Advanced Spectroscopy and APBS Modeling for Determination of the Role of His190 and Trp103 in Mouse Thymidylate Synthase Interaction with Selected dUMP Analogues
Abstract
1. Introduction
- Revealing that both reactions leading to dTMP production, (1) methylene group transfer from a cofactor to the C(5) atom of dUMP with the concomitant proton abstraction from C(5) and (2) the hydride transfer from a cofactor to the exocyclic methylene group (donated in reaction (1)), display a higher complexity that lies in a strong liability of the C(6)-S bond between the thiol of catalytic cysteine and C(6) of dUMP [8,9,10,11,12,13];
- The observation of the enhanced emission quenching of the human TS in comparison to E. coli TS by 5-fluoro-dUMP (FdUMP) [14] with yet unsolved reason;
2. Results
2.1. Fluorescence Spectra
2.2. MDF and Chemometrics
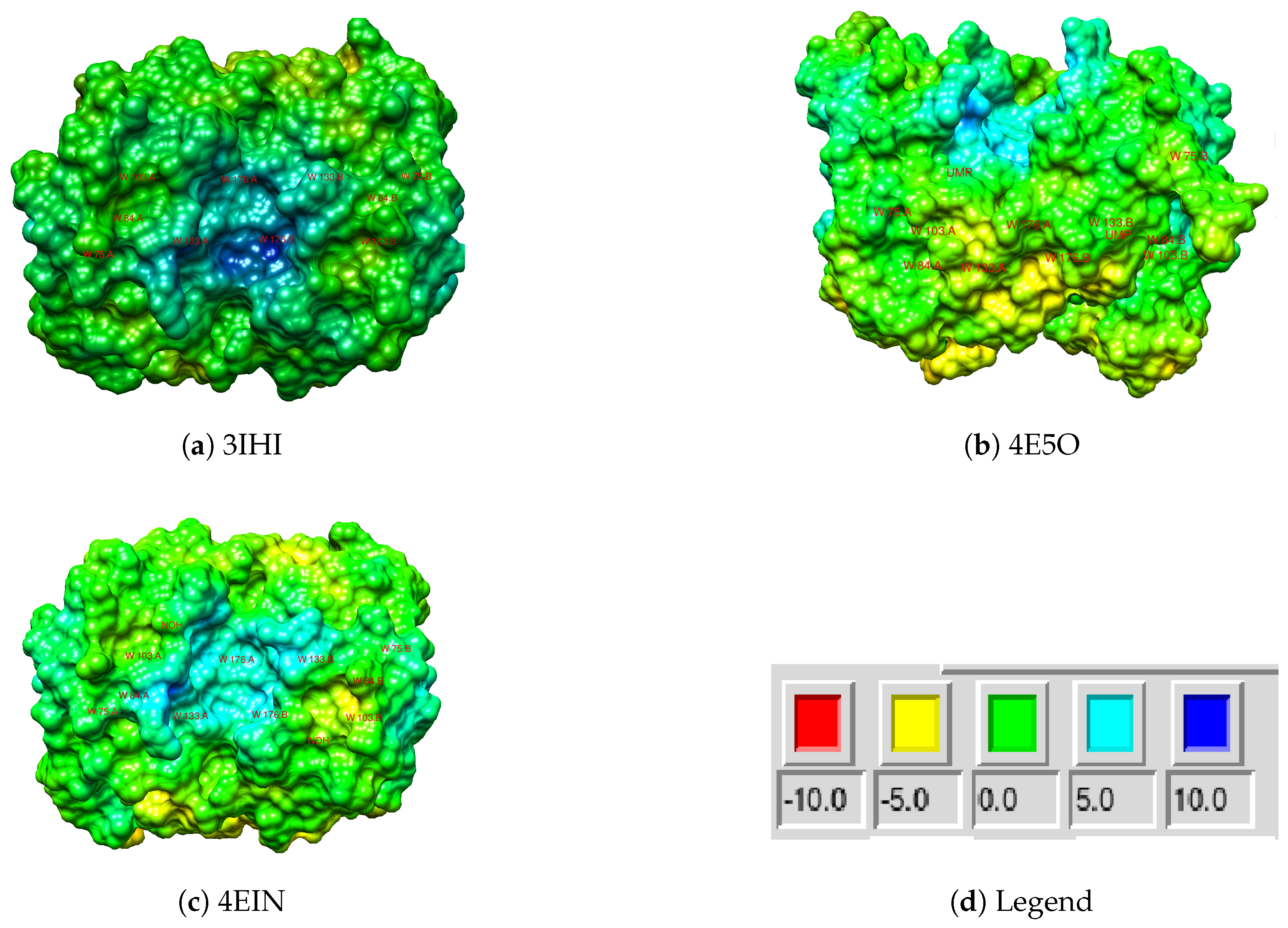
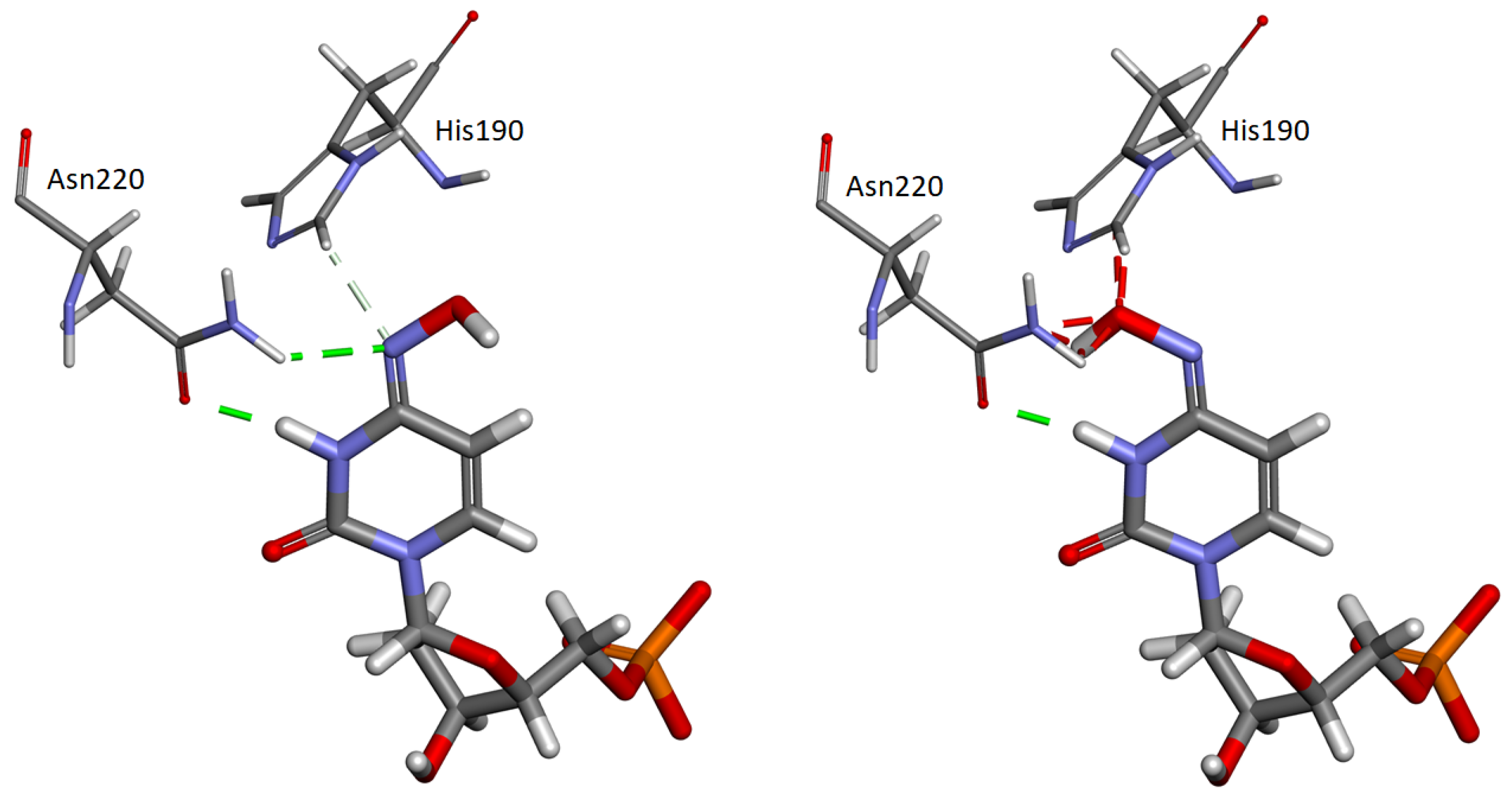
2.3. Electrostatic Potential
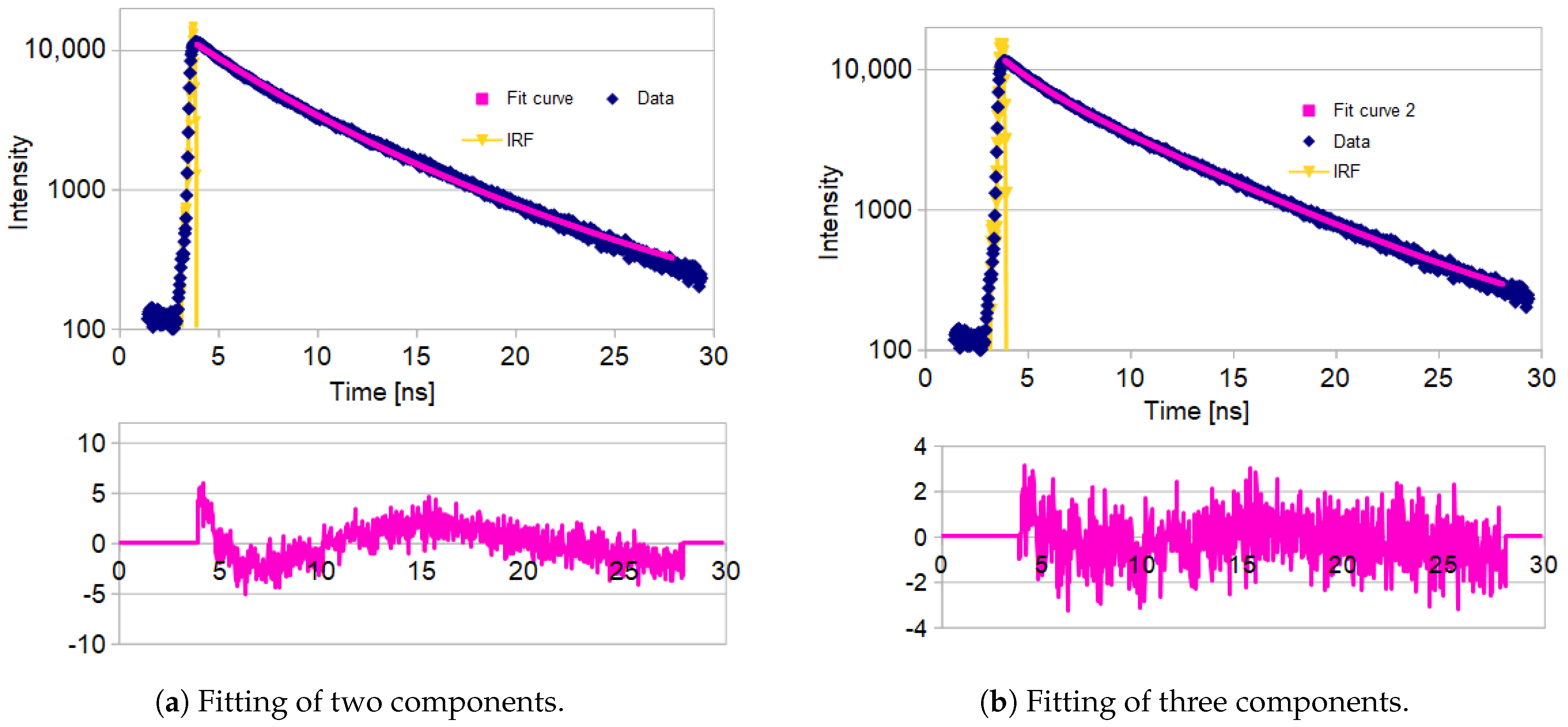
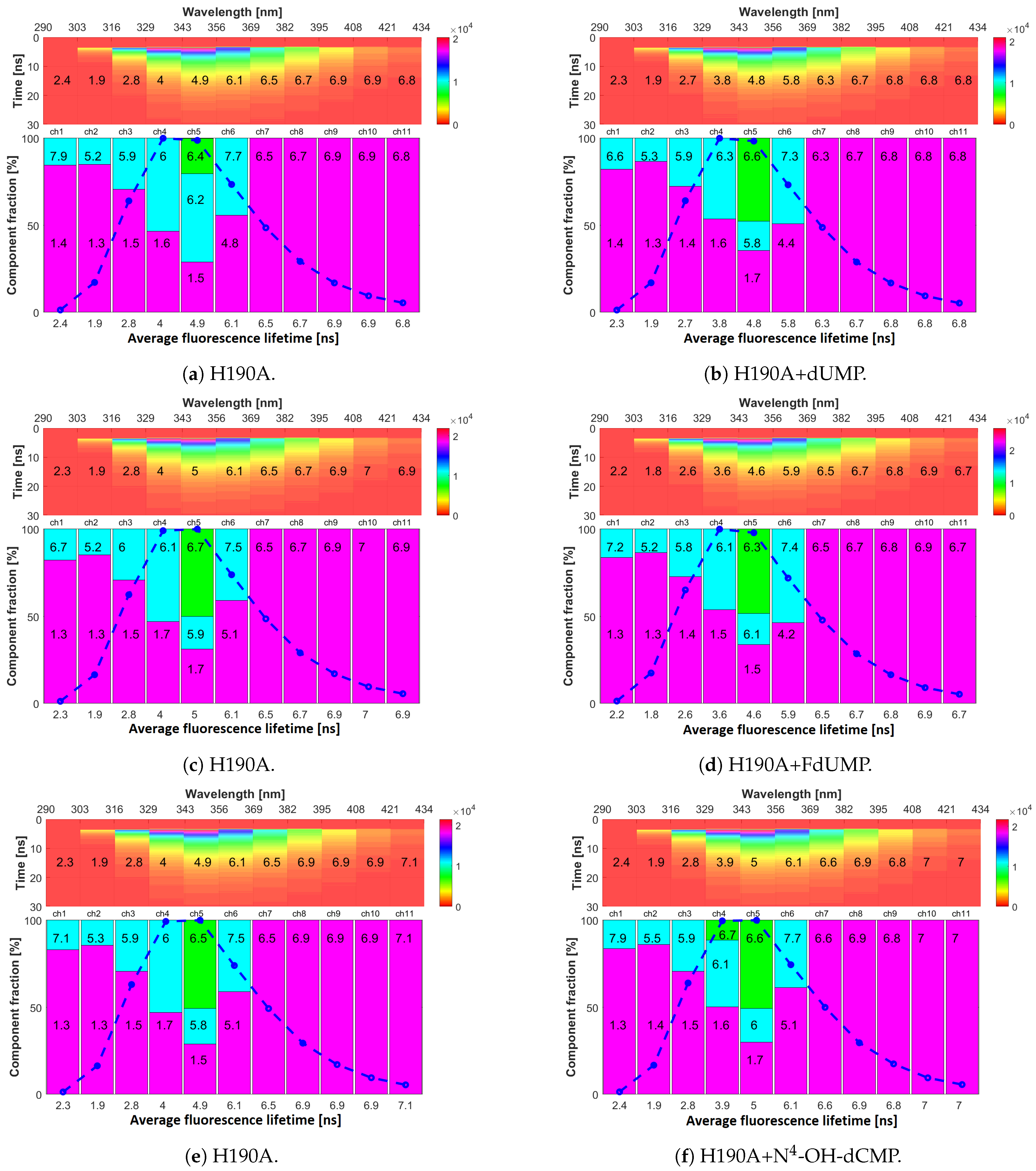
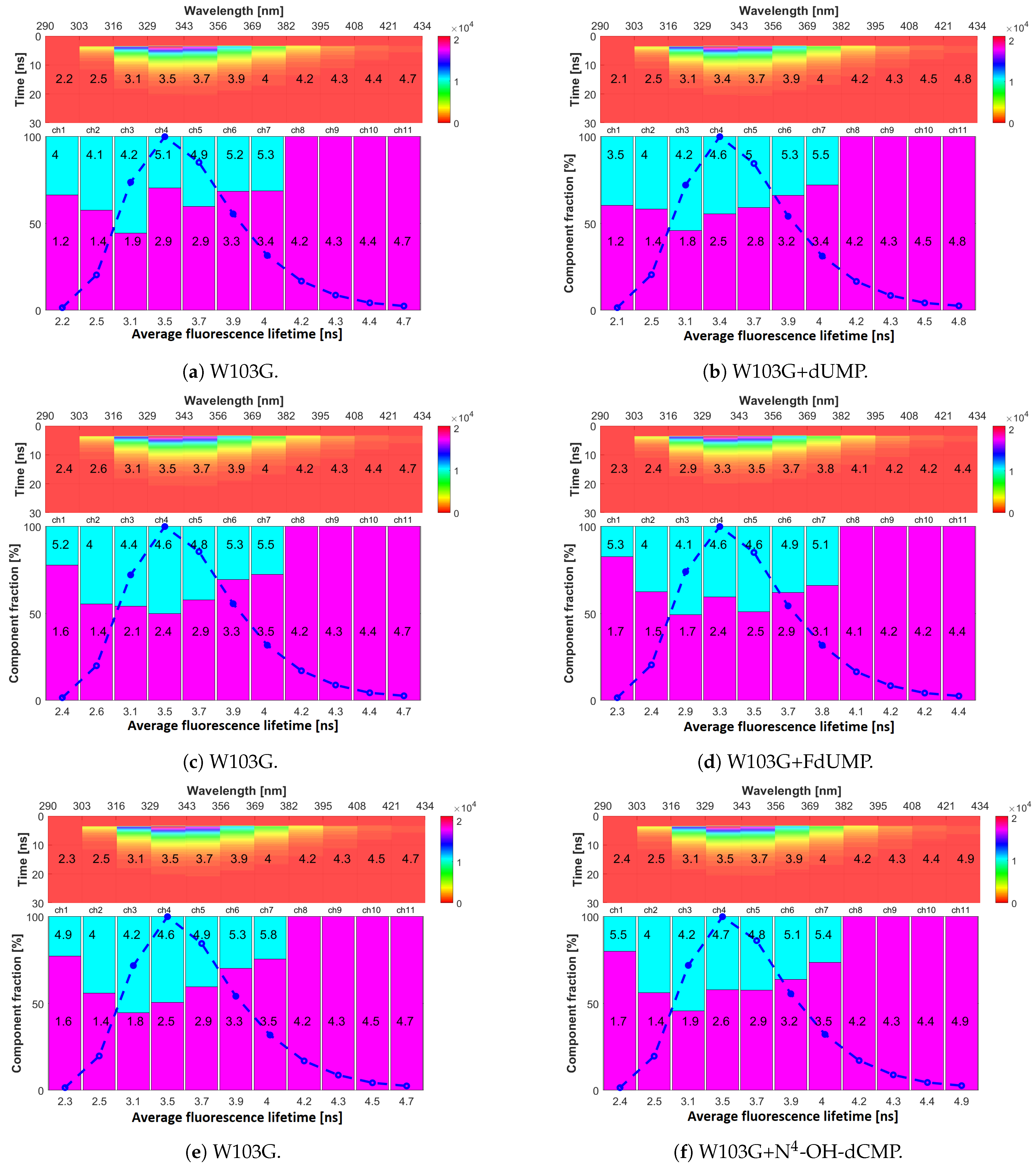
2.4. Fluorescence Lifetimes
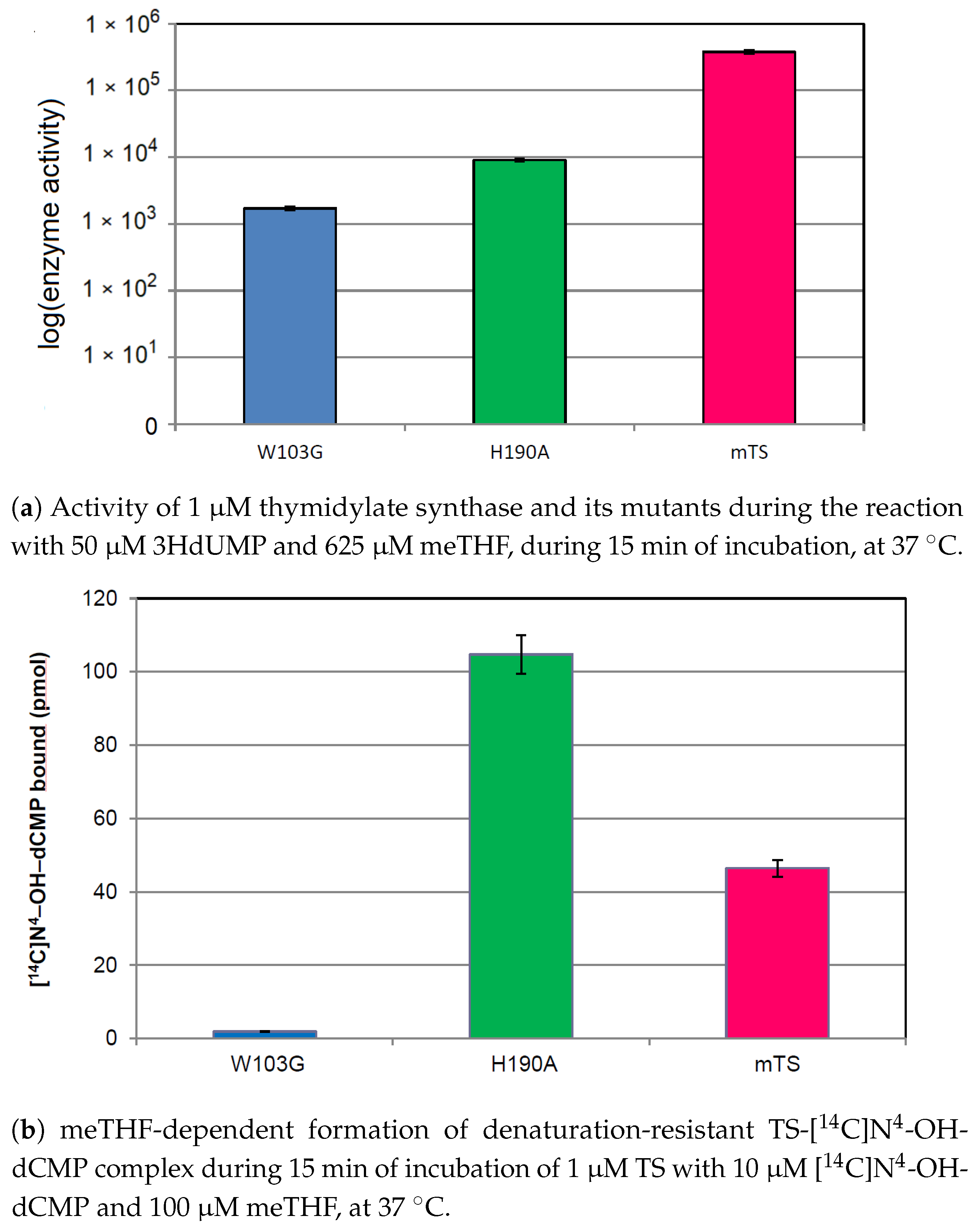
2.5. Interaction of His190 with N-OH-dCMP
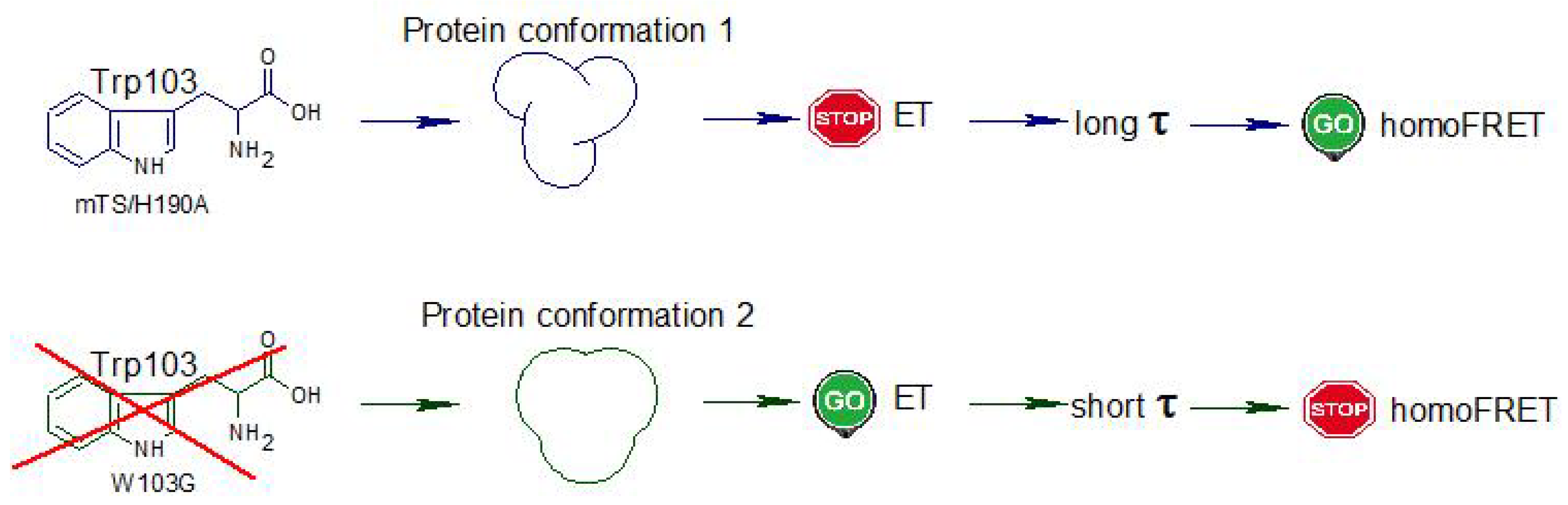
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Expression and Purification of Thymidylate Synthase and Its Mutants
4.3. Buffers and Solutions
4.4. Fluorescence Spectroscopy
4.5. Multi-Dimensional Fluorescence Spectroscopy
4.5.1. Instrumentation
4.5.2. Data Pre-Processing and Analysis
4.6. Fluorescence Lifetime Spectroscopy
4.6.1. Instrumentation
4.6.2. Data Analysis
4.7. Electrostatic Potential Computations
4.8. Tryptophan Accessibility
4.9. mTHF-Dependent Covalent N-OH-dCMP Binding by the Enzyme
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARMES | Anisotropy-Resolved Multi-Dimensional Emission Spectroscopy |
| Asn/N | asparagine |
| C | -cysteine |
| CT | charge transfer |
| DHF | dihydrofolate |
| dTMP | deoxythymidine monophosphate |
| dUMP | deoxyuridine monophospate |
| EcTS | E. coli thymidylate synthase |
| EEM | emission-excitation matrices |
| E.P. | electrostatic potential |
| FdUMP | 5- fluoro deoxyuridine monophospate |
| HH | horizontal–horizontal |
| His/H | histidine |
| hTS | human thymidylate synthase |
| HV | vertical–horizontal |
| KIE | kinetic isotop effect |
| K | Michaelis–Menten constant |
| L | emitting excited state of tryptophan |
| mTHF | R-N methylenetetrahydrofolate |
| mTS | mouse thymidylate synthase |
| N4 | N-OH-dCMP |
| PARAFAC | Parallel Factor Analysis—a multi-way method originating from psychometrics |
| PMT | photomultiplier tube |
| pTSFS | Polarised Total Synchronous Fluorescence Spectra |
| QM | quantum mechanics |
| QY | quantum yield |
| TCSPC | Time-Correlated Single Photon Counting |
| Trp/W | tryptophan |
| TS | thymidylate synthase |
| Tyr | tyrosine |
| VH | vertical–horizontal |
| VV | vertical–vertical |
Appendix A
| PaC1 (nm) | PAC1 Fit Model (%) | PaC2 (nm) | PaC2 Fit Model (%) | Variance Explained (%) | CONCORDIA (%) | Split-Half Analysis |
|---|---|---|---|---|---|---|
| 280/326 | 30.2 | 282/356 | 69.8 | 99.7 | 98.4 | 78.6 |
| PaC1 (nm) | PAC1 Fit Model (%) | PaC2 (nm) | PaC2 Fit Model (%) | PaC3 (nm) | PaC3 Fit Model (%) | Variance Explained (%) | CONCORDIA (%) | Split-Half Analysis |
|---|---|---|---|---|---|---|---|---|
| 284/362 | 68.9 | 278/334 | 25.68 | 294/334 | 5.4 | 99.9 | −702.25 | 11.1 |
References
- Friedkin, M.; De Roberts, W. Conversion of uracil deoxyriboside to thymidine of deoxyribonucleic acid. J. Biol. Chem. 1956, 220, 653–660. [Google Scholar] [CrossRef]
