Intra-Articular Administration of Cramp into Mouse Knee Joint Exacerbates Experimental Osteoarthritis Progression
Abstract
1. Introduction
2. Results
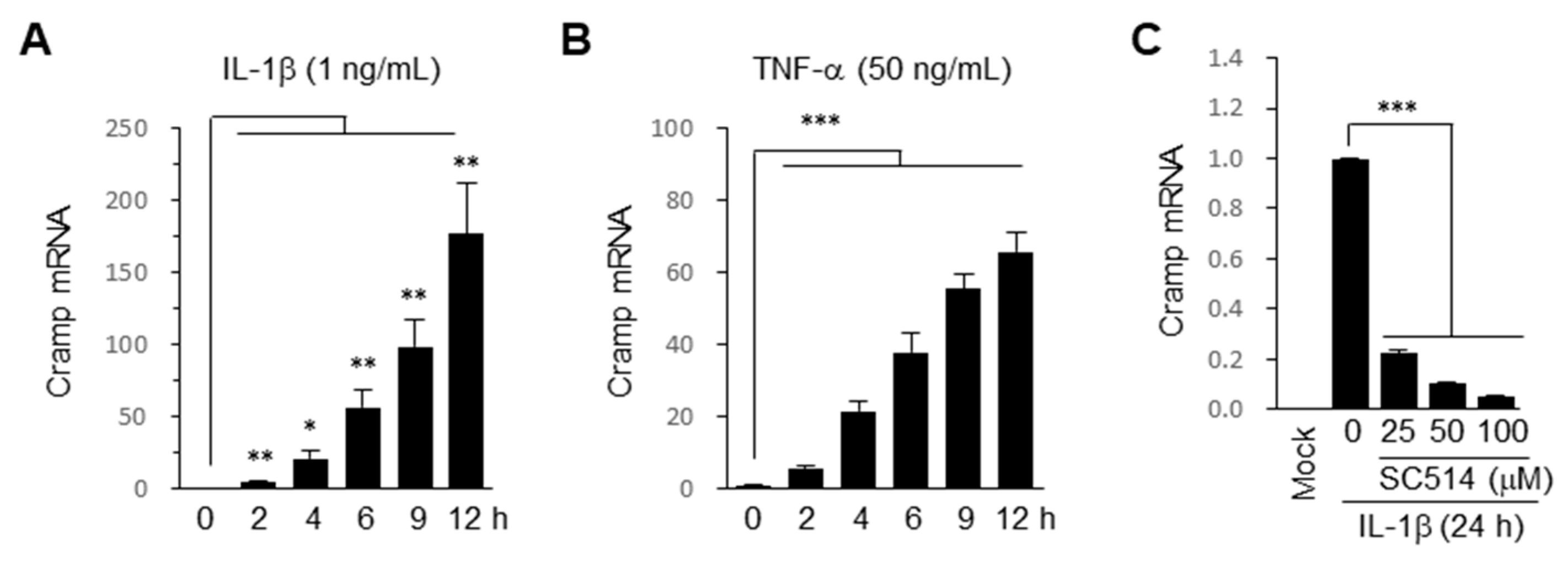
2.1. Cramp Is Induced by Pro-Inflammatory Cytokines via NF-κB Pathway in Chondrocytes
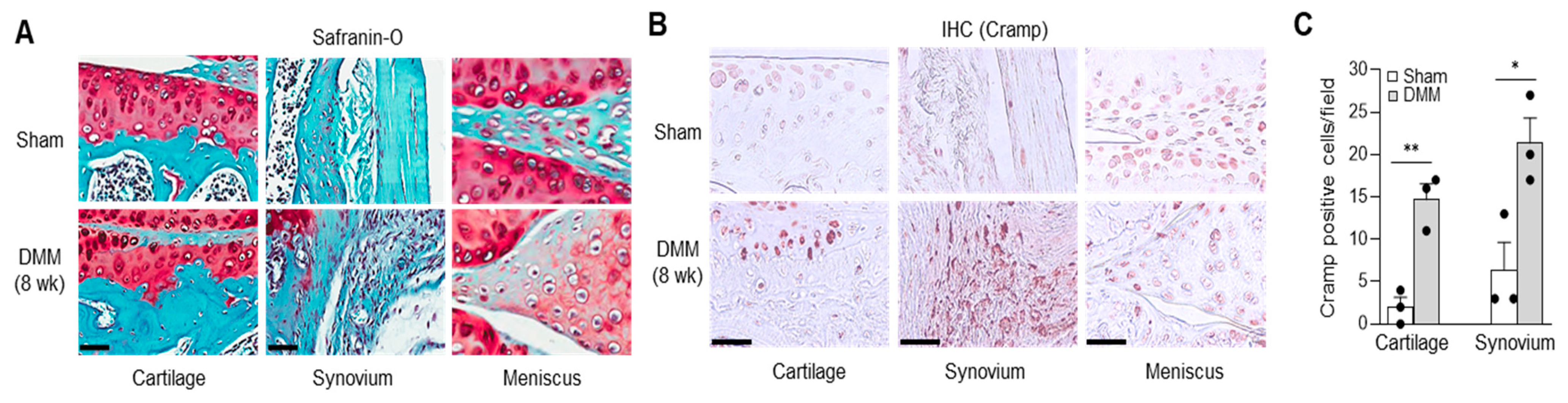
2.2. Cramp Expression Is Elevated in Cartilage and Synovium of Experimental OA
2.3. Cramp Increases Mmp3 and Mmp13 Expression in Chondrocytes
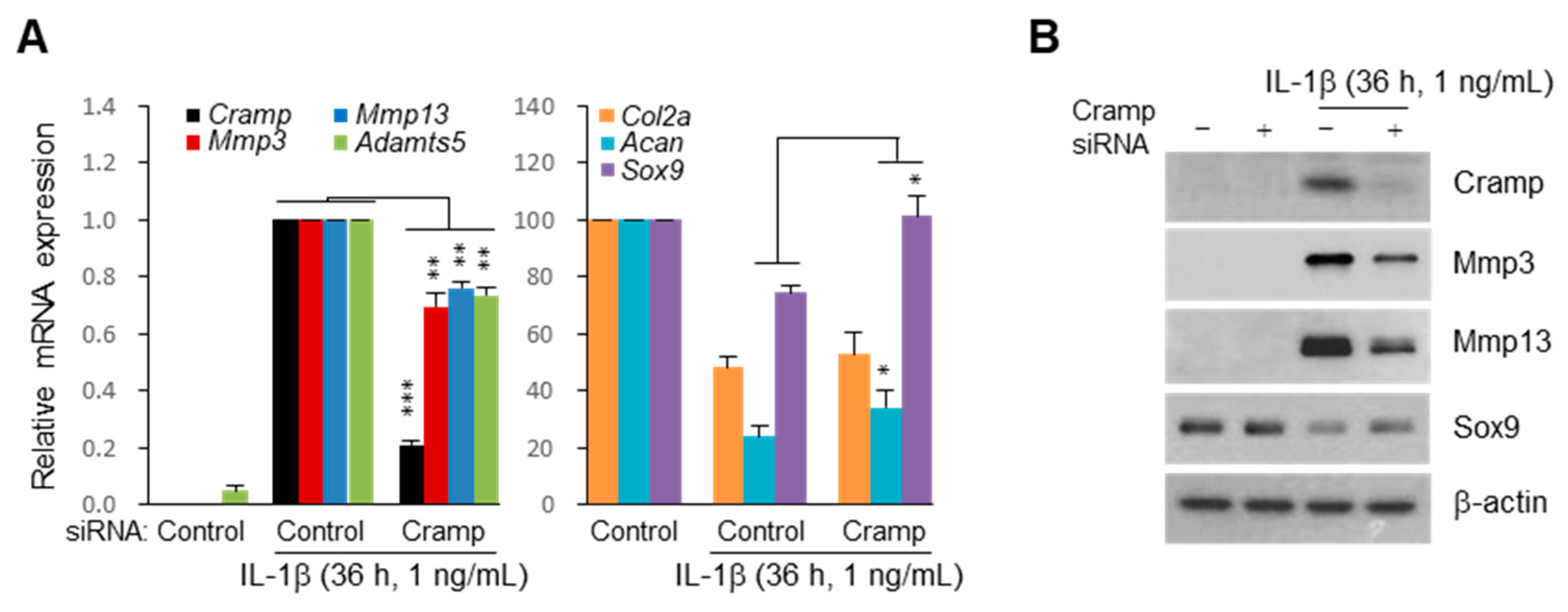
2.4. siRNA-Mediated Knockdown of Cramp Reduces IL-1β-Induced Catabolic Gene Expression
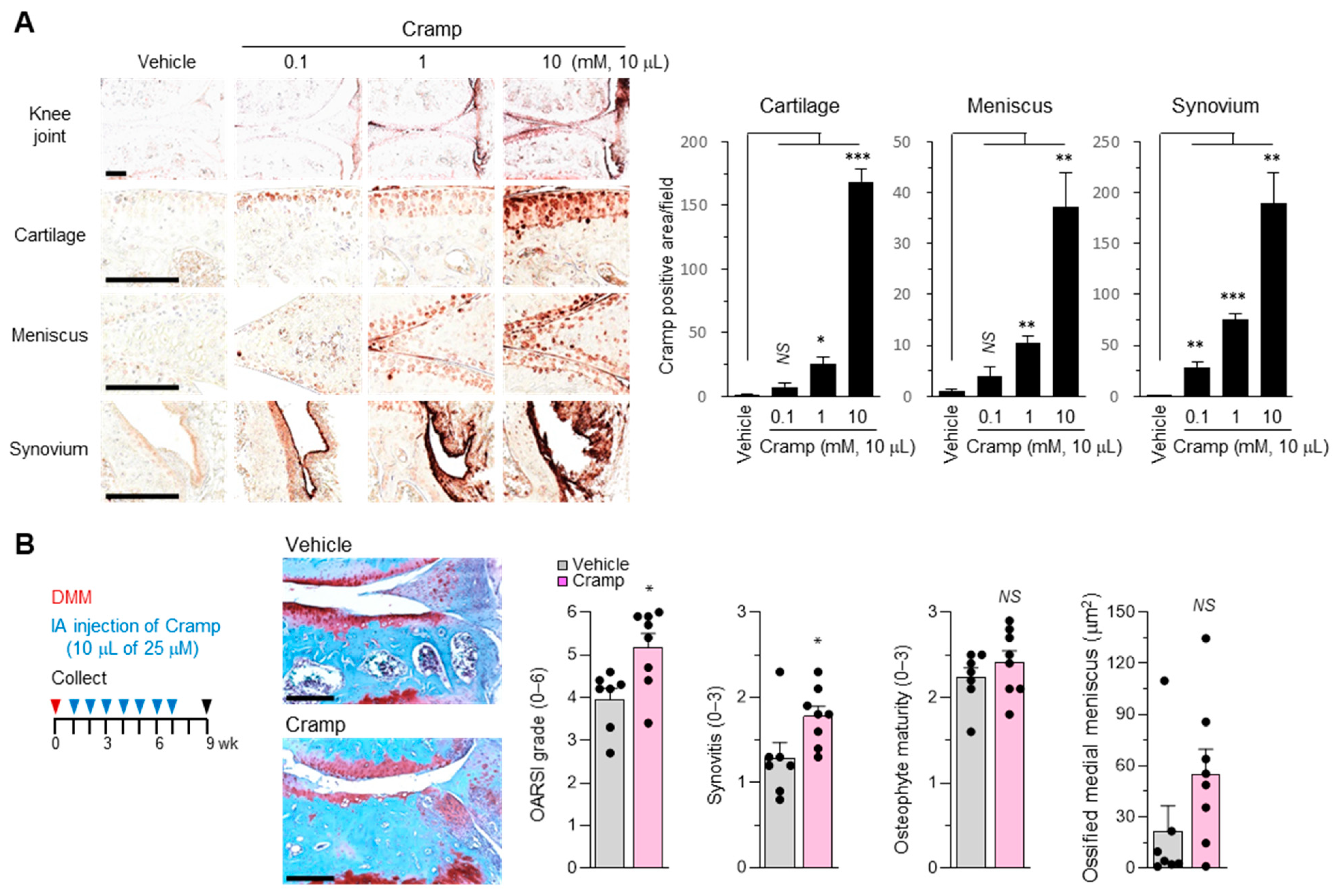
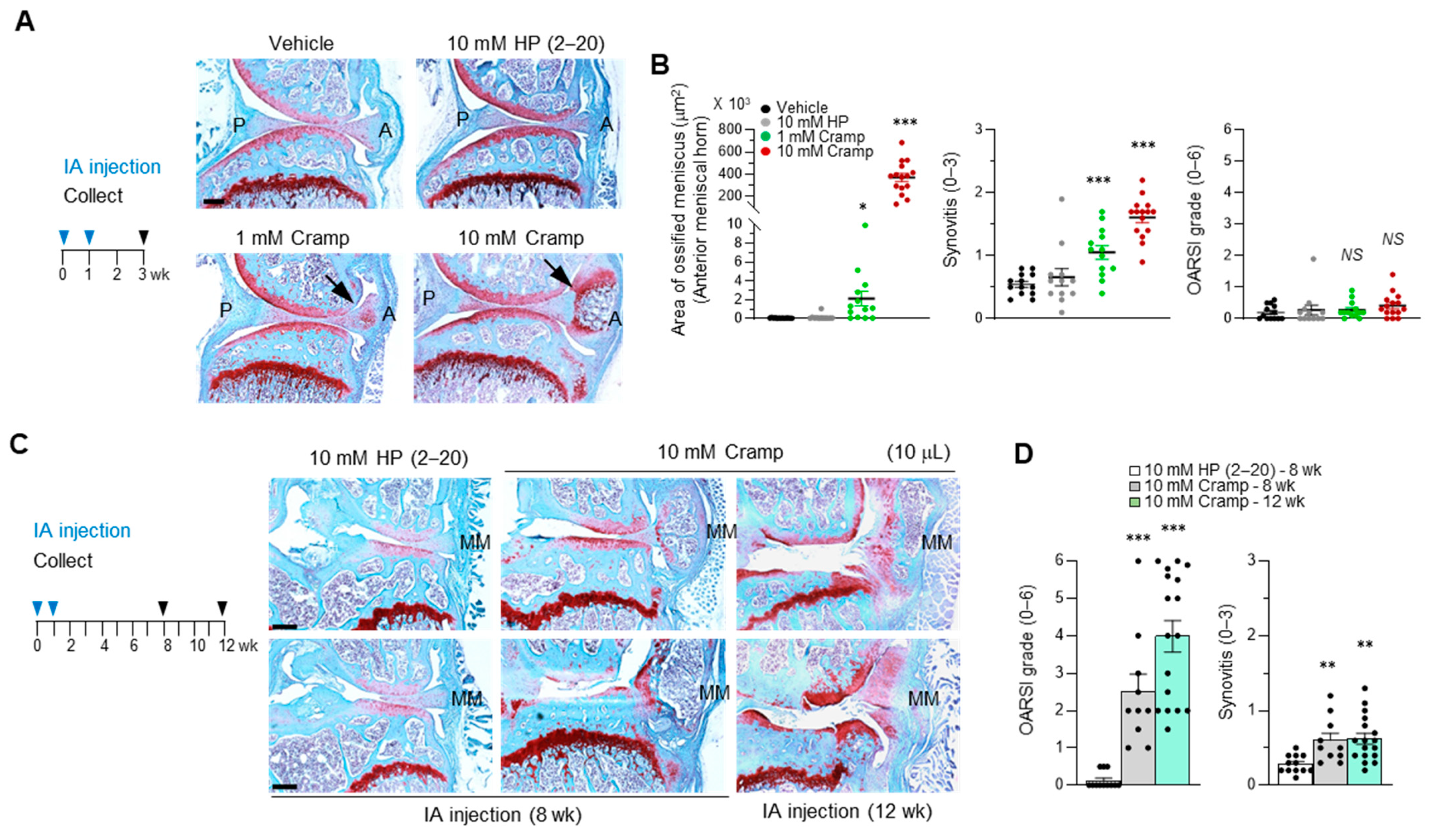
2.5. Cramp Exacerbates the Progression of Experimental OA
2.6. Introduction of Excessive Cramp Causes Meniscus Ossification and Tears
3. Discussion
4. Materials and Methods
4.1. Chondrocyte Culture, Peptide Treatment, and siRNA Transfection
4.2. RNA Analysis
4.3. Western Blot Analysis
4.4. IA Injection in Mice, and Experimental OA
4.5. Histology and Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Englund, M.; Roos, E.M.; Lohmander, L.S. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: A sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003, 48, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Englund, M.; Felson, D.T.; Guermazi, A.; Roemer, F.W.; Wang, K.; Crema, M.D.; Lynch, J.A.; Sharma, L.; Segal, N.A.; Lewis, C.E.; et al. Risk factors for medial meniscal pathology on knee MRI in older US adults: A multicentre prospective cohort study. Ann. Rheum. Dis. 2011, 70, 1733–1739. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Gale, D.; Hunter, D.J.; Aliabadi, P.; Clancy, M.; Felson, D.T. