Molecular Dissection of Escherichia coli CpdB: Roles of the N Domain in Catalysis and Phosphate Inhibition, and of the C Domain in Substrate Specificity and Adenosine Inhibition
Abstract
:1. Introduction
2. Results
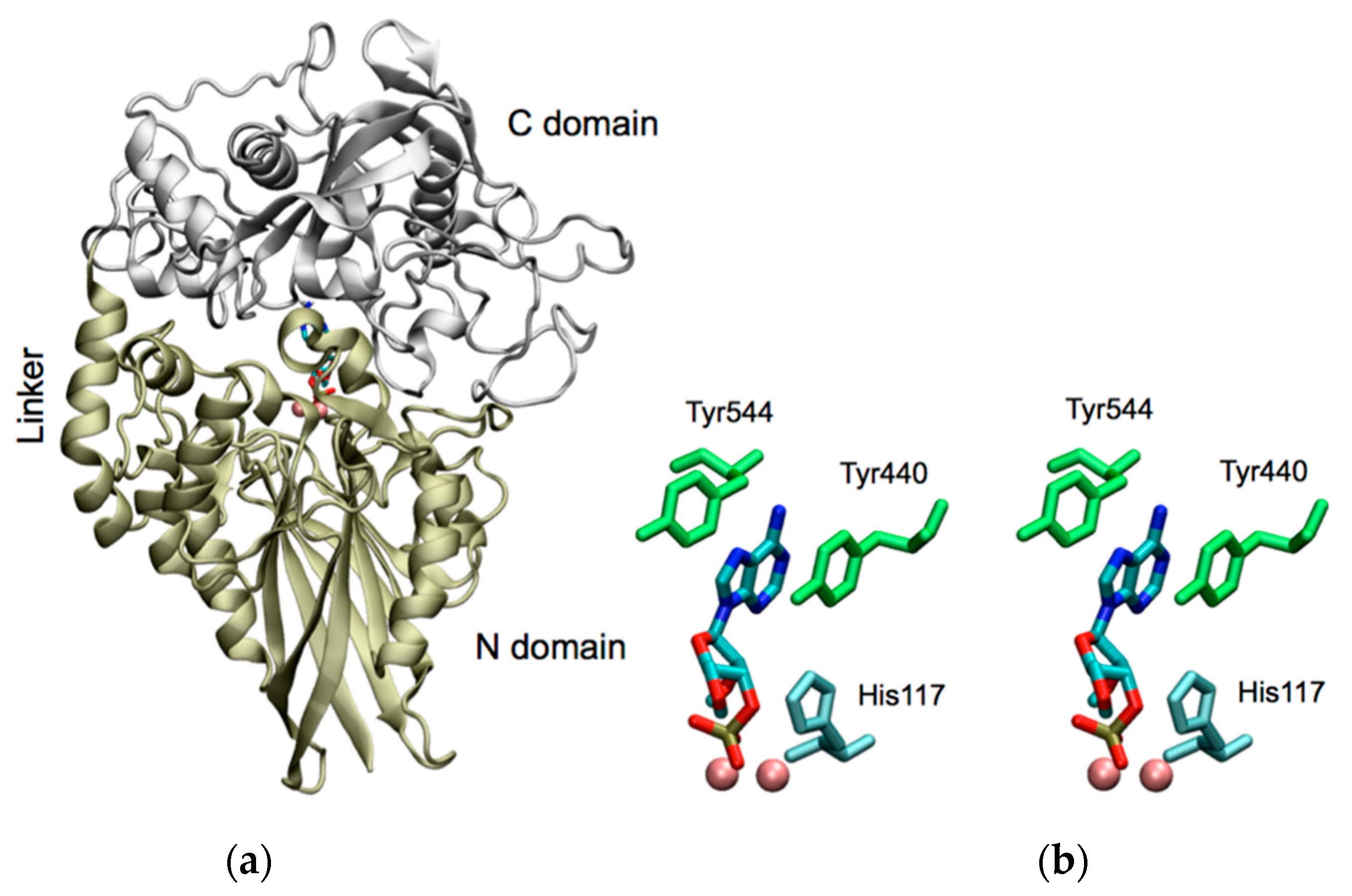
2.1. Molecular Modeling of CpdB Reveals a Two-Domain Conformation with Overall Similitude to 5′-Nucleotidase UshA and with Sequential and Spatial Conservation of Catalytic Histidine and Substrate-Binding Aromatic Residues
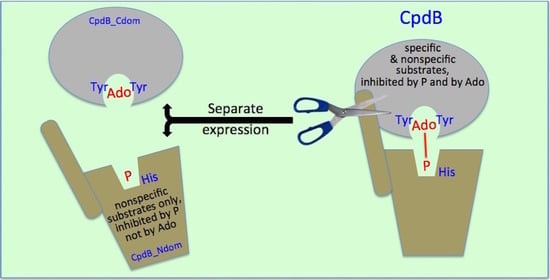
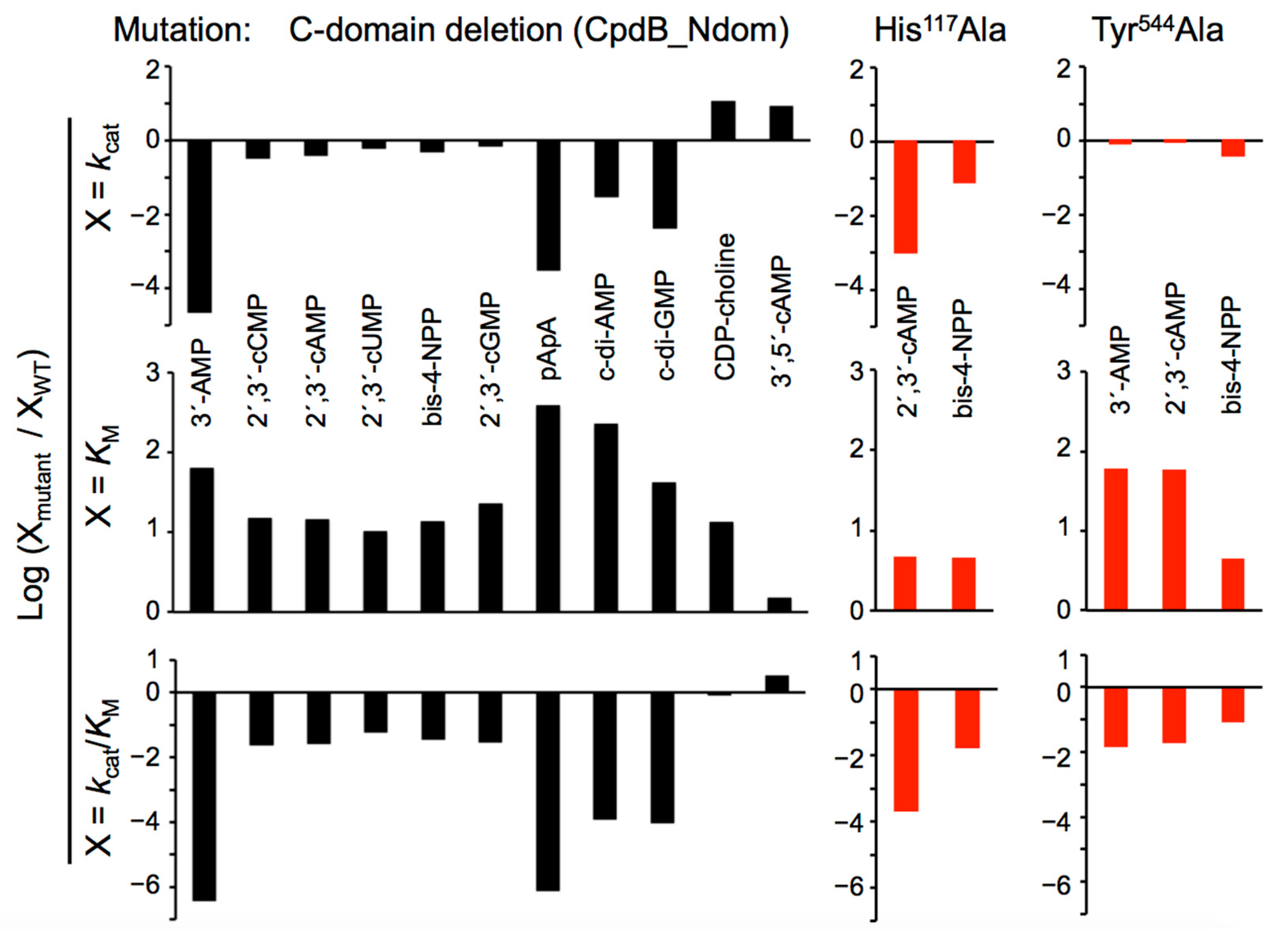
2.2. Catalytic Properties of the N and C Domains of Mature CpdB Expressed Separately, and of Point Mutants of Mature CpdB: His117Ala in the Catalytic Site (N Domain) and Tyr544Ala in the Substrate Binding Site (C Domain)
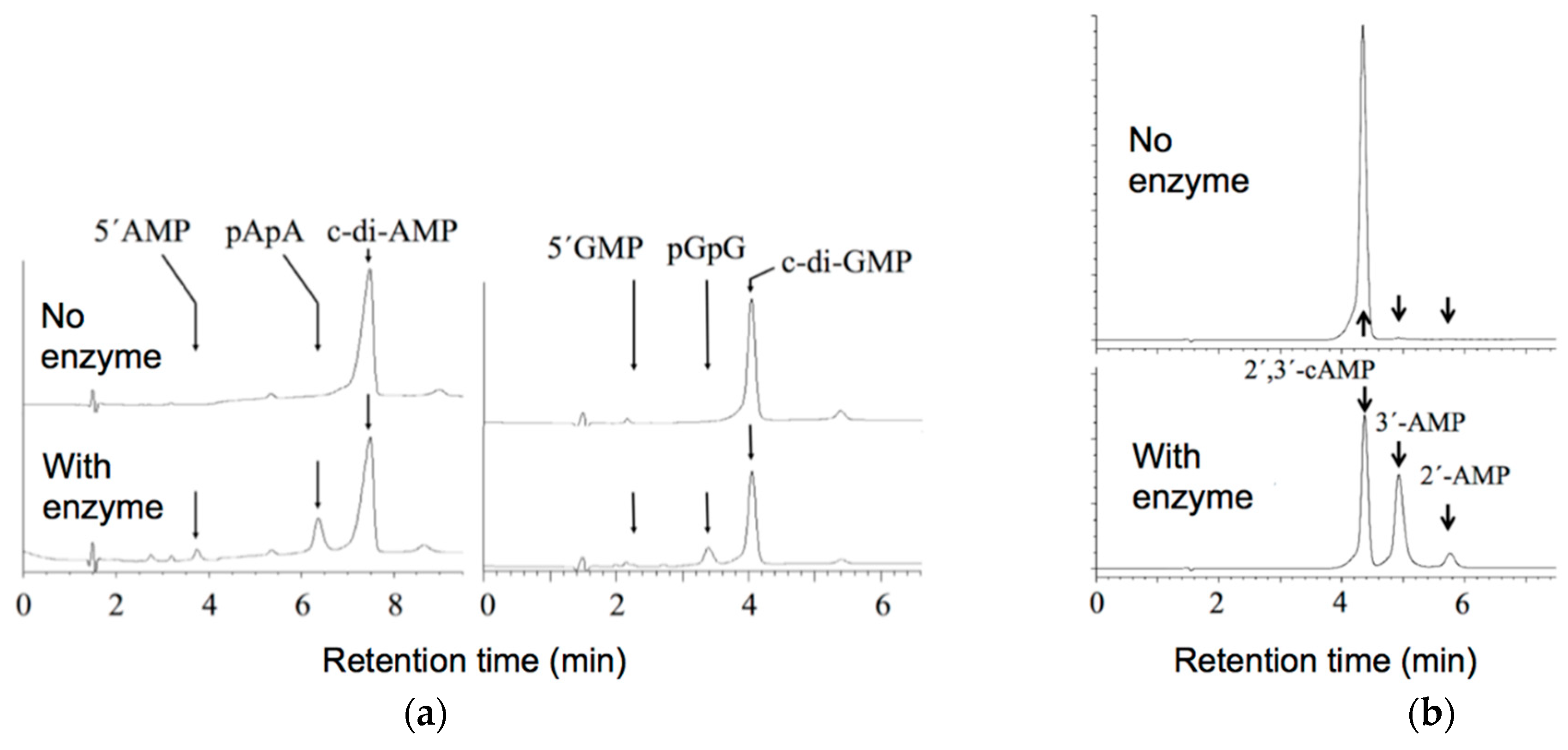
2.3. Alteration of the Reaction Products of the Hydrolysis of Cyclic (di)Nucleotides by the Deletion of the C Domain of Mature CpdB
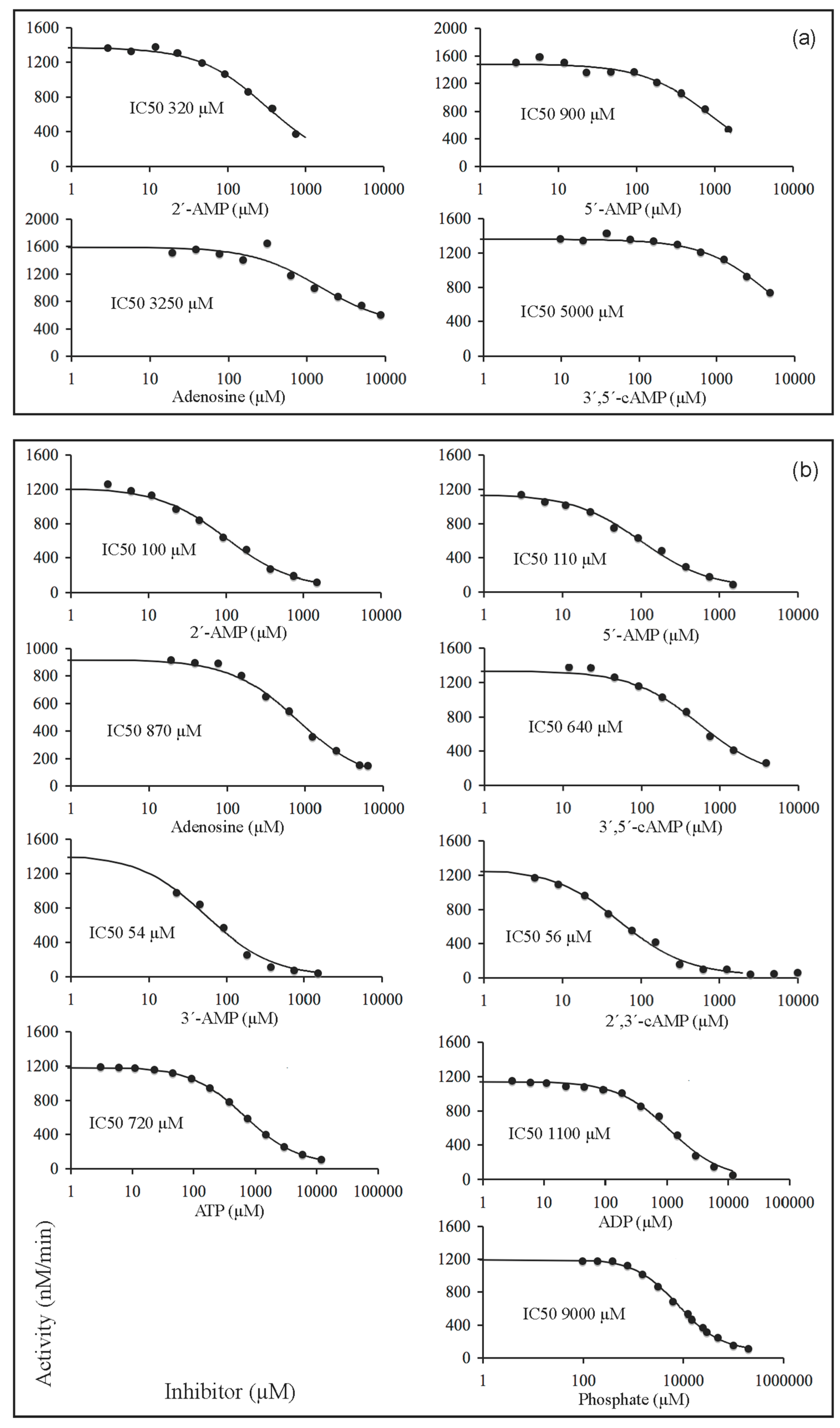
2.4. Inhibition of the Activity of Mature CpdB and CpdB_Ndom on 3′-AMP or bis-4-NPP by Alternative Substrates and Non-Substrates
3. Discussion
4. Materials and Methods
4.1. Homology Modeling of CpdB and Docking of 3′-AMP to the Active Site
4.2. Construction of Plasmids Encoding Point Mutants, the N Domain (CpdB_Ndom) and the C Domain (CpdB_Cdom) of the CpdB Protein
4.3. Expression and Purification of Recombinant Proteins
4.4. Enzyme Activity Assays
4.5. Analyses of Saturation and Inhibition Kinetics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| bis-4-NPP | Bis-4-nitrophenylphosphate |
| c-di-AMP | 3′,5′-cyclic diadenosine monophosphate |
| c-di-GMP | 3′,5′-cyclic diguanosine monophosphate |
| CpdB_Cdom | C-terminal domain of CpdB |
| CpdB_Ndom | N-terminal domain of CpdB |
| HPLC | High-performance liquid chromatography |
| GST | Glutathione S-transferase |
| EDTA | Ethylenediaminetetraacetate |
| pApA | 5′-phosphoadenylyl-3′→5′-adenosine |
| PDB | Protein Data Bank |
| pGpG | 5′-phosphoguanylyl-3′→5′-guanosine |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| Tris | Tris(hydroxymethyl)aminomethane |
References
- Anraku, Y. A new cyclic phosphodiesterase having a 3′-nucleotidase activity from Escherichia coli B. I. Purification and some properties of the enzyme. J. Biol. Chem. 1964, 239, 3412–3419. [Google Scholar] [CrossRef]