- Blakley, R.L.; McDougall, B.M. The biosynthesis of thymidylic acid. III. Purification of thymidylate synthetase and its spectroscopic assay. J. Biol. Chem. 1962, 237, 812–818. [Google Scholar] [CrossRef]
- Blakley, R.L.; Ramasastri, B.V.; McDougall, B.M. The biosynthesis of thymidylic acid. V. Hydrogen isotope studies with dihydrofolic reductase and thymidylate synthetase. J. Biol. Chem. 1963, 238, 3075–3079. [Google Scholar] [CrossRef]
- Smith-Lomax, M.I.; Greenberg, G.R. An exchange between the hydrogen atom on carbon 5 of deoxyuridylate and water catalyzed by thymidylate synthetase. J. Biol. Chem. 1967, 242, 1302–1306. [Google Scholar] [CrossRef]
- Smith-Lomax, M.I.; Greenberg, G.R. A new assay of thymidylate synthetase activity based on the release of tritium from deoxyuridylate = 53H. J. Biol. Chem. 1967, 242, 109–113. [Google Scholar] [CrossRef]
- Carreras, C.W.; Santi, D.V. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 1995, 64, 721–762. [Google Scholar] [CrossRef]
- Rode, W.; Leś, A. Molecular mechanism of thymidylate synthase-catalyzed reaction and interaction of the enzyme with 2-and/or 4-substitued analogues of dUMP and 5-fluoro-dUMP. Acta Biochim. Pol. 1996, 43, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Whang, Z.; Ferrer, S.; Kohen, A. Qm/MM calculations suggest a novel intermediate following the proton abstraction catalyzed by thymidylate synthase. Biochemistry 2013, 52, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Kholodar, S.A.; Kohen, A. Noncovalent Intermediate of Thymidylate Synthase: Fact or Fiction? J. Am. Chem. Soc. 2016, 138, 8056–8059. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Strutzenberg, T.S.; Gurevic, I.; Kohen, A. Concerted versus stepwise mechanism in thymidylate synthase. J. Am. Chem. Soc. 2014, 136, 9850–9853. [Google Scholar] [CrossRef]
- Islam, Z.; Strutzenberg, T.S.; Ghosh, A.K.; Kohen, A. Activation of Two Sequential H-transfers in the Thymidylate Synthase Catalyzed Reaction. ACS Catal. 2015, 5, 6061–6068. [Google Scholar] [CrossRef]
- Świderek, K.; Arafet, K.; Kohen, A.; Molner, V. Benchmarking Quantum Mechanics/Molecular Mechanics (QM/MM) Methods on the Thymidylate Synthase-Catalyzed Hydride Transfer. J. Chem. Theory Comput. 2017, 13, 1375–1388. [Google Scholar] [CrossRef]
- Kanaan, N.; Martí, S.; Moliner, V.; Kohen, A. A Quantum Mechanics/Molecular Mechanics Study of the Catalytic Mechanism of the Thymidylate Synthase. Biochemistry 2007, 46, 3704–3713. [Google Scholar] [CrossRef] [PubMed]
- Felder, T.; Dunlap, R.B.; Dix, D.; Spencer, T. Differences in natural ligand and f luoropyrimidine binding to human thymidylate synthase identified by transient-state spectroscopic and continuous variation methods. Biochim. Biophys. Acta 2002, 1597, 149–156. [Google Scholar] [CrossRef]
- Sobich, J.; Prokopowicz, M.; Maj, P.; Wilk, P.; Zieliński, Z.; Frączyk, T.; Rode, W. Thymidylate synthase-catalyzed, tetrahydrofolate-dependent selfinactivation by 5-FdUMP. Arch. Biochem. Biophys. 2019, 15, 108106. [Google Scholar] [CrossRef] [PubMed]
- Dowierciał, A.; Jarmuła, A.; Wilk, P.; Rypniewski, W.; Kierdaszuk, B.; Rode, W. Crystal structures of complexes of mouse thymidylate synthase crystallized with N4-OH-dCMP alone or in the presence of N5,10-methylenetetrahydrofolate. Pteridines 2013, 24, 93–98. [Google Scholar] [CrossRef]
- Groza, R.C.; Calvet, A.; Ryder, A.G. A fluorescence anisotropy method for measuring protein concentration in complex cell culture media. Anal. Chim. Acta 2014, 821, 54–61. [Google Scholar] [CrossRef]
