Aged Mice Devoid of the M3 Muscarinic Acetylcholine Receptor Develop Mild Dry Eye Disease
Abstract
1. Introduction
2. Results
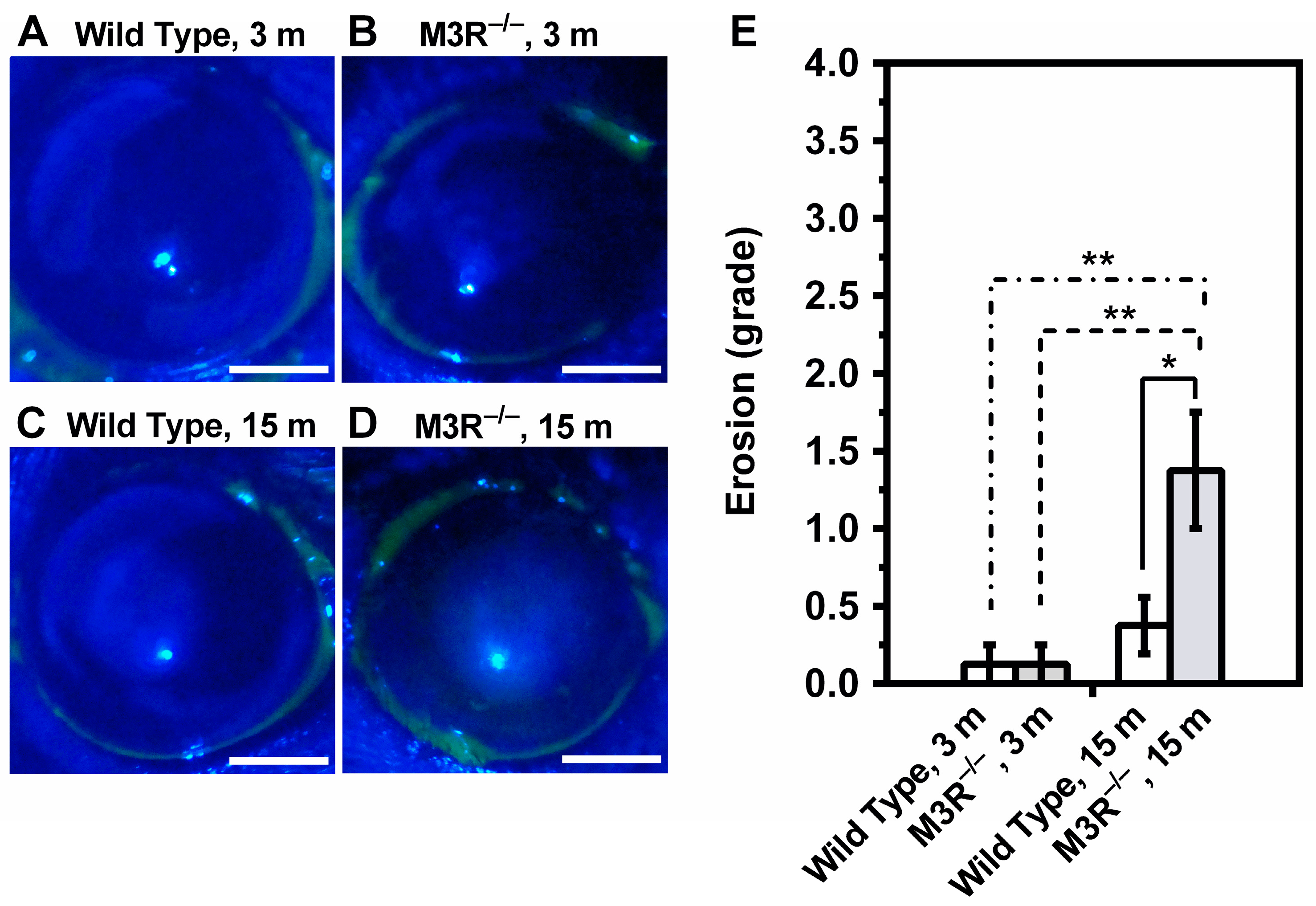
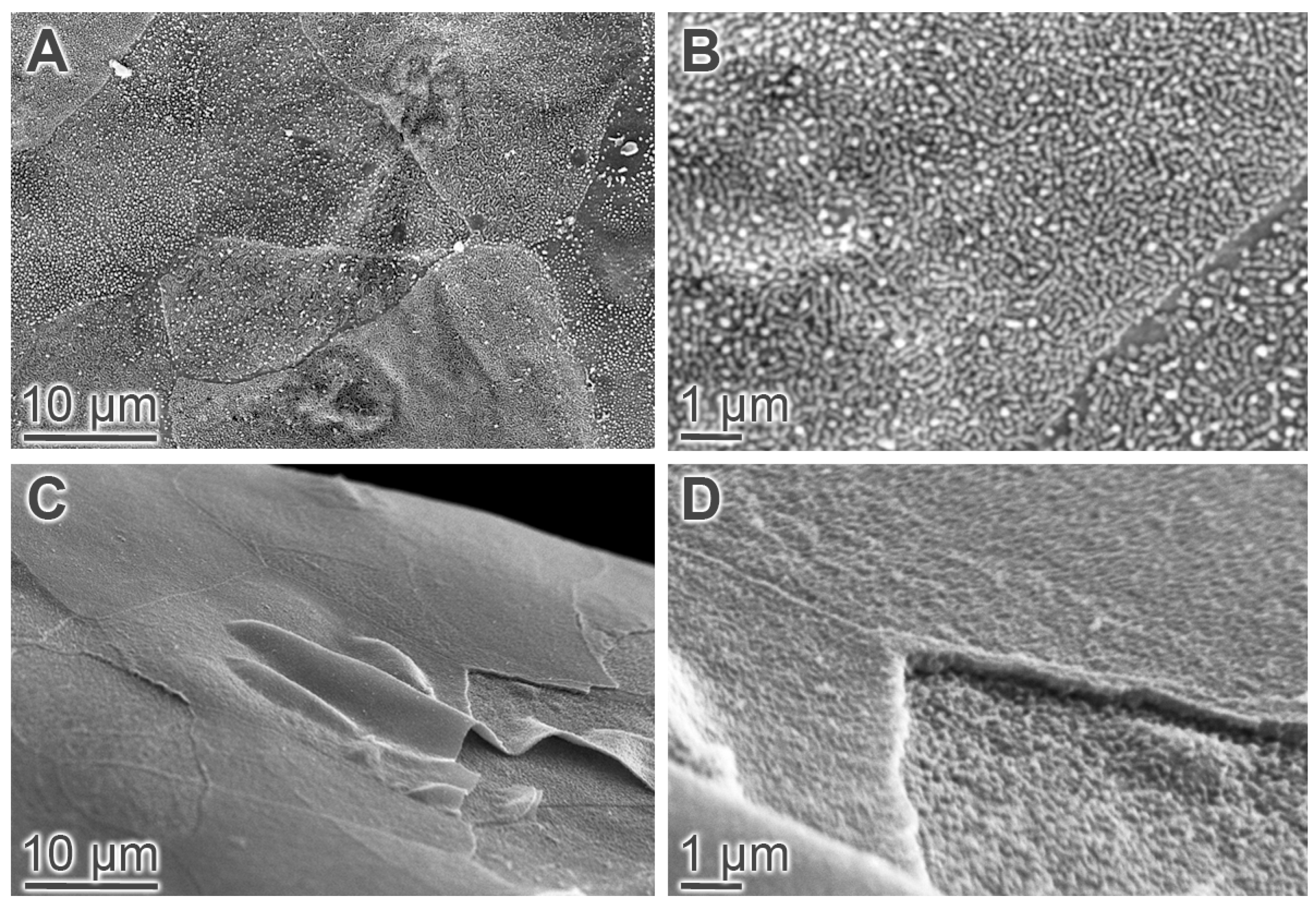
2.1. Tear Production and Ocular Surface Characteristics
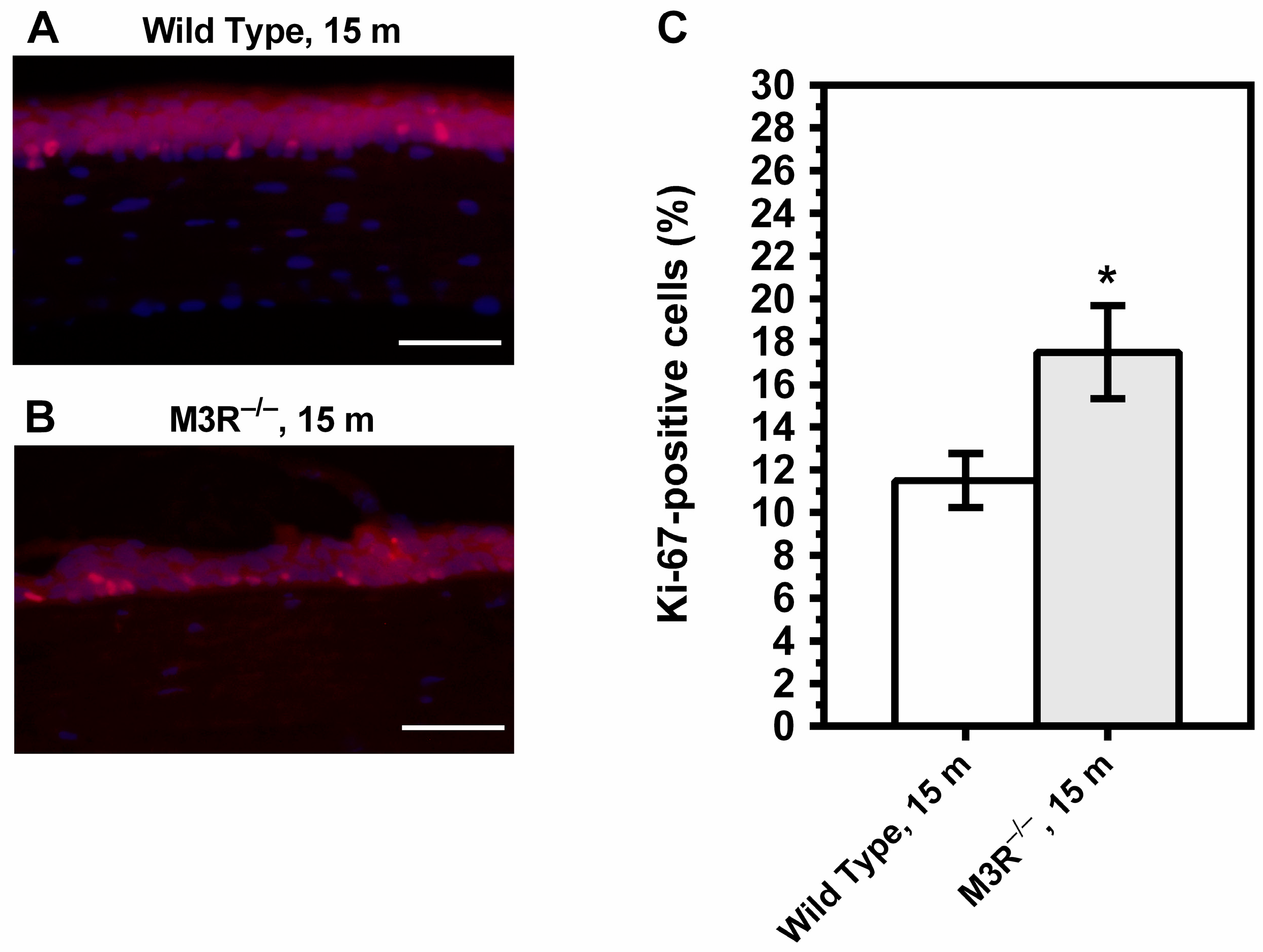
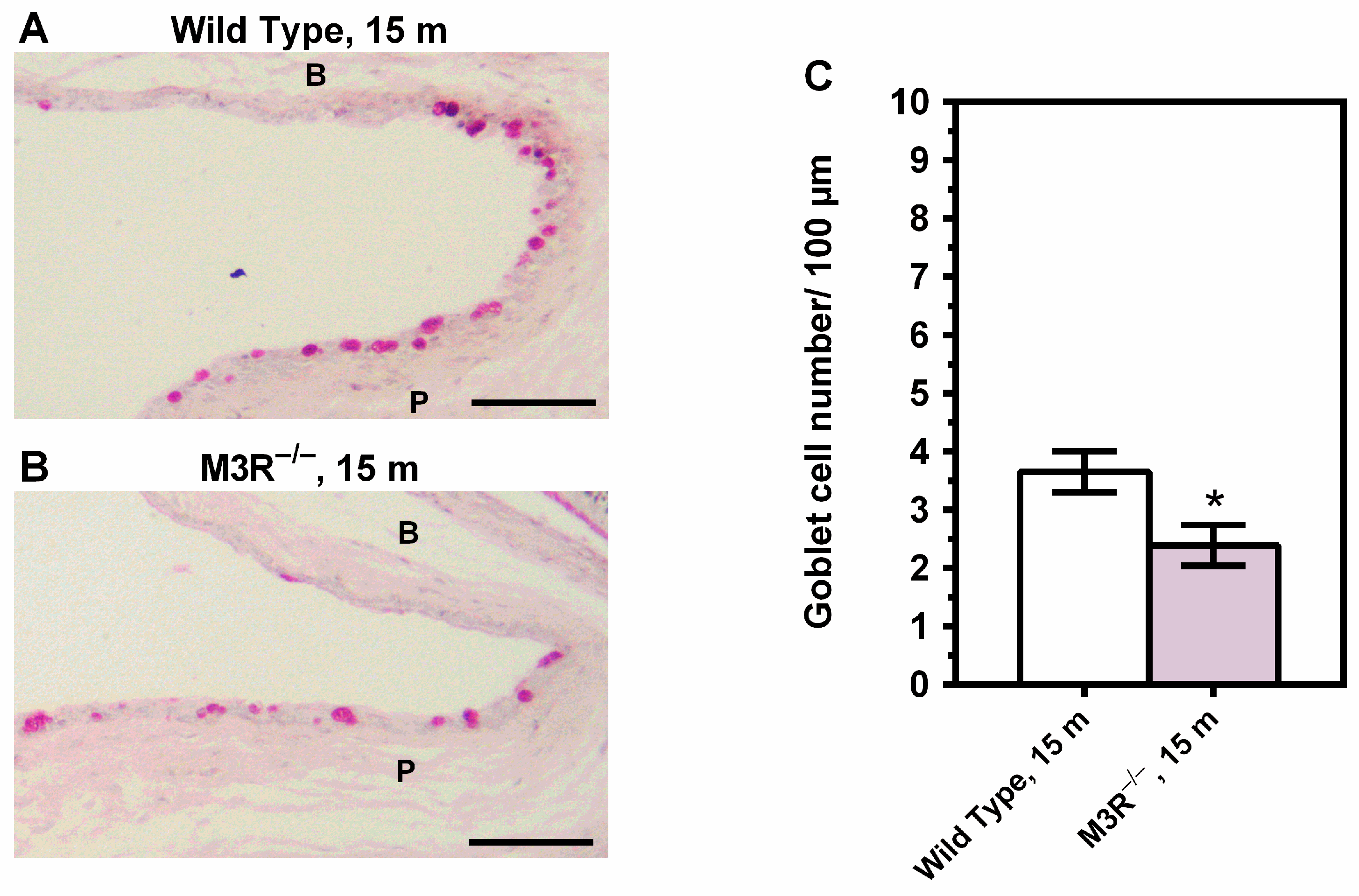
2.2. Corneal Epithelial Cell Proliferation and Conjunctival Goblet Cell Density
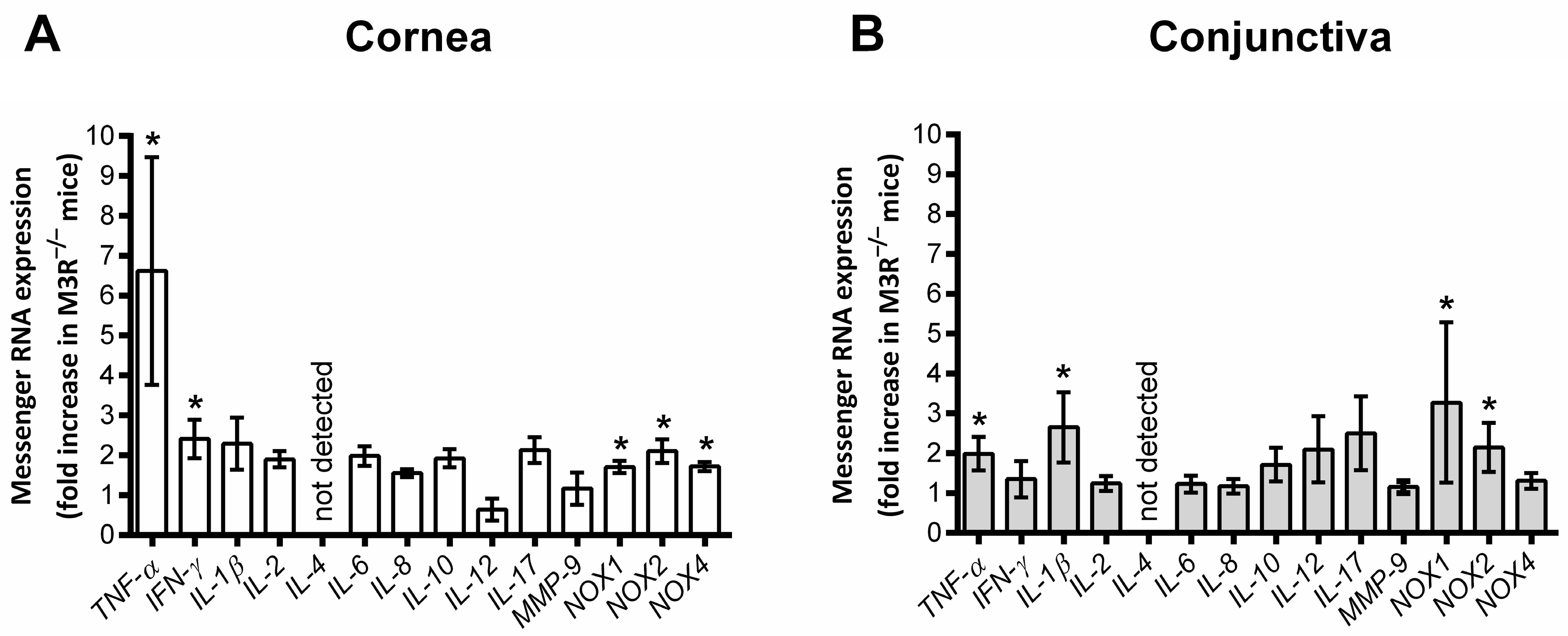
2.3. Expression of Inflammatory and Prooxidant Redox Genes
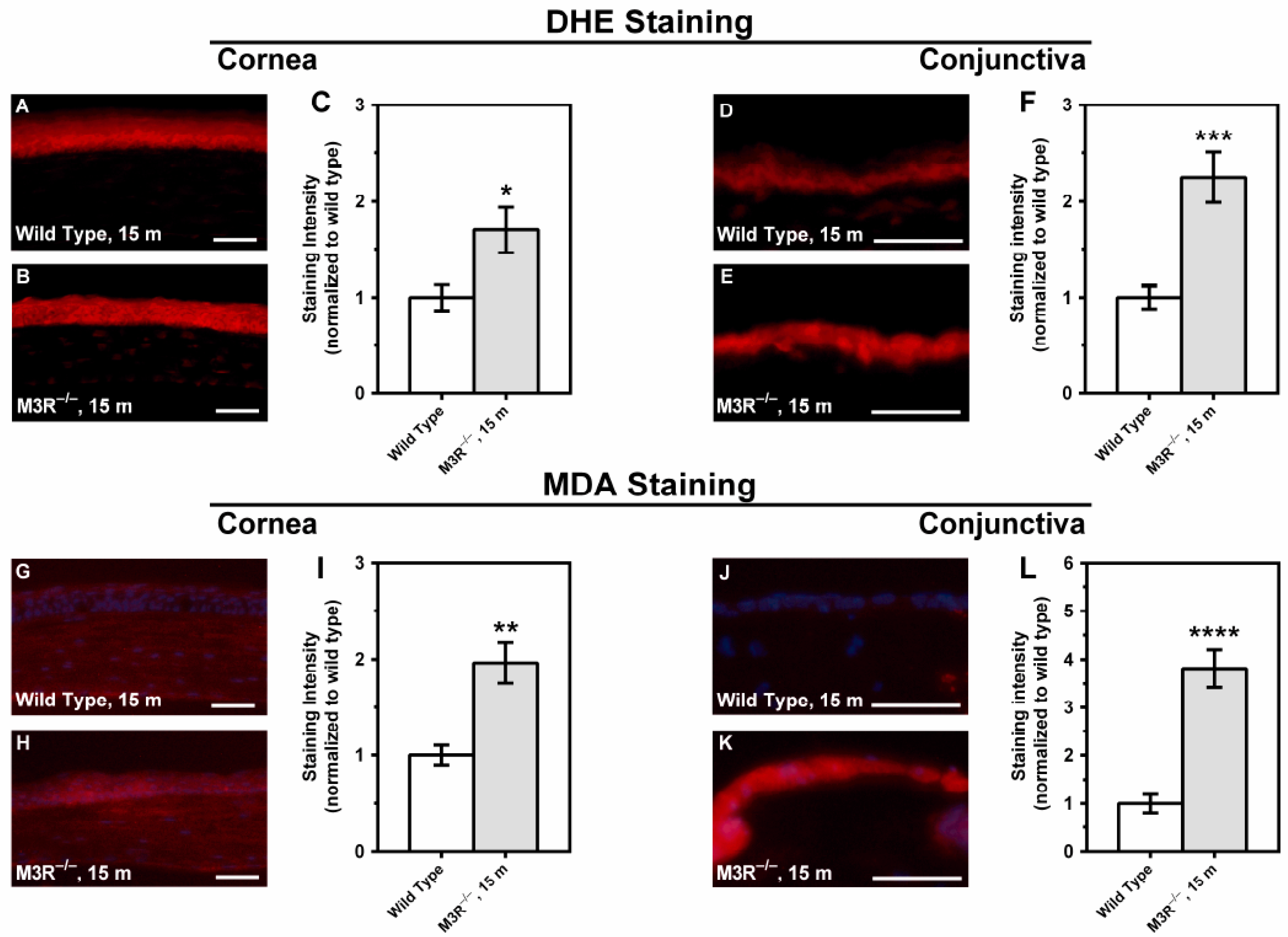
2.4. ROS Formation in the Corneal and Conjunctival Epithelium
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Quantification of Tear Production
4.3. Characterization of the Corneal Surface
4.4. Environmental Scanning Electron Microscopy
4.5. Quantification of Proliferating Corneal Epithelial Cells and Conjunctival Goblet Cells
4.6. Quantification of Inflammatory and Prooxidant Redox Genes by Real-Time PCR
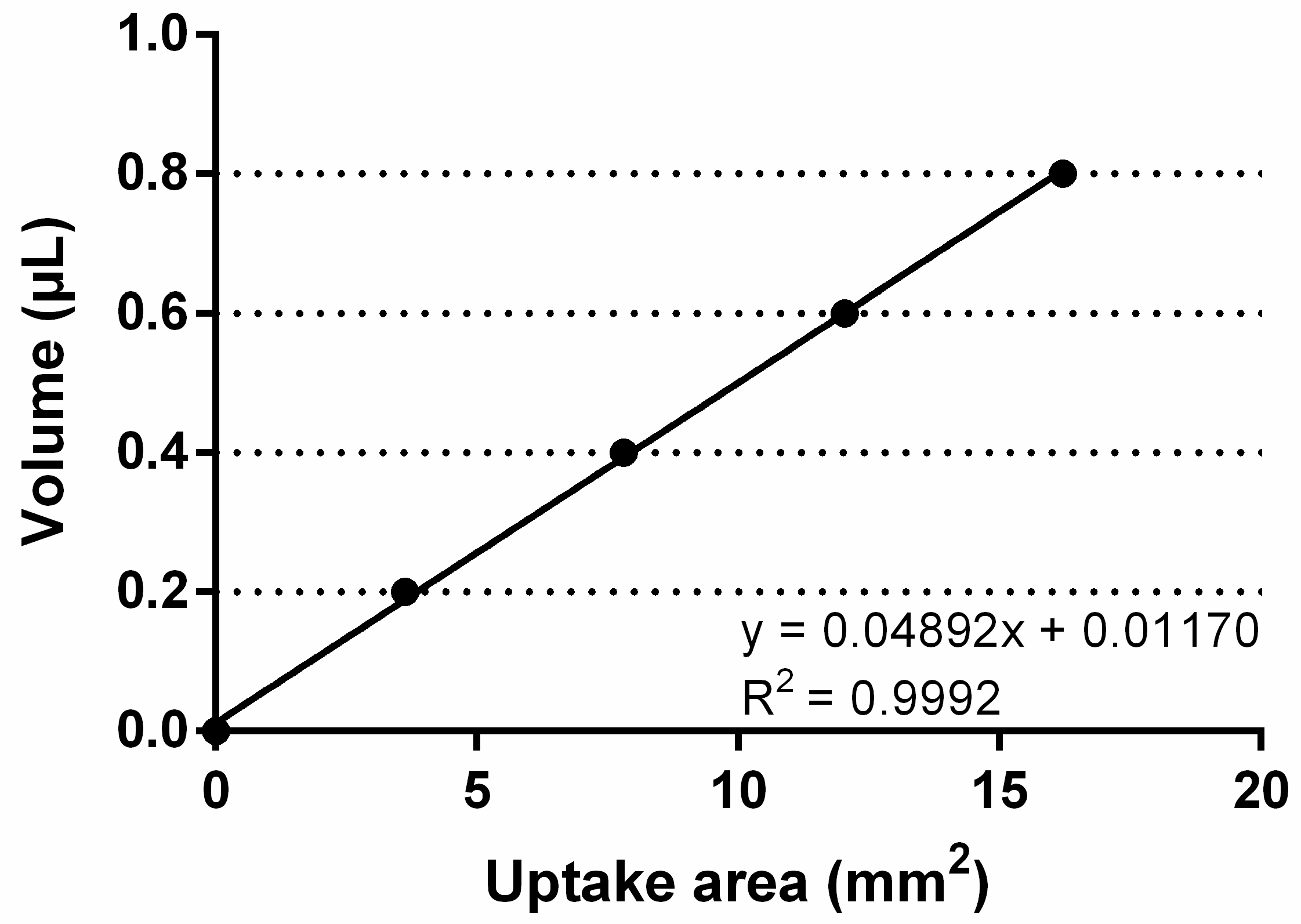
4.7. Quantification of Reactive Oxygen Species
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.P.; Santo, R.M. The impact of dry eye disease treatment on patient satisfaction and quality of life: A review. Ocul. Surf. 2019, 17, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Gumus, K.; Feuerman, J.; Alex, A. Tear Volume-based Diagnostic Classification for Tear Dysfunction. Int. Ophthalmol. Clin. 2017, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, P.J.; Van Der Heijde, G.L.; Breedveld, F.C.; Markusse, H.M. Parasympathetic dysfunction in rheumatoid arthritis patients with ocular dryness. Ann. Rheum. Dis. 1996, 55, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Heigle, T.; Pflugfelder, S.C. Nasolacrimal Stimulation of Aqueous Tear Production. Cornea 1997, 16, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Zheng, Y.; Deng, L.; Huang, X. Prevalence of dry eye disease in the elderly. Medicine 2020, 99, e22234. