Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee
Abstract
1. Introduction
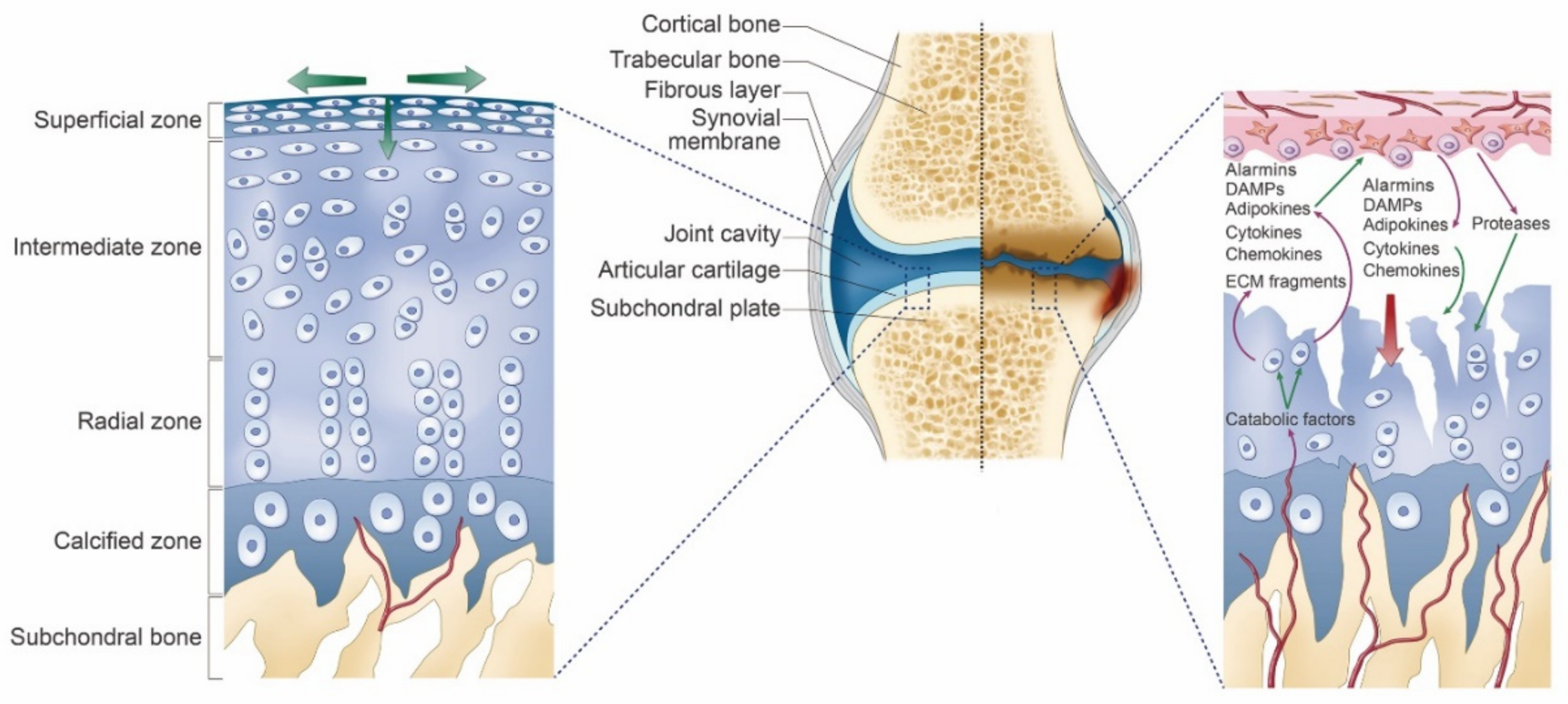
2. Osteoarthritis of the Knee
3. Mechanisms/Pathophysiology
4. Diagnosis
5. Risk Factor
6. Current Point-of-Care Treatment
Conventional Management
7. Interventional Management
7.1. Intra-Articular Corticosteroid Injection
7.2. Intra-Articular Hyaluronic Acid Injection (Viscosupplementation)
7.3. Intra-Articular Platelet-Rich Plasma Injection
8. Surgery
8.1. Total Knee Replacement Surgery (Total Knee Arthroplasty)
8.2. Partial Knee Replacement Surgery (Unicompartmental Knee Arthroplasty)
8.3. Knee Osteotomy (High Tibial Osteotomy or Femoral Osteotomy)
8.4. Knee Arthroscopy
8.5. Knee Cartilage Repair and Cartilage Restoration
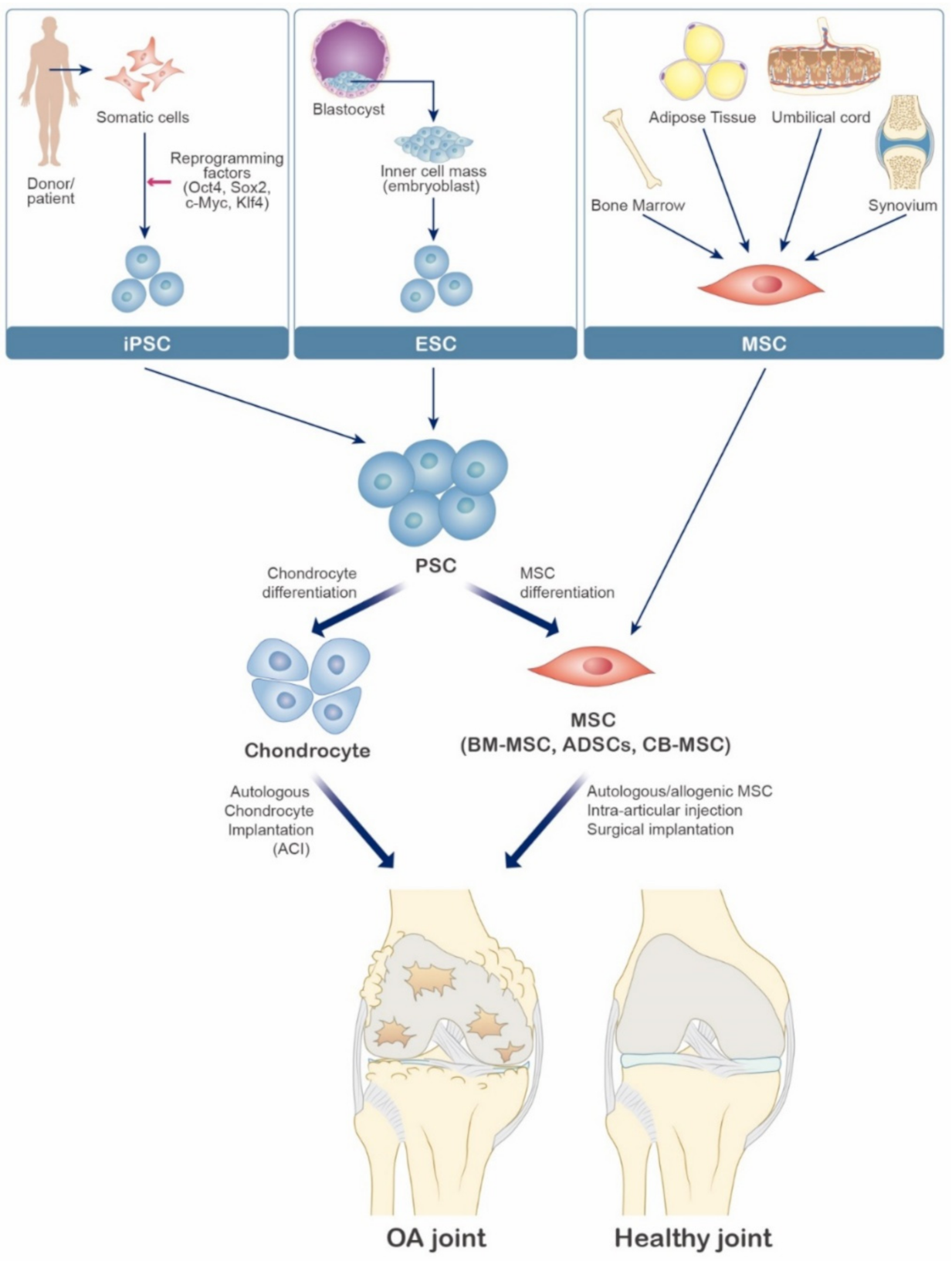
9. Cellular & Experimental Therapy
9.1. Cellular Therapy
9.2. Induced Pluripotent Stem Cells for Osteoarthritis
9.3. Mesenchymal Stem Cells for Osteoarthritis
| Cell Source | No. of Participants | Mean Follow-Up (Months) | Delivery Methods | Clinical Outcome | Reference/NCT |
|---|---|---|---|---|---|
| ESCs | N/A | N/A | N/A | N/A | N/A |
| iPSCs | N/A | N/A | N/A | N/A | N/A |
| BMSCs | 45 | 75 | Two-stage surgical approaches | No risk of serious complications | [91] N/A |
| BMSCs | 4 | 12 | IA injection | Improved pain, walking, and stairs climbing | [92] 00550524 |
| BMSCs | 56 | 24 | IA injection | Better clinical outcomes and MRI in MSCs group | [93] N/A |
| BMSCs | 12 | 12 | IA injection | Improvement of cartilage quality on MRI | [94,95] 03956719 |
| BMSCs | 3 | 60 | IA injection | Better than the baseline level | [96] 00550524 |
| AMSCs (ASF) | 18 | 6 | IA injection | Better clinical outcomes | [97] 01300598 |
| AMSCs (ASF) | 100 | 26 | IA injection | Improved pain VAS scores | [98] N/A |
| AMSCs (GSF) | 40 | 29 | IA injection and surgical implantation | Improved clinical outcomes | [99] N/A |
| AMSCs (ASF) | 18 | 6 | IA injection | Improved clinical outcomes | [100] 01585857 |
| AMSCs (ASF) | 24 | 6 | IA injection | Improved clinical outcomes | [101] 02658344 |
10. Noncellular Therapy
Gene Therapy
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Jeffrey, M.R.; Ball, J. The Epidemiology of Chronic Rheumatism: A Symposium; Blackwell Scientific Publications: Oxford, UK, 1963. [Google Scholar]
- Hannan, M.T.; Felson, D.T.; Pincus, T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J. Rheumatol. 2000, 27, 1513–1517. [Google Scholar]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.R. Management of osteoarthritic knee pain. J. Am. Osteopath. Assoc. 2005, 105, S23–S28. [Google Scholar]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Netter, F.H.; Hansen, J.T. Atlas of Human Anatomy, 3rd ed.; Icon Learning Systems: Teterboro, NJ, USA, 2003. [Google Scholar]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta 2012, 1824, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.; Blanchet, T.; Ma, H.L.; Flannery, C.R.; Peluso, D.; Kanki, K.; Yang, Z.; et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005, 434, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Little, C.B.; Barai, A.; Burkhardt, D.; Smith, S.M.; Fosang, A.J.; Werb, Z.; Shah, M.; Thompson, E.W. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009, 60, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
