Trace Identification and Visualization of Multiple Benzimidazole Pesticide Residues on Toona sinensis Leaves Using Terahertz Imaging Combined with Deep Learning
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Dynamic Simulation of Pesticides
2.2. Spectral Analysis of Multiple BZM Pesticide Mixtures
2.3. Clustering Analysis Based on Absorption Peaks and Full Spectra
2.4. Identification of Multiple Pesticide Residues on T. sinensis Leaves
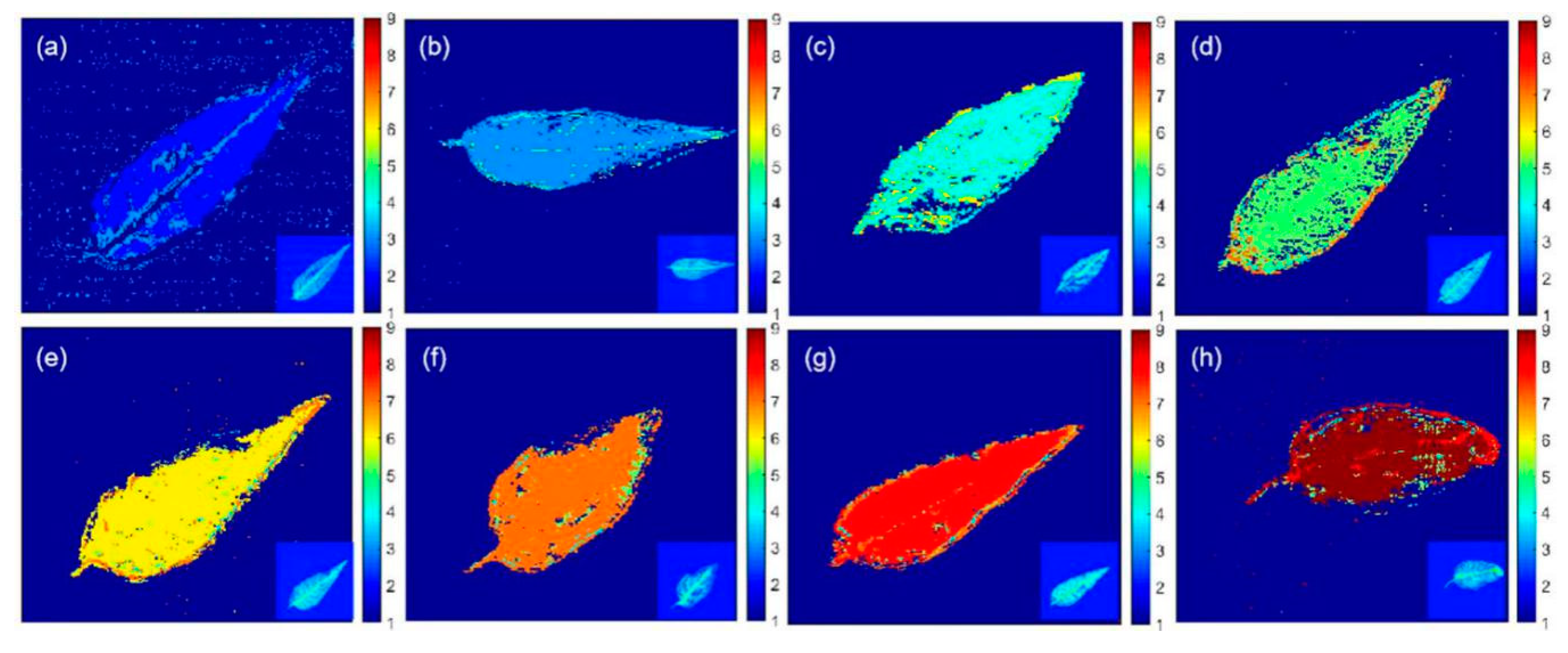
2.5. Visualization of Pesticide Residue Distributions on T. sinensis Leaves
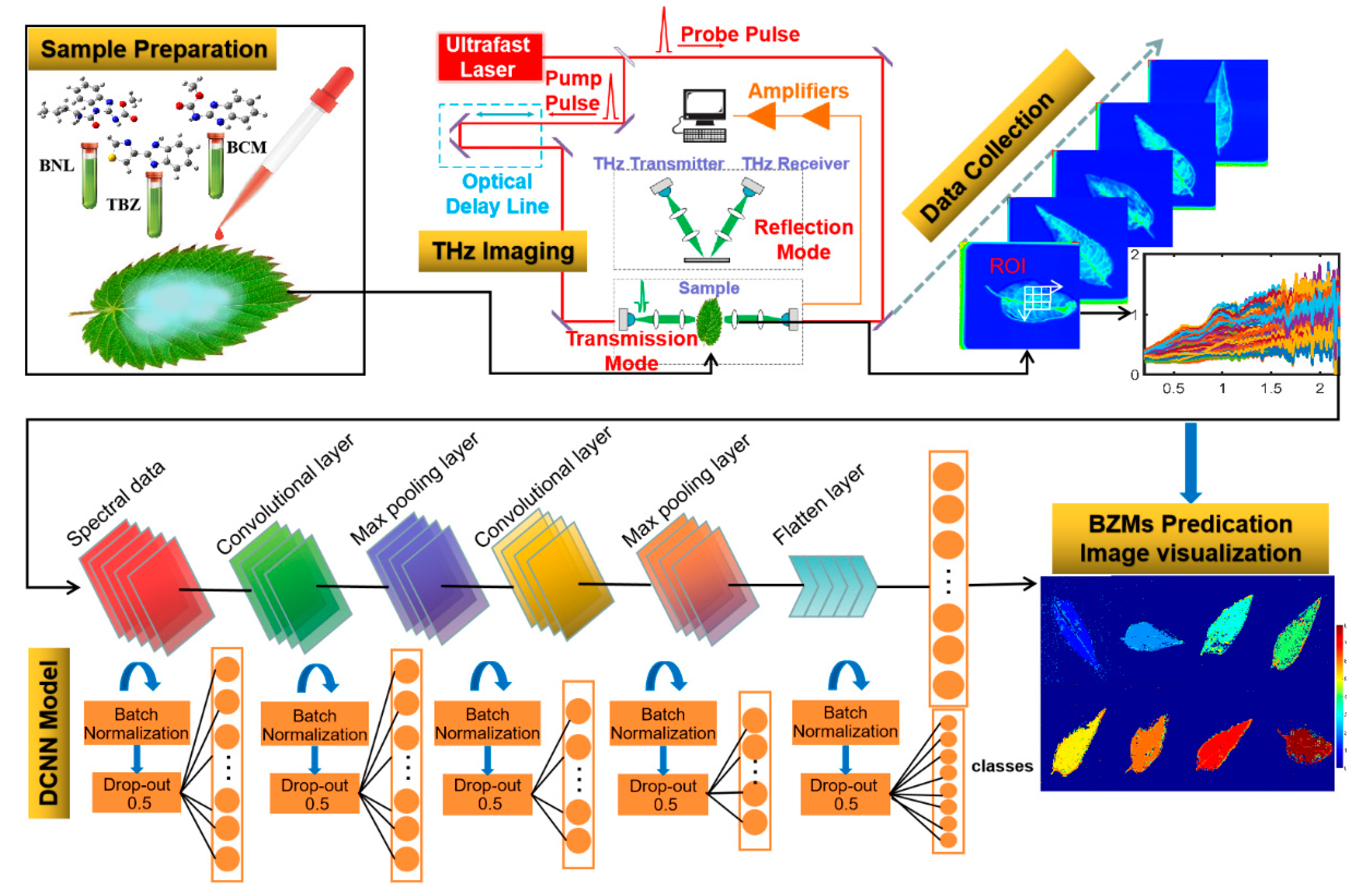
3. Materials and Methods
3.1. Sample Preparation
3.1.1. Preparation of BZM Pesticide Pellets
3.1.2. Preparation of Toona sinensis Leaf Samples
3.2. THz Data Acquisition and Extraction
3.2.1. THz Spectra and Image Acquisition
3.2.2. Spectral Extraction from THz Images
3.3. Neural Network Classification Models
3.3.1. Deep Convolution Neural Network
3.3.2. Back-Propagation Neural Networks
3.4. Data Analysis and Image Visualization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farahat, A.A.; Ismail, M.A.; Kumar, A.; Wenzler, T.; Brun, R.; Paul, A.; Wilson, W.D.; Boykin, D.W. Indole and benzimidazole bichalcophenes: Synthesis, DNA binding and antiparasitic activity. Eur. J. Med. Chem. 2018, 143, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.B.; Li, T.; Xiao, X.M.; Wu, J.; Li, S.K.; Wang, J.X.; Zhou, M.G. Pharmacological characteristics of the novel fungicide pyrisoxazole against sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2018, 149, 61–66. [Google Scholar] [CrossRef]
- Prasad, K.D.; Rani, M.S.; Anusha, D. Synthesis, characterization of benzimidazole derivatives carrying pyridine moiety. Asian J. Res. Chem. 2018, 11, 241–243. [Google Scholar] [CrossRef]
