PF-3845, a Fatty Acid Amide Hydrolase Inhibitor, Directly Suppresses Osteoclastogenesis through ERK and NF-κB Pathways In Vitro and Alveolar Bone Loss In Vivo
Abstract
:1. Introduction
2. Results
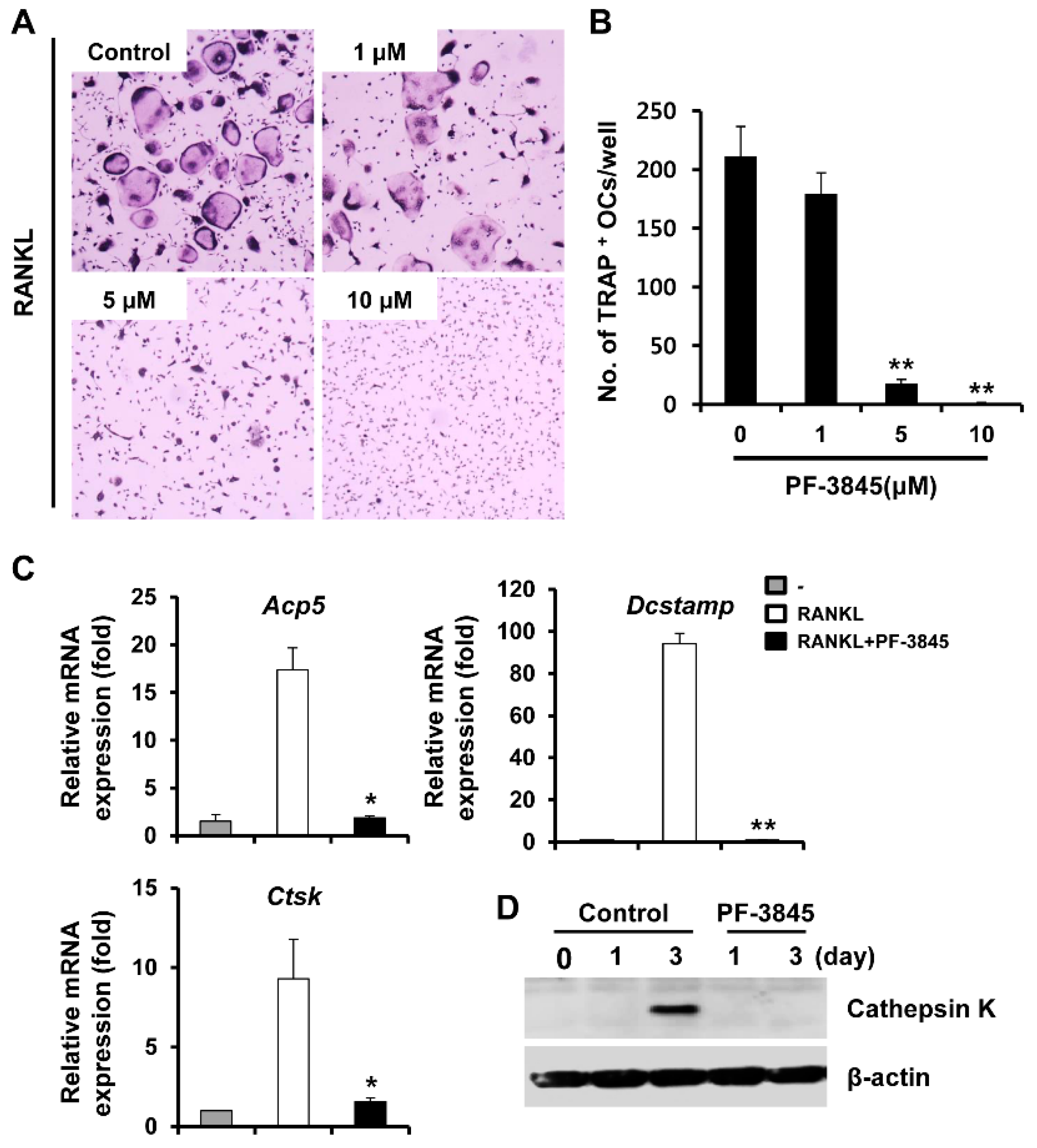
2.1. Effect of PF-3845 on Osteoclast Differentiation
2.2. Effect of PF-3845 on RANKL-Mediated NFATc1 Induction and Actin Ring Formation
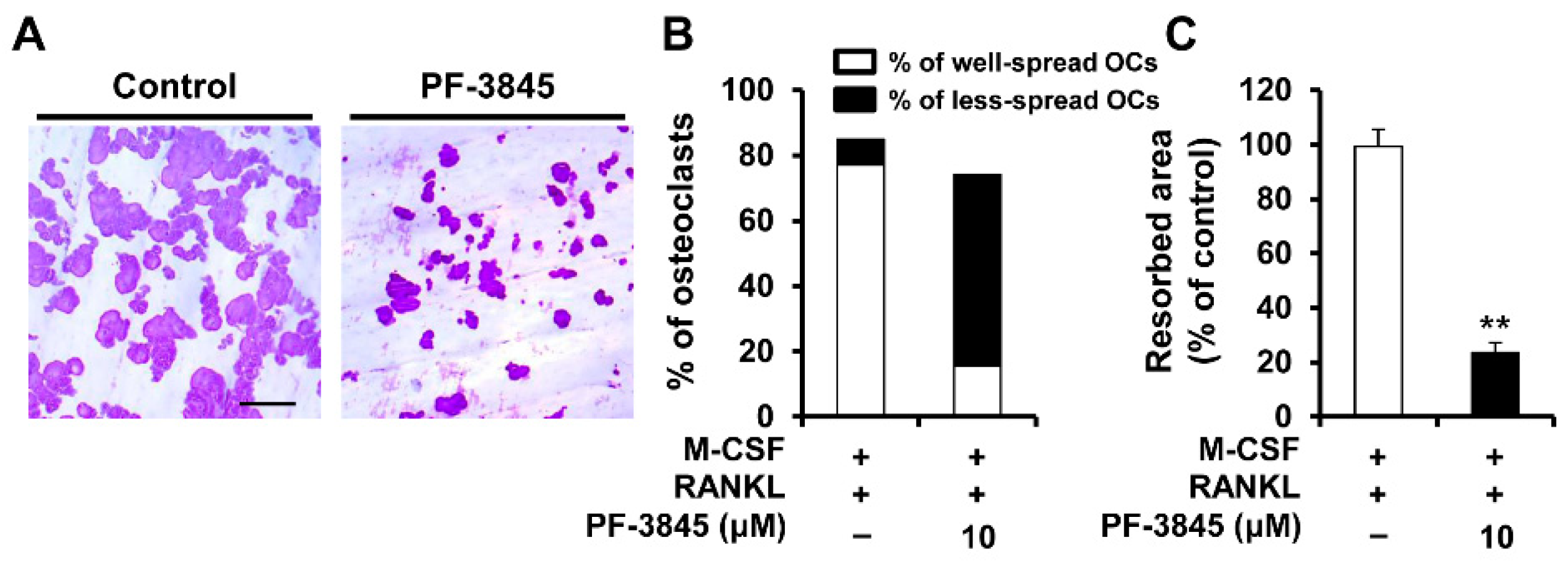
2.3. Effect of PF-3845 on Bone-Resorbing Activity
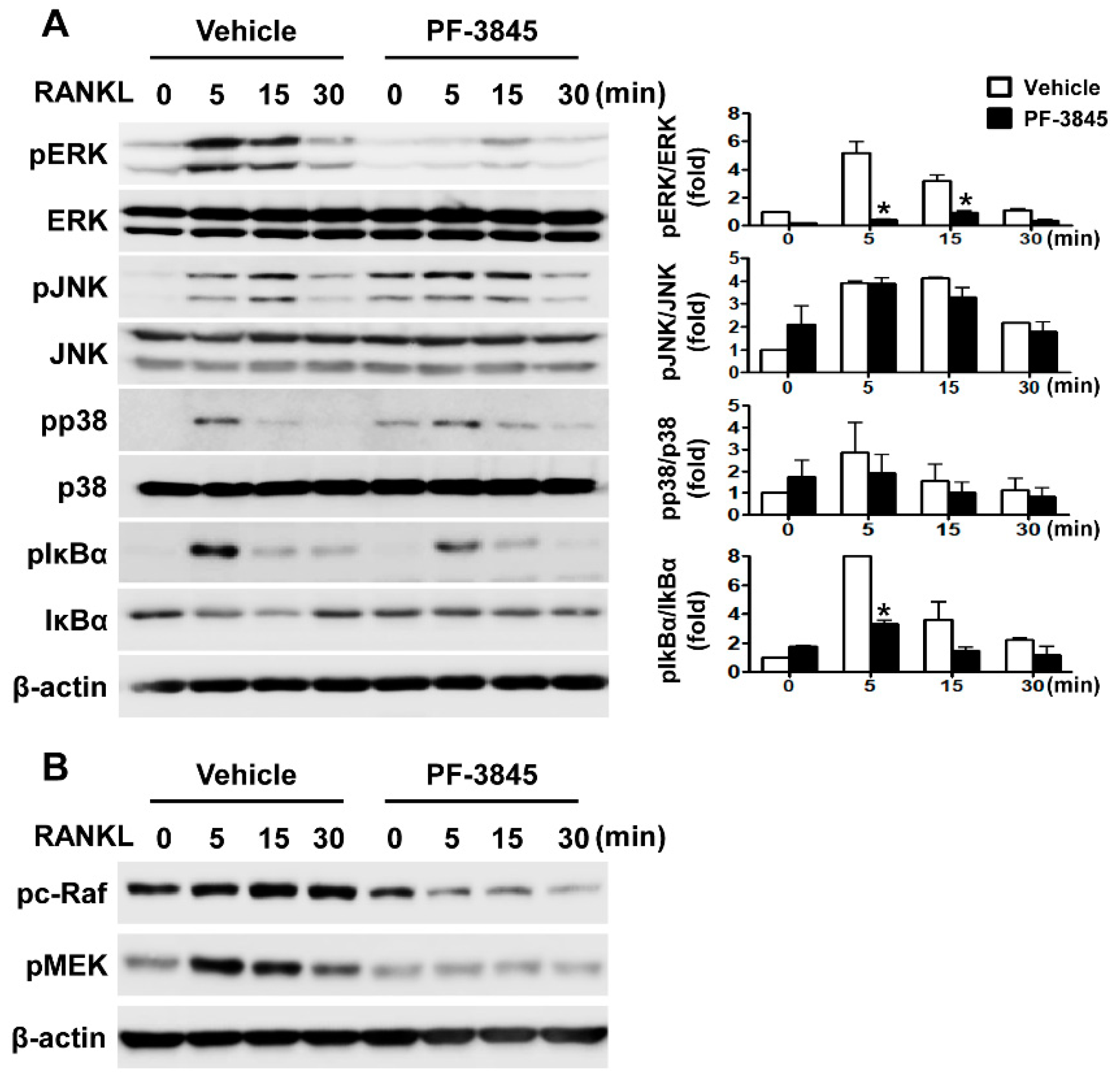
2.4. Effect of PF-3845 on RANKL-Mediated Signal Transduction
2.5. Effect of PF-3845 on Alveolar Bone Loss in Experimental Periodontitis
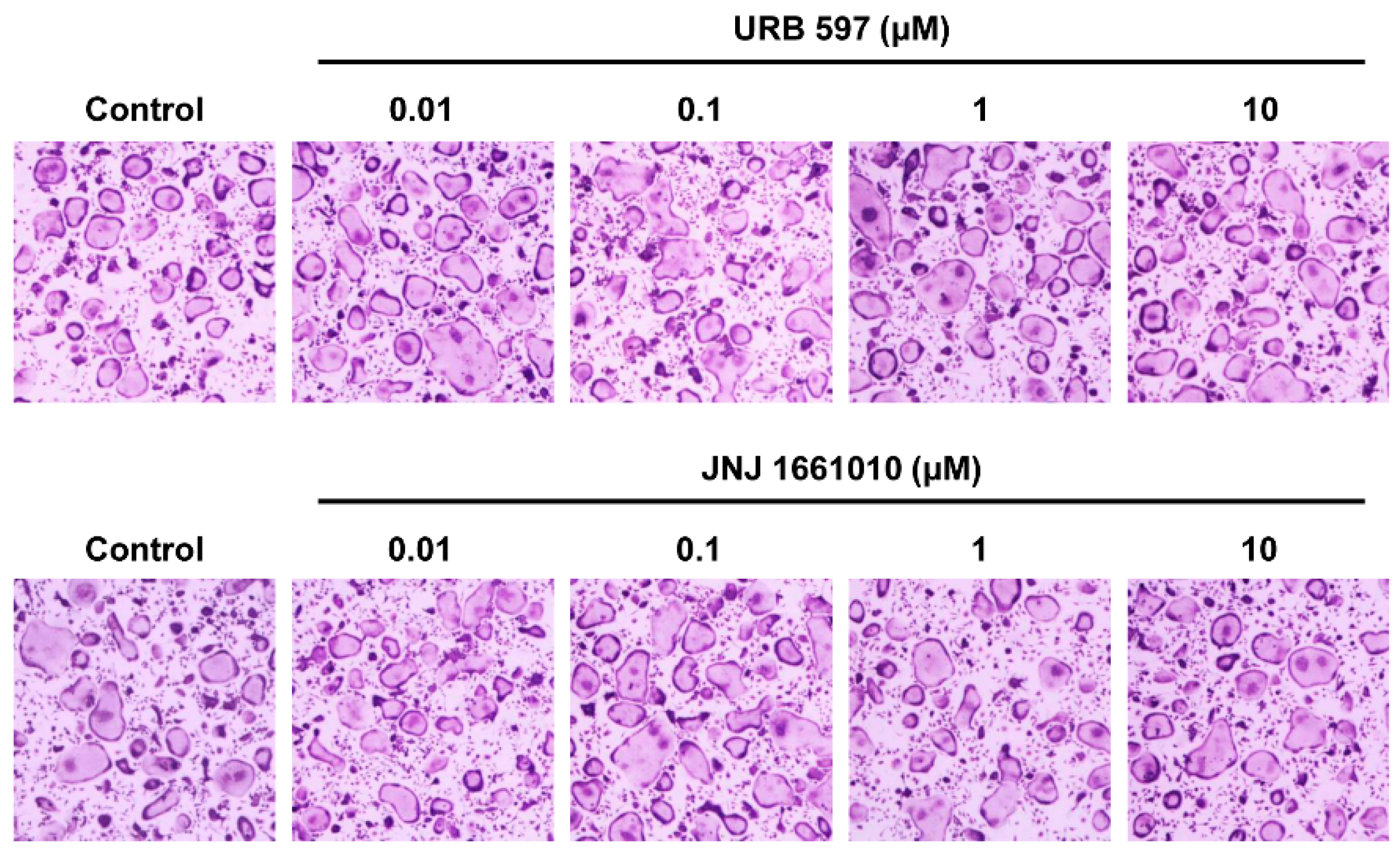
2.6. Effect of Other FAAH Inhibitors on Osteoclast Differentiation
3. Discussion
4. Materials and Methods
4.1. Mice and Reagents
4.2. Osteoclast Differentiation
4.3. Quantitative Real-Time PCR Analysis
4.4. Immunoblot Analysis
4.5. Resorption Pit Assay
4.6. Immunofluorescence
4.7. Ligature-Induced Experimental Periodontitis
4.8. Micro-Computed Tomography (Micro-CT) and Histomorphometric Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N. Periodontal diseases: Epidemiology. Ann. Periodontol. 1996, 1, 1–36. [Google Scholar] [CrossRef]
- Irfan, U.M.; Dawson, D.V.; Bissada, N.F. Epidemiology of periodontal disease: A review and clinical perspectives. J. Int. Acad. Periodontol. 