better antitumor efficacy in A549 lung cancer cells compared to EGCG alone via increased ROS/RNS modulation of Nrf2/keap1 signaling and the loss of the mitochondrial membrane potential, leading to apoptosis. Additionally, Radhakrishnan et al. [142] indicated that bombesin-conjugated EGCG-loaded SLNs resulted in increased cytotoxicity in MDA-MB-231 breast cancer cells and reduced the tumor size of B16F10 cells compared to EGCG or the bombesin-conjugated EGCG (EB-SLN) group. Similarly, Hajipour et al. [143] reported that arginyl-glycyl-aspartic acid (RGD)-containing ECGC-loaded nanostructured lipid carriers (EGCG-loaded NLC-RGD) enhanced the cytotoxic and apoptotic effects of doxorubicin (DOX) in MDA-MB-231 breast cancer cells compared to EGCG-loaded NLCs. Notably, Chavva et al. [144] demonstrated that EGCG–gold nanoparticles (E-GNPs) showed better cytotoxicity and cellular uptake in PC-3 and MDA-MB-231 cells compared to free EGCG or citrate GNPs via the inhibition of NF-κB, BCL-2, and BCL-xL and the activation of Bax and cleaved caspase-7/3. Overall, though some nanoparticles with EGCG have been found to be effective in vitro and in vivo by compensating for the weaknesses of EGCG, further study with EGCG-NPs is required to establish the pharmacodynamic and pharmacokinetic profiles for future clinical application.
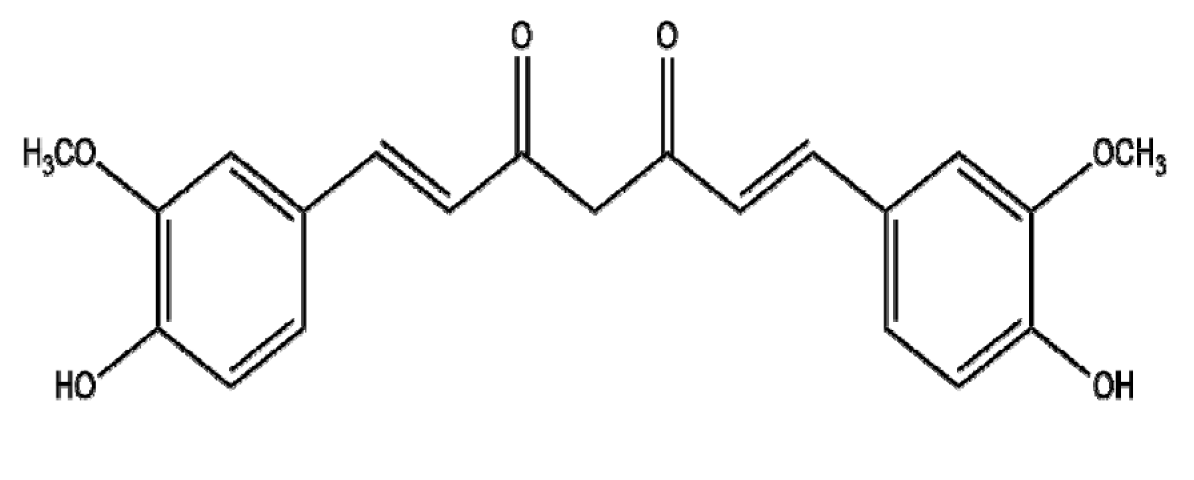
4.5. Ferulic Acid
Ferulic acid (FA), which is commonly found in vegetables, sweet corn, bamboo shoots, and rice grain [145], is known to have cytotoxic and apoptotic effects via the inhibition of PI3K/AKT signaling in pancreatic and cervical cancers [146,147]. Recently, to improve the EPR effect of FA, Cui et al. [148] studied the antitumor effects of FA-modified selenium nanoparticles (FA-Se NPs) in HepG2 cells and reported increased ROS production, MMP disruption, and caspase-9/3 activation compared to FA alone, while ZnO nanoparticles (ZnONPs)-FA (FAC), compared to ZnONPs or FA alone, suppressed hepatocellular cancer progression via ROS production; the inhibition of MMP-2 Bcl-2, and Bcl-xL; and the activation of Bax, Bad, cleaved caspase-3, and cleaved PARP [149]. Furthermore, Thakkar et al. [150] reported that the chemopreventive effect of FA and aspirin (ASP) encapsulated by chitosan-coated solid lipid nanoparticles (c-SLNs) occurred via the inhibition of PCNA and Ki67 and the activation of p-RB, p21, and p-ERK1/2 in MIA, PaCa-2, and Panc-1 pancreatic cancer cells. Further study is required in humans to determine the PD and PK profiles of FA-NPs, both alone and in combination with classical anticancer drugs, for future clinical trials.
4.6. Gambogic Acid
Gambogic acid (GA), a component of the dry resin (gamboge) from the Garcinia hanburyi tree, is known to have antitumor effects in prostate [151], lung [152], stomach [152], and liver [153] cancers. GA induces apoptosis in cancer cells by increasing ROS production and inhibiting the NF-κB, MAPK/ERK, and PI3K/AKT signaling pathways. Studying polymeric NPs with GA, Xu et al. showed that the apoptotic effect of L-methionine poly(ester amide) (Met-PEA-PEG) via high intracellular ROS production in PC-3 cells was better than that of GA alone [154]. Furthermore, Wang et al. [155] demonstrated the improved apoptotic effect of hyaluronic acid-grafted polyethylenimine-poly(D,L-lactide-co-glycolide) (HA-PEI-PLGA) NPs via the activation of caspase-3/8 and the inhibition of survivin and Bcl-2 in triple-negative breast cancer (TNBC) compared to GA alone. Similarly, He et al. [156] reported that GA-loaded folate-conjugated Arg-based poly(ester urea urethane)s NPs (GA-loaded FA-Arg-PEUU NPs) induced more powerful apoptotic and anti-metastatic effects via the inhibition of MMP-2/9 and mitochondrial membrane potential and the activation of DNA fragmentation compared to GA alone in HeLa and A549 cells. Notably, Fang et al. [157] showed that magnetic NPs containing Fe3O4 as a carrier for GA (GA MNPs-Fe3O4) had a stronger apoptotic effect in LoVo colon cancer cells compared to GA alone via the activation of caspase-9/3 and the suppression of p-PI3K, p-AKT, and p-BAD. Overall, polymeric or metal NPs with GA can enhance drug delivery and thereby increase anticancer activity compared to GA alone and should be studied in future clinical trials.