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Mircheff, A.K.; Geerling, G. Animal Models of Dry Eye. Integr. Intraoper. Ocul. Coherence Tomogr. Pediatr. Ocul. Surg. 2008, 41, 298–312. [Google Scholar] [CrossRef]
- Shinomiya, K.; Ueta, M.; Kinoshita, S. A new dry eye mouse model produced by exorbital and intraorbital lacrimal gland excision. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Bao, J.; Li, C.; Tan, G.; Wu, A.; Ye, L.; Ye, L.; Zhou, Q.; Shao, Y. A murine model of dry eye induced by topical administration of erlotinib eye drops. Int. J. Mol. Med. 2017, 41, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hirayama, M.; Kawakita, T.; Tsubota, K. A Ligation of the Lacrimal Excretory Duct in Mouse Induces Lacrimal Gland Inflammation with Proliferative Cells. Stem Cells Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Dursun, D.; Wang, M.; Monroy, D.; Li, D.-Q.; Lokeshwar, B.L.; E Stern, M.; Pflugfelder, S.C. A mouse model of keratoconjunctivitis sicca. Investig. Ophthalmol. Vis. Sci. 2002, 43, 632–638. [Google Scholar]
- Wess, J.; Eglen, R.M.; Gautam, D. Muscarinic acetylcholine receptors: Mutant mice provide new insights for drug development. Nat. Rev. Drug Discov. 2007, 6, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.C.; Kobilka, B.K.; Gautam, D.; Sexton, P.M.; Christopoulos, A.; Wess, J. Muscarinic acetylcholine receptors: Novel opportunities for drug development. Nat. Rev. Drug Discov. 2014, 13, 549–560. [Google Scholar] [CrossRef]
- Pradidarcheep, W.; Labruyère, W.T.; Dabhoiwala, N.F.; Lamers, W.H. Lack of Specificity of Commercially Available Antisera: Better Specifications Needed. J. Histochem. Cytochem. 2008, 56, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Bacman, S.R.; Leiros, C.P.; Sterin-Borda, L.; Hubscher, O.; Arana, R.; Borda, E. Autoantibodies against lacrimal gland M3 muscarinic acetylcholine receptors in patients with primary Sjogren’s syndrome. Investig. Ophthalmol. Vis. Sci. 1998, 39, 151–156. [Google Scholar]