2001, 3, 14–21. [Google Scholar] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [PubMed] [Green Version]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.C.; Spiers, J.G.; Sernia, C.; Lavidis, N.A. Inhibition of Fatty Acid Amide Hydrolase by PF-3845 Alleviates the Nitrergic and Proinflammatory Response in Rat Hippocampus Following Acute Stress. Int. J. Neuropsychopharmacol. 2018, 21, 786–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchantchou, F.; Tucker, L.B.; Fu, A.H.; Bluett, R.J.; McCabe, J.T.; Patel, S.; Zhang, Y. The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology 2014, 85, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Wasilewski, A.; Krajewska, U.; Owczarek, K.; Lewandowska, U.; Fichna, J. Fatty acid amide hydrolase (FAAH) inhibitor PF-3845 reduces viability, migration and invasiveness of human colon adenocarcinoma Colo-205 cell line: An in vitro study. Acta Biochim. Pol. 2017, 64, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef]
- Henriksen, K.; Sorensen, M.G.; Nielsen, R.H.; Gram, J.; Schaller, S.; Dziegiel, M.H.; Everts, V.; Bollerslev, J.; Karsdal, M.A. Degradation of the organic phase of bone by osteoclasts: A secondary role for lysosomal acidification. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006, 21, 58–66. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Staser, K.; Rhodes, S.D.; Liu, Y.; Wu, X.; Park, S.J.; Yuan, J.; Yang, X.; Li, X.; Jiang, L.; et al. Erk1 positively regulates osteoclast differentiation and bone resorptive activity. PLoS ONE 2011, 6, e24780. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, S.; Zhang, Y.; Li, X.; Li, Y.; Wu, X.; Yuan, J.; Robling, A.G.; Kapur, R.; Chan, R.J.; et al. Rac1 mediates the osteoclast gains-in-function induced by haploinsufficiency of Nf1. Hum. Mol. Genet. 2008, 17, 936–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.W.; Lee, D.; Minematsu, H.; Kim, A.D.; Shin, M.; Cho, S.K.; Kim, D.W.; Yang, J.; Lee, F.Y. Targeting extracellular signal-regulated kinase (ERK) signaling has therapeutic implications for inflammatory osteolysis. Bone 2010, 46, 695–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, T. The dendritic cell-specific transmembrane protein DC-STAMP is essential for osteoclast fusion and osteoclast bone-resorbing activity. Mod. Rheumatol. 2006, 16, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Oikawa, T.; Kuroda, Y.; Matsuo, K. Regulation of osteoclasts by membrane-derived lipid mediators. Cell. Mol. Life Sci. CMLS 2013, 70, 3341–3353. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.R.; Peters, C.; Saftig, P.; Bromme, D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J. Biol. Chem. 2009, 284, 2584–2592. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Ihn, H.J.; Lee, T.; Kim, J.A.; Lee, D.; Kim, N.D.; Shin, H.I.; Bae, Y.C.; Park, E.K. OCLI-023, a Novel Pyrimidine Compound, Suppresses Osteoclastogenesis In Vitro and Alveolar Bone Resorption In Vivo. PLoS ONE 2017, 12, e0170159. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.J.; Lee, T.; Lee, D.; Kim, J.A.; Kim, K.; Lim, S.; Kim, J.Y.; Lee, Y.; Kim, S.H.; Lee, H.S.; et al. A novel benzamide derivative protects ligature-induced alveolar bone erosion by inhibiting NFATc1-mediated osteoclastogenesis. Toxicol. Appl. Pharmacol. 2018, 355, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Fine, D.; Teng, Y.T.; Van Dyke, T.E.; Hajishengallis, G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008, 35, 89–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaga, M.; Mokrowiecka, A.; Zakrzewski, P.K.; Cygankiewicz, A.; Leishman, E.; Sobczak, M.; Zatorski, H.; Malecka-Panas, E.; Kordek, R.; Storr, M.; et al. Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH). J. Crohn’s Colitis 2014, 8, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Siniscalco, D.; Luongo, L.; De Petrocellis, L.; Bellini, G.; Petrosino, S.; Torella, M.; Santoro, C.; Nobili, B.; Perrotta, S.; et al. The endovanilloid/endocannabinoid system in human osteoclasts: Possible involvement in bone formation and resorption. Bone 2009, 44, 476–484. [Google Scholar] [CrossRef]
- Idris, A.I.; van ‘t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar] [CrossRef] [Green Version]
- Idris, A.I.; Ralston, S.H. Role of cannabinoids in the regulation of bone remodeling. Front. Endocrinol. 2012, 3, 136. [Google Scholar] [CrossRef] [Green Version]
