Cannabidiol and Other Cannabinoids in Demyelinating Diseases
Abstract
1. Introduction
| Compounds | Therapeutic Potential | References |
| CBD | Inflammation | Mecha et al., 2012 [13] |
| Epilepsy | Burstein et al., 2015 [11] | |
| Cancer | Mori et al., 2017 [9] | |
| Anxiety | Kis et al., 2019 [12] | |
| Neuroprotection | García-Gutiérrez et al., 2020 [10] | |
| Myelination | Li et al., 2020 [8] | |
| CBDA | Inflammation | Pellati et al., 2018 [14] |
| Cancer | ||
| Antimicrobial | ||
| CBDVA-C3 | Convulsion | Anderson et al., 2019 [15] |
| CBDV | Convulsion | Zamberletti et al., 2019 [16] |
| Epilepsy | Morano et al., 2020 [17] | |
| Autism spectrum disorder | ||
| H2-CBD | Inflammation | Ben-Shabat et al., 2006 [18] |
| H4-CBD | Inflammation | Ben-Shabat et al., 2006 [18] |
| HU-446 | Inflammation | Kozela et al., 2016 [19] |
| HU-465 | Inflammation | Kozela et al., 2016 [19] |
| DMH-CBD | Inflammation Cancer Pain Neuroprotection | Burstein et al., 2015 [11] Juknat et al., 2016 [20] |
| HU-330 | Inflammation | Sumariwalla et al., 2004 [21] |
| Immunosuppresion | ||
| HU-410 | Inflammation | Mechoulam et al., 2008 [22] |
| HU-427 | Inflammation | Mechoulam et al., 2008 [22] |
| HU-432 | Inflammation | Mechoulam et al., 2008 [22] |
| HU-331 | Cancer | Kogan et al., 2003 [23] |
| VCE-004.8/EHP-101 | Inflammation Fibrosis Neuroprotection Remyelination | Del Rio et al., 2016 [24] Navarrete et al., 2018 [25] García-Martin et al., 2018 [26] García-Martin et al., 2019 [27] Navarrete et al., 2020 [28] |
2. Cannabidiol: General Pharmacology and Therapeutic Profile
The Endocannabinoid System
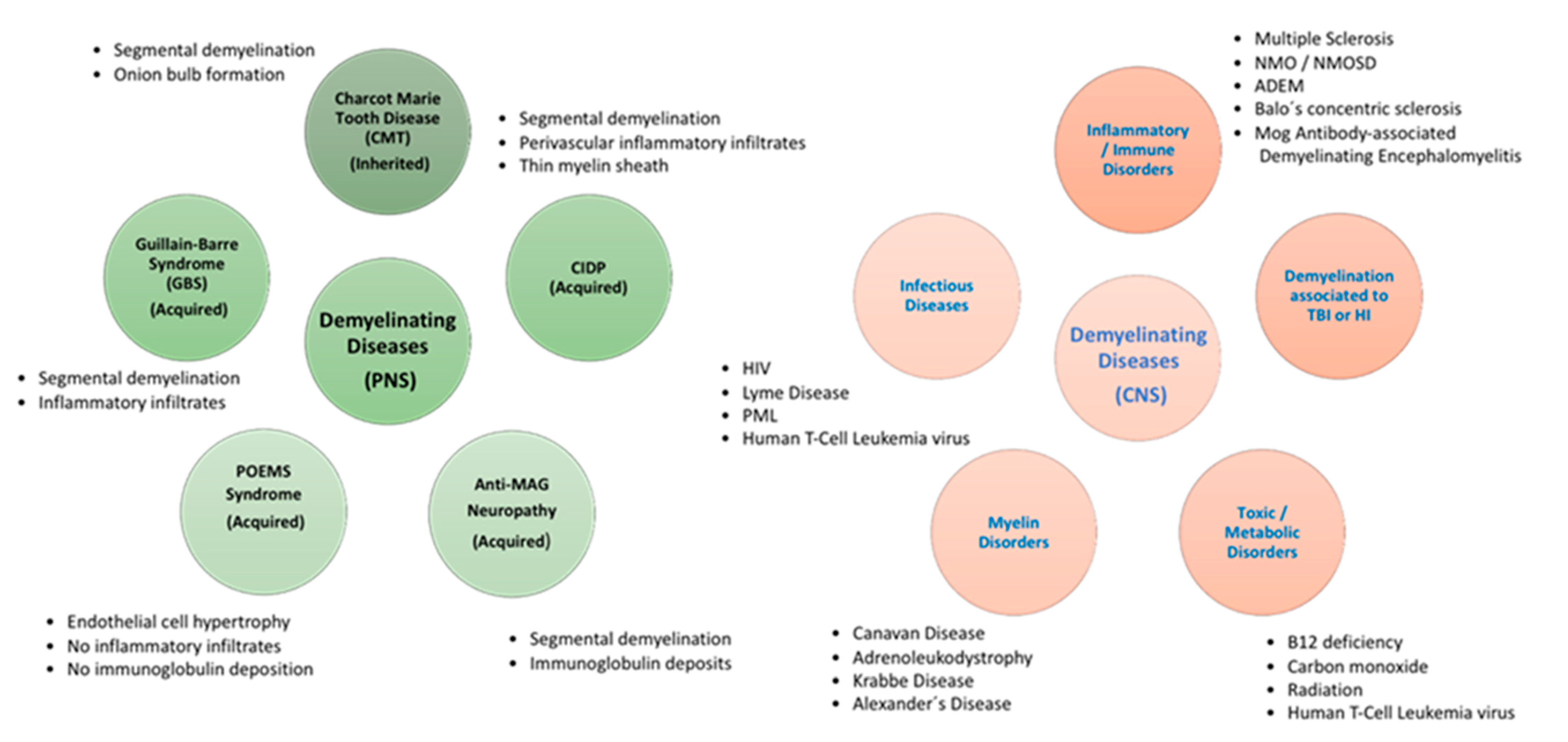
3. Demyelinating Diseases
4. Cannabidiol and Demyelinating Diseases
5. Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol
6. Clinical Trials of Cannabidiol Focused on Demyelinating Disorders
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| ADEM | Acute Disseminated Encephalomyelitis |
| AEA | Anandamide |
| AHL | Acute Hemorrhagic Leukoencephalitis |
| AIDS | Acquired Immunodeficiency Syndrome |
| BBB | Blood-Brain Barrier |
| BCCAO | Bilateral Common Carotid Artery Occlusion |
| BDNF | Brain-Derived Neurotrophic Factor |
| CB1R | Cannabinoid receptor type 1 |
| CB2R | Cannabinoid receptor type 2 |
| CBD | Cannabidiol |
| CBDA | Cannabidiolic Acid |
| CBDM | Cannabidiol Monomethyl Ether |
| CBDV | Cannabidivarin |
| CBDVA | Cannabidivarinic Acid |
| CIDP | Chronic Inflammatory Demyelinating Polyradiculoneuropathy |
| CMT | Charcot Marie Tooth Disease |
| CNP | Central Neuropathic Pain |
| CNS | Central Nervous System |
| COX-2 | Cyclooxygenase-2 |
| CSF | Cerebrospinal Fluid |
| EAE | Experimental Autoimmune Encephalomyelitis |
| eCBome | Endocannabidiome |
| ECS | Endocannabinoid System |
| FAAH | Fatty Acid Amide Hydrolase |
| FDA | Food and Drug Administration |
| GBS | Guillain-Barre Syndrome |
| GPCR | G protein-coupled receptor |
| GPR55 | G protein-coupled receptor 55 |
| HI | Hypoxia-Ischemia |
| HIF | Hypoxia-Inducible Factor |
| IIDDs | Idiopathic Inflammatory-Demyelinating Diseases |
| IL-6 | Interleukin-6 |
| iNOS | Inducible Nitric Oxide Synthase |
