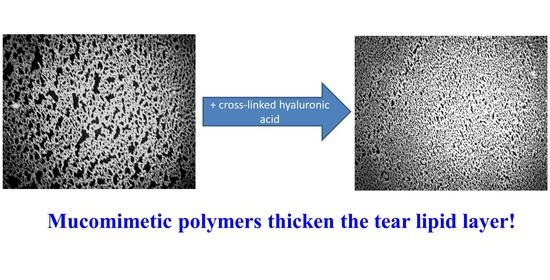
Interactions of Meibum and Tears with Mucomimetic Polymers: A Hint towards the Interplay between the Layers of the Tear Film
Abstract
1. Introduction
2. Results
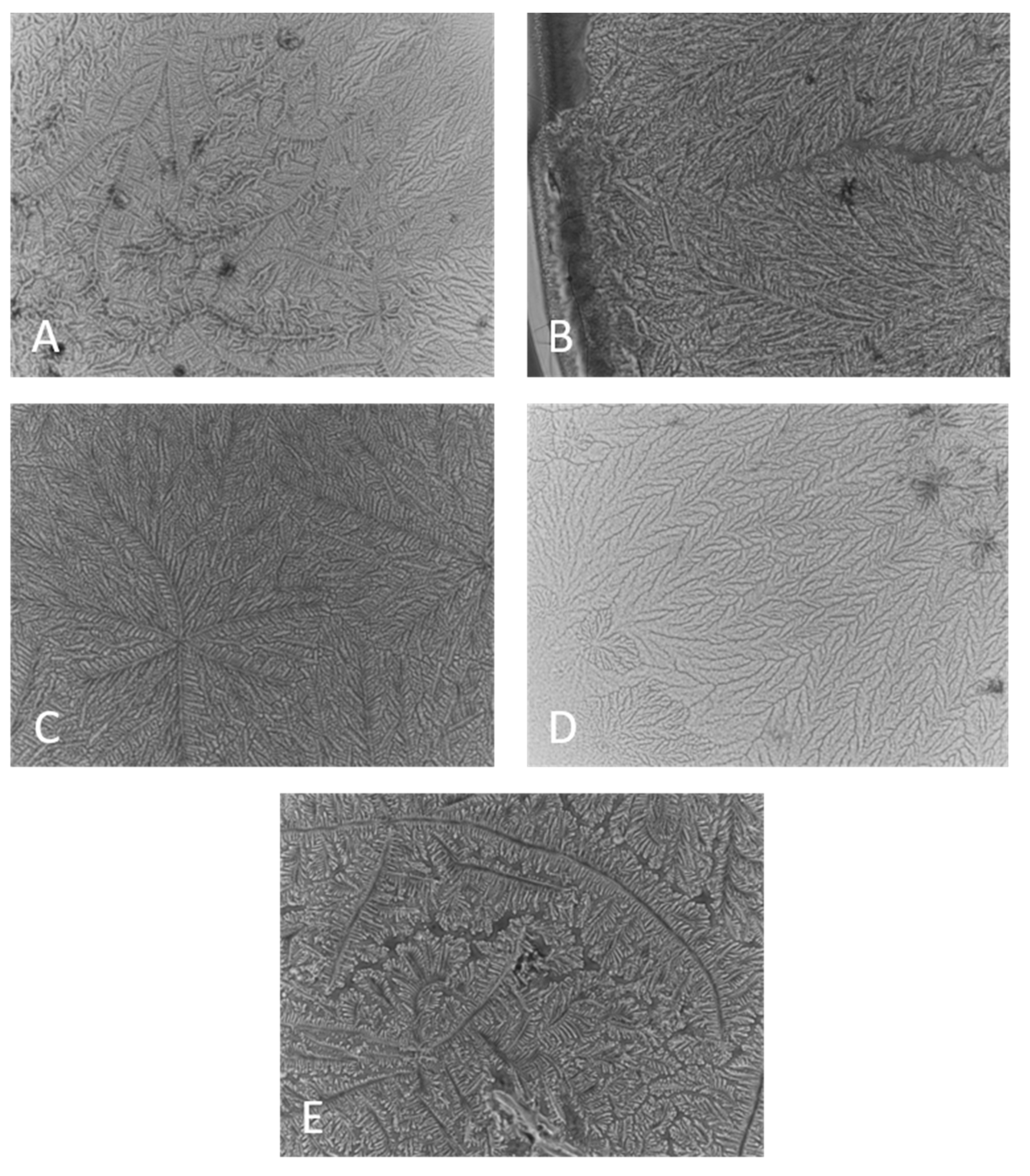
2.1. Ferning Patterns of Polymer Solutions, Pure and Included in Human Tears
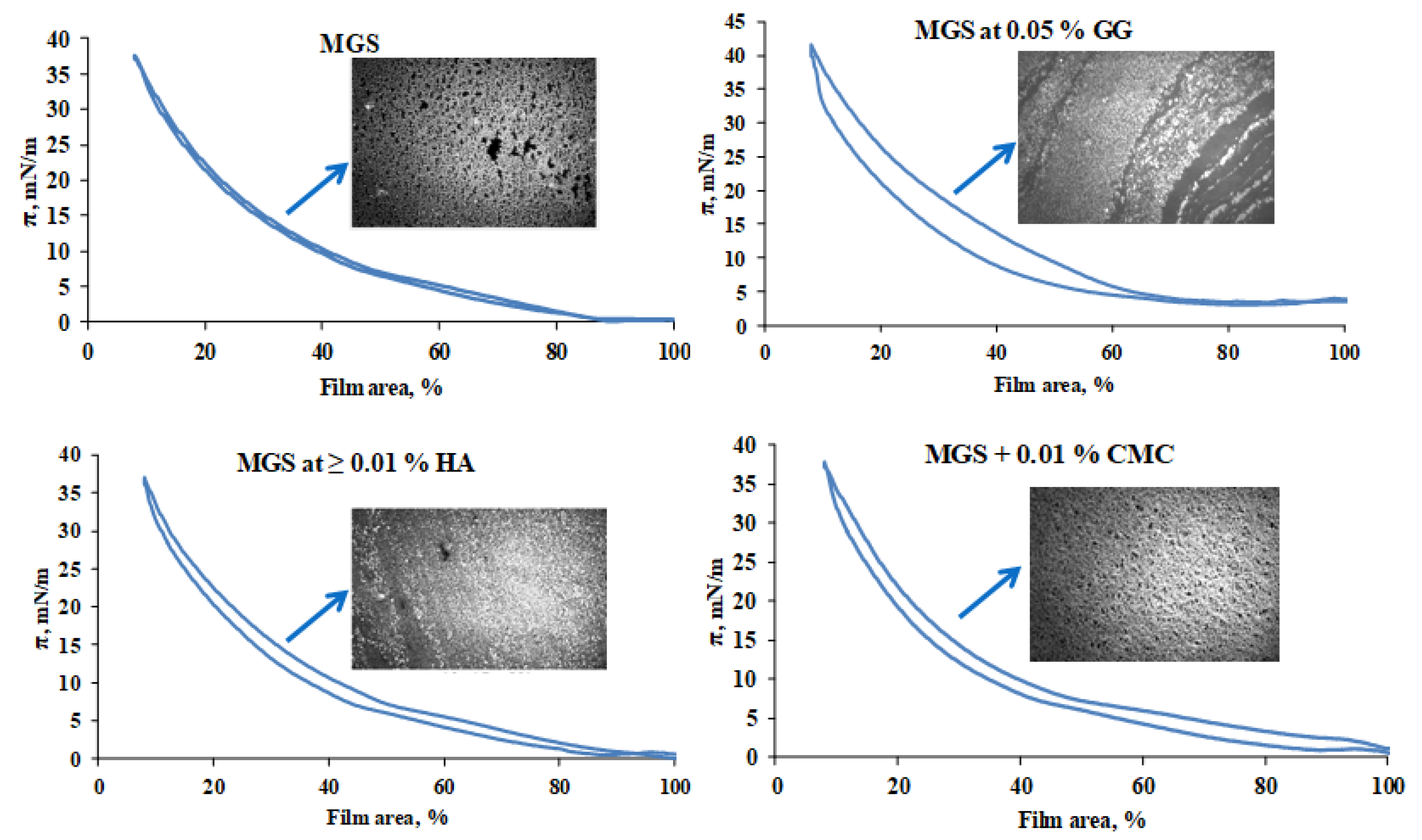
2.2. Effect of MMP on the Surface Pressure/Area Isotherms of Meibomian Films
2.2.1. Effects of HA, CMC and GG
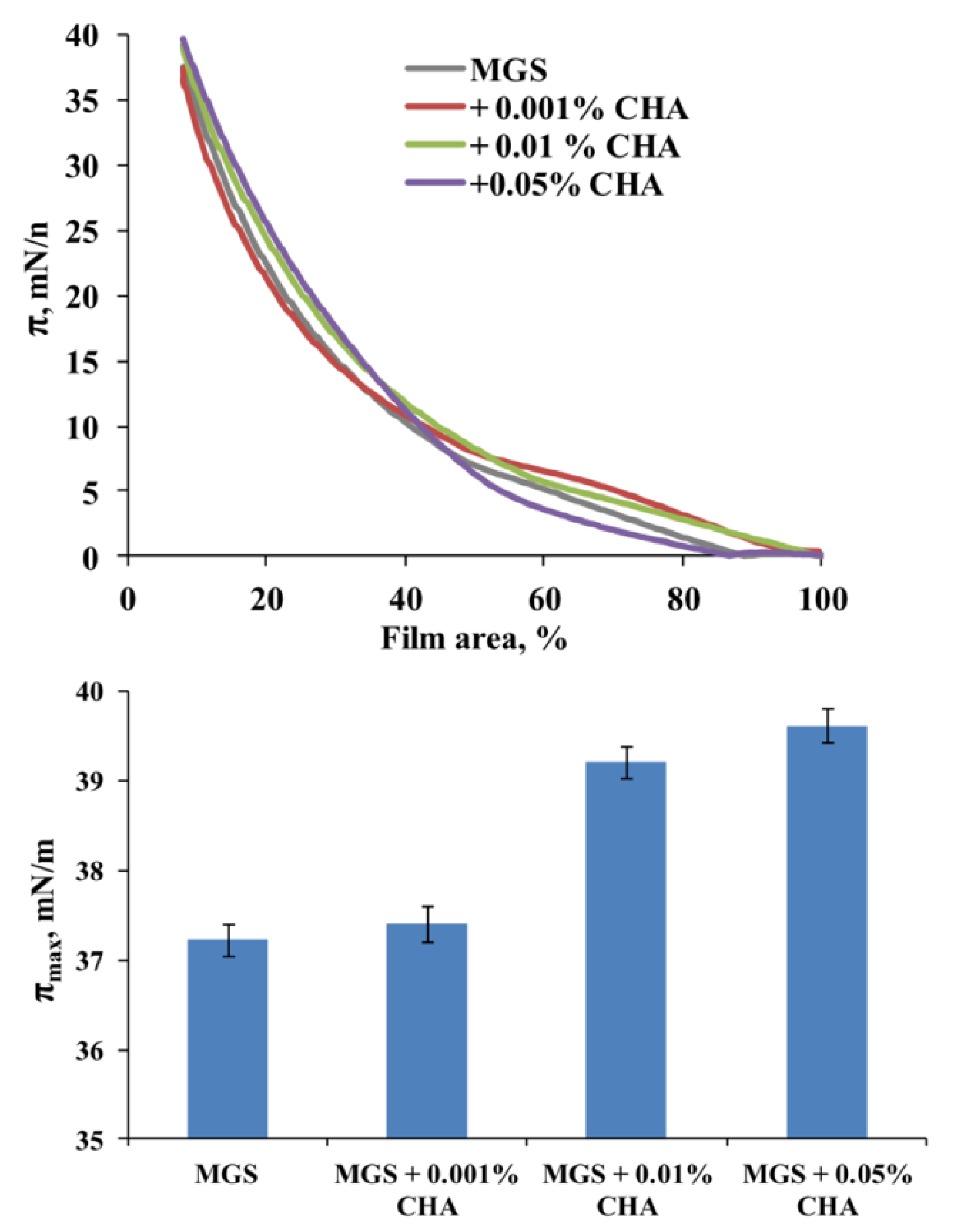
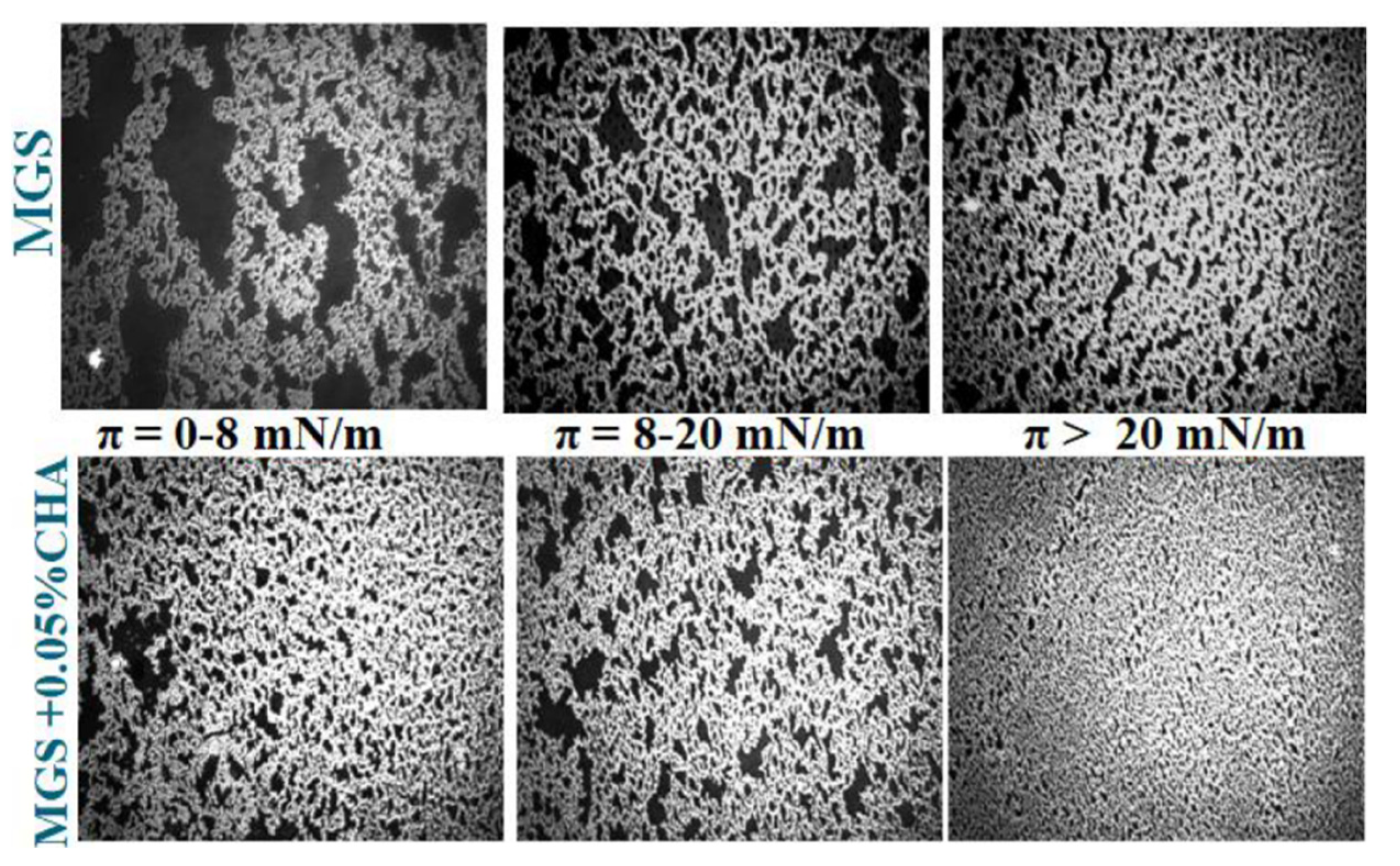
2.2.2. Effects of CHA
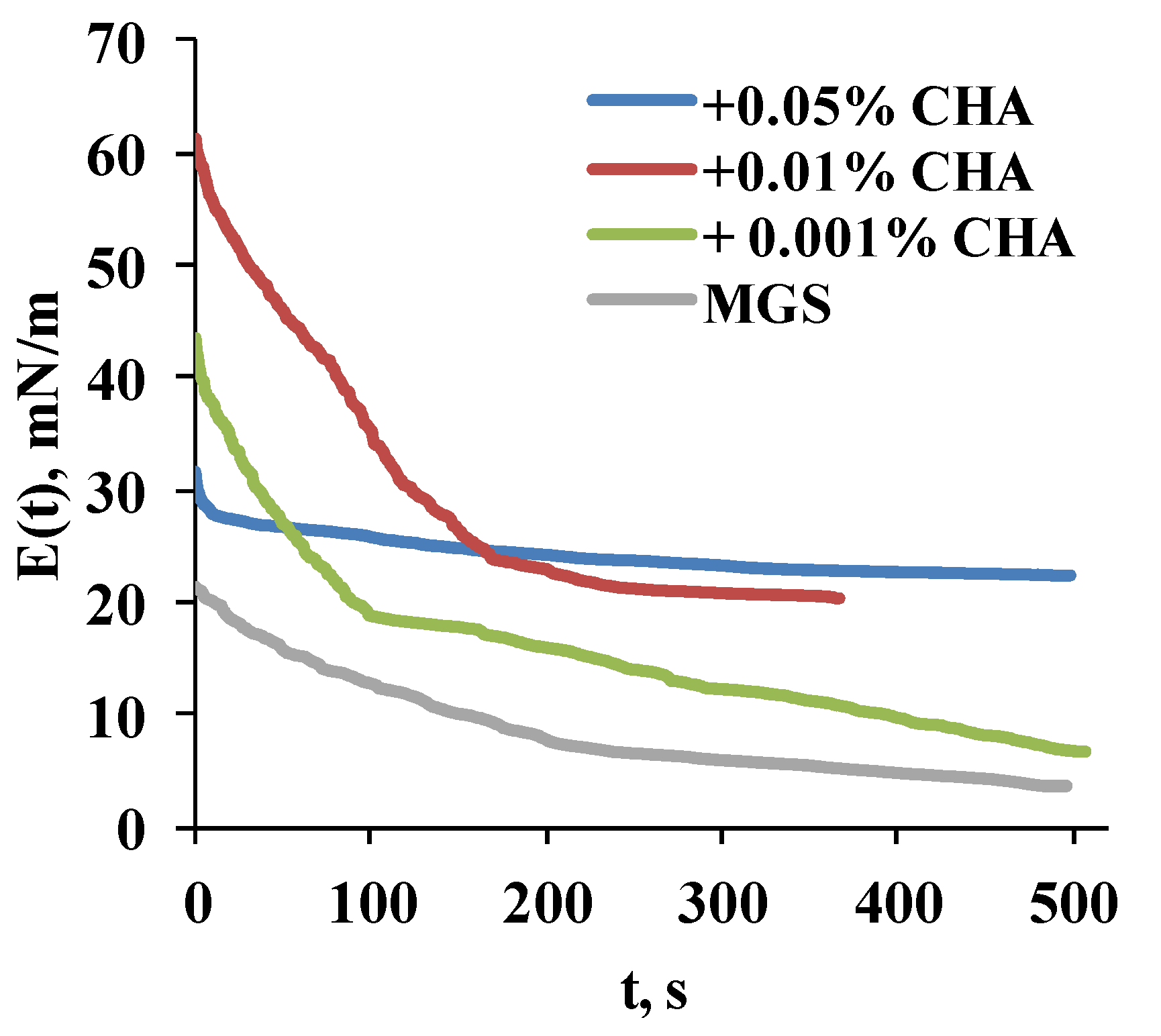
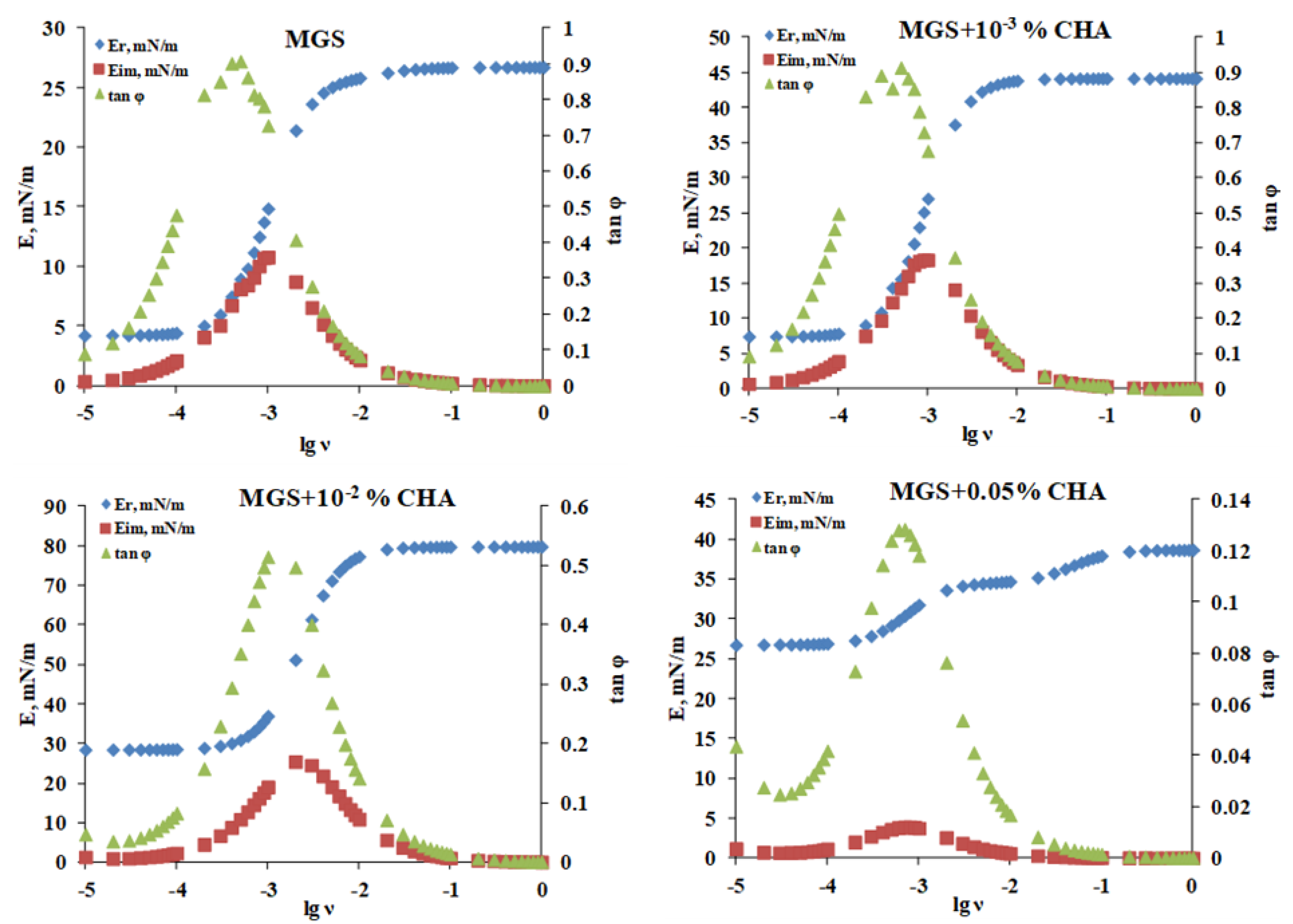
2.2.3. Stress Relaxations
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Ferning Patterns
4.2.2. Langmuir Surface Balance Studies
Compression Isotherms
Stress-Relaxation Studies via the Small Deformations Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp. Eye Res. 2017, 163, 17–28. [Google Scholar] [CrossRef]
- McDonald, M.; Patel, D.A.; Keith, M.S.; Snedecor, S.J. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul. Surf. 2016, 14, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.H.; Kunnen, C.M.; Duchoslav, E.; Dolla, N.K.; Kelso, M.J.; Papas, E.B.; Lazon de la Jara, P.; Willcox, M.D.; Blanksby, S.J.; Mitchell, T.W. A comparison of patient matched meibum and tear lipidomes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7417–7424. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.; Lu, H.; Butovich, I.A. The spectrophotometric sulfo-phospho-vanillin assessment of total lipids in human meibomian gland secretions. Lipids 2013, 48, 513–525. [Google Scholar] [CrossRef]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Milliner, S.E. Differences in human meibum lipid composition with meibomian gland dysfunction using NMR and principal component analysis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, M.R.; Wang, J.; Shen, M. Tear film measurement by optical reflectometry technique. J. Biomed. Opt. 2014, 19, 027001. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A. Tear film lipids. Exp. Eye Res. 2013, 117, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Bell, J.; Wells, E.; Neravetla, S.; Greenstone, V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3805–3817. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Tonchev, V.; Nencheva, Y.; Krastev, R. Surface relaxations as a tool to distinguish the dynamic interfacial properties of films formed by normal and diseased meibomian lipids. Soft Matter 2014, 10, 5579–5588. [Google Scholar] [CrossRef]
- Rosenfeld, L.; Cerretani, C.; Leiske, D.L.; Toney, M.F.; Radke, C.J.; Fuller, G.G. Structural and rheological properties of meibomian lipid. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2720–2732. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update. Int. J. Mol. Sci. 2019, 20, 6132. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Dimitrov, T.; Andreev, K.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of hyaluronic acid and benzalkonium chloride with meibomian and corneal cell lipids. Soft Matter 2013, 9, 10841–10856. [Google Scholar] [CrossRef]
- Svitova, T.F.; Lin, M.C. Dynamic interfacial properties of human tear-lipid films and their interactions with model-tear proteins in vitro. Adv. Colloid Interface Sci. 2016, 233, 4–24. [Google Scholar] [CrossRef]
- Wieland, D.C.F.; Degen, P.; Zander, T.; Gayer, S.; Raj, A.; An, J.; Dėdinaitė, A.; Claesson, P.; Willumeit-Römer, R. Structure of DPPC–hyaluronan interfacial layers—Effects of molecular weight and ion composition. Soft Matter 2016, 12, 729–740. [Google Scholar] [CrossRef]
- Li, Y.; Sang, X.; Yang, L.; Wang, X.R.; Liu, J.H.; He, X.J.; Liu, Y.; Lu, X.H.; Wang, Z.C. Low concentration of sodium hyaluronate temporarily elevates the tear film lipid layer thickness in dry eye patients with lipid deficiency. Int. J. Ophthalmol. 2018, 11, 389–394. [Google Scholar] [CrossRef]
