Genetic Regulation of Tryptase Production and Clinical Impact: Hereditary Alpha Tryptasemia, Mastocytosis and Beyond
Abstract
:1. Mast Cells and Mast Cell Diseases
2. Biological Functions of Human Tryptase
3. Regulation of Tryptase Expression and Secretion
4. Clinical Significance of Elevated Serum Tryptase Levels
5. Tryptase in MC Diseases
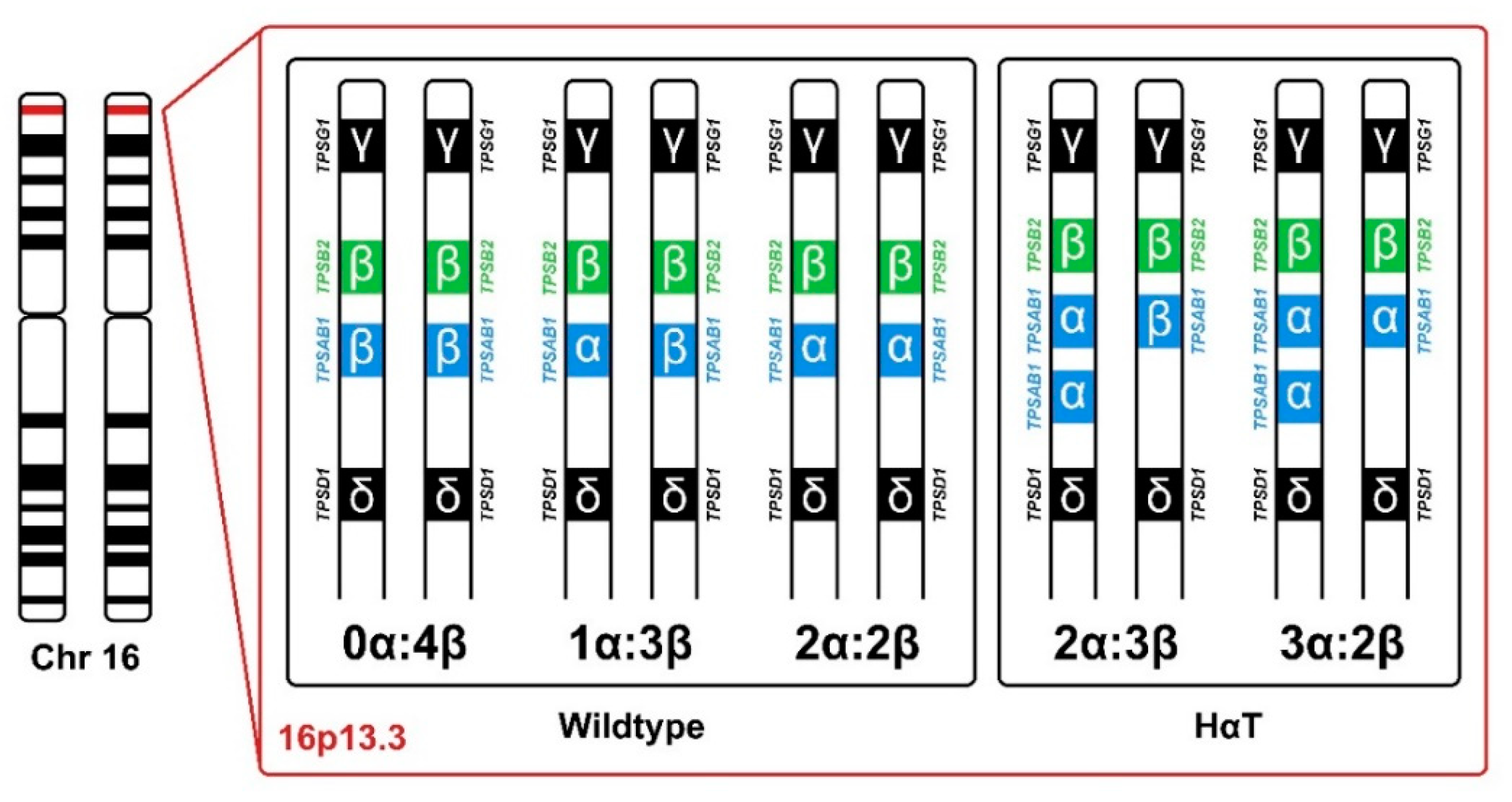
6. Genetic Background of Tryptase
7. Hereditary Alpha Tryptasemia
8. HαT—In Vitro Experiments and Possible Mechanisms of MC (Hyper)Activation
9. HαT in Mast Cell Diseases
10. Therapeutic Approaches in HαT-Mediated (Co-Triggered) Pathologies
11. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehrlich, P. Beiträge zur kenntniss der granulirten bindegewebszellen und der eosinophilen leukocythen. Arch. Anat. Physiol. 1879, 3, 166–169. [Google Scholar]
- Ehrlich, P. Beiträge zur kenntniss der anilinfärbungen und ihrer verwendung in der mikroskopischen technik. Arch. Mikr. Anat. 1877, 13, 263–278. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef]
- Galli, S.J. New concepts about the mast cell. N. Engl. J. Med. 1993, 328, 257–265. [Google Scholar] [CrossRef]
- Kirshenbaum, A.S.; Goff, J.P.; Semere, T.; Foster, B.; Scott, L.M.; Metcalfe, D.D. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13). Blood 1999, 94, 2333–2342. [Google Scholar] [CrossRef]
- Valent, P. The riddle of the mast cell: Kit (CD117)-ligand as the missing link? Immunol. Today 1994, 15, 111–114. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Wershil, B.K. The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am. J. Pathol. 1993, 142, 965–974. [Google Scholar]
- Valent, P.; Spanblochl, E.; Sperr, W.R.; Sillaber, C.; Zsebo, K.M.; Agis, H.; Strobl, H.; Geissler, K.; Bettelheim, P.; Lechner, K. Induction of differentiation of human mast cells from bone marrow and peripheral blood mononuclear cells by recombinant human stem cell factor/kit-ligand in long-term culture. Blood 1992, 80, 2237–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirshenbaum, A.S.; Kessler, S.W.; Goff, J.P.; Metcalfe, D.D. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J. Immunol. 1991, 146, 1410–1415. [Google Scholar]
- Toru, H.; Eguchi, M.; Matsumoto, R.; Yanagida, M.; Yata, J.; Nakahata, T. Interleukin-4 promotes the development of tryptase and chymase double-positive human mast cells accompanied by cell maturation. Blood 1998, 91, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, M.; Fukamachi, H.; Takei, M.; Hagiwara, T.; Uzumaki, H.; Tokiwa, T.; Saito, H.; Iikura, Y.; Nakahata, T. Interferon-gamma promotes the survival and Fc epsilon RI-mediated histamine release in cultured human mast cells. Immunology 1996, 89, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, M.; Fukamachi, H.; Ohgami, K.; Kuwaki, T.; Ishii, H.; Uzumaki, H.; Amano, K.; Tokiwa, T.; Mitsui, H.; Saito, H.; et al. Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood 1995, 86, 3705–3714. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 163–172. [Google Scholar] [CrossRef]
- Galli, S.J. New insights into “the riddle of the mast cells”: Microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab. Invest. 1990, 62, 5–33. [Google Scholar] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.; Takeishi, T.; Thompson, H.; Langley, K.E.; Zsebo, K.M.; Metcalfe, D.D.; Geissler, E.N.; Galli, S.J. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc. Natl. Acad. Sci. USA 1991, 88, 6382–6386. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.; Shih, L.S.; Newlands, G.F.; Takeishi, T.; Langley, K.E.; Zsebo, K.M.; Miller, H.R.; Geissler, E.N.; Galli, S.J. The rat c-kit ligand, stem cell factor, induces the development of connective tissue-type and mucosal mast cells in vivo. Analysis by anatomical distribution, histochemistry, and protease phenotype. J. Exp. Med. 1991, 174, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Castells, M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol. Allergy Clin. North Am. 2006, 26, 465–485. [Google Scholar] [CrossRef]
- Artuc, M.; Hermes, B.; Steckelings, U.M.; Grutzkau, A.; Henz, B.M. Mast cells and their mediators in cutaneous wound healing--active participants or innocent bystanders? Exp. Dermatol. 1999, 8, 1–16. [Google Scholar] [CrossRef]
- Wodnar-Filipowicz, A.; Heusser, C.H.; Moroni, C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature 1989, 339, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Fodinger, M.; Fritsch, G.; Winkler, K.; Emminger, W.; Mitterbauer, G.; Gadner, H.; Valent, P.; Mannhalter, C. Origin of human mast cells: Development from transplanted hematopoietic stem cells after allogeneic bone marrow transplantation. Blood 1994, 84, 2954–2959. [Google Scholar] [CrossRef] [Green Version]
- Paolino, G.; Corsetti, P.; Moliterni, E.; Corsetti, S.; Didona, D.; Albanesi, M.; Mattozzi, C.; Lido, P.; Calvieri, S. Mast cells and cancer. G Ital. Dermatol. Venereol. 2019, 154, 650–668. [Google Scholar] [CrossRef]
- Cardamone, C.; Parente, R.; Feo, G.D.; Triggiani, M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol. Lett. 2016, 178, 10–14. [Google Scholar] [CrossRef]
- Wulff, B.C.; Wilgus, T.A. Mast cell activity in the healing wound: More than meets the eye? Exp. Dermatol. 2013, 22, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Tete, S.; Tripodi, D.; Rosati, M.; Conti, F.; Maccauro, G.; Saggini, A.; Salini, V.; Cianchetti, E.; Caraffa, A.; Antinolfi, P.; et al. Role of mast cells in innate and adaptive immunity. J. Biol. Regul. Homeost. Agents 2012, 26, 193–201. [Google Scholar]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta 2012, 1822, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Kunder, C.A.; St John, A.L.; Abraham, S.N. Mast cell modulation of the vascular and lymphatic endothelium. Blood 2011, 118, 5383–5393. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 2010, 40, 1843–1851. [Google Scholar] [CrossRef] [Green Version]
- Metz, M.; Maurer, M. Mast cells—Key effector cells in immune responses. Trends Immunol. 2007, 28, 234–241. [Google Scholar] [CrossRef]