- Bacman, S.R.; Berra, A.; Sterin-Borda, L.; Borda, E.E. Human primary Sjögren’s syndrome autoantibodies as mediators of nitric oxide release coupled to lacrimal gland muscarinic acetylcholine receptors. Curr. Eye Res. 1998, 17, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Li, D.-Q.; Farley, W.; Luo, L.H.; Heuckeroth, R.O.; Milbrandt, J.; Pflugfelder, S.C. Neurturin-Deficient Mice Develop Dry Eye and Keratoconjunctivitis Sicca. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4223–4229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef]
- Jakubik, J.; El-Fakahany, E.E. Current Advances in Allosteric Modulation of Muscarinic Receptors. Biomolecules 2020, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Miyakawa, T.; Duttaroy, A.; Yamanaka, A.; Moriguchi, T.; Makita, R.; Ogawa, M.; Chou, C.J.; Xia, B.; Crawley, J.N.; et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nat. Cell Biol. 2001, 410, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Wess, J. Muscarinic acetylcholine receptor knockout mice: Novel Phenotypes and Clinical Implications. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 423–450. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Wakamatsu, T.H.; Dogru, M.; Ogawa, Y.; Igarashi, A.; Ibrahim, O.M.; Inaba, T.; Shimizu, T.; Noda, S.; Obata, H.; et al. Age-Related Dysfunction of the Lacrimal Gland and Oxidative Stress. Am. J. Pathol. 2012, 180, 1879–1896. [Google Scholar] [CrossRef] [PubMed]
- McClellan, A.J.; Volpe, E.A.; Zhang, X.; Darlington, G.J.; Li, D.-Q.; Pflugfelder, S.C.; de Paiva, C.S. Ocular Surface Disease and Dacryoadenitis in Aging C57BL/6 Mice. Am. J. Pathol. 2014, 184, 631–643. [Google Scholar] [CrossRef]