- Fukuoka, S.; Arita, R. Increase in tear film lipid layer thickness after instillation of 3% diquafosol ophthalmic solution in healthy human eyes. Ocul. Surf. 2017, 15, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A. Tear-film-oriented diagnosis for dry eye. Jpn. J. Ophthalmol. 2019, 63, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Masmali, A.M.; Purslow, C.; Murphy, P.J. The tear ferning test: A simple clinical technique to evaluate the ocular tear film. Clin. Exp. Optom. 2014, 97, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Golding, T.R.; Brennan, N.A. The basis of tear ferning. Clin. Exp. Optom. 1989, 72, 102–112. [Google Scholar] [CrossRef]
- Huehnerfuss, H.; Alpers, W. Molecular aspects of the system water/monomolecular surface film and the occurrence of a new anomalous dispersion regime at 1.43 GHz. J. Phys. Chem. 1983, 87, 5251–5258. [Google Scholar] [CrossRef]
- Tiffany, J.M.; Winter, N.; Bliss, G. Tear film stability and tear surface tension. Curr. Eye Res. 1989, 8, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Bron, A.J.; Georgiev, G.A. The precorneal tear film as a fluid shell: The effect of blinking and saccades on tear film distribution and dynamics. Ocul. Surf. 2014, 12, 252–266. [Google Scholar] [CrossRef]