- Greiner, G.; Witzeneder, N.; Berger, A.; Schmetterer, K.; Eisenwort, G.; Schiefer, A.I.; Roos, S.; Popow-Kraupp, T.; Mullauer, L.; Zuber, J.; et al. CCL2 is a KIT D816V-dependent modulator of the bone marrow microenvironment in systemic mastocytosis. Blood 2017, 129, 371–382. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef]
- Horny, H.P.A.C.; Arber, D. Mastocytosis. In World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Eds.; IARC: Lyon, France, 2017. [Google Scholar]
- Xu, Y.; Chen, G. Mast cell and autoimmune diseases. Mediat. Inflamm. 2015, 2015, 246126. [Google Scholar] [CrossRef]
- Brown, M.A.; Hatfield, J.K. Mast cells are important modifiers of autoimmune disease: With so much evidence, why is there still controversy? Front. Immunol. 2012, 3, 147. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, D.D.; Peavy, R.D.; Gilfillan, A.M. Mechanisms of mast cell signaling in anaphylaxis. J. Allergy Clin. Immunol. 2009, 124, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.M.; Wilson, T.M.; Metcalfe, D.D. The mast cell and allergic diseases: Role in pathogenesis and implications for therapy. Clin. Exp. Allergy 2008, 38, 4–18. [Google Scholar] [CrossRef]
- Bradding, P.; Walls, A.F.; Holgate, S.T. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006, 117, 1277–1284. [Google Scholar] [CrossRef]
- Glenner, G.G.; Cohen, L.A. Histochemical demonstration of a species-specific trypsin-like enzyme in mast cells. Nature 1960, 185, 846–847. [Google Scholar] [CrossRef]
- Benditt, E.P.; Arase, M. An enzyme in mast cells with properties like chymotrypsin. J. Exp. Med. 1959, 110, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Benditt, E.P.; Arase, M. Enzyme kinetics in a histochemical system. J. Histochem. Cytochem. 1958, 6, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Gomori, G. Chloroacyl esters as histochemical substrates. J. Histochem. Cytochem. 1953, 1, 469–470. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, L.B.; Irani, A.M.; Roller, K.; Castells, M.C.; Schechter, N.M. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J. Immunol. 1987, 138, 2611–2615. [Google Scholar]
- Schwartz, L.B.; Lewis, R.A.; Austen, K.F. Tryptase from human pulmonary mast cells. Purification and characterization. J. Biol. Chem. 1981, 256, 11939–11943. [Google Scholar] [CrossRef]
- Foster, B.; Schwartz, L.B.; Devouassoux, G.; Metcalfe, D.D.; Prussin, C. Characterization of mast-cell tryptase-expressing peripheral blood cells as basophils. J. Allergy Clin. Immunol. 2002, 109, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.C.; Irani, A.M.; Schwartz, L.B. Evaluation of human peripheral blood leukocytes for mast cell tryptase. J. Immunol. 1987, 138, 2184–2189. [Google Scholar] [PubMed]
- Schwartz, L.B.; Lewis, R.A.; Seldin, D.; Austen, K.F. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J. Immunol. 1981, 126, 1290–1294. [Google Scholar]
- Schwartz, L.B.; Austen, K.F. Enzymes of the mast cell granule. J. Invest. Dermatol. 1980, 74, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wong, G.W.; Ghildyal, N.; Gurish, M.F.; Sali, A.; Matsumoto, R.; Qiu, W.T.; Stevens, R.L. The tryptase, mouse mast cell protease 7, exhibits anticoagulant activity in vivo and in vitro due to its ability to degrade fibrinogen in the presence of the diverse array of protease inhibitors in plasma. J. Biol. Chem. 1997, 272, 31885–31893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.B.; Bradford, T.R.; Littman, B.H.; Wintroub, B.U. The fibrinogenolytic activity of purified tryptase from human lung mast cells. J. Immunol. 1985, 135, 2762–2767. [Google Scholar]
- Kaminska, R.; Helisalmi, P.; Harvima, R.J.; Naukkarinen, A.; Horsmanheimo, M.; Harvima, I.T. Focal dermal-epidermal separation and fibronectin cleavage in basement membrane by human mast cell tryptase. J. Invest. Dermatol. 1999, 113, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Lohi, J.; Harvima, I.; Keski-Oja, J. Pericellular substrates of human mast cell tryptase: 72,000 dalton gelatinase and fibronectin. J. Cell Biochem. 1992, 50, 337–349. [Google Scholar] [CrossRef]
- Kielty, C.M.; Lees, M.; Shuttleworth, C.A.; Woolley, D. Catabolism of intact type VI collagen microfibrils: Susceptibility to degradation by serine proteinases. Biochem. Biophys. Res. Commun. 1993, 191, 1230–1236. [Google Scholar] [CrossRef]
- Lee, M.; Sommerhoff, C.P.; von Eckardstein, A.; Zettl, F.; Fritz, H.; Kovanen, P.T. Mast cell tryptase degrades HDL and blocks its function as an acceptor of cellular cholesterol. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 2086–2091. [Google Scholar] [CrossRef]
- Sonneck, K.; Florian, S.; Bohm, A.; Krauth, M.T.; Kondo, R.; Hauswirth, A.W.; Gleixner, K.V.; Aichberger, K.J.; Derdak, S.; Pickl, W.F.; et al. Evaluation of biologic activity of tryptase secreted from blast cells in acute myeloid leukemia. Leuk. Lymphoma 2006, 47, 897–906. [Google Scholar] [CrossRef]
- Ruoss, S.J.; Hartmann, T.; Caughey, G.H. Mast cell tryptase is a mitogen for cultured fibroblasts. J. Clin. Invest. 1991, 88, 493–499. [Google Scholar] [CrossRef]
- Bagher, M.; Larsson-Callerfelt, A.K.; Rosmark, O.; Hallgren, O.; Bjermer, L.; Westergren-Thorsson, G. Mast cells and mast cell tryptase enhance migration of human lung fibroblasts through protease-activated receptor 2. Cell Commun. Signal 2018, 16, 59. [Google Scholar] [CrossRef]
- Desai, A.; Jung, M.Y.; Olivera, A.; Gilfillan, A.M.; Prussin, C.; Kirshenbaum, A.S.; Beaven, M.A.; Metcalfe, D.D. IL-6 promotes an increase in human mast cell numbers and reactivity through suppression of suppressor of cytokine signaling 3. J. Allergy Clin. Immunol. 2016, 137, 1863–1871. [Google Scholar] [CrossRef] [Green Version]
- Malamud, V.; Vaaknin, A.; Abramsky, O.; Mor, M.; Burgess, L.E.; Ben-Yehudah, A.; Lorberboum-Galski, H. Tryptase activates peripheral blood mononuclear cells causing the synthesis and release of TNF-alpha, IL-6 and IL-1 beta: Possible relevance to multiple sclerosis. J. Neuroimmunol. 2003, 138, 115–122. [Google Scholar] [CrossRef]
- Compton, S.J.; Cairns, J.A.; Holgate, S.T.; Walls, A.F. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: Tryptase induces expression of mRNA for IL-1 beta and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J. Immunol. 1998, 161, 1939–1946. [Google Scholar] [PubMed]