- Anraku, Y. A new cyclic phosphodiesterase having a 3′-nucleotidase activity from Escherichia coli B. II. Further studies on substrate specificity and mode of action of the enzyme. J. Biol. Chem. 1964, 239, 3420–3424. [Google Scholar] [CrossRef]
- Liu, J.; Burns, D.M.; Beacham, I.R. Isolation and sequence analysis of the gene (cpdB) encoding periplasmic 2′,3′-cyclic phosphodiesterase. J. Bacteriol. 1986, 165, 1002–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Villamizar, I.; Cabezas, A.; Pinto, R.M.; Canales, J.; Ribeiro, J.M.; Cameselle, J.C.; Costas, M.J. The characterization of Escherichia coli CpdB as a recombinant protein reveals that, besides having the expected 3′-nucleotidase and 2′,3′-cyclic mononucleotide phosphodiesterase activities, it is also active as cyclic dinucleotide phosphodiesterase. PLoS ONE 2016, 11, e0157308. [Google Scholar] [CrossRef]
- Romling, U.; Liang, Z.X.; Dow, J.M. Progress in understanding the molecular basis underlying functional diversification of cyclic dinucleotide turnover proteins. J. Bacteriol. 2017, 199, e00790-16. [Google Scholar] [CrossRef] [Green Version]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [Green Version]
- Andrade, W.A.; Firon, A.; Schmidt, T.; Hornung, V.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Trieu-Cuot, P.; Golenbock, D.T.; Kaminski, P.A. Group B Streptococcus degrades cyclic-di-AMP to modulate STING-dependent type I interferon production. Cell Host Microbe 2016, 20, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrell, J.W. Ribonuclease I released from Escherichia coli by osmotic shock. Arch. Biochem. Biophys. 1971, 142, 693–700. [Google Scholar] [CrossRef]
- Cuchillo, C.M.; Nogues, M.V.; Raines, R.T. Bovine pancreatic ribonuclease: Fifty years of the first enzymatic reaction mechanism. Biochemistry 2011, 50, 7835–7841. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, B.M.; Martin, K.S.; Garcia-Rodriguez, J.M.; Jung, C.; Briggs, L.; Southwell, J.E.; Jia, X.; Weinert, E.E. RNase I regulates Escherichia coli 2′,3′-cyclic nucleotide monophosphate levels and biofilm formation. Biochem. J. 2018, 475, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, L.; Si, W.; Wang, C.; Zhu, F.; Li, G.; Liu, S. Physiology and pathogenicity of cpdB deleted mutant of avian pathogenic Escherichia coli. Res. Vet. Sci. 2017, 111, 21–25. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.; Wang, X.; Si, W.; Wang, H.; Wang, C.; Liu, S.; Li, G. Decrease of colonization in the chicks’ cecum and internal organs of Salmonella enterica serovar Pullorum by deletion of cpdB by Red system. Microb. Pathog. 2015, 80, 21–26. [Google Scholar] [CrossRef]
- Osaki, M.; Takamatsu, D.; Shimoji, Y.; Sekizaki, T. Characterization of Streptococcus suis genes encoding proteins homologous to sortase of gram-positive bacteria. J. Bacteriol. 2002, 184, 971–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, D.S.; MacInnes, J.I. Differential expression of Haemophilus parasuis genes in response to iron restriction and cerebrospinal fluid. Can. J. Vet. Res. 2007, 71, 181–188. [Google Scholar] [PubMed]
- Li, W.; Liu, L.; Chen, H.; Zhou, R. Identification of Streptococcus suis genes preferentially expressed under iron starvation by selective capture of transcribed sequences. FEMS Microbiol. Lett. 2009, 292, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Tseng, S.P.; Lin, Y.Y.; Tsai, J.C.; Hsueh, P.R.; Chen, H.J.; Hung, W.C.; Teng, L.J. Distribution of emm types and genetic characterization of the mgc locus in group G Streptococcus dysgalactiae subsp. equisimilis from a hospital in northern Taiwan. J. Clin. Microbiol. 2010, 48, 2975–2977. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Xu, T.; Fang, Q.; Yu, L.; Zhu, J.; Chen, L.; Liu, J.; Zhou, R. The surface-exposed protein SntA contributes to complement evasion in zoonotic Streptococcus suis. Front. Immunol. 2018, 9, 1063. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, K.; Sabareesh, V.; Singh, N.; Saigal, K.; Mechold, U.; Sinha, K.M. Two-step synthesis and hydrolysis of cyclic di-AMP in Mycobacterium tuberculosis. PLoS ONE 2014, 9, e86096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, R.J.; Dey, B.; Zheng, Y.; Cheung, L.S.; Zhou, J.; Sayre, D.; Kumar, P.; Guo, H.; Lamichhane, G.; Sintim, H.O.; et al. Inhibition of innate immune cytosolic surveillance by an M. tuberculosis phosphodiesterase. Nat. Chem. Biol. 2017, 13, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Host-Pathogen interactions: Nucleotide circles of life and death. Nat. Chem. Biol. 2017, 13, 130–131. [Google Scholar] [CrossRef]