- Gosset, M.; Berenbaum, F.; Levy, A.; Pigenet, A.; Thirion, S.; Cavadias, S.; Jacques, C. Mechanical stress and prostaglandin E2 synthesis in cartilage. Biorheology 2008, 45, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Gill, S.E.; Massena de Camin, S.; Wilson, D.; McWilliams, D.F.; Walsh, D.A. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis. 2007, 66, 1423–1428. [Google Scholar] [CrossRef]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; Macchi, V.; Fontanella, C.G.; Carniel, E.L.; Olivotto, E.; Filardo, G.; Sarasin, G.; Porzionato, A.; Granzotto, M.; Pozzuoli, A.; et al. Infrapatellar Fat Pad Gene Expression and Protein Production in Patients with and without Osteoarthritis. Int. J. Mol. Sci 2020, 21, 6016. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Cho, M.L.; Choi, H.Y.; Yoon, C.S.; Jhun, J.Y.; Oh, H.J.; Kim, H.Y. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheumatol. 2006, 54, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Morrell, M.R.; Heinze, E.; Concoff, A.L.; Wollaston, S.J.; Arnold, E.L.; Singh, R.; Charles, C.; Skovrun, M.L.; FitzGerald, J.D.; et al. Validation of American College of Rheumatology classification criteria for knee osteoarthritis using arthroscopically defined cartilage damage scores. Semin. Arthritis Rheum. 2005, 35, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Schiphof, D.; van Middelkoop, M.; de Klerk, B.M.; Oei, E.H.; Hofman, A.; Koes, B.W.; Weinans, H.; Bierma-Zeinstra, S.M. Crepitus is a first indication of patellofemoral osteoarthritis (and not of tibiofemoral osteoarthritis). Osteoarthr. Cartil. 2014, 22, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ike, R.; O’Rourke, K.S. Compartment-directed physical examination of the knee can predict articular cartilage abnormalities disclosed by needle arthroscopy. Arthritis Rheumatol. 1995, 38, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Hernandez-Vaquero, D.; Fernandez-Carreira, J.M. Relationship between radiological grading and clinical status in knee osteoarthritis. A multicentric study. BMC Musculoskelet Disord. 2012, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, C.R.; Takahashi, T.; Rogan, M.; Snyckers, C.H.; Vermaak, D.P.; Jones, G.G.; Porteous, A.; Maposa, I.; Pandit, H. The Knee Osteoarthritis Grading System for Arthroplasty. J. Arthroplast. 2019, 34, 450–455. [Google Scholar] [CrossRef]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Intern. Med. 2011, 2, 205–212. [Google Scholar]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Jevsevar, D.S.; Brown, G.A.; Jones, D.L.; Matzkin, E.G.; Manner, P.A.; Mooar, P.; Schousboe, J.T.; Stovitz, S.; Sanders, J.O.; Bozic, K.J.; et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: Treatment of osteoarthritis of the knee, 2nd edition. J. Bone Jt. Surg. Am. 2013, 95, 1885–1886. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Schmid, C.H.; Kent, D.M.; Vaysbrot, E.E.; Wong, J.B.; McAlindon, T.E. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: A systematic review and network meta-analysis. Ann. Intern. Med. 2015, 162, 46–54. [Google Scholar] [CrossRef]
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J. Orthop. 2014, 5, 351–361. [Google Scholar] [CrossRef]
- Georgiev, T. Multimodal approach to intraarticular drug delivery in knee osteoarthritis. Rheumatol. Int. 2020, 40, 1763–1769. [Google Scholar] [CrossRef]
- Rozental, T.D.; Sculco, T.P. Intra-articular corticosteroids: An updated overview. Am. J. Orthop. 2000, 29, 18–23. [Google Scholar]
- Osteoarthritis: National Clinical Guideline for Care and Management in Adults, London. 2008. Available online: https://www.ncbi.nlm.nih.gov/books/NBK48984/ (accessed on 3 March 2021).
- McArthur, B.A.; Dy, C.J.; Fabricant, P.D.; Valle, A.G. Long term safety, efficacy, and patient acceptability of hyaluronic acid injection in patients with painful osteoarthritis of the knee. Patient Prefer. Adherence 2012, 6, 905–910. [Google Scholar]
- Strauss, E.J.; Hart, J.A.; Miller, M.D.; Altman, R.D.; Rosen, J.E. Hyaluronic acid viscosupplementation and osteoarthritis: Current uses and future directions. Am. J. Sports Med. 2009, 37, 1636–1644. [Google Scholar] [CrossRef]
- Pourcho, A.M.; Smith, J.; Wisniewski, S.J.; Sellon, J.L. Intraarticular platelet-rich plasma injection in the treatment of knee osteoarthritis: Review and recommendations. Am. J. Phys. Med. Rehabil. 2014, 93, S108–S121. [Google Scholar] [CrossRef] [PubMed]
- Leopold, S.S. Minimally invasive total knee arthroplasty for osteoarthritis. N. Engl. J. Med. 2009, 360, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Skou, S.T.; Roos, E.M.; Laursen, M.B.; Rathleff, M.S.; Arendt-Nielsen, L.; Simonsen, O.; Rasmussen, S. A Randomized, Controlled Trial of Total Knee Replacement. N. Engl. J. Med. 2015, 373, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, X.; Lee, W.G.; Tu, Y.; Li, H.; Cai, X.; Yang, H. Infrapatellar fat pad resection or preservation during total knee arthroplasty: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2020, 15, 297. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C.; Gomez-Cardero, P. Unicompartmental knee arthroplasty: Current indications, technical issues and results. Efort Open Rev. 2018, 3, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Gao, Y.; Zhang, J.; Jin, Z. High Tibial Osteotomy: Review of Techniques and Biomechanics. J. Healthc. Eng. 2019, 2019, 8363128. [Google Scholar] [CrossRef]
- Chua, M.J.; Hart, A.J.; Mittal, R.; Harris, I.A.; Xuan, W.; Naylor, J.M. Early mobilisation after total hip or knee arthroplasty: A multicentre prospective observational study. PLoS ONE 2017, 12, e0179820. [Google Scholar] [CrossRef]