- Fabiani, C.; Barabino, S.; Rashid, S.; Dana, M.R. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp. Eye Res. 2009, 89, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Li, J.; Yang, Q.; Wu, A.; Qu, D.-Y.; Wang, Y.; Ye, L.; Bao, J.; Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 2018, 8, 17828. [Google Scholar] [CrossRef]
- Corrales, R.M.; De Paiva, C.S.; Li, D.-Q.; Farley, W.J.; Henriksson, J.T.; Bergmanson, J.P.G.; Pflugfelder, S.C. Entrapment of Conjunctival Goblet Cells by Desiccation-Induced Cornification. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3492–3499. [Google Scholar] [CrossRef] [PubMed]
- Gilbard, J.P.; Rossi, S.R.; Gray, K.L.; Hanninen, L.A.; Kenyon, K.R. Tear film osmolarity and ocular surface disease in two rabbit models for keratoconjunctivitis sicca. Investig. Ophthalmol. Vis. Sci. 1988, 29, 374–378. [Google Scholar]
- Niederkorn, J.Y.; Stern, M.E.; Pflugfelder, S.C.; De Paiva, C.S.; Corrales, R.M.; Gao, J.; Siemasko, K. Desiccating Stress Induces T Cell-Mediated Sjögren’s Syndrome-Like Lacrimal Keratoconjunctivitis. J. Immunol. 2006, 176, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Villarreal, A.L.; Corrales, R.M.; Rahman, H.T.; Chang, V.Y.; Farley, W.J.; Stern, M.E.; Niederkorn, J.Y.; Li, D.-Q.; Pflugfelder, S.C. Dry Eye–Induced Conjunctival Epithelial Squamous Metaplasia Is Modulated by Interferon-γ. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2553–2560. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; De Paiva, C.S.; Corrales, R.M.; Volpe, E.A.; McClellan, A.J.; Farley, W.J.; Li, D.-Q.; Pflugfelder, S.C. Interferon-γ Exacerbates Dry Eye–Induced Apoptosis in Conjunctiva through Dual Apoptotic Pathways. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6279–6285. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Q.; Lokeshwar, B.L.; Solomon, A.; Monroy, D.; Ji, Z.; Pflugfelder, S.C. Regulation of MMP-9 Production by Human Corneal Epithelial Cells. Exp. Eye Res. 2001, 73, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Sivak, J.; Fini, M. MMPs in the eye: Emerging roles for matrix metalloproteinases in ocular physiology. Prog. Retin. Eye Res. 2002, 21, 1–14. [Google Scholar] [CrossRef]