- Woodward, J.J.; Iavarone, A.T.; Portnoy, D.A. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 2010, 328, 1703–1705. [Google Scholar] [CrossRef] [Green Version]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011, 478, 515–518. [Google Scholar] [CrossRef]
- Parvatiyar, K.; Zhang, Z.; Teles, R.M.; Ouyang, S.; Jiang, Y.; Iyer, S.S.; Zaver, S.A.; Schenk, M.; Zeng, S.; Zhong, W.; et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat. Immunol. 2012, 13, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Bai, Y.; Zhang, Y.; Gabrielle, V.D.; Jin, L.; Bai, G. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection. Mol. Microbiol. 2014, 93, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, B.; Bishai, W.R. Crosstalk between Mycobacterium tuberculosis and the host cell. Semin. Immunol. 2014, 26, 486–496. [Google Scholar] [CrossRef] [Green Version]
- Danilchanka, O.; Mekalanos, J.J. Cyclic dinucleotides and the innate immune response. Cell 2013, 154, 962–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaux, L.; Kaminski, P.A.; Trieu-Cuot, P.; Firon, A. Cyclic di-AMP in host-pathogen interactions. Curr. Opin. Microbiol. 2018, 41, 21–28. [Google Scholar] [CrossRef]
- Eaglesham, J.B.; Kranzusch, P.J. Conserved strategies for pathogen evasion of cGAS-STING immunity. Curr. Opin. Immunol. 2020, 66, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Glaser, L.; Melo, A.; Paul, R. Uridine diphosphate sugar hydrolase. Purification of enzyme and protein inhibitor. J. Biol. Chem. 1967, 242, 1944–1954. [Google Scholar] [CrossRef]
- Melo, A.; Glaser, L. Nucleotide diphosphate hexose pyrophosphatases. Biochem. Biophys. Res. Commun. 1966, 22, 524–531. [Google Scholar] [CrossRef]
- Neu, H.C. The 5′-nucleotidase of Escherichia coli. I. Purification and properties. J. Biol. Chem. 1967, 242, 3896–3904. [Google Scholar] [CrossRef]
- Neu, H.C. The 5′-nucleotidases and cyclic phosphodiesterases (3′-nucleotidases) of the Enterobacteriaceae. J. Bacteriol. 1968, 95, 1732–1737. [Google Scholar] [CrossRef] [Green Version]
- Neu, H.C.; Heppel, L.A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J. Biol. Chem. 1965, 240, 3685–3692. [Google Scholar] [CrossRef]