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N. Engl. J. Med. 2008, 359, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Heinegard, D.; Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Nakasa, T.; Hikata, T.; Asahara, H. Molecular network of cartilage homeostasis and osteoarthritis. Med. Res. Rev. 2008, 28, 464–481. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. 2009, 11, 224. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Yan, Z.P.; Chen, X.Y.; Ni, G.X. Infrapatellar Fat Pad and Knee Osteoarthritis. Aging Dis. 2020, 11, 1317–1328. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Little, C.B.; Hunter, D.J. Post-traumatic osteoarthritis: From mouse models to clinical trials. Nat. Rev. Rheumatol. 2013, 9, 485–497. [Google Scholar] [CrossRef]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta 2012, 1824, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-kappaB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; MaruYama, T.; Chun, C.H.; Park, Y. Alleviation of Murine Osteoarthritis by Cartilage-Specific Deletion of IkappaBzeta. Arthritis Rheumatol. 2018, 70, 1440–1449. [Google Scholar] [CrossRef]
- Kobayashi, H.; Chang, S.H.; Mori, D.; Itoh, S.; Hirata, M.; Hosaka, Y.; Taniguchi, Y.; Okada, K.; Mori, Y.; Yano, F.; et al. Biphasic regulation of chondrocytes by Rela through induction of anti-apoptotic and catabolic target genes. Nat. Commun. 2016, 7, 13336. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, D.; Lin, Y.; Yuan, Q.; Zhou, X. Anterior Cruciate Ligament Transection-Induced Cellular and Extracellular Events in Menisci: Implications for Osteoarthritis. Am. J. Sports Med. 2018, 46, 1185–1198. [Google Scholar] [CrossRef]
- Gamer, L.W.; Xiang, L.; Rosen, V. Formation and maturation of the murine meniscus. J. Orthop. Res. 2017, 35, 1683–1689. [Google Scholar] [CrossRef]