| MAG | Anti-Myelin Associated Glycoprotein |
| MAGL | Monoacylglycerol Lipase |
| MAP-2 | Microtubule-Associated Protein 2 |
| MOG | Myelin Oligodendrocyte Glycoprotein |
| MRI | Magnetic Resonance Imaging |
| MS | Multiple Sclerosis |
| NMO | Neuromyelitis Optica |
| NMOSD | Neuromyelitis Optica Spectrum Disorder |
| NRS | Numerical Rate Scale |
| NVU | Neurovascular Unit |
| OGD | Oxygen-Glucose Deprivation |
| OPCs | Oligodendrocyte Progenitor Cells |
| PDD | Peripheral Demyelinating Diseases |
| PML | Progressive Multifocal Leukoencephalopathy |
| PNS | Peripheral Nervous System |
| POEMS | Polyneuropathy, Organomegaly, Endocrinopathy, M protein and Skin changes Syndrome |
| PP | Primary Progressive |
| PPARγ | Peroxisome Proliferator-Activated Receptor-Gamma |
| ROS | Reactive Oxygen Species |
| RR | Relapsing-Remitting |
| SC | Schwann Cell |
| SP | Secondary Progressive |
| TBI | Traumatic Brain Injury |
| TNF-α | Tumor Necrosis Factor-α |
| TRPV1 | Transient Receptor Potential Cation Channel Subfamily V Member 1 |
| VEGF | Vascular Endothelial Growth Factor |
| Δ9-THC | Δ9-tetrahydrocannabinol |
References
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, W.B. On the Preparations of the Indian Hemp, or Gunjah (Cannabis indica), Their Effects on the Animal System in Health, and Their Utility in the Treatment of Tetanus and Other Convulsive Diseases. Br. Foreign Med. Rev. 1840, 10, 225–228. [Google Scholar]
- Mechoulam, R.; Shvo, Y. Hashish. I. The structure of cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation and structure of DELTA+−tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 1971, 93, 217–224. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. The absolute configuration of δ1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett. 1967, 12, 1109–1111. [Google Scholar] [CrossRef]
- Borgelt, L.M.; Franson, K.L.; Nussbaum, A.M.; Wang, G.S. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 2013, 33, 195–209. [Google Scholar] [CrossRef]
- Davis, M.P. Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain. Expert Opin. Investig. Drugs 2008, 17, 85–95. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112163. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol—From Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef]
- Mecha, M.; Torrao, A.S.; Mestre, L.; Carrillo-Salinas, F.J.; Mechoulam, R.; Guaza, C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e331. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. Biomed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Low, I.K.; Banister, S.D.; McGregor, I.S.; Arnold, J.C. Pharmacokinetics of Phytocannabinoid Acids and Anticonvulsant Effect of Cannabidiolic Acid in a Mouse Model of Dravet Syndrome. J. Nat. Prod. 2019, 82, 3047–3055. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Woolley-Roberts, M.; Bingham, S.; Rubino, T.; Parolaro, D. Cannabidivarin Treatment Ameliorates Autism-Like Behaviors and Restores Hippocampal Endocannabinoid System and Glia Alterations Induced by Prenatal Valproic Acid Exposure in Rats. Front. Cell. Neurosci. 