- Ruiz, A.; Hurtado, C.; Meireles Ribeiro, J.; Sillero, A.; Günther Sillero, M.A. Hydrolysis of bis(5′-nucleosidyl) polyphosphates by Escherichia coli 5′-nucleotidase. J. Bacteriol. 1989, 171, 6703–6709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves-Pereira, I.; Canales, J.; Cabezas, A.; Cordero, P.M.; Costas, M.J.; Cameselle, J.C. CDP-alcohol hydrolase, a very efficient activity of the 5′-nucleotidase/UDP-sugar hydrolase encoded by the ushA gene of Yersinia intermedia and Escherichia coli. J. Bacteriol. 2008, 190, 6153–6161. [Google Scholar] [CrossRef] [Green Version]
- Knöfel, T.; Sträter, N. X-ray structure of the Escherichia coli periplasmic 5′-nucleotidase containing a dimetal catalytic site. Nat. Struct. Biol. 1999, 6, 448–453. [Google Scholar] [CrossRef]
- Knöfel, T.; Sträter, N. Mechanism of hydrolysis of phosphate esters by the dimetal center of 5′-nucleotidase based on crystal structures. J. Mol. Biol. 2001, 309, 239–254. [Google Scholar] [CrossRef]
- Knöfel, T.; Sträter, N.E. coli 5′-nucleotidase undergoes a hinge-bending domain rotation resembling a ball-and-socket motion. J. Mol. Biol. 2001, 309, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Heienbrok, R.; Maier, T.; Strater, N. Trapping a 96 degrees domain rotation in two distinct conformations by engineered disulfide bridges. Protein Sci. 2004, 13, 1811–1822. [Google Scholar] [CrossRef]
- Schultz-Heienbrok, R.; Maier, T.; Sträter, N. A large hinge bending domain rotation is necessary for the catalytic function of Escherichia coli 5′-nucleotidase. Biochemistry 2005, 44, 2244–2252. [Google Scholar] [CrossRef]
- Sträter, N. Ecto-5′-nucleotidase: Structure function relationships. Purinergic Signal. 2006, 2, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Krug, U.; Patzschke, R.; Zebisch, M.; Balbach, J.; Sträter, N. Contribution of the two domains of E. coli 5′-nucleotidase to substrate specificity and catalysis. FEBS Lett. 2013, 5793, 010. [Google Scholar] [CrossRef] [Green Version]