4.7. Ginsenosides
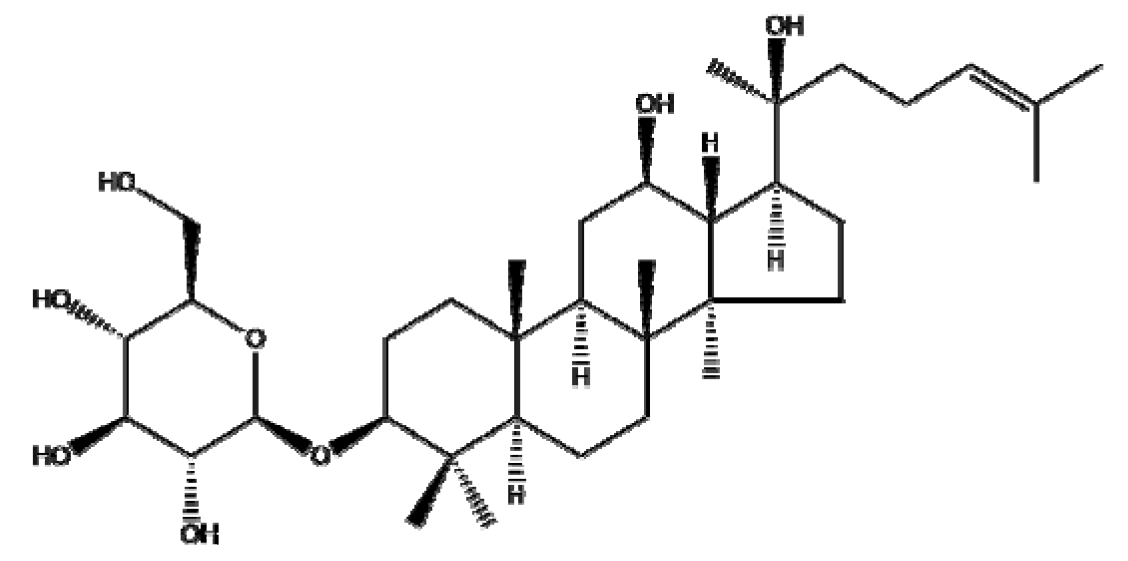
Ginsenosides or panaxosides are a class of steroid glycosides and triterpene saponins derived from Panax ginseng. The major protopanaxadiols include ginsenoside Rb1, Rb2, Rg3, Rh2, and Rh3, while the major protopanaxatriols are ginsenoside Rg1, Rg2, and Rh1 [158,159]. To increase their low oral bioavailability and achieve efficient drug release, several NPs, including liposomes, emulsions, and micelles, have been conjugated with ginsenosides for cancer therapy [160]. Ren et al. [161] reported that Fe3O4 NPs with ginsenoside Rg3 (NpRg3) significantly inhibited HCC development and metastasis via the remodeling of unbalanced gut microbiota and metabolism compared to Rg3 or Fe3O4 alone. Similarly, Qiu et al. [162] reported that Rg3 co-loaded with poly(ethylene glycol)-block-poly(L-glutamic acid-co-L-phenylalanine) (mPEG-b-P(Glu-co-Phe)) NPs (Rg3-NPs) significantly reduced proliferating cell nuclear antigen (PCNA) and increased caspase-7/9/3 compared to Rg3 alone in a colon cancer xenograft mouse model. Kim et al. [44] revealed that Rh2 hyaluronic acid-functionalized zinc oxide (Rh2HAZnO) enhanced p53, pp38, Bax, PARP, and ROS production and reduced the expression of Bcl-2 compared to Rh2 alone in A549 lung cancer, HT29 colon cancer, and MCF7 breast cancer cells. Additionally, Dong et al. [163] demonstrated that folic acid (FA)-modified BSA and Rg5 NPs (FA-Rg5-BSA NPs) significantly enhanced cellular uptake and apoptotic cell death compared to the Rg5 or Rg5-BSA NP group in MCF-7 cells and a xenograft mouse model. Overall, several ginsenosides encapsulated by NPs have been found to exert anticancer effects by increasing the drug delivery efficiency. However, further study in humans is recommended to analyze the pharmacodynamic and pharmacokinetic profiles for clinical applications and the scale-up of ginsenosides, either alone or in combination with classical anticancer drugs.
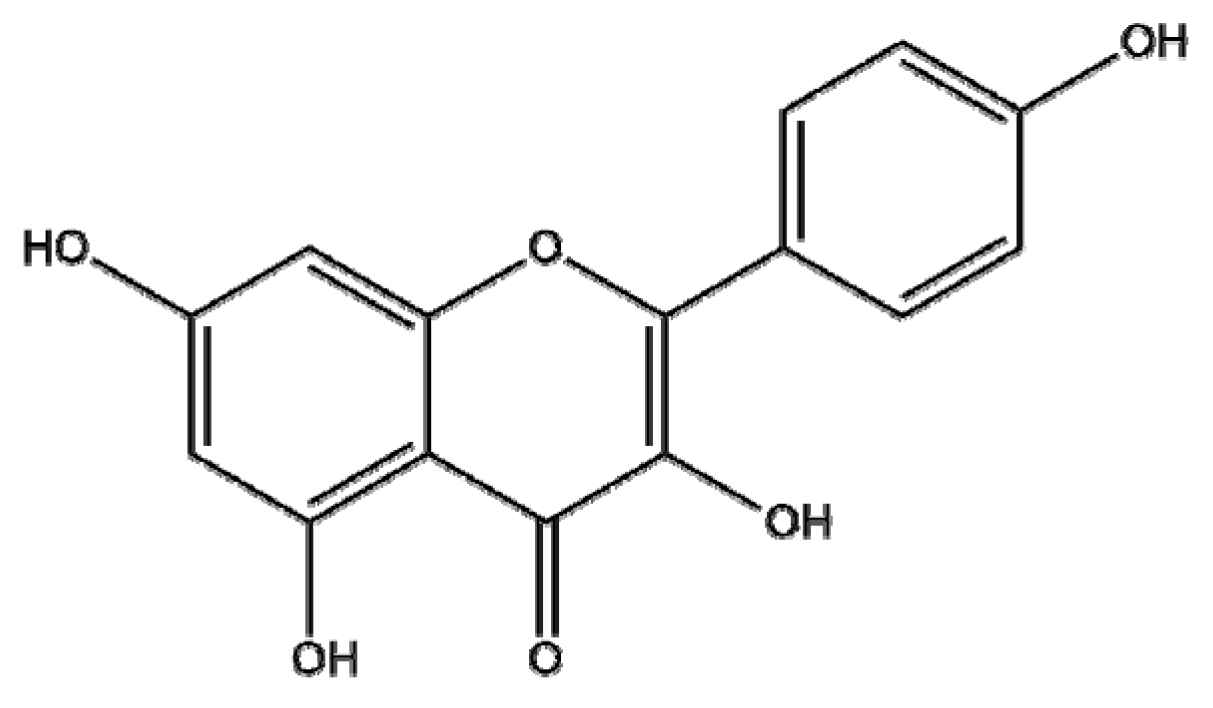
4.8. Kaempferol
Kaempferol (3,4′,5,7-tetrahydroxyflavone; KPF), a natural flavonol, is present in many edible plants, such as tea, kale, beans, leek, tomato, strawberries, broccoli, cabbage, and grapes, and common medicinal plants (Aloe vera, Ginkgo biloba, Rosmarinus officinalis, Crocus sativus, Hypericum perforatum) [164]. Though KPF is known to have antioxidant, anti-inflammatory, estrogenic, anxiolytic, analgesic, antimicrobial, cardioprotective, neuroprotective, antidiabetic, and anticancer activities [164,165], it has some shortcomings, such as poor drug delivery due to low water solubility and bioavailability in clinical application [95]. To improve the bioavailability of KPF, it has been used in several types of NPs in cancer cells. Luo et el. [166] comparatively evaluated the efficacy of five different types of NPs with the incorporation of KPF, such as poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO poly(DL-lactic acid-co-glycolic acid) (PLGA), PLGA-polyethylenimine (PEI), glycol chitosan, and poly(amidoamine) (PAMAM) dendrimer in ovarian cancer cells. In A2780/CP70 and OVCAR-3 ovarian cancer cells, PEO-PPO-PEO and PLGA NPs reduced cell viability, whereas PLGA-PEI, glycol chitosan, and PAMAM dendrimer NPs did not show significant efficacy, implying that PLGA NPs may be a promising candidate for cancer treatment. Additionally, Govindaraju et al. reported that KPF-conjugated gold nanoclusters (K-AuNCs) had stronger cytotoxicity in A549 cells compared to KPF alone, and they did not damage HK-2 normal kidney cells [167]. Notably, Chuang et al. [168] suggested that gelatin nanoparticles (GNPs) with KPF encapsulation (GNP-KA) inhibited the viability and migration of human umbilical vein endothelial cells (HUVECs) and suppressed vessel formation in mice cornea compared to KPF alone via the inhibition of MMP-2, MMP-9, and VEGF. Interestingly, Colombo et al. [169] reported that a KPF-loaded mucoadhesive nanoemulsion (KPF-MNE) more effectively permeated the mucosa, and the retention of KPF in the nasal mucosa was significantly improved, making it an effective therapeutic candidate for glioma treatment. Overall, NPs with materials such as polymers, metals, lipids, and gelatin encapsulated with KPF enhanced the antitumor efficacy of KPF without harming normal cells, implying that polymeric, lipid, metal, and gelatin NPs with KPF are potent antitumor candidates for cancer therapy.