2019, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Morano, A.; Fanella, M.; Albini, M.; Cifelli, P.; Palma, E.; Giallonardo, A.T.; Di Bonaventura, C. Cannabinoids in the Treatment of Epilepsy: Current Status and Future Prospects. Neuropsychiatr. Dis. Treat. 2020, 16, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Hanus, L.O.; Katzavian, G.; Gallily, R. New cannabidiol derivatives: Synthesis, binding to cannabinoid receptor, and evaluation of their antiinflammatory activity. J. Med. Chem. 2006, 49, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Haj, C.; Hanus, L.; Chourasia, M.; Shurki, A.; Juknat, A.; Kaushansky, N.; Mechoulam, R.; Vogel, Z. HU-446 and HU-465, Derivatives of the Non-psychoactive Cannabinoid Cannabidiol, Decrease the Activation of Encephalitogenic T Cells. Chem. Biol. Drug Des. 2016, 87, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Juknat, A.; Kozela, E.; Kaushansky, N.; Mechoulam, R.; Vogel, Z. Anti-inflammatory effects of the cannabidiol derivative dimethylheptyl-cannabidiol—Studies in BV-2 microglia and encephalitogenic T cells. J. Basic Clin. Physiol Pharmcol. 2016, 27, 289–296. [Google Scholar] [CrossRef]
- Sumariwalla, P.F.; Gallily, R.; Tchilibon, S.; Fride, E.; Mechoulam, R.; Feldmann, M. A novel synthetic, nonpsychoactive cannabinoid acid (HU-320) with antiinflammatory properties in murine collagen-induced arthritis. Arthritis Rheum. 2004, 50, 985–998. [Google Scholar] [CrossRef]
- Mechoulam, R.; Kogan, N.; Gallily, R.; Breuer, A. Novel Cannabidiol Derivatives and Their Use as Anti-inflammatory Agents. WO Patent 2008/107879 A1, 5 March 2008. [Google Scholar]
- Kogan, N.M.; Rabinowitz, R.; Levi, P.; Gibson, D.; Sandor, P.; Schlesinger, M.; Mechoulam, R. Synthesis and antitumor activity of quinonoid derivatives of cannabinoids. J. Med. Chem. 2004, 47, 3800–3806. [Google Scholar] [CrossRef]
- Del Río, C.; Navarrete, C.; Collado, J.A.; Bellido, M.L.; Gómez-Cañas, M.; Pazos, M.R.; Fernández-Ruiz, J.; Pollastro, F.; Appendino, G.; Calzado, M.A.; et al. The cannabinoid quinol VCE-004.8 alleviates bleomycin-induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator-activated receptor-γ and CB2 pathways. Sci. Rep. 2016, 6, 21703. [Google Scholar] [CrossRef]
- Navarrete, C.; Carrillo-Salinas, F.; Palomares, B.; Mecha, M.; Jiménez-Jiménez, C.; Mestre, L.; Feliú, A.; Bellido, M.L.; Fiebich, B.L.; Appendino, G.; et al. Hypoxia mimetic activity of VCE-004.8, a cannabidiol quinone derivative: Implications for multiple sclerosis therapy. J. Neuroinflamm. 2018, 15, 64. [Google Scholar] [CrossRef]
- García-Martín, A.; Garrido-Rodríguez, M.; Navarrete, C.; Del Río, C.; Bellido, M.L.; Appendino, G.; Calzado, M.A.; Muñoz, E. EHP-101, an oral formulation of the cannabidiol aminoquinone VCE-004.8, alleviates bleomycin-induced skin and lung fibrosis. Biochem. Pharm. 2018, 157, 304–313. [Google Scholar] [CrossRef]