- Johnstone, A.F.M.; Strickland, J.D.; Crofton, K.M.; Gennings, C.; Shafer, T.J. Effects of an environmentally-relevant mixture of pyrethroid insecticides on spontaneous activity in primary cortical networks on microelectrode arrays. Neurotoxicology 2017, 60, 234–239. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Song, L.; Han, Y.T.; Yang, J.; Qin, Y.H.; Zeng, W.B.; Xu, S.Q.; Pan, C.P. Rapid single-step cleanup method for analyzing 47 pesticide residues in pepper, chili peppers and its sauce product by high performance liquid and gas chromatography-tandem mass spectrometry. Food Chem. 2019, 279, 237–245. [Google Scholar] [CrossRef]
- Barbieri, M.V.; Postigo, C.; Guillem-Argiles, N.; Monllor-Alcaraz, L.S.; Simionato, J.I.; Stella, E.; Barcelo, D.; de Alda, M.L. Analysis of 52 pesticides in fresh fish muscle by quechers extraction followed by lc-ms/ms determination. Sci. Total Environ. 2019, 653, 958–967. [Google Scholar] [CrossRef]
- Kumar, V.; Upadhay, N.; Wasit, A.B.; Singh, S.; Kaur, P. Spectroscopic methods for the detection of organophosphate pesticides—A preview. Curr. World Environ. 2013, 8, 313–318. [Google Scholar] [CrossRef]
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of pesticide residues in food using surface-enhanced raman spectroscopy: A review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef] [PubMed]
- Mittleman, D.M. Perspective: Terahertz science and technology. J. Appl. Phys. 2017, 122, 230901. [Google Scholar] [CrossRef]
- Qu, F.; Lin, L.; Cai, C.; Dong, T.; He, Y.; Nie, P. Molecular characterization and theoretical calculation of plant growth regulators based on terahertz time-domain spectroscopy. Appl. Sci. Basel 2018, 8, 420. [Google Scholar] [CrossRef]
- Qu, F.; Lin, L.; He, Y.; Nie, P.; Cai, C.; Dong, T.; Pan, Y.; Tang, Y.; Luo, S. Spectral characterization and molecular dynamics simulation of pesticides based on terahertz time-domain spectra analyses and density functional theory (dft) calculations. Molecules 2018, 23, 1607. [Google Scholar] [CrossRef]