- Cairns, J.A.; Walls, A.F. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J. Immunol. 1996, 156, 275–283. [Google Scholar]
- Margulis, A.; Nocka, K.H.; Brennan, A.M.; Deng, B.; Fleming, M.; Goldman, S.J.; Kasaian, M.T. Mast cell-dependent contraction of human airway smooth muscle cell-containing collagen gels: Influence of cytokines, matrix metalloproteases, and serine proteases. J. Immunol. 2009, 183, 1739–1750. [Google Scholar] [CrossRef] [Green Version]
- Kauhanen, P.; Kovanen, P.T.; Reunala, T.; Lassila, R. Effects of skin mast cells on bleeding time and coagulation activation at the site of platelet plug formation. Thromb. Haemost. 1998, 79, 843–847. [Google Scholar] [CrossRef]
- Cairns, J.A. Mast cell tryptase and its role in tissue remodelling. Clin. Exp. Allergy 1998, 28, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Pejler, G. Biology of mast cell tryptase. An inflammatory mediator. FEBS J. 2006, 273, 1871–1895. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.L.; Niles, A.; Burdick, K.; Maffitt, M.; Backes, B.J.; Ellman, J.A.; Kuntz, I.; Haak-Frendscho, M.; Craik, C.S. Definition of the extended substrate specificity determinants for beta-tryptases I and II. J. Biol. Chem. 2001, 276, 34941–34947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.B.; Min, H.K.; Ren, S.; Xia, H.Z.; Hu, J.; Zhao, W.; Moxley, G.; Fukuoka, Y. Tryptase precursors are preferentially and spontaneously released, whereas mature tryptase is retained by HMC-1 cells, Mono-Mac-6 cells, and human skin-derived mast cells. J. Immunol. 2003, 170, 5667–5673. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, L.B.; Sakai, K.; Bradford, T.R.; Ren, S.; Zweiman, B.; Worobec, A.S.; Metcalfe, D.D. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J. Clin. Invest. 1995, 96, 2702–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, P.J.; Bergner, A.; Macedo-Ribeiro, S.; Huber, R.; Matschiner, G.; Fritz, H.; Sommerhoff, C.P.; Bode, W. Human beta-tryptase is a ring-like tetramer with active sites facing a central pore. Nature 1998, 392, 306–311. [Google Scholar] [CrossRef]
- Maun, H.R.; Liu, P.S.; Franke, Y.; Eigenbrot, C.; Forrest, W.F.; Schwartz, L.B.; Lazarus, R.A. Dual functionality of beta-tryptase protomers as both proteases and cofactors in the active tetramer. J. Biol. Chem. 2018, 293, 9614–9628. [Google Scholar] [CrossRef] [Green Version]
- Hallgren, J.; Lindahl, S.; Pejler, G. Structural requirements and mechanism for heparin-dependent activation and tetramerization of human betaI- and betaII-tryptase. J. Mol. Biol. 2005, 345, 129–139. [Google Scholar] [CrossRef]
- Hallgren, J.; Spillmann, D.; Pejler, G. Structural requirements and mechanism for heparin-induced activation of a recombinant mouse mast cell tryptase, mouse mast cell protease-6: Formation of active tryptase monomers in the presence of low molecular weight heparin. J. Biol. Chem. 2001, 276, 42774–42781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallgren, J.; Karlson, U.; Poorafshar, M.; Hellman, L.; Pejler, G. Mechanism for activation of mouse mast cell tryptase: Dependence on heparin and acidic pH for formation of active tetramers of mouse mast cell protease 6. Biochemistry 2000, 39, 13068–13077. [Google Scholar] [CrossRef]
- Ren, S.; Sakai, K.; Schwartz, L.B. Regulation of human mast cell beta-tryptase: Conversion of inactive monomer to active tetramer at acid pH. J. Immunol. 1998, 160, 4561–4569. [Google Scholar] [PubMed]
- Sakai, K.; Ren, S.; Schwartz, L.B. A novel heparin-dependent processing pathway for human tryptase. Autocatalysis followed by activation with dipeptidyl peptidase I. J. Clin. Invest. 1996, 97, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B.; Bradford, T.R. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J. Biol. Chem. 1986, 261, 7372–7379. [Google Scholar] [CrossRef]
- Wolters, P.J.; Pham, C.T.; Muilenburg, D.J.; Ley, T.J.; Caughey, G.H. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J. Biol. Chem. 2001, 276, 18551–18556. [Google Scholar] [CrossRef] [Green Version]
- Le, Q.T.; Min, H.K.; Xia, H.Z.; Fukuoka, Y.; Katunuma, N.; Schwartz, L.B. Promiscuous processing of human alphabeta-protryptases by cathepsins L., B., and C. J. Immunol. 2011, 186, 7136–7143. [Google Scholar] [CrossRef] [Green Version]
- Le, Q.T.; Gomez, G.; Zhao, W.; Hu, J.; Xia, H.Z.; Fukuoka, Y.; Katunuma, N.; Schwartz, L.B. Processing of human protryptase in mast cells involves cathepsins L., B., and C. J. Immunol. 2011, 187, 1912–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selwood, T.; Wang, Z.M.; McCaslin, D.R.; Schechter, N.M. Diverse stability and catalytic properties of human tryptase alpha and beta isoforms are mediated by residue differences at the S1 pocket. Biochemistry 2002, 41, 3329–3340. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Yu, X.; Hughes, J.D.; Le, Q.T.; Jamil, A.; Bai, Y.; Ho, N.; Zhao, M.; Liu, Y.; O’Connell, M.P.; et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat. Genet. 2016, 48, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, U.; Zettl, F.; Huber, R.; Bode, W.; Sommerhoff, C. The crystal structure of human alpha1-tryptase reveals a blocked substrate-binding region. J. Mol. Biol. 2002, 321, 491–502. [Google Scholar] [CrossRef]
- Huang, C.; Li, L.; Krilis, S.A.; Chanasyk, K.; Tang, Y.; Li, Z.; Hunt, J.E.; Stevens, R.L. Human tryptases alpha and beta/II are functionally distinct due, in part, to a single amino acid difference in one of the surface loops that forms the substrate-binding cleft. J. Biol. Chem. 1999, 274, 19670–19676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maaninka, K.; Lappalainen, J.; Kovanen, P.T. Human mast cells arise from a common circulating progenitor. J. Allergy Clin. Immunol. 2013, 132, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Butterfield, J.H.; Nilsson, K.; Siegbahn, A. Stem cell factor is a chemotactic factor for human mast cells. J. Immunol. 1994, 153, 3717–3723. [Google Scholar]
- Agis, H.; Willheim, M.; Sperr, W.R.; Wilfing, A.; Kromer, E.; Kabrna, E.; Spanblochl, E.; Strobl, H.; Geissler, K.; Spittler, A.; et al. Monocytes do not make mast cells when cultured in the presence of SCF. Characterization of the circulating mast cell progenitor as a c-kit+, CD34+, Ly-, CD14-, CD17-, colony-forming cell. J. Immunol. 1993, 151, 4221–4227. [Google Scholar] [PubMed]