- Groza, R.C.; Li, B.; Ryder, A.G. Anisotropy resolved multidimensional emission spectroscopy(ARMES): A new tool for protein analysis. Anal. Chim. Acta 2015, 886, 133–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Casamayou-Boucau, Y.; Ryder, A.G. Accurate anisotropy recovery from fluorophore mixtures using multivariate curve resolution (MCR). Anal. Chim. Acta 2018, 1000, 132–143. [Google Scholar] [CrossRef]
- Melnikau, D.; Elcoroaristizabal, S.; Ryder, A.G. An excitation emission fluorescence lifetime spectrometer using a frequency doubled supercontinuum laser source. Methods Appl. Fluoresc. 2018, 6, 045007. [Google Scholar] [CrossRef]
- Alcala, J.R.; Gratton, E.; Prendergast, F.G. Fluorescence lifetime distributions in proteins. Biophys. J. 1987, 51, 597–604. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Energy transfer. In Principles of Fluorescence Spectroscopy; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 368–394. [Google Scholar]
- Chen, Y.; Barkley, M.D. Toward Understanding Tryptophan Fluorescence in Proteins. Biochemistry 1998, 37, 9976–9982. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.V.; McHenry, C.S. 5-Fluoro-2′-Deoxyuridylate: Covalent Complex with Thymidylate Synthetase. Proc. Natl. Acad. Sci. USA 1972, 69, 1855–1857. [Google Scholar] [CrossRef] [PubMed]
- Bunz, F. Thymidylate synthase and 5-fluorouracil. Cancer Biol. Ther. 2008, 7, 995–996. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rode, W.; Jarmuła, A. Mechanizm reakcji katalizowanej przez syntazę tymidylanową. Post. Bioch. 2015, 61, 274–283. [Google Scholar]
- Maj, P.; Jarmuła, A.; Wilk, P.; Prokopowicz, M.; Rypniewski, W.; Zieliński, Z.; Dowierciał, A.; Bzowska, M.; Rode, W. Molecular mechanism of thymidylate synthase inhibition by N4-hydroxy-2′-deoxycytidine 5′-monophosphate in view of spectrophotometric and crystallographic studies. in preparation.
- Bro, R.; Kiers, H.A.L. A new efficient method for determining the number of components in PARAFAC models. J. Chemom. 2003, 17, 274–286. [Google Scholar] [CrossRef]
- Gryczynski, I.; Wiczk, W.; Johnson, M.L.; Lakowicz, J.R. Lifetime distributions and anisotropy decays of indole fluorescence in cyclohexane/ethanol mixtures by frequency-domain fluorometry. Biophys. Chem. 1988, 32, 173–185. [Google Scholar] [CrossRef]
- Fraczkiewicz, R.; Braun, W. Exact and Efficient Analytical Calculation of the Accessible Surface Areas and Their Gradients for Macromolecules. J. Comp. Chem. 1998, 19, 319–333. [Google Scholar] [CrossRef]
- Fuentes, L.; Oyola, J.; Fernández, M.; Quiñones, E. Conformational Changes in Azurin from Pseudomona aeruginosa Induced through Chemical and Physical Protocols. Biophys J. 2004, 87, 1873–1880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fritz, T.A.; Liu, L.; Finer-Moore, J.S.; Stroud, R.M. Tryptophan 80 and Leucine 143 Are Critical for the Hydride Transfer Step of Thymidylate Synthase by Controlling Active Site Access. Biochemistry 2002, 41, 7021–7029. [Google Scholar] [CrossRef]
- Burstein, E.A.; Vedenkina, N.S.; Ivkova, M.N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem. Photobiol. 1973, 18, 263–279. [Google Scholar] [CrossRef]
- Burstein, E.A.; Abornev, S.M.; Reshetnyak, Y.K. Decomposition of Protein Tryptophan Fluorescence Spectra into Log-Normal Components. I. Decomposition Algorithms. Biophys. J. 2001, 81, 1699–1709. [Google Scholar] [CrossRef]
- Reshetnyak, Y.K.; Burstein, E.A. Decomposition of Protein Tryptophan Fluorescence Spectra into Log-Normal Components. II. The Statistical Proof of Discreteness of Tryptophan Classes in Proteins. Biophys. J. 2001, 81, 1710–1734. [Google Scholar] [CrossRef]