- Sun, Q.S.; He, Y.Z.; Liu, K.; Fan, S.T.; Parrott, E.P.J.; Pickwell-MacPherson, E. Recent advances in terahertz technology for biomedical applications. Quant. Imaging Med. Surg. 2017, 7, 345–355. [Google Scholar] [CrossRef]
- Dhillon, S.S.; Vitiello, M.S.; Linfield, E.H.; Davies, A.G.; Hoffmann, M.C.; Booske, J.; Paoloni, C.; Gensch, M.; Weightman, P.; Williams, G.P.; et al. The 2017 terahertz science and technology roadmap. J. Phys. D Appl. Phys. 2017, 50, 043001. [Google Scholar] [CrossRef]
- Maeng, I.; Baek, S.H.; Kim, H.Y.; Ok, G.-S.; Choi, S.-W.; Chun, H.S. Feasibility of using terahertz spectroscopy to detect seven different pesticides in wheat flour. J. Food Prot. 2014, 77, 2081–2087. [Google Scholar] [CrossRef]
- Massaouti, M.; Daskalaki, C.; Gorodetsky, A.; Koulouklidis, A.D.; Tzortzakis, S. Detection of harmful residues in honey using terahertz time-domain spectroscopy. Appl. Spectrosc. 2013, 67, 1264–1269. [Google Scholar] [CrossRef]
- Baek, S.H.; Kang, J.H.; Hwang, Y.H.; Ok, K.M.; Kwak, K.; Chun, H.S. Detection of methomyl, a carbamate insecticide, in food matrices using terahertz time-domain spectroscopy. J. Infrared Millim. Terahertz Waves 2016, 37, 486–497. [Google Scholar] [CrossRef]
- Kistenev, Y.V.; Borisov, A.V.; Knyazkova, A.I.; Sandykova, E.A.; Nikolaev, V.V.; Vrazhnov, D.A. Applications of thz laser spectroscopy and machine learning for medical diagnostics. EPJ Web Conf. 2018, 195, 1006. [Google Scholar] [CrossRef][Green Version]
- Xing, H.; Zhang, G.G.; Ma, S. Deep learning. Int. J. Semant. Comput. 2016, 10, 417–439. [Google Scholar]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar]
- Deng, L.; Yu, D. Deep learning: Methods and applications. Found. Trends Signal Process. 2014, 7, 197–387. [Google Scholar] [CrossRef]