- Ihn, H.J.; Kim, K.; Cho, H.S.; Park, E.K. Pentamidine Inhibits Titanium Particle-Induced Osteolysis In Vivo and Receptor Activator of Nuclear Factor-kappaB Ligand-Mediated Osteoclast Differentiation In Vitro. Tissue Eng. Regen. Med. 2019, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Baek, C.W.; Kim, H.J.; Kim, E.J.; Byeon, G.J.; Yoon, J.U. Remifentanil Negatively Regulates RANKL-Induced Osteoclast Differentiation and Bone Resorption by Inhibiting c-Fos/NFATc1 Expression. Tissue Eng. Regen. Med. 2018, 15, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.J.; Lee, T.; Lee, D.; Bae, J.S.; Kim, S.H.; Jang, I.H.; Bae, Y.C.; Shin, H.I.; Park, E.K. Inhibitory Effect of KP-A038 on Osteoclastogenesis and Inflammatory Bone Loss Is Associated With Downregulation of Blimp1. Front. Pharmacol. 2019, 10, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedse, G.; Bluett, R.J.; Patrick, T.A.; Romness, N.K.; Gaulden, A.D.; Kingsley, P.J.; Plath, N.; Marnett, L.J.; Patel, S. Therapeutic endocannabinoid augmentation for mood and anxiety disorders: Comparative profiling of FAAH, MAGL and dual inhibitors. Transl. Psychiatry 2018, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booker, L.; Kinsey, S.G.; Abdullah, R.A.; Blankman, J.L.; Long, J.Z.; Ezzili, C.; Boger, D.L.; Cravatt, B.F.; Lichtman, A.H. The fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the nervous system to reverse LPS-induced tactile allodynia in mice. Br. J. Pharmacol. 