- Reshetnyak, Y.K.; Koshevnik, Y.; Burstein, E.A. Decomposition of Protein Tryptophan Fluorescence Spectra into Log-Normal Components. III. Correlation between Fluorescence and Microenvironment Parameters of Individual Tryptophan Residues. Biophys. J. 2001, 81, 1735–1758. [Google Scholar] [CrossRef]
- Garg, D.; Skouloubris, S.; Briffotaux, J.; Myllykallio, H.; Wade, R.C. Conservation and Role of Electrostatics in Thymidylate Synthase. Sci. Rep. 2015, 5, 17356. [Google Scholar] [CrossRef] [PubMed]
- Maley, F.; Pederen-Lane, J.; Changchien, L. Complete restoration of activity to inactive mutants of Escherichia coli thymidylate synthase: Evidence that E. coli thymidylate synthase is a half-the-sites activity enzyme. Biochemistry 1995, 34, 1469–1474. [Google Scholar] [CrossRef]
- Saxl, R.L.; Changchien, L.; Hardy, L.W.; Maley, F. Parameters affecting the restoration of tactivity to inactive mutanst of thymidylate syntase via subunit exchange: Further evidence that thymidylate synthase is a half-the-sites activity enzyme. Biochemistry 2001, 40, 5275–5282. [Google Scholar] [CrossRef] [PubMed]
- Świniarska, M.; Leś, A.; Rode, W.; Cieśla, J.; Millán-Pacheco, C.; Blake, I.O.; Pastor, N. Segmental motions of rat thymidylate synthase leading to half-the-sites behavior. Biopolymers 2010, 93, 549–559. [Google Scholar]
- Sillen, A.; Díaz, J.F.; Engelborghs, Y. A step toward the prediction of the fluorescence lifetimes of tryptophan residues in proteins based on structural and spectral data. Prot. Sci. 2000, 9, 158–169. [Google Scholar] [CrossRef]
- Włodarczyk, K.; Kierdaszuk, B. A new approach to interpretation of heterogeneity of fluorescence decay: Effect of induced tautomeric shift and enzyme → ligand fluorescence resonance energy transfer. Biophys. Chem. 2006, 123, 146–153. [Google Scholar] [CrossRef]
- Rode, W.; Zieliński, Z.; Dzik, J.M.; Kulikowski, T.; Bretner, M.; Kierdaszuk, B.; Cieśla, J.; Shugar, D. Mechanism of Inhibition of Mammalian Tumor and Other Thymidylate Synthases by N4-Hydroxy-dCMP, N4-Hydroxy-5-fluoro-dCMP, and Related Analogues. Biochemistry 1990, 29, 10835–10842. [Google Scholar] [CrossRef] [PubMed]
- Dowierciał, A.; Jarmuła, A.; Wilk, P.; Rypniewski, W.; Kowalska, M.; Frączyk, T.; Cieśla, J.; Rode, W. Mouse thymidylate synhtase does not show the inactive conformation, observed for the human enzyme. Struct. Chem. 2017, 28, 667–674. [Google Scholar] [CrossRef]
- Cieśla, J.; Mitkowski, P.; Gójdź, A.; Jarmuła, A.; Rode, W. Overproduction in bacteria of mouse thymidylate synthase forms with point mutations in N4-hydroxy-dCMP binding site. New Biotechnol. 2016, 33, S62–S63. [Google Scholar] [CrossRef]
- Pierce, A.C.; Sandretto, K.L.; Bemis, G.W. Kinase inhibitors and the case for CH…O hydrogen bonds in protein-ligand binding. Proteins 2002, 49, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Chen, Y.; Ma, K.; Zagorski, M.G.; Sönnichsen, F.D.; McLaughlin, M.L.; Brakley, M.D. Intramolecular Quenching of Tryptophan Fluorescence by the Peptide Bond in Cyclic Hexapeptides. J. Am. Chem. Soc. 2002, 5, 17356. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.P.; Barkley, M.D. Conformational Effects on Tryptophan Fluorescence in Cyclic Hexapeptides. Biophys. J. 2004, 86, 3828–3835. [Google Scholar] [CrossRef]
- Hellings, M.; De Maeyer, M.; Verheyden, S.; Hao, Q.; Van Damme, E.J.M.; Pemans, W.J.; Engelborghs, Y. The Dead-End Elimination Method, Tryptophan Rotamers, and Fluorescence Lifetimes. Biophys. J. 2003, 85, 1894–1902. [Google Scholar] [CrossRef]