- Dey, D.; Chatterjee, B.; Dalai, S.; Munshi, S.; Chakravorti, S. A deep learning framework using convolution neural network for classification of impulse fault patterns in transformers with increased accuracy. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3894–3897. [Google Scholar] [CrossRef]
- Feng, J.; Cai, S.; Ma, X. Enhanced sentiment labeling and implicit aspect identification by integration of deep convolution neural network and sequential algorithm. Clust. Comput. J. Netw. Softw. Tools Appl. 2019, 22, S5839–S5857. [Google Scholar] [CrossRef]
- Al-Saffar, A.A.M.; Tao, H.; Talab, M.A. Review of Deep Convolution Neural Network in Image Classification; IEEE: New York, NY, USA, 2017; pp. 26–31. [Google Scholar]
- Pandey, S.; Hindoliya, D.A.; Mod, R. Artificial neural networks for predicting indoor temperature using roof passive cooling techniques in buildings in different climatic conditions. Appl. Soft Comput. 2012, 12, 1214–1226. [Google Scholar] [CrossRef]
- Tapkin, S.; Cevik, A.; Usar, U. Prediction of marshall test results for polypropylene modified dense bituminous mixtures using neural networks. Expert Syst. Appl. 2010, 37, 4660–4670. [Google Scholar] [CrossRef]
- Feil, B.; Balasko, B.; Abonyi, J. Visualization of fuzzy clusters by fuzzy sammon mapping projection: Application to the analysis of phase space trajectories. Soft Comput. 2007, 11, 479–488. [Google Scholar] [CrossRef]
- Pan, W.-T. Combining fuzzy sammon mapping and fuzzy clustering approach to perform clustering effect analysis: Take the banking service satisfaction as an example. Expert Syst. Appl. 2010, 37, 4139–4145. [Google Scholar] [CrossRef]

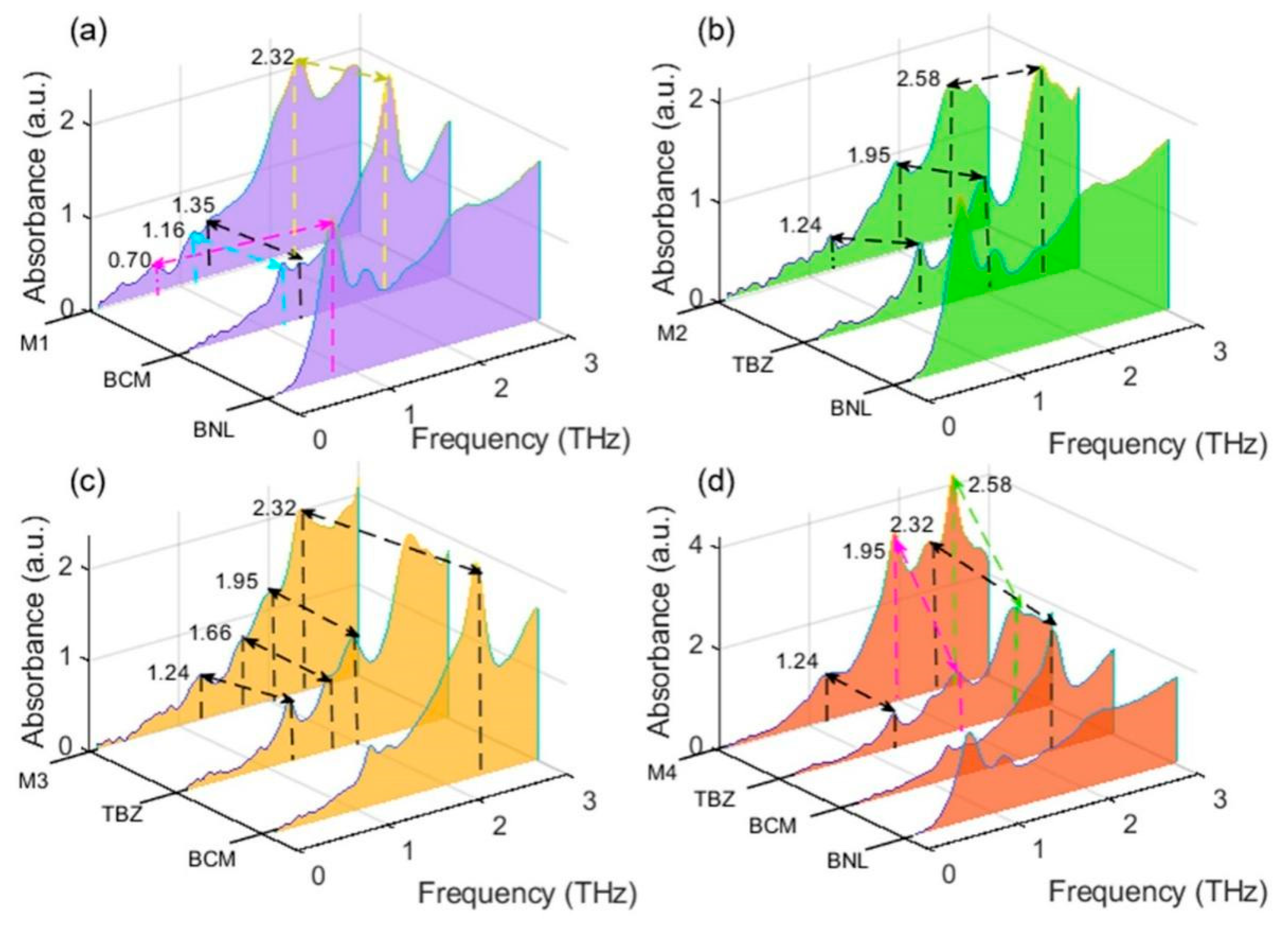


| DFT Simulation (THz) | THz Experiment (THz) | Shift (THz) | Vibration Modes |
|---|---|---|---|
| benomyl | |||
| 0.66 | 0.70 | −0.04 | υring |
| 1.13 | 1.07 | 0.05 | δ(C-N)oop |
| 2.18 | 2.20 | −0.02 | δ(C-O)ip |