- Krug, U.; Alexander, N.S.; Stein, R.A.; Keim, A.; McHaourab, H.S.; Strater, N.; Meiler, J. Characterization of the domain orientations of E. coli 5′-nucleotidase by fitting an ensemble of conformers to DEER distance distributions. Structure 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koonin, E.V. Conserved sequence pattern in a wide variety of phosphoesterases. Protein Sci. 1994, 3, 356–358. [Google Scholar] [CrossRef] [Green Version]
- Fontes, R.; Ribeiro, J.M.; Sillero, A. Inhibition and activation of enzymes. The effect of a modifier on the reaction rate and on kinetic parameters. Acta Biochim. Pol. 2000, 47, 233–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Rodrigues, J.R.; Couto, A.; Cabezas, A.; Pinto, R.M.; Ribeiro, J.M.; Canales, J.; Costas, M.J.; Cameselle, J.C. Bifunctional homodimeric triokinase/FMN cyclase: Contribution of protein domains to the activities of the human enzyme and molecular dynamics simulation of domain movements. J. Biol. Chem. 2014, 289, 10620–10636. [Google Scholar] [CrossRef] [Green Version]
- Neu, H.C. The cyclic phosphodiesterases (3′-nucleotidases) of the Enterobacteriaceae. Biochemistry 1968, 7, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Unemoto, T.; Takahashi, F.; Hayashi, M. Relationship between the active sites of 2′,3′-cyclic phosphodiesterase with 3′-nucleotidase activity purified from Vibrio alginolyticus. Biochim. Biophys. Acta 1969, 185, 134–142. [Google Scholar] [CrossRef]
- Unemoto, T.; Hayashi, M. Chloride ion as a modifier of 2′,3′-cyclic phosphodiesterase purified from halophilic Vibrio alginolyticus. Biochim. Biophys. Acta 1969, 171, 89–102. [Google Scholar] [CrossRef]
- Beacham, I.R.; Garrett, S. Isolation of Escherichia coli mutants (cpdB) deficient in periplasmic 2′:3′-cyclic phosphodiesterase and genetic mapping of the cpdB locus. J. Gen. Microbiol. 1980, 119, 31–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, F.; Qi, Y.; Murugan, E.; Pasunooti, S.; Ji, Q. 2′,3′-cAMP hydrolysis by metal-dependent phosphodiesterases containing DHH, EAL, and HD domains is non-specific: Implications for PDE screening. Biochem. Biophys. Res. Commun. 2010, 398, 500–505. [Google Scholar] [CrossRef]