- García-Martín, A.; Garrido-Rodríguez, M.; Navarrete, C.; Caprioglio, D.; Palomares, B.; DeMesa, J.; Rollland, A.; Appendino, G.; Muñoz, E. Cannabinoid derivatives acting as dual PPARγ/CB2 agonists as therapeutic agents for systemic sclerosis. Biochem. Pharmcol. 2019, 163, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, C.; García-Martin, A.; Garrido-Rodríguez, M.; Mestre, L.; Feliú, A.; Guaza, C.; Calzado, M.A.; Muñoz, E. Effects of EHP-101 on inflammation and remyelination in murine models of Multiple sclerosis. Neurobiol. Dis. 2020, 143, 104994. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabidiol as a potential medicine. In Cannabinoids as Therapeutics, Milestones in Drug Therapy MDT; Mechoulam, R., Ed.; Birkhäuser: Basel, Switzerland, 2005. [Google Scholar]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharm. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C.; Glass, M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 2007, 5, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmcol. 2007, 150, 613–623. [Google Scholar] [CrossRef]
- Lunn, C.A.; Fine, J.S.; Rojas-Triana, A.; Jackson, J.V.; Fan, X.; Kung, T.T.; Gonsiorek, W.; Schwarz, M.A.; Lavey, B.; Kozlowski, J.A.; et al. A novel cannabinoid peripheral cannabinoid receptor-selective inverse agonist blocks leukocyte recruitment in vivo. J. Pharmacol. Exp. Ther. 2006, 316, 780–788. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmcol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef]
- Franco, R.; Villa, M.; Morales, P.; Reyes-Resina, I.; Gutierrez-Rodriguez, A.; Jimenez, J.; Jagerovic, N.; Martinez-Orgado, J.; Navarro, G. Increased expression of cannabinoid CB2 and serotonin 5-HT1A heteroreceptor complexes in a model of newborn hypoxic-ischemic brain damage. Neuropharmacology 2019, 152, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Whalley, B.J.; Bazelot, M.; Rosenberg, E.; Tsien, R. A role of GPR55 in the antiepileptic properties of cannabidiol (CBD). Neurology 2018, 90 (Suppl. 15), P2.277. [Google Scholar]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmcol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Stetten, N.; Pomeranz, J.; Moorhouse, M.; Yurasek, A.; Blue, A.V. The level of evidence of medical marijuana use for treating disabilities: A scoping review. Disabil. Rehabil. 2020, 42, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Shani, A.; Edery, H.; Grunfeld, Y. Chemical basis of hashish activity. Science 1970, 169, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Gaoni, Y. A Total Synthesis of dl-Δ1-Tetrahydrocannabinol, the Active Constituent of Hashish. J. Am. Chem. Soc. 1965, 87, 3273–3275. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Dysarz, F.A., III; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmcol. 1988, 34, 605–613. [Google Scholar]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Dinh, T.P.; Freund, T.F.; Piomelli, D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem. Phys. Lipids 2002, 121, 149–158. [Google Scholar] [CrossRef]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef]
- Di Iorio, G.; Lupi, M.; Sarchione, F.; Matarazzo, I.; Santacroce, R.; Petruccelli, F.; Martinotti, G.; Di Giannantonio, M. The endocannabinoid system: A putative role in neurodegenerative diseases. Int. J. High Risk Behav. Addict. 2013, 2, 100–106. [Google Scholar] [CrossRef]