- Moors, S.L.C.; Hellings, M.; De Maeyer, M.; Engelborghs, Y.; Ceulemans, A. Tryptophan Rotamers as Evidenced by X-Ray, Fluorescence Lifetimes, and Molecular Dynamics Modeling. Biophys. J. 2006, 91, 816–823. [Google Scholar] [CrossRef]
- Callis, P.R.; Liu, T. Quantitative Prediction of Fluorescence Quantum Yields for Tryptophan in Proteins. J. Phys. Chem. B 2004, 108, 4248–4259. [Google Scholar] [CrossRef]
- Vivian, J.T.; Callis, P.R. Mechanisms of Tryptophan Fluorescence Shifts in Proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef]
- Lakowicz, J.R. On Spectral Relaxation in Proteins. Photochem. Photobiol. 2000, 72, 421–437. [Google Scholar] [CrossRef]
- Hudson, B.S. An Ionization/Recombination Mechanism for Complexity of the Fluorescence of Tryptophan in Proteins. Acc. Chem. Res. 1999, 32, 297–300. [Google Scholar] [CrossRef]
- Callis, P.R. 1La and 1Lb Transitions of Tryptophan: Applications of Theory and Experimental Observations to Fluorescence of Proteins. Methods Enzymol. 1997, 278, 113–150. [Google Scholar]
- Dev, I.K.; Dallas, W.S.; Ferone, R.; Hanlon, M.; McKnee, D.D.; Yates, B.B. Mode of Binding of Folate Analogs to Thymidylate Synthase. Evidence For Two Asymmetric However, Interactive Substrate Binding Sites. J. Biol. Chem. 1994, 269, 1873–1882. [Google Scholar] [CrossRef]
- Gibson, L.M.; Celeste, L.R.; Lovelace, L.L.; Lebiona, L. Structures of human thymidylate synthase R163K with dUMP, FdUMP and glutathione show asymmetric ligand binding. Acta Cryst. 2011, 67, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.T.; Barbour, K.W.; Dunlap, R.B.; Berger, F.G. Biphasic binding of 5-fluoro-2′-deoxyuridylate to human thymidylate synthase. Mol. Pharmacol. 1995, 48, 72–79. [Google Scholar]
- Goldstein, S.; Pogolotti, A.L., Jr.; Garvey, E.P.; Santi, D.V. Interaction of N4-hydroxy-2′-deoxycytidilic acid with thymidylate synthetase. Med. Chem. 1984, 27, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Rode, W.; Cieśla, J.; Zieliński, Z.; Kędzierska, B. Purification and properties of mouse thymus thymidylate synthase. Comparison of the enzyme from mammalian normal and tumour tissues. Int. J. Biochem. 1986, 18, 361–368. [Google Scholar] [CrossRef]
- Rode, W.; Kulikowski, T.; Kędzierska, B.; Shugar, D. Studies on the interaction with thymidylate synthase of analogues of 20-deoxyuridine-50-phosphate and 5-fluoro-20-deoxyuridine-50-phosphate with modified phosphate groups. Biochem. Pharmacol. 1987, 36, 203–210. [Google Scholar] [CrossRef]
- Dzik, J.M.; Kulikowski, T.; Zieliński, Z.; Cieśla, J.; Rode, W.; Shugar, D. Interaction of 5-fluoro-4-thio-2′-deoxyuridine 5′-phosphate with mammalian tumour thymidylate synthase: Role of the pyrimidine N(3)-H dissociation. Biochem. Biophys. Res. Commun 1987, 149, 1200–1207. [Google Scholar] [CrossRef]
- Ludwiczak, J.; Maj, P.; Wilk, P.; Frączyk, T.; Ruman, T.; Kierdaszuk, B.; Jarmuła, A.; Rode, W. Phosphorylation of thymidylate synthase affects slow-binding inhibition by 5-fluoro-dUMP and N(4)-hydroxy-dCMP. Mol. Biosyst. 2016, 12, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Galivan, J.H.; Maley, F.; Baugh, C.M. Demonstration of separate binding sites for the folate coenzymes and deoxynucleotides with inactivated Lactobacillus casei thymidylate synthetase. Biochem. Biophys. Res. Commun. 1976, 71, 527–534. [Google Scholar] [CrossRef]