4.9. Lycopene
Lycopene (LYC), a member of the carotenoid family that naturally occurs in tomatoes and guava watermelon, is known to have anticancer activity in prostate, colorectal, and gastric cancers [170]. To enhance its efficacy and bioavailability, several types of NPs have been used with lycopene in cancer therapy [171]. Vasconcelos et al. [172] demonstrated that poly(ε-caprolactone) lipid-core NPs containing lycopene-rich extract from red guava (nano-LEG) were more effective and safe in MCF-7 breast cancer cells compared to the LYC control via the inhibition of NF-kB activation and ROS production. Similarly, Jain et al. [173] reported that lycopene-loaded solid lipid NPs (LYC-SLNs) exerted a synergistic effect with methotrexate in MCF-7 breast cancer cells, and cellular uptake of LYC-SLNs was higher compared to LYC alone. Furthermore, Jain et al. [173] demonstrated that LYC-loaded whey protein NPs (LYC-WPI-NPs) enhanced the bioavailability and prophylactic effect of LYC in MCF-7 breast cancer cells compared to LYC alone. Similarly, the results reported by Huang et al. [174] suggest that a nanoemulsion of LYC and gold nanoparticles (AN) has potential as a potent therapeutic agent against colon cancer. The AN–LYC nanoemulsion significantly reduced the expression of procaspases-8, -3, and -9, PARP-1, Bcl-2, Akt, NF-κB, and pro-MMP-2/9 and enhanced the expression of Bax and E-cadherin. Overall, lycopene encapsulated with lipid, metal, and polymeric NPs appear to be more potent antitumor agents than lycopene alone in prostate, ovarian, lung, and breast cancers.
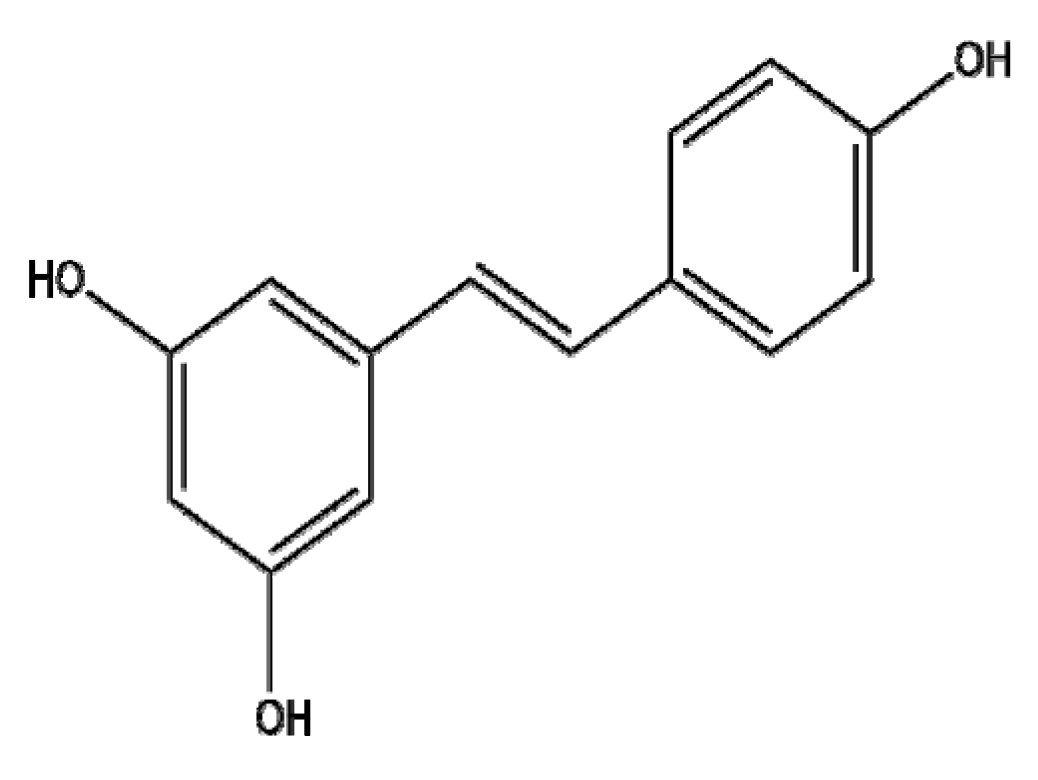
4.10. Resveratrol
Resveratrol (RES), a non-flavonoid polyphenol and a phytoestrogen, is known to have antioxidant, anti-inflammatory, cardioprotective, anticancer, and anti-multidrug resistance (MDR) effects [175,176]. However, since the poor bioavailability of resveratrol in humans has been a critical issue, recent studies have focused on its delivery system, formulation, and interaction with other compounds [177]. Senthil Kumar et al. [178] demonstrated that chitosan-coated-transresveratrol (RSV) and ferulic acid (FER)-loaded solid lipid nanoparticles (SLNs) conjugated with folic acid (FA) (C-RSV-FER-FA-SLNs) exhibited enhanced cytotoxic activity and G0/G1 stage arrest compared to free RSV-FER or RSV-FER-SLNs in HT-29 colon cancer cells. Furthermore, Park et al. [179] reported that resveratrol-capped gold nanoparticles (Rev-AuNPs) inhibited breast cancer progression via the suppression of NF-kB, AP-1, Akt, ERK, MMP-9, and COX-2 and the upregulation of HO-1 compared to RES alone in MCF-7 breast cancer cells. Furthermore, Song et al. [180] demonstrated that epidermal growth factor-modified docetaxel and RES co-encapsulated lipid-polymeric hybrid NPs (EGF DTX/RSV LPNs) significantly inhibited the viability and proliferation of HCC827 and NCIH2135 non-small cell lung cancer cells (NSCLCs) compared to DTX/RSV LPNs or free DTX/RSVDTX. Similarly, Wang et al. [181] reported that resveratrol-loaded solid lipid nanoparticles (RES-SLNs) induced enhanced apoptosis via the upregulated ratio of Bax/Bcl-2 and the suppression of cyclin D1 and c-Myc compared to RES alone in MDA-MB-231 cells. Karthikeyan et al. [182] reported that the anticancer effects of RES-loaded gelatin nanoparticles (RSV-GNPs) were improved in NCI-H460 cells compared to resveratrol alone via the upregulation of Bax, p53, p21, and caspase-3; the downregulation of Bcl-2 and NF-κB; and G0/G1 arrest. Similarly, Guo et al. [183] revealed that resveratrol–bovine serum albumin nanoparticles (RES-BSANP) induced apoptosis via the activation of apoptosis-inducing factor (AIF), cytochrome c, and Bax in SKOV3 ovarian cancer cells. Furthermore, Fan et al. [184] reported that resveratrol-loaded oxidized mesoporous carbon nanoparticles (oMCNs-RES) showed better cytotoxicity and promoted the drug loading efficiency and solubility of RES compared to resveratrol alone in MDA-MB-231 breast cancer cells. Overall, combining RES with NPs containing materials such as polymers, lipids, proteins, carbon nanotubes, and metals enhances the antitumor effects of RES by improving drug delivery and bioavailability, suggesting the potential for further clinical triald of RES NPs in cancer patients in the future. Overall, the potency of the nano-phytochemicals mentioned in this review has been verified, and thus, they are worthy of further development for clinical translation. Additionally, the molecular mechanism of action, pharmacokinetic profiles, toxicological profiles of nano-phytochemicals should be further explored, though nano-phytochemicals bring benefits in cancer therapy via efficient drug delivery, stability, EPR effect and low clearance by RES.
5. Clinical Implications
Since nanotechnology can offer new solutions for the development of cancer diagnosis and therapeutics, several antitumor nanodrugs have been tested in preclinical and clinical trials over the past decade [186]. To date, seven anticancer nanodrugs, namely, Doxil, DaunoXome, Eligar, Abraxane, Marquibo, Onivyde, and Mycocet, have entered the pharmaceutical market, while Astragen (for acute promyelocytic leukemia), Lipoplatin (for pancreatic, head and neck, and breast cancers), Aurimmune (CYT-6091) (for head and neck cancer), and Paclical (for ovarian cancer) are still undergoing Phase II/III clinical trials [187,188].