- Ramos-Mucci, L.; Javaheri, B.; van’t Hof, R.; Bou-Gharios, G.; Pitsillides, A.A.; Comerford, E.; Poulet, B. Meniscal and ligament modifications in spontaneous and post-traumatic mouse models of osteoarthritis. Arthritis Res. 2020, 22, 171. [Google Scholar] [CrossRef]
- Kapadia, R.D.; Badger, A.M.; Levin, J.M.; Swift, B.; Bhattacharyya, A.; Dodds, R.A.; Coatney, R.W.; Lark, M.W. Meniscal ossification in spontaneous osteoarthritis in the guinea-pig. Osteoarthr. Cartil. 2000, 8, 374–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomsen, J.S.; Straarup, T.S.; Danielsen, C.C.; Oxlund, H.; Bruel, A. Relationship between articular cartilage damage and subchondral bone properties and meniscal ossification in the Dunkin Hartley guinea pig model of osteoarthritis. Scand. J. Rheumatol. 2011, 40, 391–399. [Google Scholar] [CrossRef]
- Cheung, H.S.; Sallis, J.D.; Demadis, K.D.; Wierzbicki, A. Phosphocitrate blocks calcification-induced articular joint degeneration in a guinea pig model. Arthritis Rheum. 2006, 54, 2452–2461. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, B.; Jensen, H.E. Periarticular ossification at the elbow joint and meniscal ossification in the stifle joint of pigs—Occurrence, pathomorphology, breed differences and correlations with osteochondrosis, leg weakness and production parameters. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2002, 49, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.A. Apparent primary ossification of the menisci in a dog. J. Am. Vet. Med. Assoc. 1998, 212, 1892–1894. [Google Scholar]
- Prasad, S.V.; Fiedoruk, K.; Daniluk, T.; Piktel, E.; Bucki, R. Expression and Function of Host Defense Peptides at Inflammation Sites. Int. J. Mol. Sci. 2019, 21, 104. [Google Scholar] [CrossRef]