- Spencer, H.T.; Villafrance, J.E.; Appleman, J.R. Kinetic Scheme for Thymidylate Synthase from Escherichia coli: Determination from Measurements of Ligand Binding, Primary and Secondary Isotope Effects, and Pre-Steady-State Catalysis. Biochemistry 1997, 36, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, E.; Spencer, H.T.; Dunlap, R.B. Kinetic Studies on Drug-Resistant Variants of Escherichia coli Thymidylate Synthase: Functional Effects of Amino Acid Substitutions at Residue 4. Arch. Biochem. Biophys. 1999, 369, 257–264. [Google Scholar] [CrossRef]
- Fantz, C.; Shaw, D.; Jennings, W.; Forsthoefel, A.; Kitchens, M.; Phan, J.; Minor, W.; Lebioda, L.; Berger, F.G.; Spencer, H.T. Drug-resistant variants of Escherichia coli thymidylate synthase: Effects of substitutions at Pro-254. Mol. Pharmacol. 2000, 57, 359–366. [Google Scholar]
- Liu, T.; Callis, P.R.; Hesp, B.H.; de Groot, M.; Buma, W.J.; Broos, J. Ionization Potentials of Fluoroindoles and the Origin of Nonexponential Tryptophan Fluorescence Decay in Proteins. J. Am. Chem. Soc. 2005, 127, 4104–4113. [Google Scholar] [CrossRef] [PubMed]
- Dev, I.K.; Yates, B.B.; Atashi, J.; Dallas, W.S. Catalytic Role of Histidine 147 in Escherichia coli Thymidylate Synthase. J. Biol. Chem. 1989, 264, 19132–19137. [Google Scholar] [CrossRef]
- Abeysinghe, T.; Hong, B.; Wang, Z.; Kohen, A. Preserved hydride transfer mechanism in evolutionarily divergent thymidylate synthases. Curr. Top. Biochem. Res. 2016, 17, 19–30. [Google Scholar]
- Kealey, J.T.; Eckstein, J.; Santi, D.V. Role of the conserved tryptophan 82 of Lactobacillus casei thymidylate synthase. Chem. Biol. 1995, 2, 609–614. [Google Scholar] [CrossRef]
- Cieśla, J.; Gołos, B.; Wałajtys-Rode, E.; Jagielska, E.; Płucienniczak, A.; Rode, W. The effect of Arg209 to Lys mutation in mouse thymidylate synthase. Acta Biochim. Pol. 2002, 49, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Frączyk, T.; Ruman, T.; Wilk, P.; Palmowski, P.; Rogowska-Wrzesinska, A.; Cieśla, J.; Zieliński, Z.; Nizioł, J.; Jarmuła, A.; Maj, P.; et al. Properties of phosphorylated thymidylate synthase. Biochim. Biophys. Acta 2015, 1854, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Wilk, P.; Jarmuła, A.; Ruman, T.; Banaszak, K.; Rypniewski, W.; Cieśla, J.; Dowierciał, A.; Rode, W. Crystal structure of phosphoramide-phosphorylated thymidylate synthase reveals pSer127, reflecting probably pHis to pSer phosphotransfer. Bioorg. Chem. 2014, 52, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wolschin, F.; Weckwerth, W. Combining metal oxide affinity chromatography (MOAC) and selective mass spectrometry for robust identification of protein phosphorylation sites. Plant Methods. 2005, 52. [Google Scholar] [CrossRef]
- Wahba, A.J.; Friedkin, M. The enzymatic synthesis of thymidylate. I. Early steps in the purification of thymidylate synthetase of Escherichia coli. J. Biol. Chem. 1962, 237, 3794–3801. [Google Scholar] [CrossRef]
- Wahba, A.J.; Friedkin, M. Direct Spectrophotometric Evidence for the Oxidation of Tetrahydrofolate during the Enzymatic Synthesis of thymidylate. J. Biol. Chem. 1961, 236, PC11–PC12. [Google Scholar] [CrossRef]
- Rode, W.; Kulikowski, T.; Kędzierska, B.; Jastreboff, M.; Shugar, D. Inhibition of mammalian tumour thymidylate synthetase by 5-alkylated 2′-deoxyuridine 5′-phosphates. Biochem. Pharmacol. 1984, 33, 2699–2705. [Google Scholar] [CrossRef]
- Ryder, A.G.; Colin, A.; Stedmon, C.A.; Harrit, N.; Bro, R. Calibration, standardization, and quantitative analysis of multidimensional fluorescence (MDF) measurements on complex mixtures (IUPAC Technical Report). Pure Appl. Chem. 2017, 89, 1849–1870. [Google Scholar] [CrossRef]
- Steiner-Browne, M.; Elcoroaristizabal, S.; Ryder, A.G. Using polarized Total Synchronous Fluorescence Spectroscopy (pTSFS) with PARAFAC analysis for characterizing intrinsic protein emission. Chemom. Intell. Lab. Syst. 2019, 194, 103871. [Google Scholar] [CrossRef]