- Afonso, A.; Sobrin, L.; Monroy, D.C.; Selzer, M.; Lokeshwar, B.; Pflugfelder, S.C. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2506–2512. [Google Scholar]
- A Smith, V.; Rishmawi, H.; Hussein, H.; Easty, D.L. Tear film MMP accumulation and corneal disease. Br. J. Ophthalmol. 2001, 85, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Dursun, D.; Wang, M.; Monroy, D.; Li, D.-Q.; Lokeshwar, B.L.; Stern, M.; Pflugfelder, S.C. Experimentally induced dry eye produces ocular surface inflammation and epithelial disease. Adv. Exp. Med. Biol. 2002, 506, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Nagelhout, T.J.; Gamache, D.A.; Roberts, L.; Brady, M.T.; Yanni, J.M. Preservation of Tear Film Integrity and Inhibition of Corneal Injury by Dexamethasone in a Rabbit Model of Lacrimal Gland Inflammation–Induced Dry Eye. J. Ocul. Pharmacol. Ther. 2005, 21, 139–148. [Google Scholar] [CrossRef]
- Chotikavanich, S.; De Paiva, C.S.; Li, D.Q.; Chen, J.J.; Bian, F.; Farley, W.J.; Pflugfelder, S.C. Production and Activity of Matrix Metalloproteinase-9 on the Ocular Surface Increase in Dysfunctional Tear Syndrome. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3203–3209. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Farley, W.; Luo, L.; Chen, L.Z.; de Paiva, C.S.; Olmos, L.C.; Li, D.-Q.; Fini, M.E. Matrix Metalloproteinase-9 Knockout Confers Resistance to Corneal Epithelial Barrier Disruption in Experimental Dry Eye. Am. J. Pathol. 2005, 166, 61–71. [Google Scholar] [CrossRef]