- Gupta, S.; Bhatia, G.; Sharma, A.; Saxena, S. Host defense peptides: An insight into the antimicrobial world. J. Oral Maxillofac. Pathol. 2018, 22, 239–244. [Google Scholar] [CrossRef]
- Alford, M.A.; Baquir, B.; Santana, F.L.; Haney, E.F.; Hancock, R.E.W. Cathelicidin Host Defense Peptides and Inflammatory Signaling: Striking a Balance. Front. Microbiol. 2020, 11, 1902. [Google Scholar] [CrossRef]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef]
- Pircher, J.; Czermak, T.; Ehrlich, A.; Eberle, C.; Gaitzsch, E.; Margraf, A.; Grommes, J.; Saha, P.; Titova, A.; Ishikawa-Ankerhold, H.; et al. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat. Commun. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Doring, Y.; Drechsler, M.; Wantha, S.; Kemmerich, K.; Lievens, D.; Vijayan, S.; Gallo, R.L.; Weber, C.; Soehnlein, O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ. Res. 2012, 110, 1052–1056. [Google Scholar] [CrossRef]
- Zhang, L.J.; Guerrero-Juarez, C.F.; Hata, T.; Bapat, S.P.; Ramos, R.; Plikus, M.V.; Gallo, R.L. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 2015, 347, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Edfeldt, K.; Agerberth, B.; Rottenberg, M.E.; Gudmundsson, G.H.; Wang, X.B.; Mandal, K.; Xu, Q.; Yan, Z.Q. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yoshimura, T.; Yao, X.; Gong, W.; Huang, J.; Dzutsev, A.K.; McCulloch, J.; O’HUigin, C.; Bian, X.W.; Trinchieri, G.; et al. Distinct contributions of cathelin-related antimicrobial peptide (CRAMP) derived from epithelial cells and macrophages to colon mucosal homeostasis. J. Pathol. 2020, 253, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics are Potential Anticancer Drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef]