- Richter, D.L.; Schenck, R.C., Jr.; Wascher, D.C.; Treme, G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health 2016, 8, 153–160. [Google Scholar] [CrossRef]
- Nam, Y.; Rim, Y.A.; Lee, J.; Ju, J.H. Current Therapeutic Strategies for Stem Cell-Based Cartilage Regeneration. Stem Cells Int. 2018, 2018, 8490489. [Google Scholar] [CrossRef]
- Nazempour, A.; Van Wie, B.J. Chondrocytes, Mesenchymal Stem Cells, and Their Combination in Articular Cartilage Regenerative Medicine. Ann. Biomed. Eng. 2016, 44, 1325–1354. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef]
- Viste, A.; Piperno, M.; Desmarchelier, R.; Grosclaude, S.; Moyen, B.; Fessy, M.H. Autologous chondrocyte implantation for traumatic full-thickness cartilage defects of the knee in 14 patients: 6-year functional outcomes. Orthop. Traumatol. Surg. Res. 2012, 98, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.M.; Emans, P.J.; Coolsen, M.M.; Voss, L.; Surtel, D.A.; Cremers, A.; van Rhijn, L.W.; Welting, T.J. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Siegel, G.; Schafer, R.; Dazzi, F. The immunosuppressive properties of mesenchymal stem cells. Transplantation 2009, 87, S45–S49. [Google Scholar] [CrossRef] [PubMed]
- Craft, A.M.; Ahmed, N.; Rockel, J.S.; Baht, G.S.; Alman, B.A.; Kandel, R.A.; Grigoriadis, A.E.; Keller, G.M. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development 2013, 140, 2597–2610. [Google Scholar] [CrossRef]
- Lach, M.; Trzeciak, T.; Richter, M.; Pawlicz, J.; Suchorska, W.M. Directed differentiation of induced pluripotent stem cells into chondrogenic lineages for articular cartilage treatment. J. Tissue Eng. 2014, 5, 2041731414552701. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, P.E.; Ransohoff, J.D.; Nahid, A.; Wu, J.C. Immunogenicity of pluripotent stem cells and their derivatives. Circ. Res. 2013, 112, 549–561. [Google Scholar] [CrossRef]
- Kim, S.H.; Ha, C.W.; Park, Y.B.; Nam, E.; Lee, J.E.; Lee, H.J. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: A meta-analysis of randomized controlled trials. Arch. Orthop. Trauma Surg. 2019, 139, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Melo, L.G. Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol 2009, 482, 281–294. [Google Scholar]
- Gruber, H.E.; Deepe, R.; Hoelscher, G.L.; Ingram, J.A.; Norton, H.J.; Scannell, B.; Loeffler, B.J.; Zinchenko, N.; Hanley, E.N.; Tapp, H. Human adipose-derived mesenchymal stem cells: Direction to a phenotype sharing similarities with the disc, gene expression profiling, and coculture with human annulus cells. Tissue Eng. Part. A 2010, 16, 2843–2860. [Google Scholar] [CrossRef] [PubMed]