- Rodrigues, J.R.; Fernández, A.; Canales, J.; Cabezas, A.; Ribeiro, J.M.; Costas, M.J.; Cameselle, J.C. Characterization of Danio rerio Mn2+-dependent ADP-ribose/CDP-alcohol diphosphatase, the structural prototype of the ADPRibase-Mn-like protein family. PLoS ONE 2012, 7, e42249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Sali, A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006, 15, 2507–2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Word, J.M.; Lovell, S.C.; Richardson, J.S.; Richardson, D.C. Asparagine and glutamine: Using hydrogen atom contacts in the choice of side-chain amide orientation. J. Mol. Biol. 1999, 285, 1735–1747. [Google Scholar] [CrossRef] [Green Version]
- Sanner, M.F. A component-based software environment for visualizing large macromolecular assemblies. Structure 2005, 13, 447–462. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Lazar, I., Jr.; Lazar, I., Sr. Gel Analyzer 2010. Available online: http://www.gelanalyzer.com (accessed on 29 November 2020).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
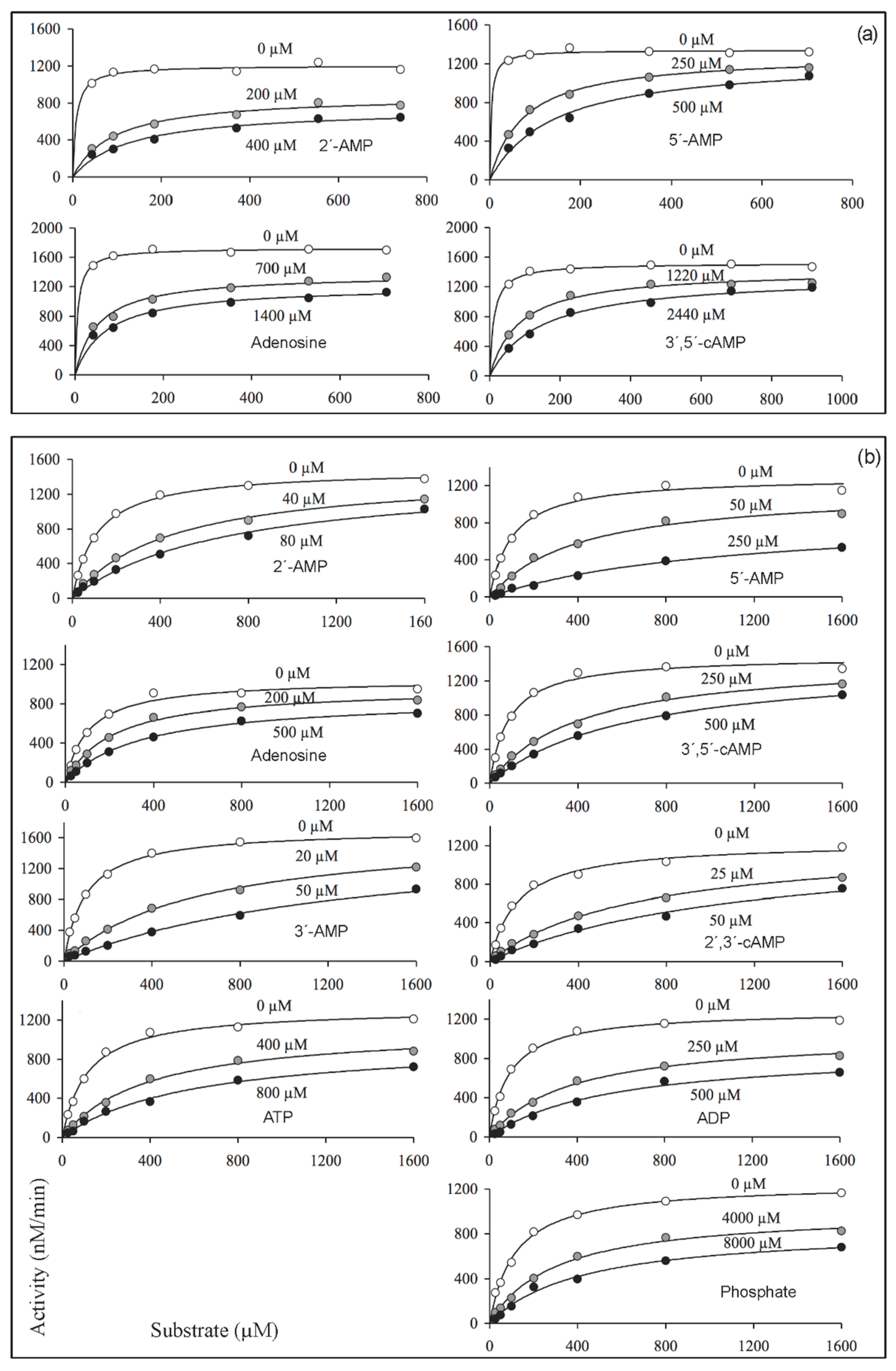

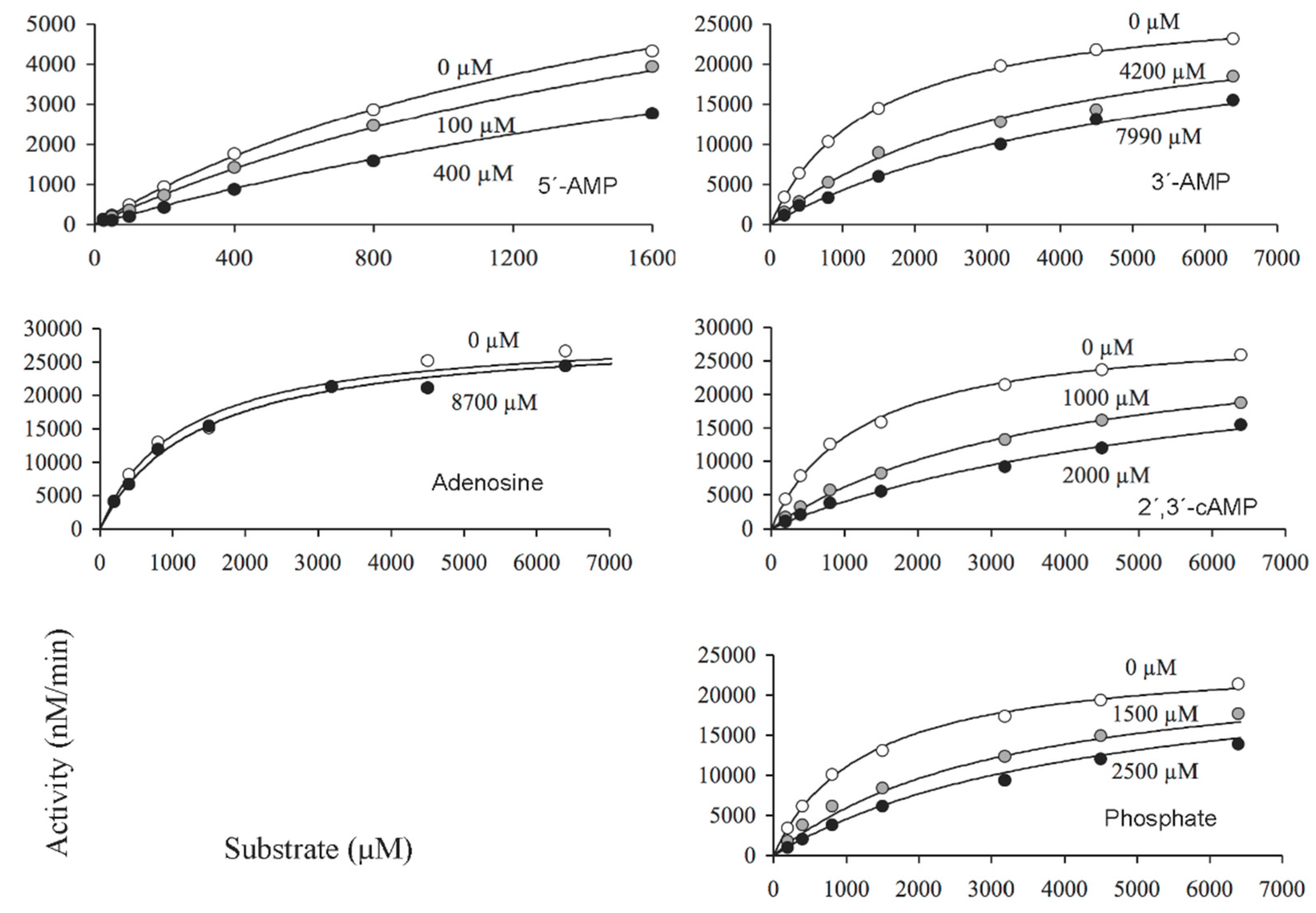

| Protein | Substrate | kcat | KM | kcat/KM |
|---|---|---|---|---|
| s−1 | µM | M−1 s−1 | ||
| CpdB_Ndom 1 | 2′,3′-cUMP | 155 ± 32 | 630 ± 170 | 250,000 ± 50,000 |
| 2′,3′-cCMP | 33 ± 5 | 180 ± 50 | 200,000 ± 80,000 | |
| 2′,3′-cAMP | 73 ± 32 | 390 ± 170 | 190,000 ± 13,000 | |
| bis-4-NPP | 164 ± 14 | 1300 ± 80 | 130,000 ± 7000 | |
| 2′,3′-cGMP | 58 ± 16 | 570 ± 160 | 100,000 ± 13,000 | |
| CDP-choline | 5.7 ± 3.5 | 2900 ± 1700 | 1950 ± 130 | |
| 3′,5′-cAMP | 4.7 ± 1.6 | 6900 ± 2600 | 700 ± 60 | |
| 3′-AMP | 0.004 ± 0.0002 | 880 ± 250 | 5.2 ± 1.0 | |
| c-di-AMP | 0.012 ± 0.007 | 3400 ± 2200 | 3.5 ± 0.1 | |
| pApA | 0.0004 ± 0.0001 | 230 ± 40 | 1.8 ± 0.2 | |
| c-di-GMP | 0.0003 ± 0.0001 | 250 ± 30 | 1.2 ± 0.4 | |
| His117Ala-CpdB 2 | bis-4-NPP | 26 ± 3 | 440 ± 90 | 60,000 ± 7000 |
| 2′,3′-cAMP | 0.18 ± 0.02 | 126 ± 7 | 1500 ± 230 | |
| Tyr544Ala-CpdB 3 | bis-4-NPP | 130 ± 34 | 430 ± 30 | 300,000 ± 100,000 |
| 3′-AMP | 160 ± 37 | 850 ± 110 | 190,000 ± 62,000 | |
| 2′,3′-cAMP | 190 ± 60 | 1600 ± 550 | 140,000 ± 73,000 | |
| Mature CpdB 4 | 2′,3′-cUMP | 260 | 62 | 4,200,000 |
| 2′,3′-cCMP | 100 | 12 | 8,500,000 | |
| 2′,3′-cAMP | 190 | 27 | 7,300,000 | |
| bis-4-NPP | 340 | 96 | 3,600,000 | |
| 2′,3′-cGMP | 84 | 25 | 3,500,000 | |
| CDP-choline | 0.51 | 219 | 2300 | |
| 3′,5′-cAMP | 0.56 | 4600 | 220 | |
| 3′-AMP | 176 | 14 | 13,000,000 | |
| c-di-AMP | 0.40 | 15 | 29,000 | |