- Luo, L.; Li, D.-Q.; Doshi, A.; Farley, W.; Corrales, R.M.; Pflugfelder, S.C. Experimental Dry Eye Stimulates Production of Inflammatory Cytokines and MMP-9 and Activates MAPK Signaling Pathways on the Ocular Surface. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Schargus, M.; Ivanova, S.; Kakkassery, V.; Dick, H.B.; Joachim, S. Correlation of Tear Film Osmolarity and 2 Different MMP-9 Tests with Common Dry Eye Tests in a Cohort of Non–Dry Eye Patients. Cornea 2015, 34, 739–744. [Google Scholar] [CrossRef]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Shibuya, M.; Nakashima, H.; Hisamura, R.; Masuda, N.; Imagawa, T.; Uehara, M.; Tsubota, K. Involvement of Oxidative Stress on Corneal Epithelial Alterations in a Blink-Suppressed Dry Eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1552–1558. [Google Scholar] [CrossRef]
- Deng, R.; Hua, X.; Li, J.; Chi, W.; Zhang, Z.; Lu, F.; Zhang, L.; Pflugfelder, S.C.; Li, D.-Q. Oxidative Stress Markers Induced by Hyperosmolarity in Primary Human Corneal Epithelial Cells. PLoS ONE 2015, 10, e0126561. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, J.-H.; Cui, L.; Li, Y.; Yang, J.M.; Yun, J.-J.; Jung, J.E.; Choi, W.; Yoon, K.C. Anti-Inflammatory and Antioxidative Effects of Camellia japonica on Human Corneal Epithelial Cells and Experimental Dry Eye: In Vivo and In Vitro Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1196–1207. [Google Scholar] [CrossRef]
- Ji, Y.W.; Byun, Y.J.; Choi, W.; Jeong, E.; Kim, J.S.; Noh, H.; Kim, E.S.; Song, Y.J.; Park, S.K.; Lee, H.K. Neutralization of Ocular Surface TNF-α Reduces Ocular Surface and Lacrimal Gland Inflammation Induced by In Vivo Dry Eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7557–7566. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.W.; Seo, Y.; Choi, W.; Yeo, A.; Noh, H.; Kim, E.K.; Lee, H.K. Dry Eye-Induced CCR7+CD11b+ Cell Lymph Node Homing Is Induced by COX-2 Activities. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6829–6838. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, A.; Ackermann, M.; Belleri, M.; Coltrini, D.; Nico, B.; Ribatti, M.; Konerding, M.A.; Presta, M.; Righi, M.G.E. Brain angioarchitecture and intussusceptive microvascular growth in a murine model of Krabbe disease. Angiogenesis 2015, 18, 499–510. [Google Scholar] [CrossRef]
- Brown, D.C.; Gatter, K.C. Ki67 protein: The immaculate deception? Histopathology 2002, 40, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Musayeva, A.; Manicam, C.; Steege, A.; Brochhausen, C.; Straub, B.K.; Bell, K.; Pfeiffer, N.; Gericke, A. Role of α1-adrenoceptor subtypes on corneal epithelial thickness and cell proliferation in mice. Am. J. Physiol. Physiol. 2018, 315, C757–C765. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Mann, C.; Zadeh, J.K.; Musayeva, A.; Wolff, I.; Wang, M.; Pfeiffer, N.; Daiber, A.; Li, H.; Xia, N.; et al. Elevated Intraocular Pressure Causes Abnormal Reactivity of Mouse Retinal Arterioles. Oxidative Med. Cell. Longev. 2019, 2019, 9736047. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, J.K.; Garcia-Bardon, A.; Hartmann, E.K.; Pfeiffer, N.; Omran, W.; Ludwig, M.; Patzak, A.; Xia, N.; Li, H.; Gericke, A. Short-Time Ocular Ischemia Induces Vascular Endothelial Dysfunction and Ganglion Cell Loss in the Pig Retina. Int. J. Mol. Sci. 2019, 20, 4685. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward | Reverse |
|---|---|---|
| TNF-α | GCC TCT TCT CAT TCC TGC TTG | CTG ATG AGA GGG AGG CCA TT |
| IFN-γ | AGC GGC TGA CTG AAC TCA GAT TGT AG | GTC ACA GTT TTC AGC TGT ATA GGG |
| IL-1β | AAG GAG AAC CAA GCA ACG ACA AAA | TGG GGA ACT CTG CAG ACT CAA ACT |
| IL-2 | CAA GTC CTG CAG GCA TGT ACA | CTG TTG ACA AGG AGC ACA AGT GT |
| IL-4 | CGCCATGCACGGAGATG | CGAGCTCACTCTCTGTGGTGTT |
| IL-6 | ACA ACC ACG GCC TTC CCT ACT T | CAC GAT TTC CCA GAG AAC ATG TG |
| IL-8 | ACGGACATGGCTGCTCAAG | GGACGAAGATGCCTAGGTTAAGG |