- Pan, L.L.; Liang, W.; Ren, Z.; Li, C.; Chen, Y.; Niu, W.; Fang, X.; Liu, Y.; Zhang, M.; Diana, J.; et al. Cathelicidin-related antimicrobial peptide protects against ischaemia reperfusion-induced acute kidney injury in mice. Br. J. Pharm. 2020, 177, 2726–2742. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, J.; Zhai, T.; Hu, J.; Luo, H.; Zhou, H.; Zhang, Q.; Zhou, Z.; Liu, F. Cathelicidin aggravates myocardial ischemia/reperfusion injury via activating TLR4 signaling and P2X7R/NLRP3 inflammasome. J. Mol. Cell. Cardiol. 2020, 139, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Pan, L.L.; Xue, R.; Ni, G.; Duan, Y.; Bai, Y.; Shi, C.; Ren, Z.; Wu, C.; Li, G.; et al. The anti-microbial peptide LL-37/CRAMP levels are associated with acute heart failure and can attenuate cardiac dysfunction in multiple preclinical models of heart failure. Theranostics 2020, 10, 6167–6181. [Google Scholar] [CrossRef] [PubMed]
- Wertenbruch, S.; Drescher, H.; Grossarth, V.; Kroy, D.; Giebeler, A.; Erschfeld, S.; Heinrichs, D.; Soehnlein, O.; Trautwein, C.; Brandenburg, L.O.; et al. The Anti-Microbial Peptide LL-37/CRAMP Is Elevated in Patients with Liver Diseases and Acts as a Protective Factor during Mouse Liver Injury. Digestion 2015, 91, 307–317. [Google Scholar] [CrossRef]
- Paulsen, F.; Pufe, T.; Conradi, L.; Varoga, D.; Tsokos, M.; Papendieck, J.; Petersen, W. Antimicrobial peptides are expressed and produced in healthy and inflamed human synovial membranes. J. Pathol. 2002, 198, 369–377. [Google Scholar] [CrossRef]