| carbendazim | |||
| 0.49 | - | - | δ(C-O)ip, δ(C-)ip |
| 1.15 | 1.16 | −0.01 | δ(C-O)ip, δ(C-)ip |
| 1.36 | 1.35 | 0.01 | δring |
| 2.32 | 2.32 | - | δ(C-O)ip |
| 2.64 | - | - | δring |
| thiabendazole | |||
| 0.23 | - | - | δ(C-C)ip |
| 0.91 | 0.92 | −0.01 | δring |
| 1.35 | 1.24 | 0.11 | δring |
| 1.56 | 1.66 | −0.10 | υ(C-C)ip |
| 1.90 | 1.95 | −0.05 | δ(C-C)ip |
| 2.62 | 2.58 | 0.04 | δ(C-H)ip |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, P.; Qu, F.; Lin, L.; He, Y.; Feng, X.; Yang, L.; Gao, H.; Zhao, L.; Huang, L. Trace Identification and Visualization of Multiple Benzimidazole Pesticide Residues on Toona sinensis Leaves Using Terahertz Imaging Combined with Deep Learning. Int. J. Mol. Sci. 2021, 22, 3425. https://doi.org/10.3390/ijms22073425
Nie P, Qu F, Lin L, He Y, Feng X, Yang L, Gao H, Zhao L, Huang L. Trace Identification and Visualization of Multiple Benzimidazole Pesticide Residues on Toona sinensis Leaves Using Terahertz Imaging Combined with Deep Learning. International Journal of Molecular Sciences. 2021; 22(7):3425. https://doi.org/10.3390/ijms22073425
Chicago/Turabian StyleNie, Pengcheng, Fangfang Qu, Lei Lin, Yong He, Xuping Feng, Liang Yang, Huaqi Gao, Lihua Zhao, and Lingxia Huang. 2021. "Trace Identification and Visualization of Multiple Benzimidazole Pesticide Residues on Toona sinensis Leaves Using Terahertz Imaging Combined with Deep Learning" International Journal of Molecular Sciences 22, no. 7: 3425. https://doi.org/10.3390/ijms22073425
APA StyleNie, P., Qu, F., Lin, L., He, Y., Feng, X., Yang, L., Gao, H., Zhao, L., & Huang, L. (2021). Trace Identification and Visualization of Multiple Benzimidazole Pesticide Residues on Toona sinensis Leaves Using Terahertz Imaging Combined with Deep Learning. International Journal of Molecular Sciences, 22(7), 3425. https://doi.org/10.3390/ijms22073425