- Ishige, I.; Nagamura-Inoue, T.; Honda, M.J.; Harnprasopwat, R.; Kido, M.; Sugimoto, M.; Nakauchi, H.; Tojo, A. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton’s jelly explants of human umbilical cord. Int. J. Hematol. 2009, 90, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Shariatzadeh, M.; Song, J.; Wilson, S.L. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019, 378, 399–410. [Google Scholar] [CrossRef]
- Iijima, H.; Isho, T.; Kuroki, H.; Takahashi, M.; Aoyama, T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: A meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen. Med. 2018, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.T.; Feng, Y.; Jia, H.H.; Zhao, M.; Yu, H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: A concise review. World J. Stem Cells 2019, 11, 222–235. [Google Scholar] [CrossRef]
- do Amaral, R.; Almeida, H.V.; Kelly, D.J.; O’Brien, F.J.; Kearney, C.J. Infrapatellar Fat Pad Stem Cells: From Developmental Biology to Cell Therapy. Stem Cells Int. 2017, 2017, 6843727. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Piccione, M.; Belluzzi, E.; Petrelli, L.; Pozzuoli, A.; Ramonda, R.; Rossato, M.; Favero, M.; Ruggieri, P.; et al. Infrapatellar Fat Pad Stem Cells Responsiveness to Microenvironment in Osteoarthritis: From Morphology to Function. Front. Cell Dev. Biol. 2019, 7, 323. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.M. Generation of mesenchymal stem-like cells for producing extracellular vesicles. World J. Stem Cells 2019, 11, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Lye, E.; Suan Yeo, K.; Khia Way Tan, E.; Salto-Tellez, M.; Liu, T.M.; Palanisamy, N.; El Oakley, R.M.; Lee, E.H.; Lim, B.; et al. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells 2007, 25, 425–436. [Google Scholar] [CrossRef]
- Hwang, N.S.; Varghese, S.; Lee, H.J.; Zhang, Z.; Ye, Z.; Bae, J.; Cheng, L.; Elisseeff, J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc. Natl. Acad. Sci. USA 2008, 105, 20641–20646. [Google Scholar] [CrossRef]
- Hwang, N.S.; Varghese, S.; Zhang, Z.; Elisseeff, J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006, 12, 2695–2706. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lee, E.H.; Guo, X.M.; Chan, J.K.; Yeow, C.H.; Choo, A.B.; Cao, T. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials 2010, 31, 6968–6980. [Google Scholar] [CrossRef]
- Jiang, B.; Fu, X.; Yan, L.; Li, S.; Zhao, D.; Wang, X.; Duan, Y.; Yan, Y.; Li, E.; Wu, K.; et al. Transplantation of human ESC-derived mesenchymal stem cell spheroids ameliorates spontaneous osteoarthritis in rhesus macaques. Theranostics 2019, 9, 6587–6600. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.D.; O’Sullivan, M.B.; Alaee, F.; Paglia, D.N.; Yoshida, R.; Guzzo, R.M.; Drissi, H. Regeneration of Articular Cartilage by Human ESC-Derived Mesenchymal Progenitors Treated Sequentially with BMP-2 and Wnt5a. Stem Cells Transl. Med. 2017, 6, 40–50. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef]