- Hoffmann, M.H.; Bruns, H.; Backdahl, L.; Neregard, P.; Niederreiter, B.; Herrmann, M.; Catrina, A.I.; Agerberth, B.; Holmdahl, R. The cathelicidins LL-37 and rCRAMP are associated with pathogenic events of arthritis in humans and rats. Ann. Rheum. Dis. 2013, 72, 1239–1248. [Google Scholar] [CrossRef]
- Schmidt, N.; Art, J.; Forsch, I.; Werner, A.; Erkel, G.; Jung, M.; Horke, S.; Kleinert, H.; Pautz, A. The anti-inflammatory fungal compound (S)-curvularin reduces proinflammatory gene expression in an in vivo model of rheumatoid arthritis. J. Pharm. Exp. 2012, 343, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Kaplan, M.J. Little peptide, big effects: The role of LL-37 in inflammation and autoimmune disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef] [PubMed]
- Kishore, N.; Sommers, C.; Mathialagan, S.; Guzova, J.; Yao, M.; Hauser, S.; Huynh, K.; Bonar, S.; Mielke, C.; Albee, L.; et al. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J. Biol. Chem. 2003, 278, 32861–32871. [Google Scholar] [CrossRef] [PubMed]
- Bylund, J.; Christophe, T.; Boulay, F.; Nystrom, T.; Karlsson, A.; Dahlgren, C. Proinflammatory activity of a cecropin-like antibacterial peptide from Helicobacter pylori. Antimicrob. Agents Chemother. 2001, 45, 1700–1704. [Google Scholar] [CrossRef]
- Park, Y.; Lee, D.G.; Hahm, K.S. HP(2-9)-magainin 2(1-12), a synthetic hybrid peptide, exerts its antifungal effect on Candida albicans by damaging the plasma membrane. J. Pept. Sci. 2004, 10, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Little, C.B.; Barai, A.; Burkhardt, D.; Smith, S.M.; Fosang, A.J.; Werb, Z.; Shah, M.; Thompson, E.W. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009, 60, 3723–3733. [Google Scholar] [CrossRef]