Recently, several phytochemicals have been reported as promising anticancer candidates with significant efficacy in vitro and in vivo. However, many clinical trials have revealed that they lack efficacy in humans, mainly due to poor bioavailability, insolubility, instability, rapid clearance, and toxicity [95]. As a nano-phytochemical, albumin-bound paclitaxcel (PTX; Abraxane) derived from Taxus brevifolia was found be more effective in delivering PTX to tumors and reducing its toxicity in normal cells compared to PTX alone [103]. Furthermore, to overcome the poor bioavailability of curcumin in humans [120,131], combining it with piperine to block the metabolic pathway of curcumin [189,190], the use of a colloidal NP THERACURMIN [191] as a standardized curcuminoid mixture, the application of its corresponding lecithin formulation (Meriva) as a phospholipid complex [192], and structural analogs [193] have been suggested for efficient cancer therapy. As in recent nano-research that found that several anticancer phytochemical-encapsulated NPs were more effective compared to NP controls [139], Gota et al. [194] reported the potency of solid lipid curcumin NPs: 22.43 ng/mL curcumin was detected at a dose of 650 mg, while it was not detected in the plasma of osteosarcoma patients. Similarly, a clinical trial (NCT03140657) showed that the solubility of curcumin in nanomicelles was increased by 100 thousand times, and another clinical trial (NCT01403545) revealed that intravenous injection of liposomal curcumin was well tolerated and safe, except for the formation of transient red blood cell echinocytes at dosages ≥ 120 mg/m2 [195], implying the potential use of curcumin NPs for the clinical treatment of cancer in the future. However, though numerous studies with nanocurcumin formulations using liposomes, polymers, conjugates, cyclodextrins, micelles, dendrimers, and other nanoparticles have shown favorable outcomes in vitro and in vivo, clinical trials with nanocurcumin have not yet been conducted in cancer patients. Hence, to develop anticancer nano-phytochemical drugs, many preclinical and clinical trials should be conducted in a large number of cancer patients to gather information on the safety, toxicity, and efficacy of nano-phytochemical formulations alone or in combination with classical anticancer agents.
6. Conclusions and Perspectives
Over the past decade, the advanced progress in nanobiotechnology and nanomaterial science has contributed to imaging diagnosis and targeted cancer therapy by providing efficient drug delivery systems. A variety of organic nanoparticles (NPs) (such as polymeric conjugates, lipid-based carriers (liposomes and micelles), dendrimers, and ferritin) and inorganic NPs (such as carbon nanotubes, quantum dots, ceramic NPs, magnetic NPs, and gold/silver NPs) encapsulated with classical anticancer drugs or phytochemicals have been developed to promote enhanced permeability and retention (EPR) in cancer. However, smart nanoencapsulation designs and synthesis of potent anticancer phytochemicals are required to better understand the physicochemical properties of NPs, such as their shape, size, charge, surface chemistry, and toxicity, since many natural compounds, such as anacardic acid, betulinic acid, curcumin, EGCG, ferulic acid, gambogic acid, ginsenosides, kaempferol, lycopene, and resveratrol, have been found to be effective in culture cells and animals with low toxicity. However, many phytochemicals have not shown significant anticancer efficacy due to their poor solubility, stability, rapid clearance through RES phagocytosis, drug distribution, degradation, and resistance in humans [196]. In contrast, phytochemical-encapsulated NPs have been shown to enhance antitumor effects in several cancers via the inhibition of PI3K/AKT, NF-κB, ERK, and anti-apoptotic proteins (such as survivin, Bcl-2, and Bcl-XL) and the activation of PARP, caspases-8, - 9, and -3, BAX, and p53 due to enhanced bioavailability, solubility, stability, and EPR effects compared to free phytochemicals or NPs alone (Figure 3). Nonetheless, the difference between nano-phytochemicals and free phytochemicals or NPs alone for a specific molecular target should be further elucidated by evaluating the synergistic or additive effects between NPs and free phytochemicals. In addition, though the EPR effect works only in solid tumors via passive targeting, while active targeting through functionalization with ligands (including proteins, antibodies, and peptides) works in hematological malignancies, the complexity and the heterogeneity of tumor phenotypes should be considered for effective clinical translation [197]. Moreover, the median and mean delivery efficiencies are reported to only be 0.70% and 1.48% of the injected dose (ID) of NPs, respectively, which is considered a major drawback for clinical translation [198,199]. Hence, for the clinical translation of nano-phytochemicals, it is crucial to design EPR-potentiating combination therapy, evaluate the extent of the EPR effect, and utilize ultrasound, radiation, hyperthermia, and photodynamic therapy as a physical targeting strategy to achieve an efficient EPR effect in EPR-insensitive tumors before and during clinical trials [105]. Additionally, the reliability and reproducibility of NP–phytochemical encapsulations should be ensured, and their possible toxicity, suitable administration routes (oral, intravenous, transdermal), and pharmacodynamic and pharmacokinetic profiles should be determined in many preclinical and randomized clinical trials because some phytochemicals can have adverse side effects [200], though they are known to generally be less toxic compared to synthetic anticancer drugs [201]. Furthermore, further study is required to elucidate the detailed molecular mechanism of nanodrugs within the tumor environment for active drug delivery; prolong the circulation time; and explore the RES evasion, sensitive drug release, and targeted co-delivery of different compounds [202]. Finally, along with the above-mentioned challenges that must be overcome, an appropriate strategy for lowering manufacturing costs and scaling up the production of anticancer phytochemical candidates should be developed to ensure their success on the pharmaceutical market.
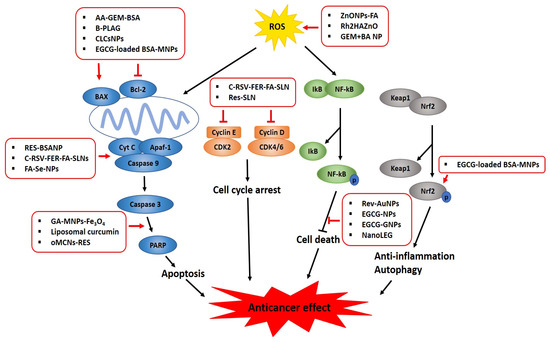
Figure 3.
Graphical abstract on the molecular mechanism of nano-phytochemicals in cancers.
Author Contributions
B.K., B.-S.S. and S.-H.K. designed and conceived the paper. J.-E.P., E.I., D.-Y.S., W.-Y.P., J.L. and Y.C. compiled papers and summarized data. E.I. and J.-E.P. drew two figures and one table. J.-E.P. and E.I. drafted the manuscript. B.-S.S. and H.-J.L. provided comments for figures and tables. S.-H.K. supervised and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MEST) (No. 2020R1A5A2019413 and 2021R1A2C2003277).
Acknowledgments
We appreciate Srivastava so much for giving a valuable idea for preparation of this paper.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Lim, Z.F.; Ma, P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.N.; Daima, H.K. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Maniam, G.; Mai, C.W.; Zulkefeli, M.; Dufès, C.; Tan, D.M.; Fu, J.Y. Challenges and Opportunities of Nanotechnology as Delivery Platform for Tocotrienols in Cancer Therapy. Front. Pharmacol. 2018, 9, 1358. [Google Scholar] [CrossRef] [PubMed]
- Landesman-Milo, D.; Ramishetti, S.; Peer, D. Nanomedicine as an emerging platform for metastatic lung cancer therapy. Cancer Metastasis Rev. 2015, 34, 291–301. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J.; et al. Chitosan-based nanoformulated (-)-epigallocatechin-3-gallate (EGCG) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis. Int. J. Nanomed. 2018, 13, 4189–4206. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Buhleier, E.; Wehner, W.; VÖGtle, F. “Cascade”- and “Nonskid-Chain-like” Syntheses of Molecular Cavity Topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- Fraley, R.; Subramani, S.; Berg, P.; Papahadjopoulos, D. Introduction of liposome-encapsulated SV40 DNA into cells. J. Biol. Chem. 1980, 255, 10431–10435. [Google Scholar] [CrossRef]
- Gabizon A, D.A. Goren D, Barenholz Y, Fuks Z, Liposomes as in vivo carriers of adriamycin: Reduced cardiac uptake and preserved antitumor activity in mice. Cancer Res. 1982, 42, 4734–4739. [Google Scholar]
- Ekimov, A.I.; Onushchenko, A.A. Quantum size effect in three-dimensional microscopic semiconductor crystals. Soviet J. Exp. Theor. Phys. Lett. 1981, 34, 345. [Google Scholar]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Baker, R. Early approval for two lipid-based drugs. BETA 1995, 4, 11363009. [Google Scholar]
- Kaposi’s sarcoma: DaunoXome approved. AIDS Treat. News 1996, 246, 3–4.