- Casamayou-Boucau, Y.; Ryder, A.G. Extended wavelength anisotropy resolved multidimensional emission spectroscopy (ARMES) measurements: Better filters, validation standards, and Rayleigh scatter removal methods. Methods Appl. Fluoresc. 2017, 5, 037001. [Google Scholar] [CrossRef]
- Bro, R. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Mortensen, P.P.; Bro, R. Real-time monitoring and chemical profiling of a cultivation process. Chemom. Intell. Lab. Syst. 2006, 84, 106–113. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, A.K. Parallel factor (PARAFAC) analysis on total synchronous fluorescence spectroscopy (TSFS) data sets in excitation-emission matrix fluorescence (EEMF) layout: Certain practical aspects. Chemom. Intell. Lab. Syst. 2015, 147, 121–130. [Google Scholar] [CrossRef]
- Bahram, M.; Bro, R.; Stedmon, C.; Afkham, A. Handling of Rayleigh and Raman scatter for PARAFAC modeling of fluorescence data using interpolation. J. Chemometr. 2006, 20, 99–105. [Google Scholar] [CrossRef]
- Becker, W. SPCImage NG Data Analysis Software. In The bh TCSPC Handbook; Becker and Hickl GmbH: Berlin, Germany, 2019; pp. 779–884. [Google Scholar]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatic calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavaihala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff19SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Jakalian, A.; Bush, B.; Jack, D.; Bayly, C. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: I Method. J. Comp. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II Parametrization and validation. J. Comp. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
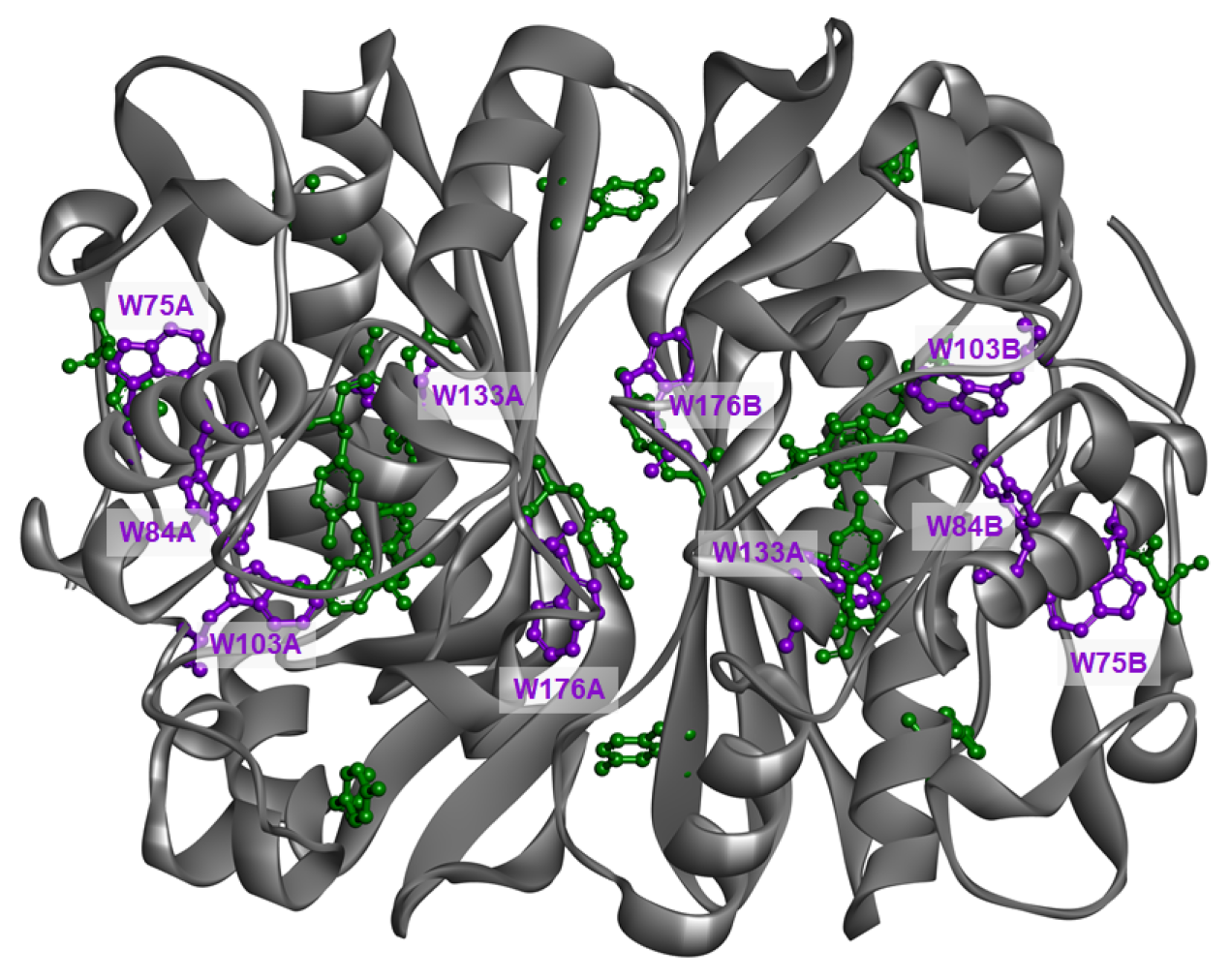
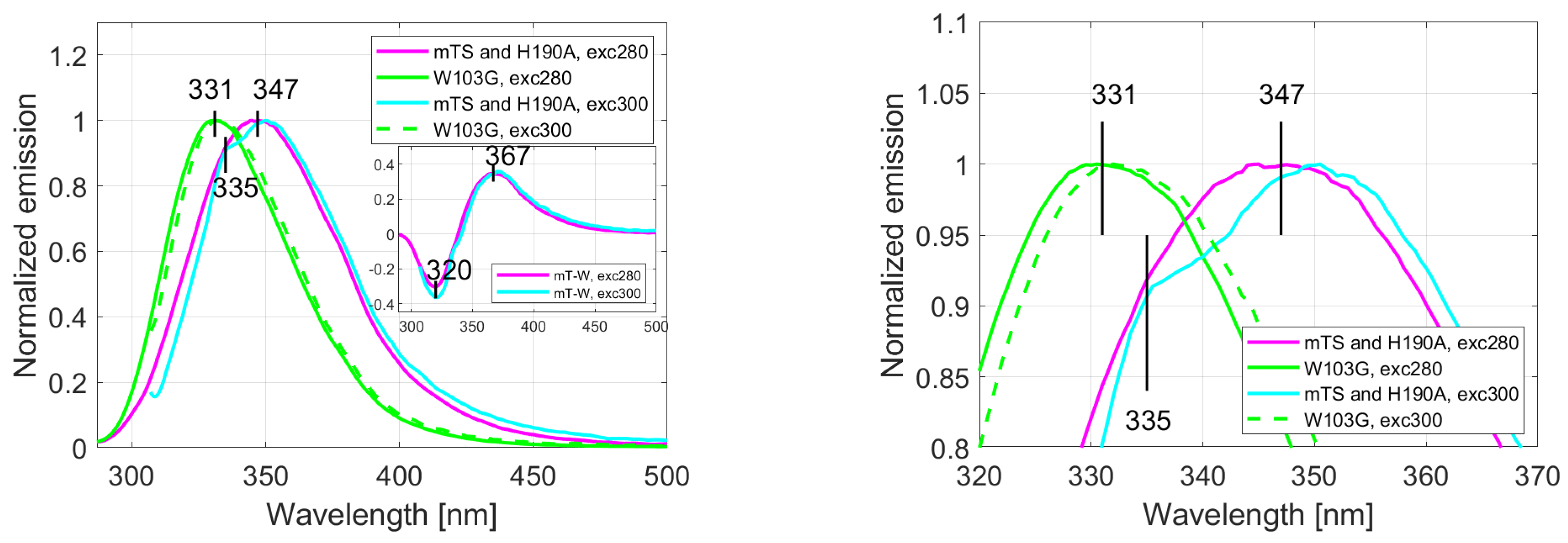
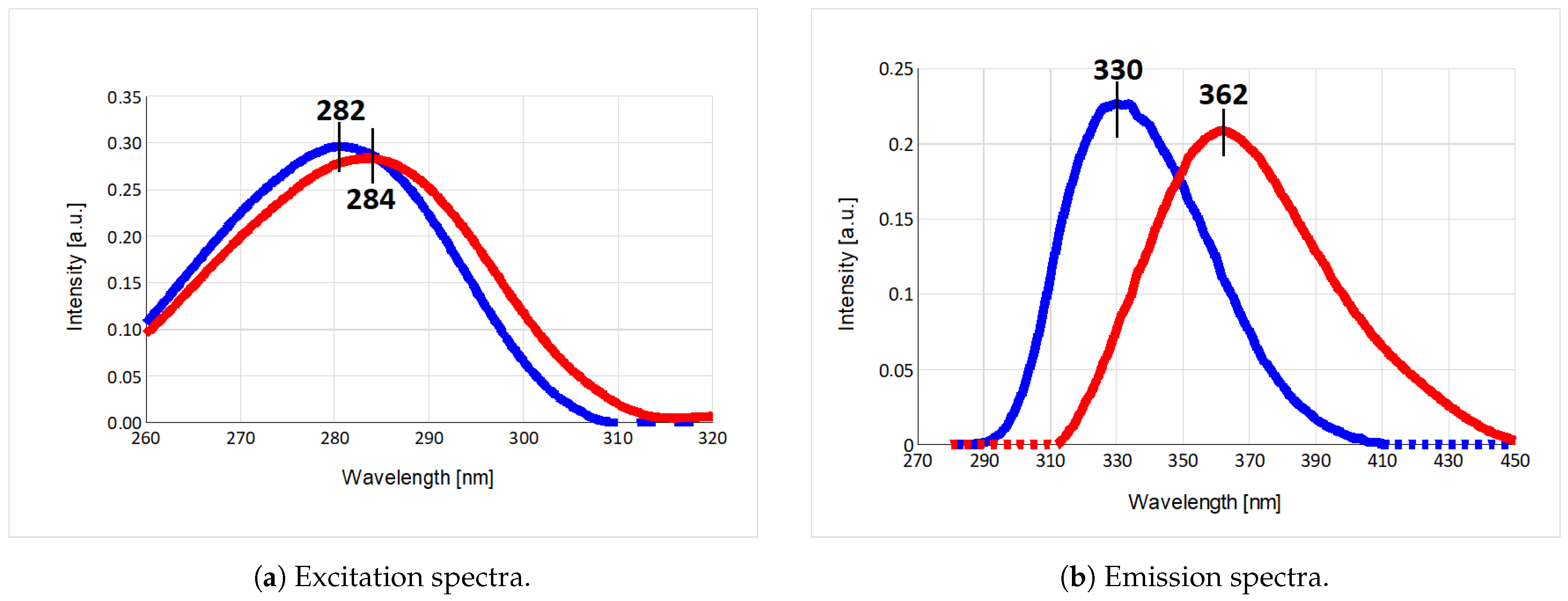
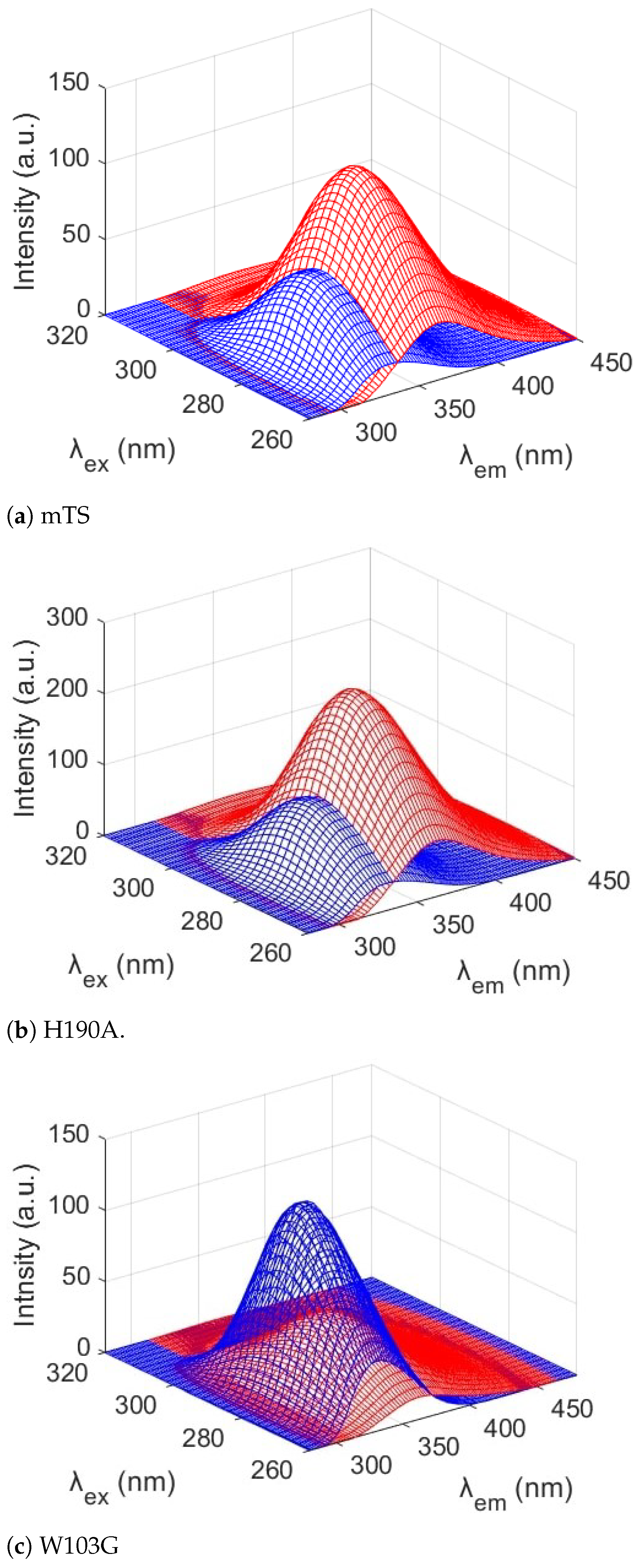

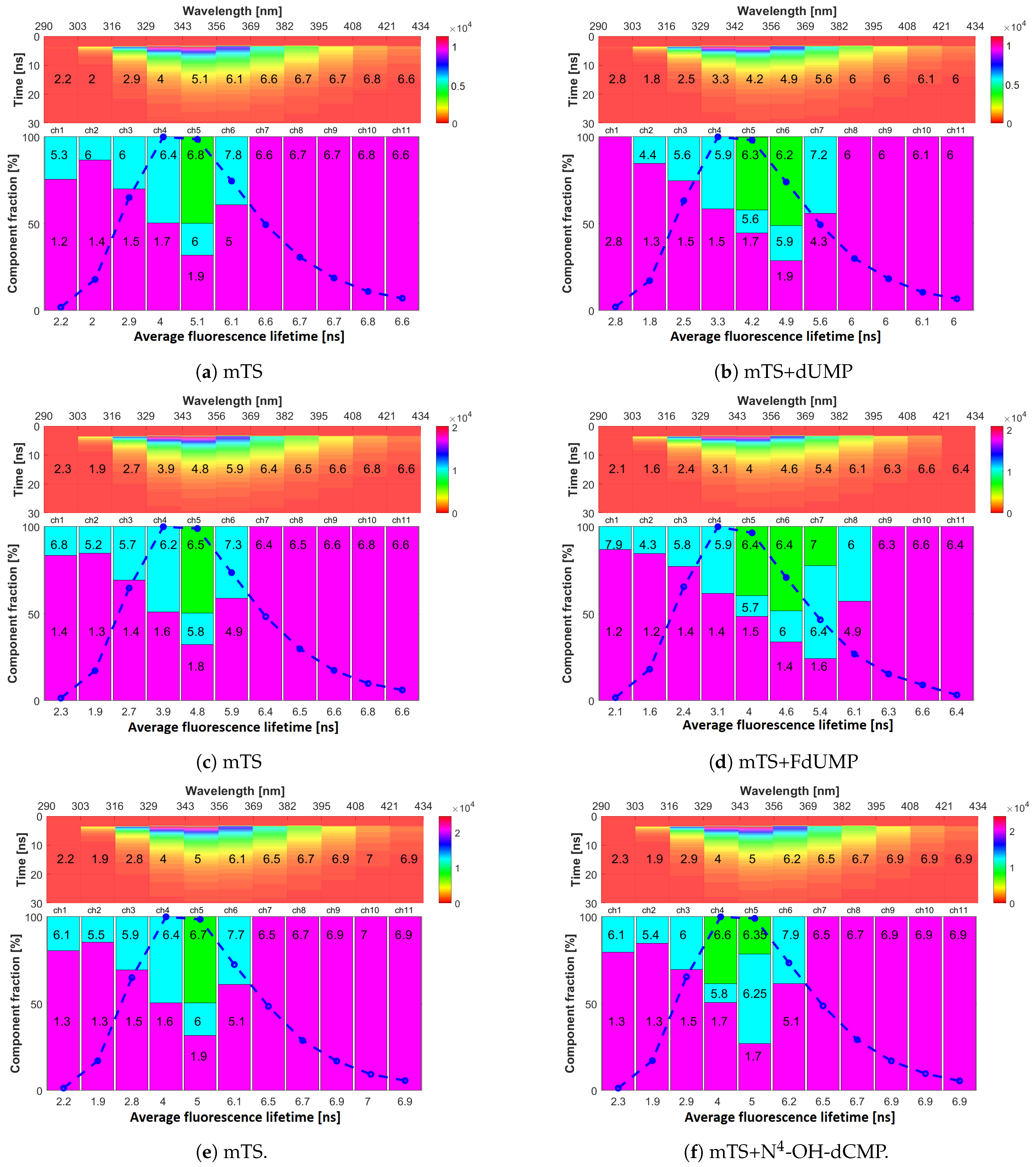
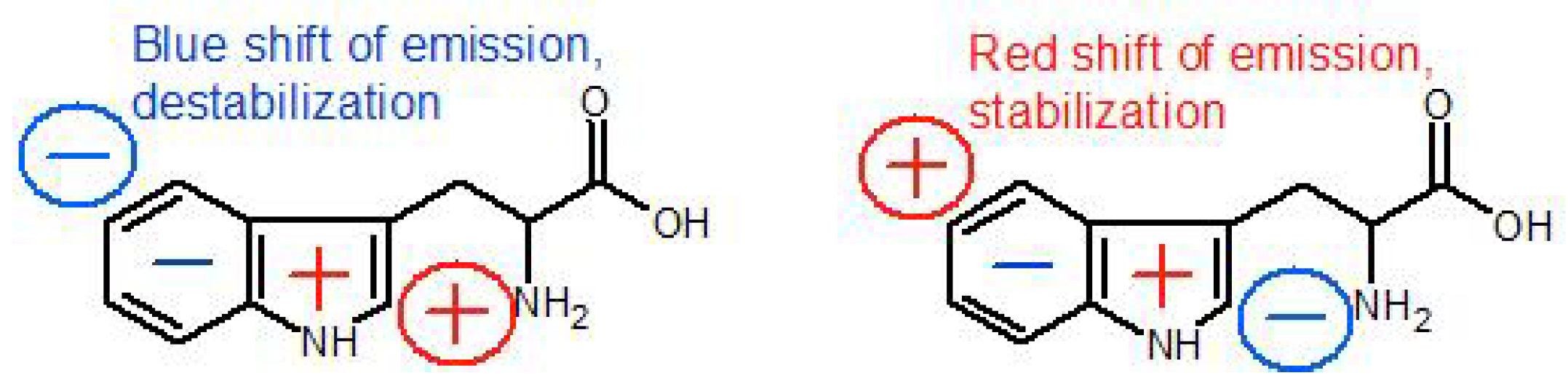
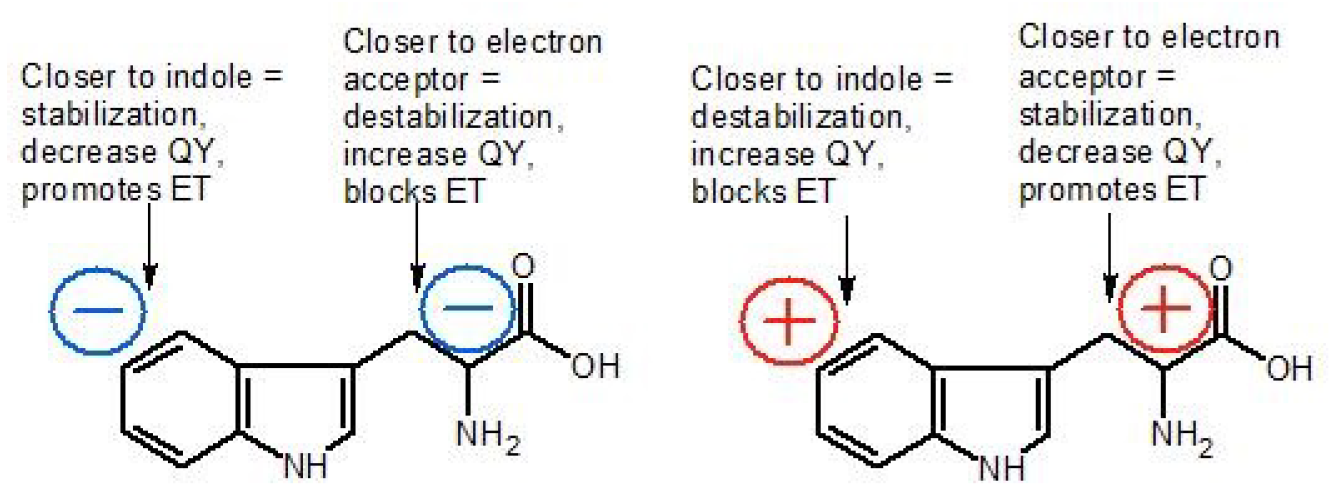
| Parameter | Trp75 | Trp84 | Trp103 | Trp133 | Trp176 |
|---|---|---|---|---|---|
| mTS (3IHI) | |||||
| Acc | 12.1 (II) | 0.9 (S) | 7.1 (I) | 1.14 (S) | 0.8 (S) |
| 12.1 (II) | 0.86 (S) | 5.9 (I) | 1.2 (S) | 0.65 (S) | |
| Den2 | 110 (II) | 133 (I) | 106 (II) | 143 (S) | 126 (I) |
| 111 (II) | 137 (I) | 121 (I/II) | 145 (S) | 125 (I) | |
| E.P. | 2.4 → 7.2 | 2.1 → 1.5 | 10 → 5.3 | 16.85 → 17.65 | 2 → 3 |
| 2.7 → 7.2 | 0.1 → 1 | 9 → 4 | 21 → 22 | 2 → 4.5 | |
| +4.8 | −0.6 | −4.7 | +0.8 | +1 | |
| +4.5 | +0.9 | −5 | +1 | +2.5 | |
| mTS + dUMP (4E5O) | |||||
| Acc | 11.7 (II) | 1.3 (S) | 7.15 (I) | 1.2 (S) | 1.6 (S) |
| 7.9 (I/II) | 0.9 (S) | 7 (I) | 1.3 (S) | 1.1 (S) | |
| Den2 | 119 (II) | 134 (I) | 128 (I) | 142 (S) | 132 (I) |
| 111 (II) | 137 (I) | 127 (I) | 140 (S) | 131 (I) | |
| E.P. | 0.8 → 6.4 | −3.6 → 2 | 3.6 →−1.6 | 6.9 → 7.1 | −0.6→ 1.6 |
| 1.3 → 6.4 | 1.4 → 2.65 | 2.45 →−0.6 | 19.45 → 20 | 0.75 → 1.65 | |
| +5.6 | +5.6 | −5.2 | +0.2 | +2.2 | |
| +5.1 | +1.25 | −3.05 | +0.55 | +0.9 | |
| mTS + N4 (4EIN) | |||||
| Acc | 12.1 (II) | 0.64 (S) | 7.6 (I/II) | 2.8 (I) | 0.7 (S) |
| 12 (II) | 0.86 (S) | 8.7 (I/II) | 2.7 (I) | 0.8 (S) | |
| Den2 | 109 (II) | 138 (I) | 121 (I/II) | 142 (S) | 127 (I) |
| 113 (II) | 134 (I) | 118 (II) | 140 (S) | 126 (I) | |
| E.P. | 1.4 → 5.6 | −1.7 →−0.2 | 2.2 →−3 | 19.5 → 20.3 | 2.4 → 5.2 |
| 1.4 → 5.6 | −0.6 → 0 | 2 →−1.4 | 24.6 → 25.4 | −1.7 → 0.05 | |
| +4.2 | +1.5 | −5.2 | +0.8 | +2.8 | |
| +4.2 | +0.6 | −3.4 | +0.8 | +1.75 | |