- Kwok, J.; Onuma, H.; Olmer, M.; Lotz, M.K.; Grogan, S.P.; D’Lima, D.D. Histopathological analyses of murine menisci: Implications for joint aging and osteoarthritis. Osteoarthr. Cartil. 2016, 24, 709–718. [Google Scholar] [CrossRef]
- Varoga, D.; Pufe, T.; Harder, J.; Schroder, J.M.; Mentlein, R.; Meyer-Hoffert, U.; Goldring, M.B.; Tillmann, B.; Hassenpflug, J.; Paulsen, F. Human beta-defensin 3 mediates tissue remodeling processes in articular cartilage by increasing levels of metalloproteinases and reducing levels of their endogenous inhibitors. Arthritis Rheum. 2005, 52, 1736–1745. [Google Scholar] [CrossRef]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.; Blanchet, T.; Ma, H.L.; Flannery, C.R.; Peluso, D.; Kanki, K.; Yang, Z.; et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005, 434, 644–648. [Google Scholar] [CrossRef]
- Wantha, S.; Alard, J.E.; Megens, R.T.; van der Does, A.M.; Doring, Y.; Drechsler, M.; Pham, C.T.; Wang, M.W.; Wang, J.M.; Gallo, R.L.; et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ. Res. 2013, 112, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Wantha, S.; Simsekyilmaz, S.; Doring, Y.; Megens, R.T.; Mause, S.F.; Drechsler, M.; Smeets, R.; Weinandy, S.; Schreiber, F.; et al. Neutrophil-derived cathelicidin protects from neointimal hyperplasia. Sci. Transl. Med. 2011, 3, 103–198. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.D.; Buckland-Wright, J.C. Meniscal and articular cartilage changes in knee osteoarthritis: A cross-sectional double-contrast macroradiographic study. Rheumatology 2002, 41, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Gamini, R.; Olmer, M.; Ikuta, Y.; Hasei, J.; Baek, J.; Alvarez-Garcia, O.; Grogan, S.P.; D’Lima, D.D.; Asahara, H.; et al. Mohawk is a transcription factor that promotes meniscus cell phenotype and tissue repair and reduces osteoarthritis severity. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Choi, S.; Matsuzaki, T.; Alvarez-Garcia, O.; Olmer, M.; Grogan, S.P.; D’Lima, D.D.; Lotz, M.K. FOXO1 and FOXO3 transcription factors have unique functions in meniscus development and homeostasis during aging and osteoarthritis. Proc. Natl. Acad. Sci. USA 2020, 117, 3135–3143. [Google Scholar] [CrossRef]
- Choi, M.C.; Choi, W.H. Mithramycin A Alleviates Osteoarthritic Cartilage Destruction by Inhibiting HIF-2alpha Expression. Int. J. Mol. Sci. 2018, 19, 1411. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18, 17–23. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.-C.; Jo, J.; Lee, M.; Park, J.; Park, Y. Intra-Articular Administration of Cramp into Mouse Knee Joint Exacerbates Experimental Osteoarthritis Progression. Int. J. Mol. Sci. 2021, 22, 3429. https://doi.org/10.3390/ijms22073429
Choi M-C, Jo J, Lee M, Park J, Park Y. Intra-Articular Administration of Cramp into Mouse Knee Joint Exacerbates Experimental Osteoarthritis Progression. International Journal of Molecular Sciences. 2021; 22(7):3429. https://doi.org/10.3390/ijms22073429
Chicago/Turabian StyleChoi, Moon-Chang, Jiwon Jo, Myeongjin Lee, Jonggwan Park, and Yoonkyung Park. 2021. "Intra-Articular Administration of Cramp into Mouse Knee Joint Exacerbates Experimental Osteoarthritis Progression" International Journal of Molecular Sciences 22, no. 7: 3429. https://doi.org/10.3390/ijms22073429
APA StyleChoi, M.-C., Jo, J., Lee, M., Park, J., & Park, Y. (2021). Intra-Articular Administration of Cramp into Mouse Knee Joint Exacerbates Experimental Osteoarthritis Progression. International Journal of Molecular Sciences, 22(7), 3429. https://doi.org/10.3390/ijms22073429