- Sartor, O. Eligard: Leuprolide acetate in a novel sustained-release delivery system. Urology 2003, 61 (Suppl. 1), 25–31. [Google Scholar] [CrossRef]
- Saif, M.W. U.S. Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane(R)) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP 2013, 14, 686–688. [Google Scholar] [PubMed]
- The FDA approves new leukemia drug; expands use of current drug. FDA Consum 2006, 40, 5.
- FDA approves liposomal vincristine (Marqibo) for rare leukemia. Oncology 2012, 26, 841.
- Huang, H.C.; Mallidi, S.; Liu, J.; Chiang, C.T.; Mai, Z.; Goldschmidt, R.; Ebrahim-Zadeh, N.; Rizvi, I.; Hasan, T. Photodynamic Therapy Synergizes with Irinotecan to Overcome Compensatory Mechanisms and Improve Treatment Outcomes in Pancreatic Cancer. Cancer Res. 2016, 76, 1066–1077. [Google Scholar] [CrossRef]
- Sparano, J.A.; Winer, E.P. Liposomal anthracyclines for breast cancer. Semin Oncol. 2001, 28 (Suppl. 12), 32–40. [Google Scholar] [CrossRef]
- Wegmann, J.; Krucker, M.; Bachmann, S.; Fischer, G.; Zeeb, D.; Lienau, A.; Glaser, T.; Runge, F.; Luddecke, E.; Albert, K. Characterization of lycopene nanoparticles combining solid-state and suspended-state NMR spectroscopy. J. Agric. Food Chem. 2002, 50, 7510–7514. [Google Scholar] [CrossRef]
- Souto, E.B.; Anselmi, C.; Centini, M.; Muller, R.H. Preparation and characterization of n-dodecyl-ferulate-loaded solid lipid nanoparticles (SLN). Int. J. Pharm 2005, 295, 261–268. [Google Scholar] [CrossRef]
- Takahashi, M.; Inafuku, K.; Miyagi, T.; Oku, H.; Wada, K.; Imura, T.; Kitamoto, D. Efficient preparation of liposomes encapsulating food materials using lecithins by a mechanochemical method. J. Oleo Sci. 2006, 56, 35–42. [Google Scholar] [CrossRef]
- Hung, C.F.; Chen, J.K.; Liao, M.H.; Lo, H.M.; Fang, J.Y. Development and evaluation of emulsion-liposome blends for resveratrol delivery. J. Nanosci. Nanotechnol. 2006, 6, 2950–2958. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, C.; Wu, X.; Tang, X.; Ping, Q. Preparation, physical properties, and stability of gambogic acid-loaded micelles based on chitosan derivatives. Drug Dev. Ind Pharm. 2008, 34, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zang, L.; Hu, S. Amplified immune response by ginsenoside-based nanoparticles (ginsomes). Vaccine 2009, 27, 2306–2311. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing nanochemoprevention as a novel approach for cancer control: Proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef]
- Saleem, J.; Wang, L.; Chen, C. Carbon-Based Nanomaterials for Cancer Therapy via Targeting Tumor Microenvironment. Adv. Healthc Mater. 2018, 7, e1800525. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Hong, H.; Gao, T.; Cai, W. Molecular Imaging with Single-Walled Carbon Nanotubes. Nano Today 2009, 4, 252–261. [Google Scholar] [CrossRef]
- Ji, S.R.; Liu, C.; Zhang, B.; Yang, F.; Xu, J.; Long, J.; Jin, C.; Fu, D.L.; Ni, Q.X.; Yu, X.J. Carbon nanotubes in cancer diagnosis and therapy. Biochim. Biophys. Acta 2010, 1806, 29–35. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomedicine 2016, 12, 333–351. [Google Scholar] [CrossRef]
- Kavosi, A.; Hosseini Ghale Noei, S.; Madani, S.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Mirzaei, M.; Kalhori, M.R.; Yavarian, M.; Alizadeh, A.M.; et al. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci Rep. 2018, 8, 8375. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A concise review of carbon nanotube’s toxicology. Nano Rev. 2013, 4, 21521. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Pant, A.B.; Kashyap, M.P.; Kumar, V.; Lohani, M.; Jonas, L.; Rahman, Q. Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line-A549. Nanotoxicology 2011, 5, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, P.; Bao, Y.; Dou, B.; Song, D.; Li, Y. Vitamin E renders protection to PC12 cells against oxidative damage and apoptosis induced by single-walled carbon nanotubes. Toxicol. Vitr. 2012, 26, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Zhang, Y.L.; Yang, N.; Zhang, Y.X.; Liu, X.Q.; Li, C.G.; Zhao, Y.; Wang, Y.G.; Zhang, G.G.; Yang, P.; et al. A functionalized single-walled carbon nanotube-induced autophagic cell death in human lung cells through Akt-TSC2-mTOR signaling. Cell Death Dis. 2011, 2, e159. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Perumalsamy, H.; Castro-Aceituno, V.; Kim, D.; Markus, J.; Lee, S.; Kim, S.; Liu, Y.; Yang, D.C. Photoluminescent And Self-Assembled Hyaluronic Acid-Zinc Oxide-Ginsenoside Rh2 Nanoparticles And Their Potential Caspase-9 Apoptotic Mechanism Towards Cancer Cell Lines. Int. J. Nanomed. 2019, 14, 8195–8208. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.S.; Wang, Z.L. Dissolving Behavior and Stability of ZnO Wires in Biofluids: A Study on Biodegradability and Biocompatibility of ZnO Nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Singh, D.; Singh, S.; Sahu, J.; Srivastava, S.; Singh, M.R. Ceramic nanoparticles: Recompense, cellular uptake and toxicity concerns. Artif Cells Nanomed. Biotechnol. 2016, 44, 401–409. [Google Scholar] [CrossRef]
- Kresge CT, L.M. Roth WJ, Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Yousatit, S.; Pitayachinchot, H.; Wijitrat, A.; Chaowamalee, S.; Nuntang, S.; Soontaranon, S.; Rugmai, S.; Yokoi, T.; Ngamcharussrivichai, C. Natural rubber as a renewable carbon source for mesoporous carbon/silica nanocomposites. Sci. Rep. 2020, 10, 12977. [Google Scholar] [CrossRef]
- Yuan, Z.; Pan, Y.; Cheng, R.; Sheng, L.; Wu, W.; Pan, G.; Feng, Q.; Cui, W. Doxorubicin-loaded mesoporous silica nanoparticle composite nanofibers for long-term adjustments of tumor apoptosis. Nanotechnology 2016, 27, 245101. [Google Scholar] [CrossRef]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef]
- Datta, A.; Mishra, S.; Manna, K.; Saha, K.D.; Mukherjee, S.; Roy, S. Pro-Oxidant Therapeutic Activities of Cerium Oxide Nanoparticles in Colorectal Carcinoma Cells. ACS Omega 2020, 5, 9714–9723. [Google Scholar] [CrossRef]
- Iqbal, S.; Jabeen, F.; Peng, C.; Ijaz, M.U.; Chaudhry, A.S. Cinnamomum cassia ameliorates Ni-NPs-induced liver and kidney damage in male Sprague Dawley rats. Hum. Exp. Toxicol. 2020, 39, 1565–1581. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Wang, Z.; Liu, B.; Zhu, S.; Zhu, L.; Peng, B. Licorice isoliquiritigenin-encapsulated mesoporous silica nanoparticles for osteoclast inhibition and bone loss prevention. Theranostics 2019, 9, 5183–5199. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today 2015, 20, 1143–1151. [Google Scholar] [CrossRef]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Lu, S.L.; Hu, C.W.; Yeh, C.S.; Lee, G.B.; Lei, H.Y. Size-dependent attenuation of TLR9 signaling by gold nanoparticles in macrophages. J. Immunol. 2012, 188, 68–76. [Google Scholar] [CrossRef]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical aspects of silver nanoparticles. Artif Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 115–126. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed. 2015, 10, 4203–4222. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.S.; Wang, J.F.; Song, J.; Liu, Y.; Zhu, G.; Dai, Y.; Shen, Z.; Tian, R.; Song, J.; Wang, Z.; et al. Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics 2019, 9, 7200–7209. [Google Scholar] [CrossRef] [PubMed]
- Ruenraroengsak, P.; Kiryushko, D.; Theodorou, I.G.; Klosowski, M.M.; Taylor, E.R.; Niriella, T.; Palmieri, C.; Yagüe, E.; Ryan, M.P.; Coombes, R.C.; et al. Frizzled-7-targeted delivery of zinc oxide nanoparticles to drug-resistant breast cancer cells. Nanoscale 2019, 11, 12858–12870. [Google Scholar] [CrossRef]