2012, 165, 2485–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.; Hoda, M.N.; Susin, C.; Wheeler, J.N.; Marshall, B.; Perry, L.; Saad, N.; Yin, L.; Elsayed, R.; Elsalanty, M.; et al. Increased Innate Lymphoid Cells in Periodontal Tissue of the Murine Model of Periodontitis: The Role of AMP-Activated Protein Kinase and Relevance for the Human Condition. Front. Immunol. 2017, 8, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihn, H.-J.; Kim, Y.-S.; Lim, S.; Bae, J.-S.; Jung, J.-C.; Kim, Y.-H.; Park, J.-W.; Wang, Z.; Koh, J.-T.; Bae, Y.-C.; et al. PF-3845, a Fatty Acid Amide Hydrolase Inhibitor, Directly Suppresses Osteoclastogenesis through ERK and NF-κB Pathways In Vitro and Alveolar Bone Loss In Vivo. Int. J. Mol. Sci. 2021, 22, 1915. https://doi.org/10.3390/ijms22041915
Ihn H-J, Kim Y-S, Lim S, Bae J-S, Jung J-C, Kim Y-H, Park J-W, Wang Z, Koh J-T, Bae Y-C, et al. PF-3845, a Fatty Acid Amide Hydrolase Inhibitor, Directly Suppresses Osteoclastogenesis through ERK and NF-κB Pathways In Vitro and Alveolar Bone Loss In Vivo. International Journal of Molecular Sciences. 2021; 22(4):1915. https://doi.org/10.3390/ijms22041915
Chicago/Turabian StyleIhn, Hye-Jung, Yi-Seul Kim, Soomin Lim, Jong-Sup Bae, Jae-Chang Jung, Yeo-Hyang Kim, Jin-Woo Park, Zhao Wang, Jeong-Tae Koh, Yong-Chul Bae, and et al. 2021. "PF-3845, a Fatty Acid Amide Hydrolase Inhibitor, Directly Suppresses Osteoclastogenesis through ERK and NF-κB Pathways In Vitro and Alveolar Bone Loss In Vivo" International Journal of Molecular Sciences 22, no. 4: 1915. https://doi.org/10.3390/ijms22041915