- Aymerich, M.S.; Aso, E.; Abellanas, M.A.; Tolon, R.M.; Ramos, J.A.; Ferrer, I.; Romero, J.; Fernandez-Ruiz, J. Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem. Pharmcol. 2018, 157, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, A.A.; Milligan, C.E.; Pharr, E.P.; Howlett, A.C. The Endocannabinoid System and Oligodendrocytes in Health and Disease. Front. Neurosci. 2018, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Silvestri, C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.E.; Kuhlmann, T.; Antel, J.P. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim. Biophys. Acta 2011, 1812, 184–193. [Google Scholar] [CrossRef]
- Muppirala, A.N.; Limbach, L.E.; Bradford, E.F.; Petersen, S.C. Schwann cell development: From neural crest to myelin sheath. Wiley Interdiscip. Rev. Dev. Biol. 2020, e398. [Google Scholar] [CrossRef] [PubMed]
- Mehndiratta, M.M.; Gulati, N.S. Central and peripheral demyelination. J. Neurosci. Rural Pract. 2014, 5, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef]
- Stassart, R.M.; Möbius, W.; Nave, K.A.; Edgar, J.M. The Axon-Myelin Unit in Development and Degenerative Disease. Front. Neurosci. 2018, 12, 467. [Google Scholar] [CrossRef]
- Love, S. Demyelinating diseases. J. Clin. Pathol. 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Leonhard, S.E.; Mandarakas, M.R.; Gondim, F.A.A.; Bateman, K.; Ferreira, M.L.B.; Cornblath, D.R.; van Doorn, P.A.; Dourado, M.E.; Hughes, R.A.C.; Islam, B.; et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat. Rev. Neurol. 2019, 15, 671–683. [Google Scholar] [CrossRef]
- Orlikowski, D.; Porcher, R.; Sivadon-Tardy, V.; Quincampoix, J.-C.; Raphaël, J.-C.; Durand, M.-C.; Sharshar, T.; Roussi, J.; Caudie, C.; Annane, D.; et al. Guillain-Barré Syndrome following Primary Cytomegalovirus Infection: A Prospective Cohort Study. Clin. Infect. Dis. 2011, 52, 837–844. [Google Scholar] [CrossRef]
- Islam, B.; Islam, Z.; GeurtsvanKessel, C.H.; Jahan, I.; Endtz, H.P.; Mohammad, Q.D.; Jacobs, B.C. Guillain-Barré syndrome following varicella-zoster virus infection. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2018, 37, 511–518. [Google Scholar] [CrossRef]
- Tam, C.C.; O’Brien, S.J.; Petersen, I.; Islam, A.; Hayward, A.; Rodrigues, L.C. Guillain-Barré syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS ONE 2007, 2, e344. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Aliu, B.; Demeestere, D.; Seydoux, E.; Boucraut, J.; Delmont, E.; Brodovitch, A.; Oberholzer, T.; Attarian, S.; Théaudin, M.; Tsouni, P.; et al. Selective inhibition of anti-MAG IgM autoantibody binding to myelin by an antigen-specific glycopolymer. J. Neurochem. 2020, 154, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Bunschoten, C.; Jacobs, B.C.; Van den Bergh, P.Y.K.; Cornblath, D.R.; van Doorn, P.A. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019, 18, 784–794. [Google Scholar] [CrossRef]
- Brown, R.; Ginsberg, L. POEMS syndrome: Clinical update. J. Neurol. 2019, 266, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Morena, J.; Gupta, A.; Hoyle, J.C. Charcot-Marie-Tooth: From Molecules to Therapy. Int. J. Mol. Sci. 2019, 20, 3419. [Google Scholar] [CrossRef]