| Channel | mTS | H190A | W103G | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ine | a | a | a | ||||||
| no. 1 | 2.23 ± 0.04 | 1.3 ± 0.1 | 80 ± 3 | 2.3 ± 0.1 | 1.33 ± 0.03 | 83 ± 1 | 2.36 ± 0.02 | 1.58 ± 0.02 | 75.6 ± 0.5 |
| 290–303 | 6.2 ± 0.7 | 20 ± 3 | 7.3 ± 0.6 | 17 ± 1 | 5.1 ± 0.2 | 22.4 ± 0.5 | |||
| no. 2 | 1.93 ± 0.04 | 1.32 ± 0.03 | 86 ± 1 | 1.89 ± 0.01 | 1.32 ± 0.01 | 85.2 ± 0.3 | 2.55 ± 0.01 | 1.42 ± 0.03 | 55.7 ± 0.4 |
| 303–316 | 5.5 ± 0.3 | 14.5 ± 0.9 | 5.2 ± 0.1 | 14.8 ± 0.3 | 3.97 ± 0.02 | 44.3 ± 0.4 | |||
| no. 3 | 2.8 ± 0.1 | 1.46 ± 0.04 | 69.5 ± 0.3 | 2.78 ± 0.03 | 1.47 ± 0.03 | 70.7 ± 0.1 | 3.14 ± 0.01 | 2.0 ± 0.2 | 50 ± 7 |
| 316–329 | 5.9 ± 0.1 | 30.5 ± 0.3 | 5.95 ± 0.02 | 29.3 ± 0.1 | 4.3 ± 0.1 | 50 ± 7 | |||
| no. 4 | 4 ± 0.1 | 1.63 ± 0.03 | 50.6 ± 0.3 | 3.7 ± 0.02 | 1.65 ± 0.01 | 46.9 ± 0.2 | 3.50 ± 0.01 | 2.46 ± 0.01 | 50.3 ± 0.5 |
| 329–343 | 6.3 ± 0.1 | 49.4 ± 0.3 | 6.03 ± 0.04 | 53.1 ± 0.2 | 4.56 ± 0.01 | 49.7 ± 0.5 | |||
| no. 5 | 5.0 ± 0.1 | 1.8 ± 0.1 | 31 ± 1 | 4.9 ± 0.1 | 1.6 ± 0.1 | 30 ± 1 | 3.90 ± 0.01 | 2.93 ± 0.01 | 59 ± 2 |
| 343–356 | 6.0 ± 0.2 | 19 ± 1 | 6.0 ± 0.2 | 30 ± 18 | 4.87 ± 0.05 | 41 ± 2 | |||
| 6.6 ± 0.2 | 50 ± 1 | 6.5 ± 0.2 | 40 ± 17 | ||||||
| no. 6 | 6.0 ± 0.2 | 5.0 ± 0.1 | 60 ± 2 | 6.09 ± 0.01 | 5.0 ± 0.2 | 58 ± 2 | 3.90 ± 0.01 | 3.3 ± 0.5 | 70 ± 1 |
| 356–369 | 7.6 ± 0.2 | 40 ± 2 | 7.6 ± 0.1 | 42 ± 2 | 5.26 ± 0.02 | 30 ± 1 | |||
| no. 7 | 6.5 ± 0.1 | - | 100 | 6.53 ± 0.01 | - | 100 | 4.03 ± 0.02 | 3.48 ± 0.01 | 74 ± 2 |
| 369–382 | 5.6 ± 0.2 | 26 ± 2 | |||||||
| no. 8 | 6.7 ± 0.1 | - | 100 | 6.8 ± 0.1 | - | 100 | 4.22 ± 0.02 | - | 100 |
| 382–395 | |||||||||
| no. 9 | 6.7 ± 0.1 | - | 100 | 6.9 ± 0.1 | - | 100 | 4.30 ± 0.02 | - | 100 |
| 395–408 | |||||||||
| no. 10 | 6.9 ± 0.1 | - | 100 | 6.91 ± 0.05 | - | 100 | 4.47 ± 0.03 | - | 100 |
| 408–421 | |||||||||
| no. 11 | 6.8 ± 0.2 | - | 100 | 6.9 ± 0.1 | - | 100 | 4.71 ± 0.02 | - | 100 |
| 421–434 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokopowicz, M.; Jarmuła, A.; Casamayou-Boucau, Y.; Gordon, F.; Ryder, A.; Sobich, J.; Maj, P.; Cieśla, J.; Zieliński, Z.; Fita, P.; et al. Advanced Spectroscopy and APBS Modeling for Determination of the Role of His190 and Trp103 in Mouse Thymidylate Synthase Interaction with Selected dUMP Analogues. Int. J. Mol. Sci. 2021, 22, 2661. https://doi.org/10.3390/ijms22052661
Prokopowicz M, Jarmuła A, Casamayou-Boucau Y, Gordon F, Ryder A, Sobich J, Maj P, Cieśla J, Zieliński Z, Fita P, et al. Advanced Spectroscopy and APBS Modeling for Determination of the Role of His190 and Trp103 in Mouse Thymidylate Synthase Interaction with Selected dUMP Analogues. International Journal of Molecular Sciences. 2021; 22(5):2661. https://doi.org/10.3390/ijms22052661
Chicago/Turabian StyleProkopowicz, Małgorzata, Adam Jarmuła, Yannick Casamayou-Boucau, Fiona Gordon, Alan Ryder, Justyna Sobich, Piotr Maj, Joanna Cieśla, Zbigniew Zieliński, Piotr Fita, and et al. 2021. "Advanced Spectroscopy and APBS Modeling for Determination of the Role of His190 and Trp103 in Mouse Thymidylate Synthase Interaction with Selected dUMP Analogues" International Journal of Molecular Sciences 22, no. 5: 2661. https://doi.org/10.3390/ijms22052661
APA StyleProkopowicz, M., Jarmuła, A., Casamayou-Boucau, Y., Gordon, F., Ryder, A., Sobich, J., Maj, P., Cieśla, J., Zieliński, Z., Fita, P., & Rode, W. (2021). Advanced Spectroscopy and APBS Modeling for Determination of the Role of His190 and Trp103 in Mouse Thymidylate Synthase Interaction with Selected dUMP Analogues. International Journal of Molecular Sciences, 22(5), 2661. https://doi.org/10.3390/ijms22052661