| pApA | 1.30 | 0.6 | 2,300,000 | |
| c-di-GMP | 0.07 | 6 | 13,000 |
| Substrate | Inhibitor | CpdB 1 | CpdB_Ndom 2 |
|---|---|---|---|
| µM | µM | ||
| 3′-AMP | 2′-AMP | 7.1 ± 3.2 | — |
| 5′-AMP | 20 ± 8 | — | |
| Adenosine | 41 ± 17 | — | |
| 3′,5′-cAMP | 145 ± 38 | — | |
| Bis-4-NPP | 2′-AMP | 8.8 ± 2.0 | 600 3 |
| 5′-AMP | 14 | 370 ± 50 | |
| Adenosine | 160 | 48,000 ± 11,000 | |
| 3′,5′-cAMP | 60 | — | |
| 3′-AMP | 3.6 ± 0.2 | 3300 ± 550 | |
| 2′,3′-cAMP | 5.0 ± 0.4 | 460 ± 50 | |
| ATP | 113 ± 19 | 5700 3 | |
| ADP | 60 | — | |
| Phosphate | 1380 ± 340 | 1060 ± 210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Villamizar, I.; Cabezas, A.; Pinto, R.M.; Canales, J.; Ribeiro, J.M.; Rodrigues, J.R.; Costas, M.J.; Cameselle, J.C. Molecular Dissection of Escherichia coli CpdB: Roles of the N Domain in Catalysis and Phosphate Inhibition, and of the C Domain in Substrate Specificity and Adenosine Inhibition. Int. J. Mol. Sci. 2021, 22, 1977. https://doi.org/10.3390/ijms22041977
López-Villamizar I, Cabezas A, Pinto RM, Canales J, Ribeiro JM, Rodrigues JR, Costas MJ, Cameselle JC. Molecular Dissection of Escherichia coli CpdB: Roles of the N Domain in Catalysis and Phosphate Inhibition, and of the C Domain in Substrate Specificity and Adenosine Inhibition. International Journal of Molecular Sciences. 2021; 22(4):1977. https://doi.org/10.3390/ijms22041977
Chicago/Turabian StyleLópez-Villamizar, Iralis, Alicia Cabezas, Rosa María Pinto, José Canales, João Meireles Ribeiro, Joaquim Rui Rodrigues, María Jesús Costas, and José Carlos Cameselle. 2021. "Molecular Dissection of Escherichia coli CpdB: Roles of the N Domain in Catalysis and Phosphate Inhibition, and of the C Domain in Substrate Specificity and Adenosine Inhibition" International Journal of Molecular Sciences 22, no. 4: 1977. https://doi.org/10.3390/ijms22041977
APA StyleLópez-Villamizar, I., Cabezas, A., Pinto, R. M., Canales, J., Ribeiro, J. M., Rodrigues, J. R., Costas, M. J., & Cameselle, J. C. (2021). Molecular Dissection of Escherichia coli CpdB: Roles of the N Domain in Catalysis and Phosphate Inhibition, and of the C Domain in Substrate Specificity and Adenosine Inhibition. International Journal of Molecular Sciences, 22(4), 1977. https://doi.org/10.3390/ijms22041977