- Kamil, K.; Yazid, M.D.; Idrus, R.B.H.; Das, S.; Kumar, J. Peripheral Demyelinating Diseases: From Biology to Translational Medicine. Front. Neurol. 2019, 10, 87. [Google Scholar] [CrossRef]
- Cañellas, A.R.; Gols, A.R.; Izquierdo, J.R.; Subirana, M.T.; Gairin, X.M. Idiopathic inflammatory-demyelinating diseases of the central nervous system. Neuroradiology 2007, 49, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Rosenthal, J.F.; Hoffman, B.M.; Tyor, W.R. CNS inflammatory demyelinating disorders: MS, NMOSD and MOG antibody associated disease. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2020, 68, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Cortese, I.; Reich, D.S.; Nath, A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol. 2021, 17, 37–51. [Google Scholar] [CrossRef]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2020, 13, 1452. [Google Scholar] [CrossRef]
- Jang, H.; Huang, S.; Hammer, D.X.; Wang, L.; Rafi, H.; Ye, M.; Welle, C.G.; Fisher, J.A.N. Alterations in neurovascular coupling following acute traumatic brain injury. Neurophotonics 2017, 4, 045007. [Google Scholar] [CrossRef]
- Jullienne, A.; Obenaus, A.; Ichkova, A.; Savona-Baron, C.; Pearce, W.J.; Badaut, J. Chronic cerebrovascular dysfunction after traumatic brain injury. J. Neurosci. Res. 2016, 94, 609–622. [Google Scholar] [CrossRef]
- Pop, V.; Badaut, J. A neurovascular perspective for long-term changes after brain trauma. Transl. Stroke Res. 2011, 2, 533–545. [Google Scholar] [CrossRef]
- Calina, D.; Buga, A.M.; Mitroi, M.; Buha, A.; Caruntu, C.; Scheau, C.; Bouyahya, A.; El Omari, N.; El Menyiy, N.; Docea, A.O. The Treatment of Cognitive, Behavioural and Motor Impairments from Brain Injury and Neurodegenerative Diseases through Cannabinoid System Modulation—Evidence from In Vivo Studies. J. Clin. Med. 2020, 9, 2395. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; You, W.; Wang, H.; Meng, Y.; Feng, J.; Yang, X. Polarization of Microglia to the M2 Phenotype in a Peroxisome Proliferator-Activated Receptor Gamma—Dependent Manner Attenuates Axonal Injury Induced by Traumatic Brain Injury in Mice. J. Neurotrauma 2018, 35, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmcol. 2011, 163, 1507–1519. [Google Scholar] [CrossRef]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef]
- Pazos, M.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martínez-Pinilla, E.; Moreno, E.; Valdizan, E.; Romero, J.; Pazos, A.; Franco, R.; et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: Role of 5HT1A and CB2 receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. [Google Scholar] [CrossRef]
- Girolamo, F.; Coppola, C.; Ribatti, D.; Trojano, M. Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2014, 2, 84. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.Y.; Soutto, M.; Sriram, S. Preconditioning with cobalt chloride or desferrioxamine protects oligodendrocyte cell line (MO3.13) from tumor necrosis factor-α-mediated cell death. J. Neurosci. Res. 2008, 86, 2403–2413. [Google Scholar] [CrossRef]