- Yokoi, N.; Takehisa, Y.; Kinoshita, S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am. J. Ophthalmol. 1996, 122, 818–824. [Google Scholar] [CrossRef]
- Nencheva, Y.; Ramasubramanian, A.; Eftimov, P.; Yokoi, N.; Borchman, D.; Georgiev, G.A. Effects of Lipid Saturation on the Surface Properties of Human Meibum Films. Int. J. Mol. Sci. 2018, 19, 2209. [Google Scholar] [CrossRef] [PubMed]
- Loglio, G.; Tesei, U.; Cini, R. Viscoelastic dilatation processes of fluid/fluid interfaces: Time-domain representation. Colloid Polym. Sci. 1986, 264, 712–718. [Google Scholar] [CrossRef]
- Monroy, F.; Ortega, F.; Rubio, R.G. Dilatational rheology of insoluble polymer monolayers: Poly(vinylacetate). Phys. Rev. E 1998, 58, 7629–7641. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 383–389. [Google Scholar] [CrossRef]
- Kogbe, O.; Liotet, S.; Tiffany, J.M. Factors responsible for tear ferning. Cornea 1991, 10, 433–444. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Yokoi, N.; Koev, K.; Kutsarova, E.; Ivanova, S.; Kyumurkov, A.; Jordanova, A.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of benzalkonium chloride with films of meibum, corneal cells lipids, and whole tears. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Fatt, I. Observations of tear film break up on model eyes. CLAO J. Off. Publ. Contact Lens Assoc. Ophthalmol. Inc 1991, 17, 267–281. [Google Scholar]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Sundaram, H.; Mackiewicz, N.; Burton, E.; Peno-Mazzarino, L.; Lati, E.; Meunier, S. Pilot Comparative Study of the Topical Action of a Novel, Crosslinked Resilient Hyaluronic Acid on Skin Hydration and Barrier Function in a Dynamic, Three-Dimensional Human Explant Model. J. Drugs Dermatol. 2016, 15, 434–441. [Google Scholar] [PubMed]
- Santoro, S.; Russo, L.; Argenzio, V.; Borzacchiello, A. Rheological properties of cross-linked hyaluronic acid dermal fillers. J. Appl. Biomater. Biomech. 2011, 9, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Calles, J.A.; Bermúdez, J.; Vallés, E.; Allemandi, D.; Palma, S. Polymers in Ophthalmology. In Advanced Polymers in Medicine; Springer: Cham, Switzerland, 2015; pp. 147–176. [Google Scholar] [CrossRef]
- Graça, A.; Gonçalves, L.M.; Raposo, S.; Ribeiro, H.M.; Marto, J. Useful In Vitro Techniques to Evaluate the Mucoadhesive Properties of Hyaluronic Acid-Based Ocular Delivery Systems. Pharmaceutics 2018, 10, 110. [Google Scholar] [CrossRef]
- Debbasch, C.; De La Salle, S.B.; Brignole, F.; Rat, P.; Warnet, J.M.; Baudouin, C. Cytoprotective effects of hyaluronic acid and Carbomer 934P in ocular surface epithelial cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3409–3415. [Google Scholar]