- Kirshenbaum, A.S.; Goff, J.P.; Kessler, S.W.; Mican, J.M.; Zsebo, K.M.; Metcalfe, D.D. Effect of IL-3 and stem cell factor on the appearance of human basophils and mast cells from CD34+ pluripotent progenitor cells. J. Immunol. 1992, 148, 772–777. [Google Scholar] [PubMed]
- Jarboe, D.L.; Marshall, J.S.; Randolph, T.R.; Kukolja, A.; Huff, T.F. The mast cell-committed progenitor. I. Description of a cell capable of IL-3-independent proliferation and differentiation without contact with fibroblasts. J. Immunol. 1989, 142, 2405–2417. [Google Scholar]
- Chen, C.C.; Grimbaldeston, M.A.; Tsai, M.; Weissman, I.L.; Galli, S.J. Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. USA 2005, 102, 11408–11413. [Google Scholar] [CrossRef] [Green Version]
- Wershil, B.K.; Tsai, M.; Geissler, E.N.; Zsebo, K.M.; Galli, S.J. The rat c-kit ligand, stem cell factor, induces c-kit receptor-dependent mouse mast cell activation in vivo. Evidence that signaling through the c-kit receptor can induce expression of cellular function. J. Exp. Med. 1992, 175, 245–255. [Google Scholar] [CrossRef]
- Gurish, M.F.; Ghildyal, N.; McNeil, H.P.; Austen, K.F.; Gillis, S.; Stevens, R.L. Differential expression of secretory granule proteases in mouse mast cells exposed to interleukin 3 and c-kit ligand. J. Exp. Med. 1992, 175, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Ghildyal, N.; McNeil, H.P.; Gurish, M.F.; Austen, K.F.; Stevens, R.L. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J. Biol. Chem. 1992, 267, 8473–8477. [Google Scholar] [CrossRef]
- Levi-Schaffer, F.; Austen, K.F.; Gravallese, P.M.; Stevens, R.L. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc. Natl. Acad. Sci. USA 1986, 83, 6485–6488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, Y.; Yokoyama, M.; Matsuda, H.; Ohno, T.; Mori, K.J. Spleen colony-forming cell as common precursor for tissue mast cells and granulocytes. Nature 1981, 291, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Matsuda, H.; Hatanaka, K. Clonal nature of mast-cell clusters formed in W/Wv mice after bone marrow transplantation. Nature 1979, 281, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Go, S. Decreased production of mast cells in S1/S1d anemic mice. Blood 1979, 53, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, Y.; Go, S.; Hatanaka, K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood 1978, 52, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Meininger, C.J.; Yano, H.; Rottapel, R.; Bernstein, A.; Zsebo, K.M.; Zetter, B.R. The c-kit receptor ligand functions as a mast cell chemoattractant. Blood 1992, 79, 958–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irani, A.M.; Nilsson, G.; Miettinen, U.; Craig, S.S.; Ashman, L.K.; Ishizaka, T.; Zsebo, K.M.; Schwartz, L.B. Recombinant human stem cell factor stimulates differentiation of mast cells from dispersed human fetal liver cells. Blood 1992, 80, 3009–3021. [Google Scholar] [CrossRef] [Green Version]
- Oskeritzian, C.A.; Zhao, W.; Pozez, A.L.; Cohen, N.M.; Grimes, M.; Schwartz, L.B. Neutralizing endogenous IL-6 renders mast cells of the MCT type from lung, but not the MCTC type from skin and lung, susceptible to human recombinant IL-4-induced apoptosis. J. Immunol. 2004, 172, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Oskeritzian, C.A.; Wang, Z.; Kochan, J.P.; Grimes, M.; Du, Z.; Chang, H.W.; Grant, S.; Schwartz, L.B. Recombinant human (rh)IL-4-mediated apoptosis and recombinant human IL-6-mediated protection of recombinant human stem cell factor-dependent human mast cells derived from cord blood mononuclear cell progenitors. J. Immunol. 1999, 163, 5105–5115. [Google Scholar]
- Bischoff, S.C.; Sellge, G.; Lorentz, A.; Sebald, W.; Raab, R.; Manns, M.P. IL-4 enhances proliferation and mediator release in mature human mast cells. Proc. Natl. Acad. Sci. USA 1999, 96, 8080–8085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.Z.; Du, Z.; Craig, S.; Klisch, G.; Noben-Trauth, N.; Kochan, J.P.; Huff, T.H.; Irani, A.M.; Schwartz, L.B. Effect of recombinant human IL-4 on tryptase, chymase, and Fc epsilon receptor type I expression in recombinant human stem cell factor-dependent fetal liver-derived human mast cells. J. Immunol. 1997, 159, 2911–2921. [Google Scholar]
- Saito, H.; Ebisawa, M.; Tachimoto, H.; Shichijo, M.; Fukagawa, K.; Matsumoto, K.; Iikura, Y.; Awaji, T.; Tsujimoto, G.; Yanagida, M.; et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J. Immunol. 1996, 157, 343–350. [Google Scholar] [PubMed]
- Tsujino, Y.; Wada, H.; Misawa, M.; Kai, S.; Hara, H. Effects of mast cell growth factor, interleukin-3, and interleukin-6 on human primitive hematopoietic progenitors from bone marrow and cord blood. Exp. Hematol. 1993, 21, 1379–1386. [Google Scholar]
- Schernthaner, G.H.; Hauswirth, A.W.; Baghestanian, M.; Agis, H.; Ghannadan, M.; Worda, C.; Krauth, M.T.; Printz, D.; Fritsch, G.; Sperr, W.R.; et al. Detection of differentiation- and activation-linked cell surface antigens on cultured mast cell progenitors. Allergy 2005, 60, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Besemer, J.; Sillaber, C.; Butterfield, J.H.; Eher, R.; Majdic, O.; Kishi, K.; Klepetko, W.; Eckersberger, F.; Lechner, K.; et al. Failure to detect IL-3-binding sites on human mast cells. J. Immunol. 1990, 145, 3432–3437. [Google Scholar]
- Yamaguchi, M.; Sayama, K.; Yano, K.; Lantz, C.S.; Noben-Trauth, N.; Ra, C.; Costa, J.J.; Galli, S.J. IgE enhances Fc epsilon receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: Synergistic effect of IL-4 and IgE on human mast cell Fc epsilon receptor I expression and mediator release. J. Immunol. 1999, 162, 5455–5465. [Google Scholar]
- Sillaber, C.; Sperr, W.R.; Agis, H.; Spanblochl, E.; Lechner, K.; Valent, P. Inhibition of stem cell factor dependent formation of human mast cells by interleukin-3 and interleukin-4. Int. Arch. Allergy Immunol. 1994, 105, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Miettinen, U.; Ishizaka, T.; Ashman, L.K.; Irani, A.M.; Schwartz, L.B. Interleukin-4 inhibits the expression of Kit and tryptase during stem cell factor-dependent development of human mast cells from fetal liver cells. Blood 1994, 84, 1519–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallgren, J.; Gurish, M.F. Granule maturation in mast cells: Histamine in control. Eur. J. Immunol 2014, 44, 33–36. [Google Scholar] [CrossRef]