- Fatemi, A.; Wilson, M.A.; Johnston, M.V. Hypoxic-ischemic encephalopathy in the term infant. Clin. Perinatol. 2009, 36, 835–858, vii. [Google Scholar] [CrossRef]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015, 11, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Baldassarro, V.A.; Marchesini, A.; Giardino, L.; Calzà, L. Differential effects of glucose deprivation on the survival of fetal versus adult neural stem cells-derived oligodendrocyte precursor cells. Glia 2020, 68, 898–917. [Google Scholar] [CrossRef] [PubMed]
- Janowska, J.; Sypecka, J. Therapeutic Strategies for Leukodystrophic Disorders Resulting from Perinatal Asphyxia: Focus on Myelinating Oligodendrocytes. Mol. Neurobiol. 2018, 55, 4388–4402. [Google Scholar] [CrossRef] [PubMed]
- Ceprián, M.; Vargas, C.; García-Toscano, L.; Penna, F.; Jiménez-Sánchez, L.; Achicallende, S.; Elezgarai, I.; Grandes, P.; Hind, W.; Pazos, M.R.; et al. Cannabidiol Administration Prevents Hypoxia-Ischemia-Induced Hypomyelination in Newborn Rats. Front. Pharmacol. 2019, 10, 1131. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Hind, W.H.; England, T.J.; O’Sullivan, S.E. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br. J. Pharm. 2016, 173, 815–825. [Google Scholar] [CrossRef]
- Belardo, C.; Iannotta, M.; Boccella, S.; Rubino, R.C.; Ricciardi, F.; Infantino, R.; Pieretti, G.; Stella, L.; Paino, S.; Marabese, I.; et al. Oral Cannabidiol Prevents Allodynia and Neurological Dysfunctions in a Mouse Model of Mild Traumatic Brain Injury. Front. Pharmacol. 2019, 10, 352. [Google Scholar] [CrossRef]
- Pazos, M.R.; Cinquina, V.; Gómez, A.; Layunta, R.; Santos, M.; Fernández-Ruiz, J.; Martínez-Orgado, J. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 2012, 63, 776–783. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmcol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Zvi, Z.; Gaoni, Y. Hashish-13. On the nature of the Beam test. Tetrahedron 1968, 24, 5615–5624. [Google Scholar] [CrossRef]
- Wu, H.Y.; Jan, T.R. Cannabidiol hydroxyquinone-induced apoptosis of splenocytes is mediated predominantly by thiol depletion. Toxicol. Lett. 2010, 195, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Sullivan, M.T.; Collins, S.A.; Goodman, H.; Limebeer, C.L.; Mechoulam, R.; Parker, L.A. Evaluation of repeated or acute treatment with cannabidiol (CBD), cannabidiolic acid (CBDA) or CBDA methyl ester (HU-580) on nausea and/or vomiting in rats and shrews. Psychopharmacology 2020, 237, 2621–2631. [Google Scholar] [CrossRef]
- Perez, J. Combined cannabinoid therapy via an oromucosal spray. Drugs Today 2006, 42, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.; Cameron, M. Cannabinoids for Treatment of MS Symptoms: State of the Evidence. Curr. Neurol. Neurosci. Rep. 2018, 18, 50. [Google Scholar] [CrossRef]
- Patejdl, R.; Zettl, U.K. Spasticity in multiple sclerosis: Contribution of inflammation, autoimmune mediated neuronal damage and therapeutic interventions. Autoimmun. Rev. 2017, 16, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P.; Henze, T.; Zettl, U.K. Spasticity in patients with multiple sclerosis—Clinical characteristics, treatment and quality of life. Acta Neurol. Scand. 2014, 129, 154–162. [Google Scholar] [CrossRef]
- Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, A.; Thompson, A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet 2003, 362, 1517–1526. [Google Scholar] [CrossRef]