- Tseng, C.L.; Hung, Y.J.; Chen, Z.Y.; Fang, H.W.; Chen, K.H. Synergistic Effect of Artificial Tears Containing Epigallocatechin Gallate and Hyaluronic Acid for the Treatment of Rabbits with Dry Eye Syndrome. PLoS ONE 2016, 11, e0157982. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds A Compend. Clin. Res. Pract. 2016, 28, 78–88. [Google Scholar]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Weigel, P.H.; Baggenstoss, B.A. What is special about 200 kDa hyaluronan that activates hyaluronan receptor signaling? Glycobiology 2017, 27, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Simmons, P.A.; Wang, H.; Wang, T. Physicochemical Properties of Hyaluronic Acid-Based Lubricant Eye Drops. Trans. Vis. Sci. Technol. 2019, 8, 2. [Google Scholar] [CrossRef]
- Fallacara, A.; Vertuani, S.; Panozzo, G.; Pecorelli, A.; Valacchi, G.; Manfredini, S. Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study. Molecules 2017, 22, 2104. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A.; Loglio, G.; Miller, R. Dilational surface visco-elasticity of polyelectrolyte/surfactant solutions: Formation of heterogeneous adsorption layers. Adv. Colloid Interface Sci. 2011, 168, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Teukolsky, S.; Press, W.; Vetterling, W. Fast Fourier Transform; Numerical Recipes in C++; Cambridge University Press: Cambridge, UK, 2007; pp. 501–537. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eftimov, P.; Yokoi, N.; Melo, A.M.; Daull, P.; Georgiev, G.A. Interactions of Meibum and Tears with Mucomimetic Polymers: A Hint towards the Interplay between the Layers of the Tear Film. Int. J. Mol. Sci. 2021, 22, 2747. https://doi.org/10.3390/ijms22052747
Eftimov P, Yokoi N, Melo AM, Daull P, Georgiev GA. Interactions of Meibum and Tears with Mucomimetic Polymers: A Hint towards the Interplay between the Layers of the Tear Film. International Journal of Molecular Sciences. 2021; 22(5):2747. https://doi.org/10.3390/ijms22052747
Chicago/Turabian StyleEftimov, Petar, Norihiko Yokoi, Ana M. Melo, Philippe Daull, and Georgi As. Georgiev. 2021. "Interactions of Meibum and Tears with Mucomimetic Polymers: A Hint towards the Interplay between the Layers of the Tear Film" International Journal of Molecular Sciences 22, no. 5: 2747. https://doi.org/10.3390/ijms22052747
APA StyleEftimov, P., Yokoi, N., Melo, A. M., Daull, P., & Georgiev, G. A. (2021). Interactions of Meibum and Tears with Mucomimetic Polymers: A Hint towards the Interplay between the Layers of the Tear Film. International Journal of Molecular Sciences, 22(5), 2747. https://doi.org/10.3390/ijms22052747