- Davidson, S.; Mansour, A.; Gallily, R.; Smolarski, M.; Rofolovitch, M.; Ginsburg, H. Mast cell differentiation depends on T cells and granule synthesis on fibroblasts. Immunology 1983, 48, 439–452. [Google Scholar]
- Nakazawa, S.; Sakanaka, M.; Furuta, K.; Natsuhara, M.; Takano, H.; Tsuchiya, S.; Okuno, Y.; Ohtsu, H.; Nishibori, M.; Thurmond, R.L.; et al. Histamine synthesis is required for granule maturation in murine mast cells. Eur. J. Immunol. 2014, 44, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Irani, A.M.; Bradford, T.R.; Kepley, C.L.; Schechter, N.M.; Schwartz, L.B. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J. Histochem. Cytochem. 1989, 37, 1509–1515. [Google Scholar] [CrossRef] [Green Version]
- Irani, A.A.; Schechter, N.M.; Craig, S.S.; DeBlois, G.; Schwartz, L.B. Two types of human mast cells that have distinct neutral protease compositions. Proc. Natl. Acad. Sci. USA 1986, 83, 4464–4468. [Google Scholar] [CrossRef] [Green Version]
- Crivellato, E.; Ribatti, D. The fundamental contribution of William Bate Hardy to shape the concept of mast cell heterogeneity. Br. J. Haematol. 2010, 150, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Enerback, L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol. Microbiol. Scand. 1966, 66, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Alter, S.C.; Metcalfe, D.D.; Bradford, T.R.; Schwartz, L.B. Regulation of human mast cell tryptase. Effects of enzyme concentration, ionic strength and the structure and negative charge density of polysaccharides. Biochem. J. 1987, 248, 821–827. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Wang, Y.; Wang, P.; Wang, Y. SCF/C-KIT signaling modulates tryptase expression in acute myeloid leukemia cells. Int. J. Hematol. 2014, 99, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Ohneda, K.; Ohmori, S.; Yamamoto, M. Mouse tryptase gene expression is coordinately regulated by GATA1 and GATA2 in bone marrow-derived mast cells. Int. J. Mol. Sci. 2019, 20, 4603. [Google Scholar] [CrossRef] [Green Version]
- Ohneda, K.; Moriguchi, T.; Ohmori, S.; Ishijima, Y.; Satoh, H.; Philipsen, S.; Yamamoto, M. Transcription factor GATA1 is dispensable for mast cell differentiation in adult mice. Mol. Cell Biol. 2014, 34, 1812–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taura, A.; Furuta, K.; Yamaguchi, T.; Kawabata, K.; Tanaka, S. Regulation of histamine synthesis and tryptase expression through transcription factors, growth factor independent 1 (Gfi1) and Gfi1b, in murine cultured mast cells. Biol. Pharm. Bull. 2014, 37, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lee, J.H.; Lee, J.H.; Kim, D.K. Involvement of MITF-A, an alternative isoform of mi transcription factor, on the expression of tryptase gene in human mast cells. Exp. Mol. Med. 2010, 42, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohrvik, H.; Logeman, B.; Noguchi, G.; Eriksson, I.; Kjellen, L.; Thiele, D.J.; Pejler, G. Ctr2 Regulates Mast Cell Maturation by Affecting the Storage and Expression of Tryptase and Proteoglycans. J. Immunol. 2015, 195, 3654–3664. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, J.; Zhang, H.; Zhan, M.; Chen, H.; Fang, Z.; Xu, C.; Chen, H.; He, S. Induction of Mast Cell Accumulation by Tryptase via a Protease Activated Receptor-2 and ICAM-1 Dependent Mechanism. Mediat. Inflamm. 2016, 2016, 6431574. [Google Scholar] [CrossRef] [Green Version]
- Melo, F.R.; Wallerman, O.; Paivandy, A.; Calounova, G.; Gustafson, A.M.; Sabari, B.R.; Zabucchi, G.; Allis, C.D.; Pejler, G. Tryptase-catalyzed core histone truncation: A novel epigenetic regulatory mechanism in mast cells. J. Allergy Clin. Immunol. 2017, 140, 474–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, F.R.; Vita, F.; Berent-Maoz, B.; Levi-Schaffer, F.; Zabucchi, G.; Pejler, G. Proteolytic histone modification by mast cell tryptase, a serglycin proteoglycan-dependent secretory granule protease. J. Biol. Chem. 2014, 289, 7682–7690. [Google Scholar] [CrossRef] [Green Version]
- Grujic, M.; Calounova, G.; Eriksson, I.; Feyerabend, T.; Rodewald, H.R.; Tchougounova, E.; Kjellen, L.; Pejler, G. Distorted secretory granule composition in mast cells with multiple protease deficiency. J. Immunol. 2013, 191, 3931–3938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.B. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol. Allergy Clin. North Am. 2006, 26, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Y.; Schwartz, L.B.; Curry, A.; Pesola, G.R.; Knight, R.J.; Lee, H.S.; Bakalchuk, L.; Tenenbaum, C.; Westfal, R.E. Histamine and tryptase levels in patients with acute allergic reactions: An emergency department-based study. J. Allergy Clin. Immunol. 2000, 106, 65–71. [Google Scholar] [CrossRef]
- Valent, P.; Sperr, W.R.; Sotlar, K.; Reiter, A.; Akin, C.; Gotlib, J.; Horny, H.P.; Arock, M. The serum tryptase test: An emerging robust biomarker in clinical hematology. Expert Rev. Hematol. 2014, 7, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperr, W.R.; El-Samahi, A.; Kundi, M.; Girschikofsky, M.; Winkler, S.; Lutz, D.; Endler, G.; Rumpold, H.; Agis, H.; Sillaber, C.; et al. Elevated tryptase levels selectively cluster in myeloid neoplasms: A novel diagnostic approach and screen marker in clinical haematology. Eur. J. Clin. Invest. 2009, 39, 914–923. [Google Scholar] [CrossRef]
- Sperr, W.R.; Jordan, J.H.; Fiegl, M.; Escribano, L.; Bellas, C.; Dirnhofer, S.; Semper, H.; Simonitsch-Klupp, I.; Horny, H.P.; Valent, P. Serum tryptase levels in patients with mastocytosis: Correlation with mast cell burden and implication for defining the category of disease. Int. Arch. Allergy Immunol. 2002, 128, 136–141. [Google Scholar] [CrossRef]
- Valent, P.; Horny, H.P.; Escribano, L.; Longley, B.J.; Li, C.Y.; Schwartz, L.B.; Marone, G.; Nunez, R.; Akin, C.; Sotlar, K.; et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk. Res. 2001, 25, 603–625. [Google Scholar] [CrossRef]