- Pharma, G.W. Cannabis-based medicines—GW pharmaceuticals: High CBD, high THC, medicinal cannabis—GW pharmaceuticals, THC:CBD. Drugs R&D 2003, 4, 306–309. [Google Scholar]
- Wade, D.T.; Robson, P.; House, H.; Makela, P.; Aram, J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin. Rehabil. 2003, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T.; Collin, C.; Stott, C.; Duncombe, P. Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult. Scler. J. 2010, 16, 707–714. [Google Scholar] [CrossRef]
- Collin, C.; Ehler, E.; Waberzinek, G.; Alsindi, Z.; Davies, P.; Powell, K.; Notcutt, W.; O’Leary, C.; Ratcliffe, S.; Nováková, I.; et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol. Res. 2010, 32, 451–459. [Google Scholar] [CrossRef]
- Novotna, A.; Mares, J.; Ratcliffe, S.; Novakova, I.; Vachova, M.; Zapletalova, O.; Gasperini, C.; Pozzilli, C.; Cefaro, L.; Comi, G.; et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur. J. Neurol. 2011, 18, 1122–1131. [Google Scholar] [CrossRef]
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2013, 260, 984–997. [Google Scholar] [CrossRef]
- Flachenecker, P.; Henze, T.; Zettl, U.K. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice—Results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur. Neurol. 2014, 71, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P.; Henze, T.; Zettl, U.K. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur. Neurol. 2014, 72, 95–102. [Google Scholar] [CrossRef]
- Marinelli, L.; Balestrino, M.; Mori, L.; Puce, L.; Rosa, G.M.; Giorello, L.; Currà, A.; Fattapposta, F.; Serrati, C.; Gandolfo, C.; et al. A randomised controlled cross-over double-blind pilot study protocol on THC:CBD oromucosal spray efficacy as an add-on therapy for post-stroke spasticity. BMJ Open 2017, 7, e016843. [Google Scholar] [CrossRef] [PubMed]
- Markovà, J.; Essner, U.; Akmaz, B.; Marinelli, M.; Trompke, C.; Lentschat, A.; Vila, C. Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: A double-blind, placebo-controlled randomised clinical trial. Int. J. Neurosci. 2019, 129, 119–128. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarrete, C.; García-Martín, A.; Rolland, A.; DeMesa, J.; Muñoz, E. Cannabidiol and Other Cannabinoids in Demyelinating Diseases. Int. J. Mol. Sci. 2021, 22, 2992. https://doi.org/10.3390/ijms22062992
Navarrete C, García-Martín A, Rolland A, DeMesa J, Muñoz E. Cannabidiol and Other Cannabinoids in Demyelinating Diseases. International Journal of Molecular Sciences. 2021; 22(6):2992. https://doi.org/10.3390/ijms22062992
Chicago/Turabian StyleNavarrete, Carmen, Adela García-Martín, Alain Rolland, Jim DeMesa, and Eduardo Muñoz. 2021. "Cannabidiol and Other Cannabinoids in Demyelinating Diseases" International Journal of Molecular Sciences 22, no. 6: 2992. https://doi.org/10.3390/ijms22062992
APA StyleNavarrete, C., García-Martín, A., Rolland, A., DeMesa, J., & Muñoz, E. (2021). Cannabidiol and Other Cannabinoids in Demyelinating Diseases. International Journal of Molecular Sciences, 22(6), 2992. https://doi.org/10.3390/ijms22062992