- Fang, M.; Chen, M.; Liu, L.; Li, Y. Applications of Quantum Dots in Cancer Detection and Diagnosis: A Review. J. Biomed. Nanotechnol 2017, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pleskova, S.; Mikheeva, E.; Gornostaeva, E. Using of Quantum Dots in Biology and Medicine. Adv. Exp. Med. Biol. 2018, 1048, 323–334. [Google Scholar]
- Lee, J.; Hwang, G.; Hong, Y.S.; Sim, T. One step synthesis of quantum dot-magnetic nanoparticle heterodimers for dual modal imaging applications. Analyst 2015, 140, 2864–2868. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, M. Review of in vitro toxicological research of quantum dot and potentially involved mechanisms. Sci. Total Environ. 2018, 625, 940–962. [Google Scholar] [CrossRef]
- Singh, A.K.; Prakash, P.; Singh, R.; Nandy, N.; Firdaus, Z.; Bansal, M.; Singh, R.K.; Srivastava, A.; Roy, J.K.; Mishra, B.; et al. Curcumin Quantum Dots Mediated Degradation of Bacterial Biofilms. Front. Microbiol. 2017, 8, 1517. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Hussein, M.Z.; Alitheen, N.B.; Abu, N. Folic acid targeted Mn:ZnS quantum dots for theranostic applications of cancer cell imaging and therapy. Int. J. Nanomed. 2016, 11, 413–428. [Google Scholar]
- Galliani, M.; Signore, G. Poly(Lactide-Co-Glycolide) Nanoparticles Co-Loaded with Chlorophyllin and Quantum Dots as Photodynamic Therapy Agents. Chempluschem 2019, 84, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef]
- Legge, C.J.; Colley, H.E.; Lawson, M.A.; Rawlings, A.E. Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J. Oral Pathol. Med. 2019, 48, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Mohtashami, L.; Ghows, N.; Tayarani-Najaran, Z.; Iranshahi, M. Galbanic Acid-Coated Fe3O4 Magnetic Nanoparticles with Enhanced Cytotoxicity to Prostate Cancer Cells. Planta Med. 2019, 85, 169–178. [Google Scholar] [CrossRef]
- Biswas, A.; Sampathkumar, R.; Kumar, A.; Bhattacharyya, D.; Sahoo, N.K.; Lagoo, K.D.; Veerapur, R.D.; Padmanabhan, M.; Puri, R.K.; Bhattacharya, D.; et al. Design and development of an in-line sputtering system and process development of thin film multilayer neutron supermirrors. Rev. Sci. Instrum. 2014, 85, 123103. [Google Scholar] [CrossRef] [PubMed]
- Odeniyi, M.A.; Omoteso, O.A.; Adepoju, A.O.; Jaiyeoba, K.T. Starch nanoparticles in drug delivery: A review. Polim Med. 2018, 48, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Sen, K.K.; Gandhi, A. Alginate Based Nanocarriers for Drug Delivery Applications. Curr. Pharm. Des. 2016, 22, 3399–3410. [Google Scholar] [CrossRef]
- Fang, Z.; Wan, L.Y.; Chu, L.Y.; Zhang, Y.Q.; Wu, J.F. ‘Smart’ nanoparticles as drug delivery systems for applications in tumor therapy. Expert. Opin. Drug Deliv. 2015, 12, 1943–1953. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Edison, T.; LewisOscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan nanopolymers: An overview of drug delivery against cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef]
- Babu, A.; Ramesh, R. Multifaceted Applications of Chitosan in Cancer Drug Delivery and Therapy. Mar. Drugs 2017, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Hamishehkar, H.; Ghorbani, M. A novel smart PEGylated gelatin nanoparticle for co-delivery of doxorubicin and betanin: A strategy for enhancing the therapeutic efficacy of chemotherapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 833–841. [Google Scholar] [CrossRef]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eldeen, A.M.; Moustafa, D.; El-Daly, S.M.; Abo-Zeid, M.A.M.; Saleh, S.; Khoobchandani, M.; Katti, K.; Shukla, R.; Katti, K.V. Gum Arabic-encapsulated gold nanoparticles for a non-invasive photothermal ablation of lung tumor in mice. Biomed. Pharmacother 2017, 89, 1045–1054. [Google Scholar] [CrossRef]
- Babu, A.; Wang, Q.; Muralidharan, R.; Shanker, M.; Munshi, A.; Ramesh, R. Chitosan coated polylactic acid nanoparticle-mediated combinatorial delivery of cisplatin and siRNA/Plasmid DNA chemosensitizes cisplatin-resistant human ovarian cancer cells. Molecular Pharm. 2014, 11, 2720–2733. [Google Scholar] [CrossRef] [PubMed]
- Strong, L.E.; Dahotre, S.N.; West, J.L. Hydrogel-nanoparticle composites for optically modulated cancer therapeutic delivery. J. Control. Release 2014, 178, 63–68. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [PubMed]
- Scheideler, M.; Vidakovic, I.; Prassl, R. Lipid nanocarriers for microRNA delivery. Chem. Phys. Lipids 2020, 226, 104837. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery—Liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer. Res. 2003, 23, 363–398. [Google Scholar]
- Wang, G.; Wang, J.J.; Wang, Y.Z.; Feng, S.; Jing, G.; Fu, X.L. Myricetin nanoliposomes induced SIRT3-mediated glycolytic metabolism leading to glioblastoma cell death. Artif Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S180–S191. [Google Scholar] [CrossRef]
- Maroufi, N.F.; Vahedian, V.; Mazrakhondi, S.A.M.; Kooti, W.; Khiavy, H.A.; Bazzaz, R.; Ramezani, F.; Pirouzpanah, S.M.; Ghorbani, M.; Akbarzadeh, M.; et al. Sensitization of MDA-MBA231 breast cancer cell to docetaxel by myricetin loaded into biocompatible lipid nanoparticles via sub-G1 cell cycle arrest mechanism. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1–11. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: STARBURST®-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Dichwalkar, T.; Patel, S.; Bapat, S.; Pancholi, P.; Jasani, N.; Desai, B.; Yellepeddi, V.K.; Sehdev, V. Omega-3 Fatty Acid Grafted PAMAM-Paclitaxel Conjugate Exhibits Enhanced Anticancer Activity in Upper Gastrointestinal Cancer Cells. Macromol. Biosci. 2017, 17, 8. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.M.; Majoral, J.P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 9. [Google Scholar] [CrossRef]
- Aas, Z.; Babaei, E.; Hosseinpour Feizi, M.A.; Dehghan, G. Anti-proliferative and Apoptotic Effects of Dendrosomal Farnesiferol C on Gastric Cancer Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5325–5329. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [PubMed]
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 8091–8108. [Google Scholar] [CrossRef]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J. Nanomater 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Islam, W.; Fang, J.; Imamura, T.; Etrych, T.; Subr, V.; Ulbrich, K.; Maeda, H. Augmentation of the Enhanced Permeability and Retention Effect with Nitric Oxide-Generating Agents Improves the Therapeutic Effects of Nanomedicines. Mol. Cancer Ther. 2018, 17, 2643–2653. [Google Scholar] [CrossRef]
- Yang, X.; Yi, C.; Luo, N.; Gong, C. Nanomedicine to overcome cancer multidrug resistance. Curr. Drug Metab. 2014, 15, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987, 47, 3039–3051. [Google Scholar]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Theek, B.; Baues, M.; Ojha, T.; Möckel, D.; Veettil, S.K.; Steitz, J.; van Bloois, L.; Storm, G.; Kiessling, F.; Lammers, T. Sonoporation enhances liposome accumulation and penetration in tumors with low EPR. J. Control. Release 2016, 231, 77–85. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, J.; Nie, Y.; Liao, G.; Yu, Y.; Li, C. Overcoming the Reticuloendothelial System Barrier to Drug Delivery with a “Don’t-Eat-Us” Strategy. ACS Nano 2019, 13, 13015–13026. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Gómez-Vallejo, V.; Puigivila, M.; Plaza-García, S.; Szczupak, B.; Piñol, R.; Murillo, J.L.; Sorribas, V.; Lou, G.; Veintemillas, S.; Ramos-Cabrer, P.; et al. PEG-copolymer-coated iron oxide nanoparticles that avoid the reticuloendothelial system and act as kidney MRI contrast agents. Nanoscale 2018, 10, 14153–14164. [Google Scholar] [CrossRef]