- Schwartz, L.B.; Metcalfe, D.D.; Miller, J.S.; Earl, H.; Sullivan, T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N. Engl. J. Med. 1987, 316, 1622–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valent, P.; Bonadonna, P.; Hartmann, K.; Broesby-Olsen, S.; Brockow, K.; Butterfield, J.H.; Triggiani, M.; Lyons, J.J.; Oude Elberink, J.N.G.; Arock, M.; et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe systemic mast cell activation and mast cell activation syndrome. Int. Arch. Allergy Immunol. 2019, 180, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Vizcaino, L.; Gude, F.; Rey, J.; Meijide, L.; Fernandez-Merino, C.; Linneberg, A.; Vidal, C. Factors influencing serum total tryptase concentrations in a general adult population. Clin. Chem. Lab. Med. 2010, 48, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Sabato, V.; Chovanec, J.; Faber, M.; Milner, J.D.; Ebo, D.; Lyons, J.J. First Identification of an Inherited TPSAB1 Quintuplication in a Patient with Clonal Mast Cell Disease. J. Clin. Immunol. 2018, 38, 457–459. [Google Scholar] [CrossRef]
- Lyons, J.J.; Sun, G.; Stone, K.D.; Nelson, C.; Wisch, L.; O’Brien, M.; Jones, N.; Lindsley, A.; Komarow, H.D.; Bai, Y.; et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J. Allergy Clin. Immunol. 2014, 133, 1471–1474. [Google Scholar] [CrossRef] [Green Version]
- Fellinger, C.; Hemmer, W.; Wohrl, S.; Sesztak-Greinecker, G.; Jarisch, R.; Wantke, F. Clinical characteristics and risk profile of patients with elevated baseline serum tryptase. Allergol. Immunopathol. 2014, 42, 544–552. [Google Scholar] [CrossRef]
- Desai, A.; Sowerwine, K.; Liu, Y.; Lawrence, M.G.; Chovanec, J.; Hsu, A.P.; O’Connell, M.P.; Kim, J.; Boris, L.; Jones, N.; et al. GATA-2-deficient mast cells limit IgE-mediated immediate hypersensitivity reactions in human subjects. J. Allergy Clin. Immunol. 2019, 144, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Schussler, E.; Yang, A.; Lyons, J.J.; Milner, J.D.; Wang, J. Persistent tryptase elevation in a patient with Gaucher disease. J. Allergy Clin. Immunol. 2018, 6, 697–699. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Escribano, L.; Fodinger, M.; Hartmann, K.; Brockow, K.; Castells, M.; Sperr, W.R.; Kluin-Nelemans, H.C.; Hamdy, N.A.; et al. Standards and standardization in mastocytosis: Consensus statements on diagnostics, treatment recommendations and response criteria. Eur. J. Clin. Invest. 2007, 37, 435–453. [Google Scholar] [CrossRef] [PubMed]
- Hasford, J.; Baccarani, M.; Hoffmann, V.; Guilhot, J.; Saussele, S.; Rosti, G.; Guilhot, F.; Porkka, K.; Ossenkoppele, G.; Lindoerfer, D.; et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: The EUTOS score. Blood 2011, 118, 686–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasford, J.; Pfirrmann, M.; Hehlmann, R.; Allan, N.C.; Baccarani, M.; Kluin-Nelemans, J.C.; Alimena, G.; Steegmann, J.L.; Ansari, H. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J. Natl. Cancer Inst. 1998, 90, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Sokal, J.E.; Cox, E.B.; Baccarani, M.; Tura, S.; Gomez, G.A.; Robertson, J.E.; Tso, C.Y.; Braun, T.J.; Clarkson, B.D.; Cervantes, F.; et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood 1984, 63, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Sperr, W.R.; Pfeiffer, T.; Hoermann, G.; Herndlhofer, S.; Sillaber, C.; Mannhalter, C.; Kundi, M.; Valent, P. Serum-tryptase at diagnosis: A novel biomarker improving prognostication in Ph (+) CML. Am. J. Cancer Res. 2015, 5, 354–362. [Google Scholar]
- Samorapoompichit, P.; Kiener, H.P.; Schernthaner, G.H.; Jordan, J.H.; Agis, H.; Wimazal, F.; Baghestanian, M.; Rezaie-Majd, A.; Sperr, W.R.; Lechner, K.; et al. Detection of tryptase in cytoplasmic granules of basophils in patients with chronic myeloid leukemia and other myeloid neoplasms. Blood 2001, 98, 2580–2583. [Google Scholar] [CrossRef] [Green Version]
- Sperr, W.R.; Jordan, J.H.; Baghestanian, M.; Kiener, H.P.; Samorapoompichit, P.; Semper, H.; Hauswirth, A.; Schernthaner, G.H.; Chott, A.; Natter, S.; et al. Expression of mast cell tryptase by myeloblasts in a group of patients with acute myeloid leukemia. Blood 2001, 98, 2200–2209. [Google Scholar] [CrossRef] [Green Version]
- Sperr, W.R.; Hauswirth, A.W.; Valent, P. Tryptase a novel biochemical marker of acute myeloid leukemia. Leuk. Lymphoma 2002, 43, 2257–2261. [Google Scholar] [CrossRef]
- Sperr, W.R.; Stehberger, B.; Wimazal, F.; Baghestanian, M.; Schwartz, L.B.; Kundi, M.; Semper, H.; Jordan, J.H.; Chott, A.; Drach, J.; et al. Serum tryptase measurements in patients with myelodysplastic syndromes. Leuk. Lymphoma 2002, 43, 1097–1105. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Bonadonna, P.; Hartmann, K.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Siebenhaar, F.; Sperr, W.R.; Oude Elberink, J.N.G.; et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J. Allergy Clin. Immunol. 2019, 7, 1125–1133. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Arock, M.; Brockow, K.; Butterfield, J.H.; Carter, M.C.; Castells, M.; Escribano, L.; Hartmann, K.; Lieberman, P.; et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: A consensus proposal. Int. Arch. Allergy Immunol. 2012, 157, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Enrique, E.; Garcia-Ortega, P.; Sotorra, O.; Gaig, P.; Richart, C. Usefulness of UniCAP-Tryptase fluoroimmunoassay in the diagnosis of anaphylaxis. Allergy 1999, 54, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Sotlar, K.; Sperr, W.R.; Reiter, A.; Arock, M.; Horny, H.P. Chronic mast cell leukemia: A novel leukemia-variant with distinct morphological and clinical features. Leuk. Res. 2015, 39, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardanani, A.; Lim, K.H.; Lasho, T.L.; Finke, C.M.; McClure, R.F.; Li, C.Y.; Tefferi, A. WHO subvariants of indolent mastocytosis: Clinical details and prognostic evaluation in 159 consecutive adults. Blood 2010, 115, 150–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.H.; Tefferi, A.; Lasho, T.L.; Finke, C.; Patnaik, M.; Butterfield, J.H.; McClure, R.F.; Li, C.Y.; Pardanani, A. Systemic mastocytosis in 342 consecutive adults: Survival studies and prognostic factors. Blood 2009, 113, 5727–5736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Advances in the classification and treatment of mastocytosis: Current status and outlook toward the future. Cancer Res. 2017, 77, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Caplan, R.M. The natural course of urticaria pigmentosa. Analysis and follow-up of 112 cases. Arch. Dermatol. 1963, 87, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Sperr, W.R.; Kundi, M.; Alvarez-Twose, I.; van Anrooij, B.; Oude Elberink, J.N.G.; Gorska, A.; Niedoszytko, M.; Gleixner, K.V.; Hadzijusufovic, E.; Zanotti, R.; et al. International prognostic scoring system for mastocytosis (IPSM): A retrospective cohort study. Lancet Haematol. 2019, 6, e638–e649. [Google Scholar] [CrossRef]