- Narsinh, K.H.; Plews, J.; Wu, J.C. Comparison of human induced pluripotent and embryonic stem cells: Fraternal or identical twins? Mol. Ther. 2011, 19, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.H.; Mason, M.J.; Xie, W.; Volinia, S.; Singer, M.; Peterson, C.; Ambartsumyan, G.; Aimiuwu, O.; Richter, L.; Zhang, J.; et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 2009, 5, 111–123. [Google Scholar] [CrossRef]
- Puri, M.C.; Nagy, A. Concise review: Embryonic stem cells versus induced pluripotent stem cells: The game is on. Stem Cells 2012, 30, 10–14. [Google Scholar] [CrossRef]
- Lietman, S.A. Induced pluripotent stem cells in cartilage repair. World J. Orthop. 2016, 7, 149–155. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zeng, W.; Wan, R.; Wang, J.; Zhou, Q.; Qiu, S.; Singh, S.R. Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. Eur. Cell Mater. 2012, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Mahdizadeh, H.; Saric, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B., IV. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef]
- Deng, X.Y.; Wang, H.; Wang, T.; Fang, X.T.; Zou, L.L.; Li, Z.Y.; Liu, C.B. Non-viral methods for generating integration-free, induced pluripotent stem cells. Curr. Stem Cell Res. Ther. 2015, 10, 153–158. [Google Scholar] [CrossRef]
- Stadtfeld, M.; Nagaya, M.; Utikal, J.; Weir, G.; Hochedlinger, K. Induced pluripotent stem cells generated without viral integration. Science 2008, 322, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987, 20, 263–272. [Google Scholar] [CrossRef]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Keating, A. Cell Therapy for Knee Osteoarthritis: Mesenchymal Stromal Cells. Gerontology 2019, 65, 294–298. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, A.; Torok-Storb, B.; Pillai, M.M. Primary marrow-derived stromal cells: Isolation and manipulation. Methods Mol. Biol. 2013, 1035, 75–101. [Google Scholar]
- Koh, Y.G.; Choi, Y.J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef]
- Koh, Y.G.; Jo, S.B.; Kwon, O.R.; Suh, D.S.; Lee, S.W.; Park, S.H.; Choi, Y.J. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 2013, 29, 748–755. [Google Scholar] [CrossRef]
- Cianca, J.C.; Jayaram, P. Musculoskeletal Injuries and Regenerative Medicine in the Elderly Patient. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Zhao, A.G.; Sumer, H. New Approaches to Treat Osteoarthritis with Mesenchymal Stem Cells. Stem Cells Int. 2018, 2018, 5373294. [Google Scholar] [CrossRef]
- Wakitani, S.; Okabe, T.; Horibe, S.; Mitsuoka, T.; Saito, M.; Koyama, T.; Nawata, M.; Tensho, K.; Kato, H.; Uematsu, K.; et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J. Tissue Eng. Regen. Med. 2011, 5, 146–150. [Google Scholar] [CrossRef]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef]
- Wong, K.L.; Lee, K.B.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: A prospective, randomized controlled clinical trial with 2 years follow-up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentis, J.; Sanchez, A.; Garcia-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentis, J.; Sanchez, A.; Garcia-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: Two-year follow-up results. Transplantation 2014, 97, e66–e68. [Google Scholar] [CrossRef]