- Teiten, M.H.; Gaascht, F.; Dicato, M.; Diederich, M. Anticancer bioactivity of compounds from medicinal plants used in European medieval traditions. Biochem. Pharmacol. 2013, 86, 1239–1247. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert. Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, Z.; Zhou, C.; Zhu, J.; Lee, R.J.; Teng, L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 2016, 34, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, X.; Cai, H.; Zhang, P.; Kong, D.; Ge, X.; Du, M.; Liang, R.; Dong, W. Anticancer effects of plant derived Anacardic acid on human breast cancer MDA-MB-231 cells. Am. J. Transl. Res. 2018, 10, 2424–2434. [Google Scholar]
- Hamad, F.B.; Mubofu, E.B. Potential biological applications of bio-based anacardic acids and their derivatives. Int. J. Mol. Sci. 2015, 16, 8569–8590. [Google Scholar] [CrossRef]
- Kushwah, V.; Katiyar, S.S.; Dora, C.P.; Kumar Agrawal, A.; Lamprou, D.A.; Gupta, R.C.; Jain, S. Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater. 2018, 73, 424–436. [Google Scholar] [CrossRef]
- Ríos, J.L.; Máñez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef]
- Saneja, A.; Arora, D.; Kumar, R.; Dubey, R.D.; Panda, A.K.; Gupta, P.N. Therapeutic applications of betulinic acid nanoformulations. Ann. N. Y. Acad. Sci. 2018, 1421, 5–18. [Google Scholar] [CrossRef]
- Saneja, A.; Kumar, R.; Mintoo, M.J.; Dubey, R.D.; Sangwan, P.L.; Mondhe, D.M.; Panda, A.K.; Gupta, P.N. Gemcitabine and betulinic acid co-encapsulated PLGA-PEG polymer nanoparticles for improved efficacy of cancer chemotherapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 764–771. [Google Scholar] [CrossRef]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I.; et al. Thermosensitive Betulinic Acid-Loaded Magnetoliposomes: A Promising Antitumor Potential for Highly Aggressive Human Breast Adenocarcinoma Cells Under Hyperthermic Conditions. Int. J. Nanomed. 2020, 15, 8175–8200. [Google Scholar] [CrossRef]
- Kumar, P.; Gautam, A.K.; Kumar, U.; Bhadauria, A.S.; Singh, A.K.; Kumar, D.; Mahata, T.; Maity, B.; Bera, H.; Saha, S. Mechanistic exploration of the activities of poly(lactic-co-glycolic acid)-loaded nanoparticles of betulinic acid against hepatocellular carcinoma at cellular and molecular levels. Arch. Physiol. Biochem. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Pandelidou, M.; Dimas, K.; Georgopoulos, A.; Hatziantoniou, S.; Demetzos, C. Preparation and characterization of lyophilised egg PC liposomes incorporating curcumin and evaluation of its activity against colorectal cancer cell lines. J. Nanosci. Nanotechnol. 2011, 11, 1259–1266. [Google Scholar] [CrossRef]
- Arya, G.; Das, M.; Sahoo, S.K. Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomed. Pharmacother. 2018, 102, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Zafaryab, M.; Mehdi, S.H.; Ahmad, I.; Rizvi, M.M. Characterization and anti-proliferative activity of curcumin loaded chitosan nanoparticles in cervical cancer. Int. J. Biol Macromol. 2016, 93 Pt A, 242–253. [Google Scholar] [CrossRef]
- Gumireddy, A.; Christman, R.; Kumari, D.; Tiwari, A.; North, E.J.; Chauhan, H. Preparation, Characterization, and In vitro Evaluation of Curcumin- and Resveratrol-Loaded Solid Lipid Nanoparticles. AAPS PharmSciTech 2019, 20, 145. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Chu, P.Y.; Tsai, S.C.; Ko, H.Y.; Wu, C.C.; Lin, Y.H. Co-Delivery of Natural Compounds with a Dual-Targeted Nanoparticle Delivery System for Improving Synergistic Therapy in an Orthotopic Tumor Model. ACS Appl. Mater. Interfaces 2019, 11, 23880–23892. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Tu, G.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Enhanced Chemotherapeutic Efficacy of PLGA-Encapsulated Epigallocatechin Gallate (EGCG) Against Human Lung Cancer. Int. J. Nanomed. 2020, 15, 4417–4429. [Google Scholar]
- Velavan, B.; Divya, T.; Sureshkumar, A.; Sudhandiran, G. Nano-chemotherapeutic efficacy of (-) -epigallocatechin 3-gallate mediating apoptosis in A549 cells: Involvement of reactive oxygen species mediated Nrf2/Keap1signaling. Biochem. Biophys. Res. Commun. 2018, 503, 1723–1731. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Pooja, D.; Kulhari, H.; Gudem, S.; Ravuri, H.G.; Bhargava, S.; Ramakrishna, S. Bombesin conjugated solid lipid nanoparticles for improved delivery of epigallocatechin gallate for breast cancer treatment. Chem. Phys. Lipids 2019, 224, 104770. [Google Scholar] [CrossRef]
- Hajipour, H.; Hamishehkar, H.; Nazari Soltan Ahmad, S.; Barghi, S.; Maroufi, N.F.; Taheri, R.A. Improved anticancer effects of epigallocatechin gallate using RGD-containing nanostructured lipid carriers. Artif Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 283–292. [Google Scholar] [CrossRef]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin Gallate-Gold Nanoparticles Exhibit Superior Antitumor Activity Compared to Conventional Gold Nanoparticles: Potential Synergistic Interactions. Nanomaterials 2019, 9, 936. [Google Scholar] [CrossRef]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sonika; Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic Acid: A Promising Therapeutic Phytochemical and Recent Patents Advances. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Fahrioğlu, U.; Dodurga, Y.; Elmas, L.; Seçme, M. Ferulic acid decreases cell viability and colony formation while inhibiting migration of MIA PaCa-2 human pancreatic cancer cells in vitro. Gene 2016, 576, 476–482. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, S.; Tong, Y.; Peng, S. Ferulic Acid Induces Apoptosis of HeLa and Caski Cervical Carcinoma Cells by Down-Regulating the Phosphatidylinositol 3-Kinase (PI3K)/Akt Signaling Pathway. Med. Sci. Monit. 2020, 26, e920095. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yan, C.; Miao, J.; Zhang, X.; Chen, J.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Synthesis, characterization and antitumor properties of selenium nanoparticles coupling with ferulic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 104–112. [Google Scholar] [CrossRef]
- Ezhuthupurakkal, P.B.; Ariraman, S.; Arumugam, S.; Subramaniyan, N.; Muthuvel, S.K.; Kumpati, P.; Rajamani, B.; Chinnasamy, T. Anticancer potential of ZnO nanoparticle-ferulic acid conjugate on Huh-7 and HepG2 cells and diethyl nitrosamine induced hepatocellular cancer on Wistar albino rat. Nanomedicine 2018, 14, 415–428. [Google Scholar] [CrossRef]
- Thakkar, A.; Chenreddy, S.; Wang, J.; Prabhu, S. Ferulic acid combined with aspirin demonstrates chemopreventive potential towards pancreatic cancer when delivered using chitosan-coated solid-lipid nanoparticles. Cell Biosci. 2015, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Lu, L.Y.; Wang, X.Q.; Li, B.X.; Kelly, K.; Lin, H.S. Gambogic Acid Induces Cell Apoptosis and Inhibits MAPK Pathway in PTEN(-/-)/p53(-/-) Prostate Cancer Cells In Vitro and Ex Vivo. Chin. J. Integr. Med. 2018, 24, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, M.; Jiang, Y.; Wu, H.; Lu, G.; Shi, W.; Cong, D.; Song, S.; Liu, K.; Wang, H. Gambogic Acid Induces Apoptosis of Non-Small Cell Lung Cancer (NSCLC) Cells by Suppressing Notch Signaling. Med. Sci. Monit. 2018, 24, 7146–7151. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.N.; Ho, W.S. Antiproliferative activity of gambogic acid isolated from Garcinia hanburyi in Hep3B and Huh7 cancer cells. Oncol. Rep. 2013, 29, 1744–1750. [Google Scholar] [CrossRef]
