Adipose Tissue Gene Expression of Entire Male, Immunocastrated and Surgically Castrated Pigs
Abstract
1. Introduction
2. Results
2.1. RNA-Sequencing Approach
2.1.1. Description of RNA-seq Data
2.1.2. Gene Expression Level Analysis
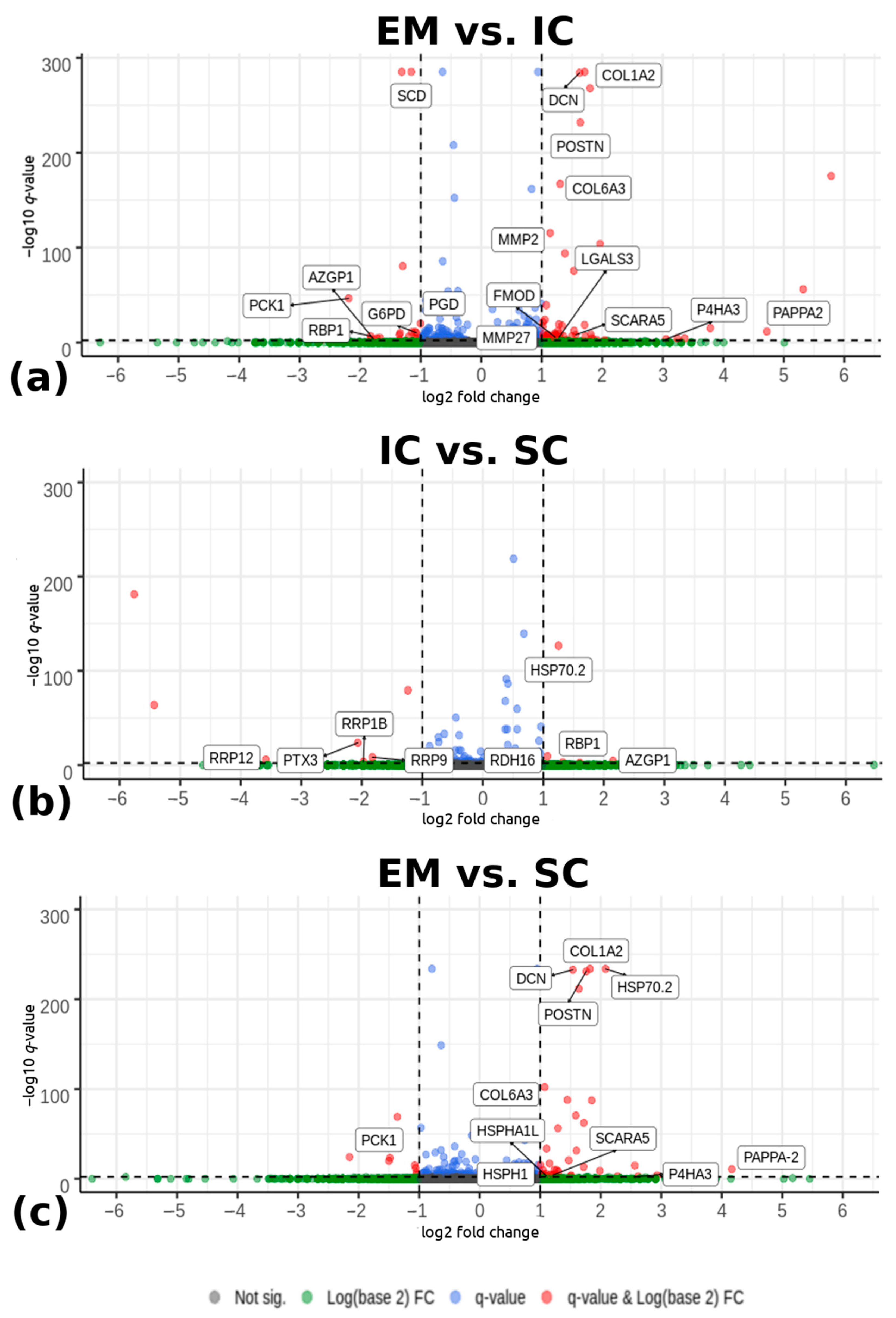
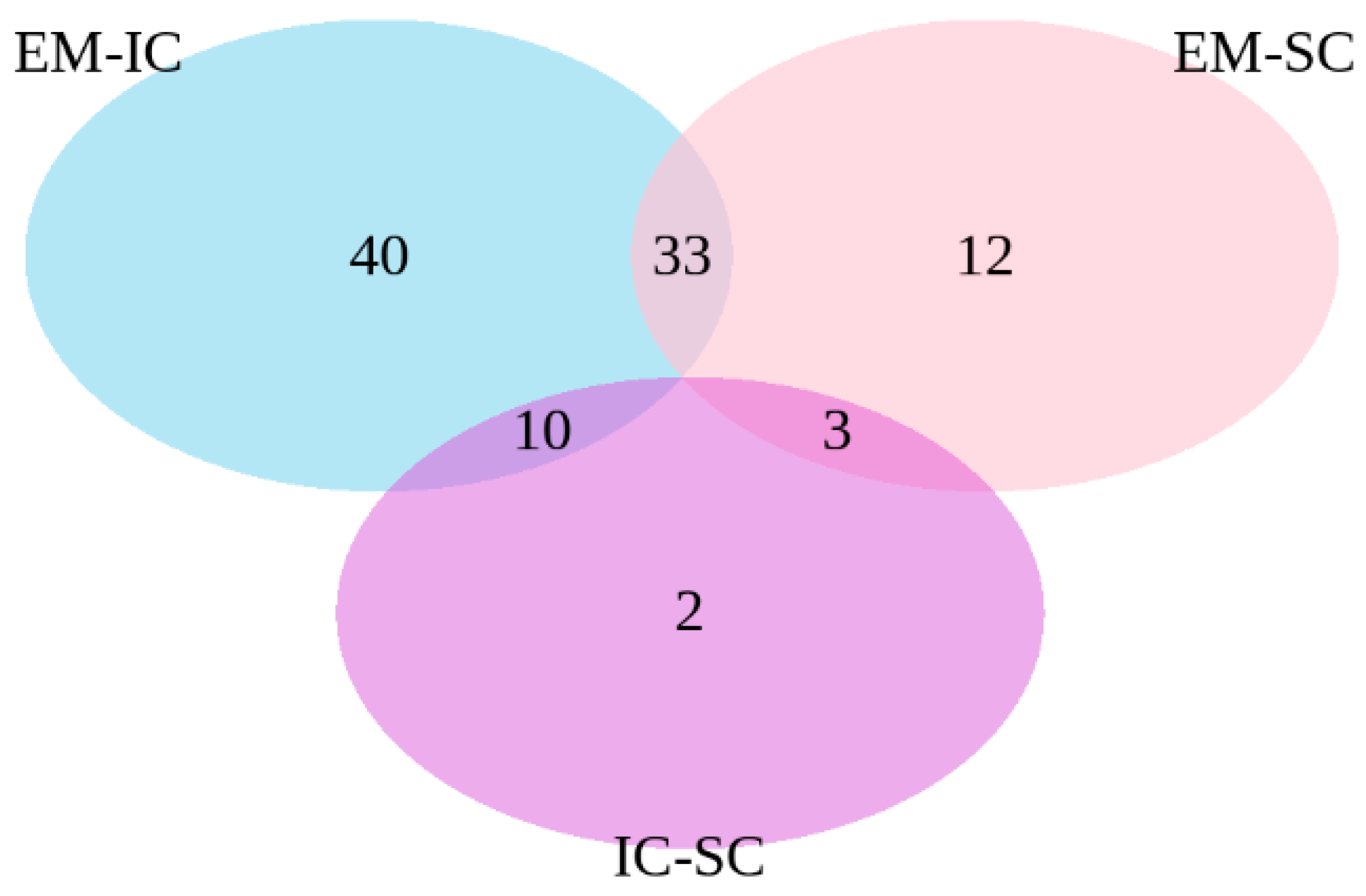