- Davatchi, F.; Sadeghi Abdollahi, B.; Mohyeddin, M.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 2016, 19, 219–225. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Chang, J.J.; Lee, J.H.; Lee, S.H. Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet Disord. 2013, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, O.R.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am. J. Sports Med. 2015, 43, 2738–2746. [Google Scholar] [CrossRef]
- Pers, Y.M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagh, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340. [Google Scholar] [CrossRef]
- Ni, Z.; Zhou, S.; Li, S.; Kuang, L.; Chen, H.; Luo, X.; Ouyang, J.; He, M.; Du, X.; Chen, L. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noel, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Gene Delivery to Joints by Intra-Articular Injection. Hum. Gene Ther. 2018, 29, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Ha, C.W.; In, Y.; Cho, S.D.; Choi, E.S.; Ha, J.K.; Lee, J.H.; Yoo, J.D.; Bin, S.I.; Choi, C.H.; et al. A Multicenter, Double-Blind, Phase III Clinical Trial to Evaluate the Efficacy and Safety of a Cell and Gene Therapy in Knee Osteoarthritis Patients. Hum. Gene Ther. Clin. Dev. 2018, 29, 48–59. [Google Scholar] [CrossRef]
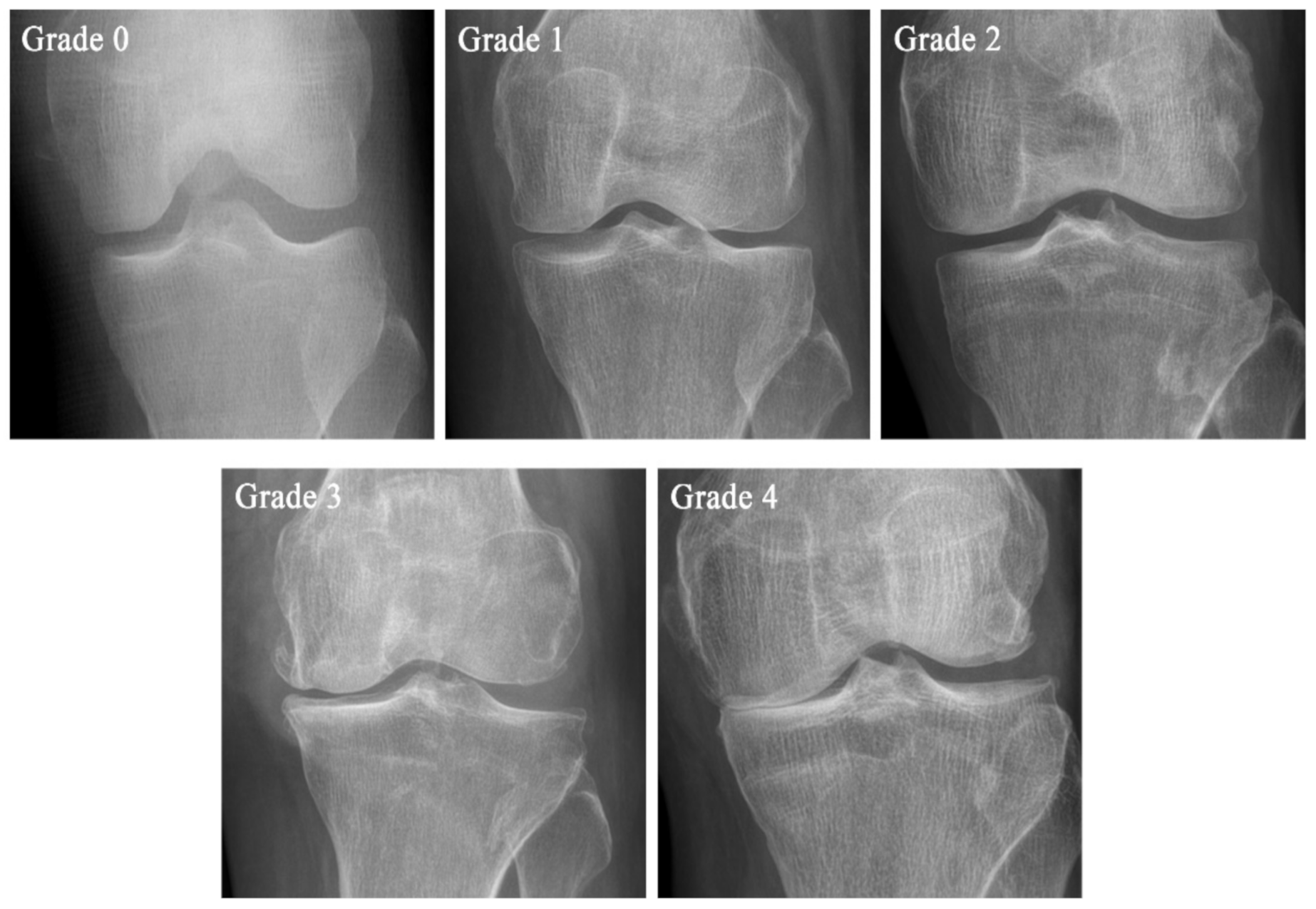
| Grade | KL Scale | Ahlbäck Classification | KOGS |
|---|---|---|---|
| Grade 0 | No pathological features of osteoarthritis (OA) | ||
| Grade 1 | Suspicious narrowing of the joint space and possible osseous lip | Joint space narrowing, with or without subchondral sclerosis. Joint space narrowing is defined by this system as a joint space <3 mm, or less than half of the space in the other compartment, or less than half of the space of the homologous compartment of the other knee | An isolated medial, lateral tibiofemoral, or patella-femoral joint OA with ligament stability and two functionally intact compartments |
| Grade 2 | Clear bone tissue and possible stenosis of the joint space | Obliteration of the joint space | Deteriorating isolated lesion with ligament stability and a correctible coronal subluxation |
| Grade 3 | Moderate multiple bone tissue, clear narrowing of the joint space, slight sclerosis, and possible deformity of the ends of the bones | Bone defect/loss < 5 mm | Includes an isolated medial or lateral tibiofemoral OA and concomitant pathologies such as anterior cruciate ligament deficiency (3A) or grooving of patella-femoral joint or patellectomy (3B) |
| Grade 4 | Large bone tissue, marked narrowing of the joint space, severe sclerosis, and clear deformities of the ends of the bones | Bone defect/loss between 5 mm and 10 mm | Includes cases of bi-compartmental tibiofemoral OA without concomitant ligament instability (4A) and with ligament instability (4B) |