- Kawakami, T.; Galli, S.J. Regulation of mast-cell and basophil function and survival by IgE. Nat. Rev. Immunol. 2002, 2, 773–786. [Google Scholar] [CrossRef]
- Akay, B.N.; Kittler, H.; Sanli, H.; Harmankaya, K.; Anadolu, R. Dermatoscopic findings of cutaneous mastocytosis. Dermatology 2009, 218, 226–230. [Google Scholar] [CrossRef]
- Akoglu, G.; Erkin, G.; Cakir, B.; Boztepe, G.; Sahin, S.; Karaduman, A.; Atakan, N.; Akan, T.; Kolemen, F. Cutaneous mastocytosis: Demographic aspects and clinical features of 55 patients. J. Eur. Acad Dermatol. Venereol. 2006, 20, 969–973. [Google Scholar] [CrossRef]
- Greiner, G.; Gurbisz, M.; Ratzinger, F.; Witzeneder, N.; Class, S.V.; Eisenwort, G.; Simonitsch-Klupp, I.; Esterbauer, H.; Mayerhofer, M.; Müllauer, L.; et al. Molecular quantification of tissue disease burden is a new biomarker and independent predictor of survival in mastocytosis. Haematologica 2020, 105, 366–374. [Google Scholar] [CrossRef]
- Arock, M.; Sotlar, K.; Akin, C.; Broesby-Olsen, S.; Hoermann, G.; Escribano, L.; Kristensen, T.K.; Kluin-Nelemans, H.C.; Hermine, O.; Dubreuil, P.; et al. KIT mutation analysis in mast cell neoplasms: Recommendations of the European Competence Network on Mastocytosis. Leukemia 2015, 29, 1223–1232. [Google Scholar] [CrossRef] [Green Version]
- Nagata, H.; Worobec, A.S.; Oh, C.K.; Chowdhury, B.A.; Tannenbaum, S.; Suzuki, Y.; Metcalfe, D.D. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc. Natl. Acad. Sci. USA 1995, 92, 10560–10564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magliacane, D.; Parente, R.; Triggiani, M. Current concepts on diagnosis and treatment of mastocytosis. Transl. Med. UniSa 2014, 8, 65–74. [Google Scholar]
- Valent, P.; Akin, C.; Gleixner, K.V.; Sperr, W.R.; Reiter, A.; Arock, M.; Triggiani, M. Multidisciplinary challenges in mastocytosis and how to address with personalized medicine approaches. Int. J. Mol. Sci. 2019, 20, 2976. [Google Scholar] [CrossRef] [Green Version]
- Castells, M.; Metcalfe, D.D.; Escribano, L. Diagnosis and treatment of cutaneous mastocytosis in children: Practical recommendations. Am. J. Clin. Dermatol. 2011, 12, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.S.; Moxley, G.; Schwartz, L.B. Cloning and characterization of a second complementary DNA for human tryptase. J. Clin. Invest. 1990, 86, 864–870. [Google Scholar] [CrossRef]
- Miller, J.S.; Westin, E.H.; Schwartz, L.B. Cloning and characterization of complementary DNA for human tryptase. J. Clin. Invest. 1989, 84, 1188–1195. [Google Scholar] [CrossRef]
- Pallaoro, M.; Fejzo, M.S.; Shayesteh, L.; Blount, J.L.; Caughey, G.H. Characterization of genes encoding known and novel human mast cell tryptases on chromosome 16p13.3. J. Biol. Chem. 1999, 274, 3355–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderslice, P.; Ballinger, S.M.; Tam, E.K.; Goldstein, S.M.; Craik, C.S.; Caughey, G.H. Human mast cell tryptase: Multiple cDNAs and genes reveal a multigene serine protease family. Proc. Natl. Acad. Sci. USA 1990, 87, 3811–3815. [Google Scholar] [CrossRef] [Green Version]
- Caughey, G.H. Tryptase genetics and anaphylaxis. J. Allergy Clin. Immunol. 2006, 117, 1411–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caughey, G.H.; Raymond, W.W.; Blount, J.L.; Hau, L.W.; Pallaoro, M.; Wolters, P.J.; Verghese, G.M. Characterization of human gamma-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. J. Immunol. 2000, 164, 6566–6575. [Google Scholar] [CrossRef] [Green Version]
- Soto, D.; Malmsten, C.; Blount, J.L.; Muilenburg, D.J.; Caughey, G.H. Genetic deficiency of human mast cell alpha-tryptase. Clin. Exp. Allergy 2002, 32, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, N.N.; Tamraz, B.; Chu, C.; Kwok, P.Y.; Caughey, G.H. Human subjects are protected from mast cell tryptase deficiency despite frequent inheritance of loss-of-function mutations. J. Allergy Clin. Immunol. 2009, 124, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Hernandez, L.; Sanz, C.; Garcia-Solaesa, V.; Padron, J.; Garcia-Sanchez, A.; Davila, I.; Isidoro-Garcia, M.; Lorente, F. Tryptase: Genetic and functional considerations. Allergol. Immunopathol. 2012, 40, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; McNeil, H.P.; Husain, A.; Liu, K.; Tedla, N.; Thomas, P.S.; Raftery, M.; King, G.C.; Cai, Z.Y.; Hunt, J.E. Delta tryptase is expressed in multiple human tissues, and a recombinant form has proteolytic activity. J. Immunol. 2002, 169, 5145–5152. [Google Scholar] [CrossRef] [Green Version]
- Wong, G.W.; Foster, P.S.; Yasuda, S.; Qi, J.C.; Mahalingam, S.; Mellor, E.A.; Katsoulotos, G.; Li, L.; Boyce, J.A.; Krilis, S.A.; et al. Biochemical and functional characterization of human transmembrane tryptase (TMT)/tryptase gamma. TMT is an exocytosed mast cell protease that induces airway hyperresponsiveness in vivo via an interleukin-13/interleukin-4 receptor alpha/signal transducer and activator of transcription (STAT) 6-dependent pathway. J. Biol. Chem. 2002, 277, 41906–41915. [Google Scholar]
- Wong, G.W.; Tang, Y.; Stevens, R.L. Cloning of the human homolog of mouse transmembrane tryptase. Int. Arch. Allergy Immunol. 1999, 118, 419–421. [Google Scholar] [CrossRef]
- Le, Q.T.; Lotfi-Emran, S.; Min, H.K.; Schwartz, L.B. A simple, sensitive and safe method to determine the human alpha/beta-tryptase genotype. PLoS ONE 2014, 9, e114944. [Google Scholar] [CrossRef] [Green Version]