| IL-10 | CAT GGG TCT TGG GAA GAG AA | AAC TGG CCA CAG TTT TCA GG |
| IL-12 | CAT CCA GCA GCT CCT CTC AGT | GCA AGG GTG GCC AAA AAG A |
| IL-17 | CCT CAC ACG AGG CAC AAG TG | TCT CCC TGG ACT CAT GTT TGC |
| MMP-9 | AAAGACCTGAAAACCTCCAACCT | GCCCGGGTGTAACCATAGC |
| NOX1 | GGAGGAATTAGGCAAAATGGATT | GCTGCATGACCAGCAATGTT |
| NOX2 | CCAACTGGGATAACGAGTTCA | GAGAGTTTCAGCCAAGGCTTC |
| NOX4 | TGTAACAGAGGGAAAACAGTTGGA | GTTCCGGTTACTCAAACTATGAAGAGT |
| TBP | CTT CGT GCA AGA AAT GCT GAA T | CAG TTG TCC GTG GCT CTC TTA TT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musayeva, A.; Jiang, S.; Ruan, Y.; Zadeh, J.K.; Chronopoulos, P.; Pfeiffer, N.; Müller, W.E.G.; Ackermann, M.; Xia, N.; Li, H.; et al. Aged Mice Devoid of the M3 Muscarinic Acetylcholine Receptor Develop Mild Dry Eye Disease. Int. J. Mol. Sci. 2021, 22, 6133. https://doi.org/10.3390/ijms22116133
Musayeva A, Jiang S, Ruan Y, Zadeh JK, Chronopoulos P, Pfeiffer N, Müller WEG, Ackermann M, Xia N, Li H, et al. Aged Mice Devoid of the M3 Muscarinic Acetylcholine Receptor Develop Mild Dry Eye Disease. International Journal of Molecular Sciences. 2021; 22(11):6133. https://doi.org/10.3390/ijms22116133
Chicago/Turabian StyleMusayeva, Aytan, Subao Jiang, Yue Ruan, Jenia Kouchek Zadeh, Panagiotis Chronopoulos, Norbert Pfeiffer, Werner E.G. Müller, Maximilian Ackermann, Ning Xia, Huige Li, and et al. 2021. "Aged Mice Devoid of the M3 Muscarinic Acetylcholine Receptor Develop Mild Dry Eye Disease" International Journal of Molecular Sciences 22, no. 11: 6133. https://doi.org/10.3390/ijms22116133
APA StyleMusayeva, A., Jiang, S., Ruan, Y., Zadeh, J. K., Chronopoulos, P., Pfeiffer, N., Müller, W. E. G., Ackermann, M., Xia, N., Li, H., & Gericke, A. (2021). Aged Mice Devoid of the M3 Muscarinic Acetylcholine Receptor Develop Mild Dry Eye Disease. International Journal of Molecular Sciences, 22(11), 6133. https://doi.org/10.3390/ijms22116133