| Grade 5 | Bone defect/loss >10 mm, often with subluxation and arthritis of the other compartment |
| Treatment | OARSI | ACR | AAOS |
|---|---|---|---|
| Exercise (Land-based) | Appropriate | Strong recommendation | Strong recommendation |
| Exercise (Water-based) | Appropriate | Strong recommendation | Strong recommendation |
| Transcutaneous electrical nerve stimulation | Uncertain | Strong recommendation against use | Inconclusive |
| Cane (Walking stick) | Appropriate | Strong recommendation | |
| Weight control | Appropriate | Strong recommendation | Moderate recommendation |
| Chondroitin or Glucosamine | Not appropriate for disease modification, Uncertain (Sx relief) | Strong recommendation against use | Recommendation against use |
| Acetaminophen | Without comorbidities: appropriate | Conditional recommendation | Inconclusive |
| Duloxetine | Appropriate | Conditional recommendation | No recommendation |
| Oral NSAIDs | Without comorbidities: appropriate; With comorbidities: Uncertain | Strong recommendation | Strong recommendation |
| Topical NSAIDs | Appropriate | Conditional recommendation against use | Strong recommendation |
| Opioids | Uncertain | No recommendation | Recommended (only tramadol) |
| Intra-articular corticosteroids | Appropriate | Strong recommendation | Inconclusive |
| Intra-articular viscosupplementation | Uncertain | Conditional recommendation against use | Recommendation against use |
| Advantages | Disadvantages | |
|---|---|---|
| Embryonic stem cells (ESCs) | Unlimited self-renewal | Ethical concerns |
| Unlimited proliferation | Tumorigenic potential | |
| Pluripotent | Difficulty in vitro work | |
| Potentially unlimited supply | Difficulty in controlling differentiation | |
| Induced pluripotent stem cells (iPSCs) | Autologous origin | Security |
| Extensive sources | Tumorigenic potential | |
| Unlimited self-renewal | Inefficiency | |
| Unlimited proliferation | Instability | |
| Pluripotent | Unclear mechanism | |
| No ethical issues | Difficulty in controlling differentiation | |
| Mesenchymal stem cells (MSCs) | High chondrogenic potential | A limited number of cells |
| Good expansion ability | More affected by donor age | |
| Easily accessible, reliable for isolation | Donor site pain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. https://doi.org/10.3390/ijms22052619
Jang S, Lee K, Ju JH. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. International Journal of Molecular Sciences. 2021; 22(5):2619. https://doi.org/10.3390/ijms22052619
Chicago/Turabian StyleJang, Sunhee, Kijun Lee, and Ji Hyeon Ju. 2021. "Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee" International Journal of Molecular Sciences 22, no. 5: 2619. https://doi.org/10.3390/ijms22052619
APA StyleJang, S., Lee, K., & Ju, J. H. (2021). Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. International Journal of Molecular Sciences, 22(5), 2619. https://doi.org/10.3390/ijms22052619