- Min, H.K.; Moxley, G.; Neale, M.C.; Schwartz, L.B. Effect of sex and haplotype on plasma tryptase levels in healthy adults. J. Allergy Clin. Immunol. 2004, 114, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Greiner, G.; Sprinzl, B.; Gorska, A.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Schmetterer, K.G.; Gisslinger, B.; Uyanik, G.; Hadzijusufovic, E.; et al. Hereditary alpha tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood 2021, 137, 238–247. [Google Scholar] [CrossRef]
- Robey, R.C.; Wilcock, A.; Bonin, H.; Beaman, G.; Myers, B.; Grattan, C.; Briggs, T.A.; Arkwright, P.D. Hereditary Alpha-Tryptasemia: UK Prevalence and Variability in Disease Expression. J. Allergy Clin. Immunol. 2020, 8, 3549–3556. [Google Scholar] [CrossRef]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Selb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable risk for severe anaphylaxis associated with increased alpha-tryptase-encoding germline copy number at TPSAB1. J. Allergy Clin. Immunol. 2020, 147, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J. Hereditary Alpha Tryptasemia: Genotyping and Associated Clinical Features. Immunol. Allergy Clin. North Am. 2018, 38, 483–495. [Google Scholar] [CrossRef]
- Alter, S.C.; Kramps, J.A.; Janoff, A.; Schwartz, L.B. Interactions of human mast cell tryptase with biological protease inhibitors. Arch. Biochem. Biophys. 1990, 276, 26–31. [Google Scholar] [CrossRef]
- Le, Q.T.; Lyons, J.J.; Naranjo, A.N.; Olivera, A.; Lazarus, R.A.; Metcalfe, D.D.; Milner, J.D.; Schwartz, L.B. Impact of naturally forming human alpha/beta-tryptase heterotetramers in the pathogenesis of hereditary alpha-tryptasemia. J. Exp. Med. 2019, 216, 2348–2361. [Google Scholar] [CrossRef]
- Boyden, S.E.; Desai, A.; Cruse, G.; Young, M.L.; Bolan, H.C.; Scott, L.M.; Eisch, A.R.; Long, R.D.; Lee, C.C.; Satorius, C.L.; et al. Vibratory Urticaria Associated with a Missense Variant in ADGRE2. N. Engl. J. Med. 2016, 374, 656–663. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, S.; Xu, L.; Yang, H.; He, S. Activation of protease-activated receptor 2-mediated signaling by mast cell tryptase modulates cytokine production in primary cultured astrocytes. Mediat. Inflamm. 2013, 2013, 140812. [Google Scholar] [CrossRef]
- Molino, M.; Barnathan, E.S.; Numerof, R.; Clark, J.; Dreyer, M.; Cumashi, A.; Hoxie, J.A.; Schechter, N.; Woolkalis, M.; Brass, L.F. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997, 272, 4043–4049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corvera, C.U.; Dery, O.; McConalogue, K.; Bohm, S.K.; Khitin, L.M.; Caughey, G.H.; Payan, D.G.; Bunnett, N.W. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J. Clin. Invest. 1997, 100, 1383–1393. [Google Scholar] [CrossRef] [Green Version]
- Carrigan, C.; Milner, J.D.; Lyons, J.J.; Vadas, P. Usefulness of testing for hereditary alpha tryptasemia in symptomatic patients with elevated tryptase. J. Allergy Clin. Immunol. 2020, 8, 2066–2067. [Google Scholar] [CrossRef]
- Greiner, G.; Gurbisz, M.; Ratzinger, F.; Witzeneder, N.; Simonitsch-Klupp, I.; Mitterbauer-Hohendanner, G.; Mayerhofer, M.; Mullauer, L.; Sperr, W.R.; Valent, P.; et al. Digital PCR: A sensitive and precise method for KIT d816v quantification in mastocytosis. Clin. Chem. 2018, 64, 547–555. [Google Scholar] [CrossRef]
- Hoermann, G.; Gleixner, K.V.; Dinu, G.E.; Kundi, M.; Greiner, G.; Wimazal, F.; Hadzijusufovic, E.; Mitterbauer, G.; Mannhalter, C.; Valent, P.; et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy 2014, 69, 810–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannetti, M.P.; Akin, C.; Hufdhi, R.; Hamilton, M.J.; Weller, E.; van Anrooij, B.; Lyons, J.J.; Hornick, J.L.; Pinkus, G.; Castells, M.; et al. Patients with mast cell activation symptoms and elevated baseline serum tryptase have unique bone marrow morphology. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Nedoszytko, B.; Bonadonna, P.; Hartmann, K.; Niedoszytko, M.; Brockow, K.; Siebenhaar, F.; Triggiani, M.; Arock, M.; et al. Diagnosis, Classification and Management of Mast Cell Activation Syndromes (MCAS) in the era of personalized medicine. Int. J. Mol. Sci. 2020, 21, 9030. [Google Scholar] [CrossRef]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- Mendoza Alvarez, L.B.; Barker, R.; Nelson, C.; DiMaggio, T.; Stone, K.D.; Milner, J.D.; Rosenthal, J.A.; Petroni, D.H.; Glover, S.C.; Lyons, J.J. Clinical response to omalizumab in patients with hereditary alpha-tryptasemia. Ann. Allergy Asthma Immunol 2020, 124, 99–100. [Google Scholar] [CrossRef] [Green Version]
- Maun, H.R.; Jackman, J.K.; Choy, D.F.; Loyet, K.M.; Staton, T.L.; Jia, G.; Dressen, A.; Hackney, J.A.; Bremer, M.; Walters, B.T.; et al. An allosteric anti-tryptase antibody for the treatment of mast cell-mediated severe asthma. Cell 2019, 179, 417–431. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprinzl, B.; Greiner, G.; Uyanik, G.; Arock, M.; Haferlach, T.; Sperr, W.R.; Valent, P.; Hoermann, G. Genetic Regulation of Tryptase Production and Clinical Impact: Hereditary Alpha Tryptasemia, Mastocytosis and Beyond. Int. J. Mol. Sci. 2021, 22, 2458. https://doi.org/10.3390/ijms22052458
Sprinzl B, Greiner G, Uyanik G, Arock M, Haferlach T, Sperr WR, Valent P, Hoermann G. Genetic Regulation of Tryptase Production and Clinical Impact: Hereditary Alpha Tryptasemia, Mastocytosis and Beyond. International Journal of Molecular Sciences. 2021; 22(5):2458. https://doi.org/10.3390/ijms22052458
Chicago/Turabian StyleSprinzl, Bettina, Georg Greiner, Goekhan Uyanik, Michel Arock, Torsten Haferlach, Wolfgang R. Sperr, Peter Valent, and Gregor Hoermann. 2021. "Genetic Regulation of Tryptase Production and Clinical Impact: Hereditary Alpha Tryptasemia, Mastocytosis and Beyond" International Journal of Molecular Sciences 22, no. 5: 2458. https://doi.org/10.3390/ijms22052458
APA StyleSprinzl, B., Greiner, G., Uyanik, G., Arock, M., Haferlach, T., Sperr, W. R., Valent, P., & Hoermann, G. (2021). Genetic Regulation of Tryptase Production and Clinical Impact: Hereditary Alpha Tryptasemia, Mastocytosis and Beyond. International Journal of Molecular Sciences, 22(5), 2458. https://doi.org/10.3390/ijms22052458