- Xu, Q.; Chu, C. Development of ROS -responsive amino acid-based poly(ester amide) nanoparticle for anticancer drug delivery. J. Biomed. Mater. Res. Part A 2021, 109, 524–537. [Google Scholar] [CrossRef]
- Wang, S.; Shao, M.; Zhong, Z.; Wang, A.; Cao, J.; Lu, Y.; Wang, Y.; Zhang, J. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017, 24, 1791–1800. [Google Scholar] [CrossRef]
- He, M.; Ro, L.; Liu, J.; Chu, C.C. Folate-decorated arginine-based poly(ester urea urethane) nanoparticles as carriers for gambogic acid and effect on cancer cells. J. Biomed. Mater. Res. A 2017, 105, 475–490. [Google Scholar] [CrossRef]
- Fang, L.; Chen, B.; Liu, S.; Wang, R.; Hu, S.; Xia, G.; Tian, Y.; Cai, X. Synergistic effect of a combination of nanoparticulate Fe3O4 and gambogic acid on phosphatidylinositol 3-kinase/Akt/Bad pathway of LOVO cells. Int. J. Nanomed. 2012, 7, 4109–4118. [Google Scholar]
- Lü, J.M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Xue, B.; Yang, C.; Miao, L.; Zhou, L.; Chen, Q.; Cai, Q.; Liu, Y.; Liu, D.; He, H.; et al. Biopharmaceutical characters and bioavailability improving strategies of ginsenosides. Fitoterapia 2018, 129, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, X.; Hong, L.; Zhao, X.; Cui, G.; Li, A.; Liu, Y.; Zhou, L.; Sun, R.; Shen, S.; et al. Nanoparticle Conjugation of Ginsenoside Rg3 Inhibits Hepatocellular Carcinoma Development and Metastasis. Small 2020, 16, e1905233. [Google Scholar] [CrossRef]
- Qiu, R.; Qian, F.; Wang, X.; Li, H.; Wang, L. Targeted delivery of 20(S)-ginsenoside Rg3-based polypeptide nanoparticles to treat colon cancer. Biomed. Microdevices 2019, 21, 18. [Google Scholar] [CrossRef]
- Dong, Y.; Fu, R.; Yang, J.; Ma, P.; Liang, L.; Mi, Y.; Fan, D. Folic acid-modified ginsenoside Rg5-loaded bovine serum albumin nanoparticles for targeted cancer therapy in vitro and in vivo. Int. J. Nanomed. 2019, 14, 6971–6988. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, B.; Li, B.; Li, Z.; Jiang, B.H.; Chen, Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar]
- Govindaraju, S.; Roshini, A.; Lee, M.H.; Yun, K. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to A549 lung cancer cells. Int. J. Nanomed. 2019, 14, 5147–5157. [Google Scholar] [CrossRef]
- Chuang, Y.L.; Fang, H.W.; Ajitsaria, A.; Chen, K.H.; Su, C.Y.; Liu, G.S.; Tseng, C.L. Development of Kaempferol-Loaded Gelatin Nanoparticles for the Treatment of Corneal Neovascularization in Mice. Pharmaceutics 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Ghoshal, G.; Kesharwani, P.; Singh, B.; Shivhare, U.S.; Katare, O.P. Lycopene loaded whey protein isolate nanoparticles: An innovative endeavor for enhanced bioavailability of lycopene and anti-cancer activity. Int. J. Pharm. 2018, 546, 97–105. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.N.; do Amaral, C.P.; de Almeida, M.P.; Borges, T.K.S.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, J.S.C.; et al. Cytotoxic activity of poly-ɛ-caprolactone lipid-core nanocapsules loaded with lycopene-rich extract from red guava (Psidium guajava L.) on breast cancer cells. Food Res. Int. 2020, 136, 109548. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Kushwah, V.; Thakur, K.; Ghoshal, G.; Singh, B.; Jain, S.; Shivhare, U.S.; Katare, O.P. Fabrication and functional attributes of lipidic nanoconstructs of lycopene: An innovative endeavour for enhanced cytotoxicity in MCF-7 breast cancer cells. Colloids Surf. B Biointerfaces 2017, 152, 482–491. [Google Scholar] [CrossRef]
- Huang, R.F.; Wei, Y.J.; Inbaraj, B.S.; Chen, B.H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, C.; Thangam, R.; Mary, S.A.; Kannan, P.R.; Arun, G.; Madhan, B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr. Polym. 2020, 231, 115682. [Google Scholar] [CrossRef]
- Park, S.Y.; Chae, S.Y.; Park, J.O.; Lee, K.J.; Park, G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial growth factor receptor-targeted and reactive oxygen species-responsive lung cancer therapy by docetaxel and resveratrol encapsulated lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y.; et al. Anticancer Effects of Resveratrol-Loaded Solid Lipid Nanoparticles on Human Breast Cancer Cells. Molecules 2017, 22, 1814. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hoti, S.L.; Prasad, N.R. Resveratrol loaded gelatin nanoparticles synergistically inhibits cell cycle progression and constitutive NF-kappaB activation, and induces apoptosis in non-small cell lung cancer cells. Biomed. Pharmacother. 2015, 70, 274–282. [Google Scholar] [CrossRef]
- Guo, L.; Peng, Y.; Li, Y.; Yao, J.; Zhang, G.; Chen, J.; Wang, J.; Sui, L. Cell death pathway induced by resveratrol-bovine serum albumin nanoparticles in a human ovarian cell line. Oncol. Lett. 2015, 9, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Kong, F.; Shetti, D.; Zhang, B.; Yang, Y.; Wei, K. Resveratrol loaded oxidized mesoporous carbon nanoparticles: A promising tool to treat triple negative breast cancer. Biochem. Biophys. Res. Commun. 2019, 519, 378–384. [Google Scholar] [CrossRef]
- Zhang, X.F.; Huang, F.H.; Zhang, G.L.; Bai, D.P.; Massimo, D.F.; Huang, Y.F.; Gurunathan, S. Novel biomolecule lycopene-reduced graphene oxide-silver nanoparticle enhances apoptotic potential of trichostatin A in human ovarian cancer cells (SKOV3). Int. J. Nanomed. 2017, 12, 7551–7575. [Google Scholar] [CrossRef]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer drug delivery in the nano era: An overview and perspectives (Review). Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G. Nanomedicines for Cancer Therapy: An Update of FDA Approved and Those under Various Stages of Development. SOJ Pharm. Pharm. Sci. 2014, 1, 13–23. [Google Scholar]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Paulraj, F.; Abas, F.; N, H.L.; Othman, I. Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules 2019, 9, 270. [Google Scholar] [CrossRef]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef]
- Storka, A.; Vcelar, B.; Klickovic, U.; Gouya, G.; Weisshaar, S.; Aschauer, S.; Bolger, G.; Helson, L.; Wolzt, M. Safety, tolerability and pharmacokinetics of liposomal curcumin in healthy humans. Int. J. Clin. Pharmacol. Ther. 2015, 53, 54–65. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Zu, Y.J.; Nie, S.F.; Cao, J.; Wang, Q.; Nie, S.P.; Deng, Z.Y.; Xie, M.Y.; Wang, S. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin. J. Nat. Med. 2015, 13, 641–652. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; He, C.; Riviere, J.E.; Monteiro-Riviere, N.A.; Lin, Z. Meta-Analysis of Nanoparticle Delivery to Tumors Using a Physiologically Based Pharmacokinetic Modeling and Simulation Approach. ACS Nano 2020, 14, 3075–3095. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Kelly, B.F.; Nappe, T.M. Cannabinoid Toxicity. In StatPearls; StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Wei, Q.Y.; He, K.M.; Chen, J.L.; Xu, Y.M.; Lau, A.T.Y. Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications. Molecules 2019, 24, 4246. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dai, W.; He, B.; Zhang, H.; Wang, X.; Wang, Y.; Zhang, Q. Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics 2017, 7, 538–558. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).