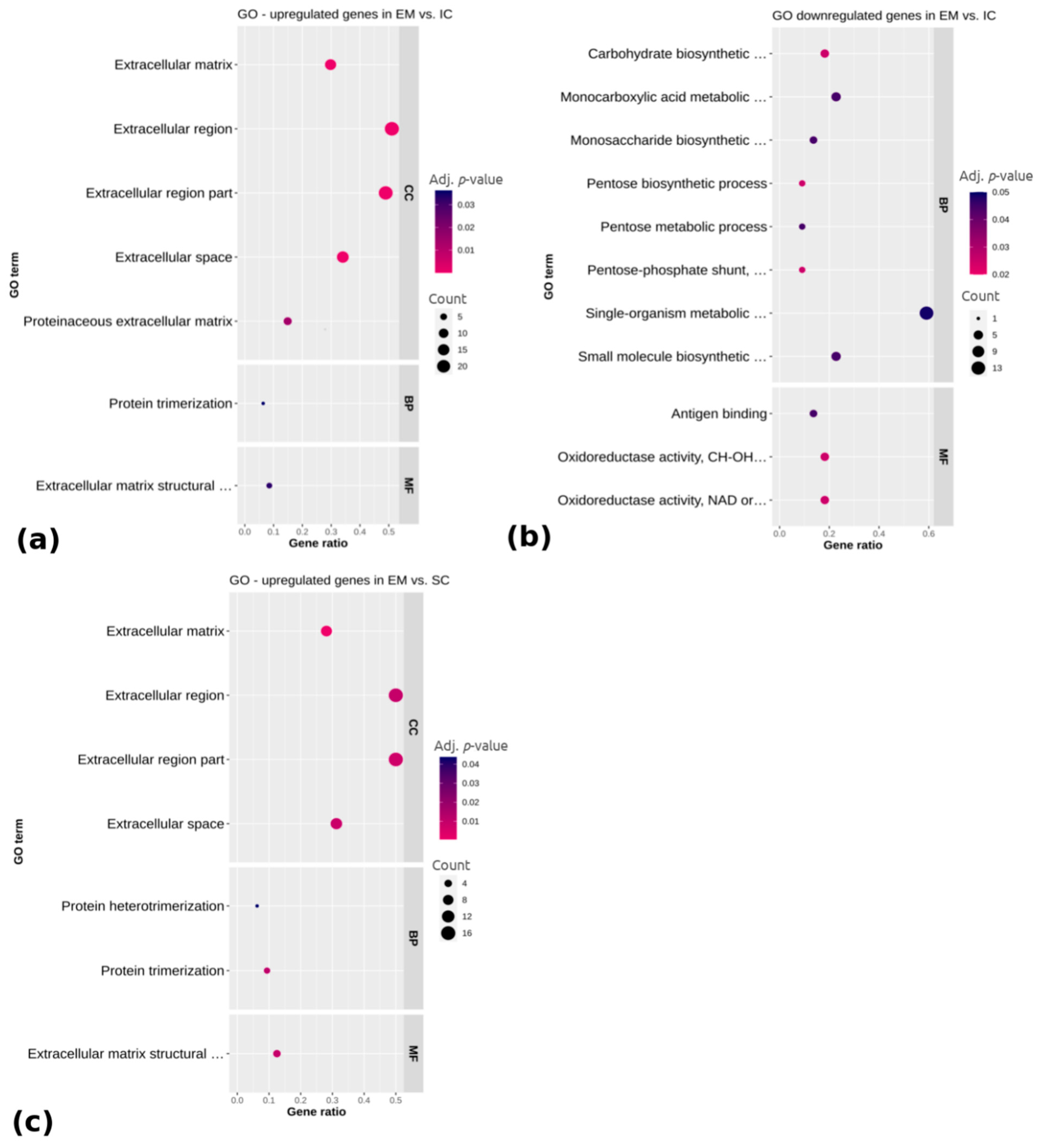
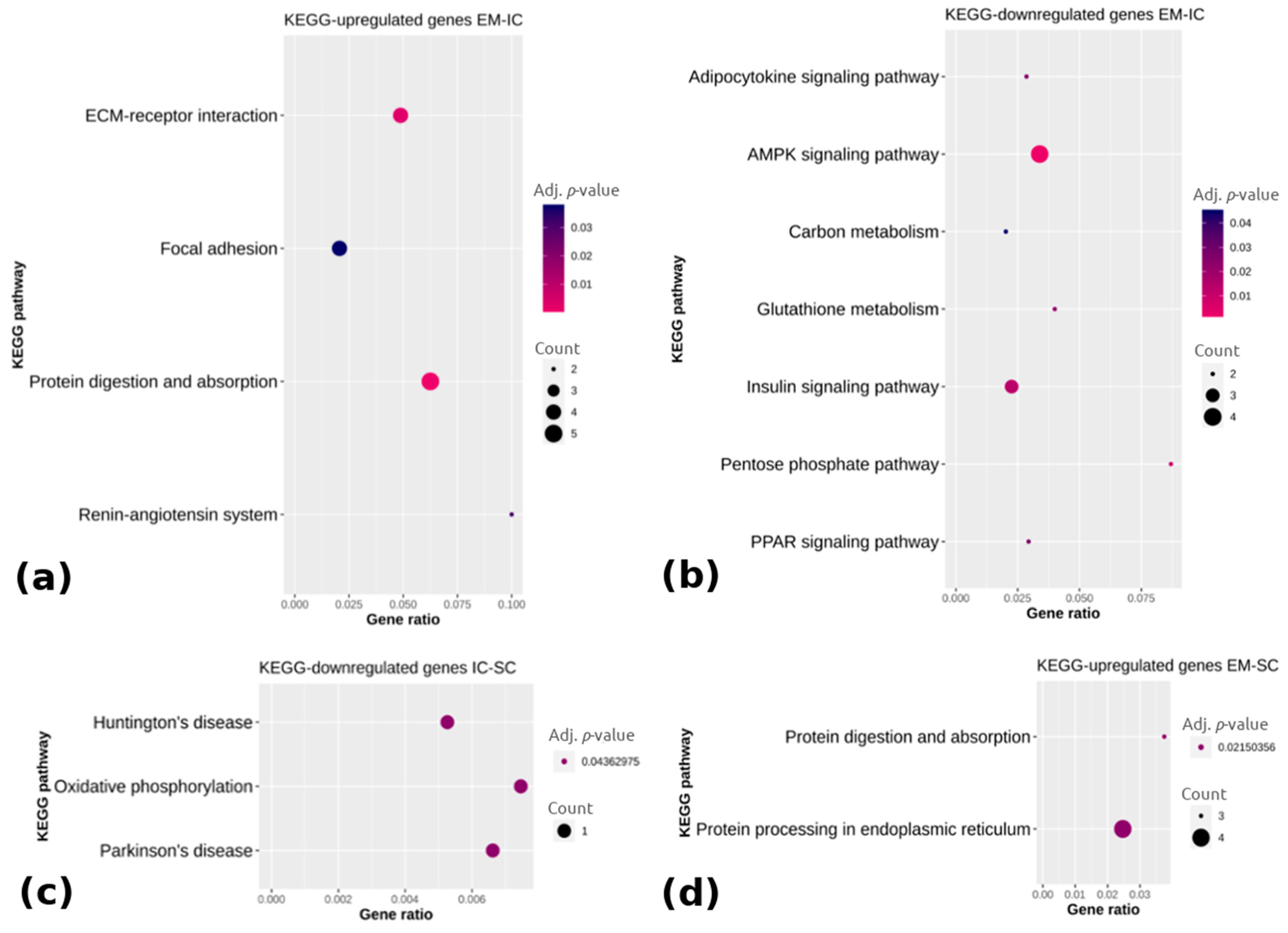
2.1.3. Differential Expression Analysis
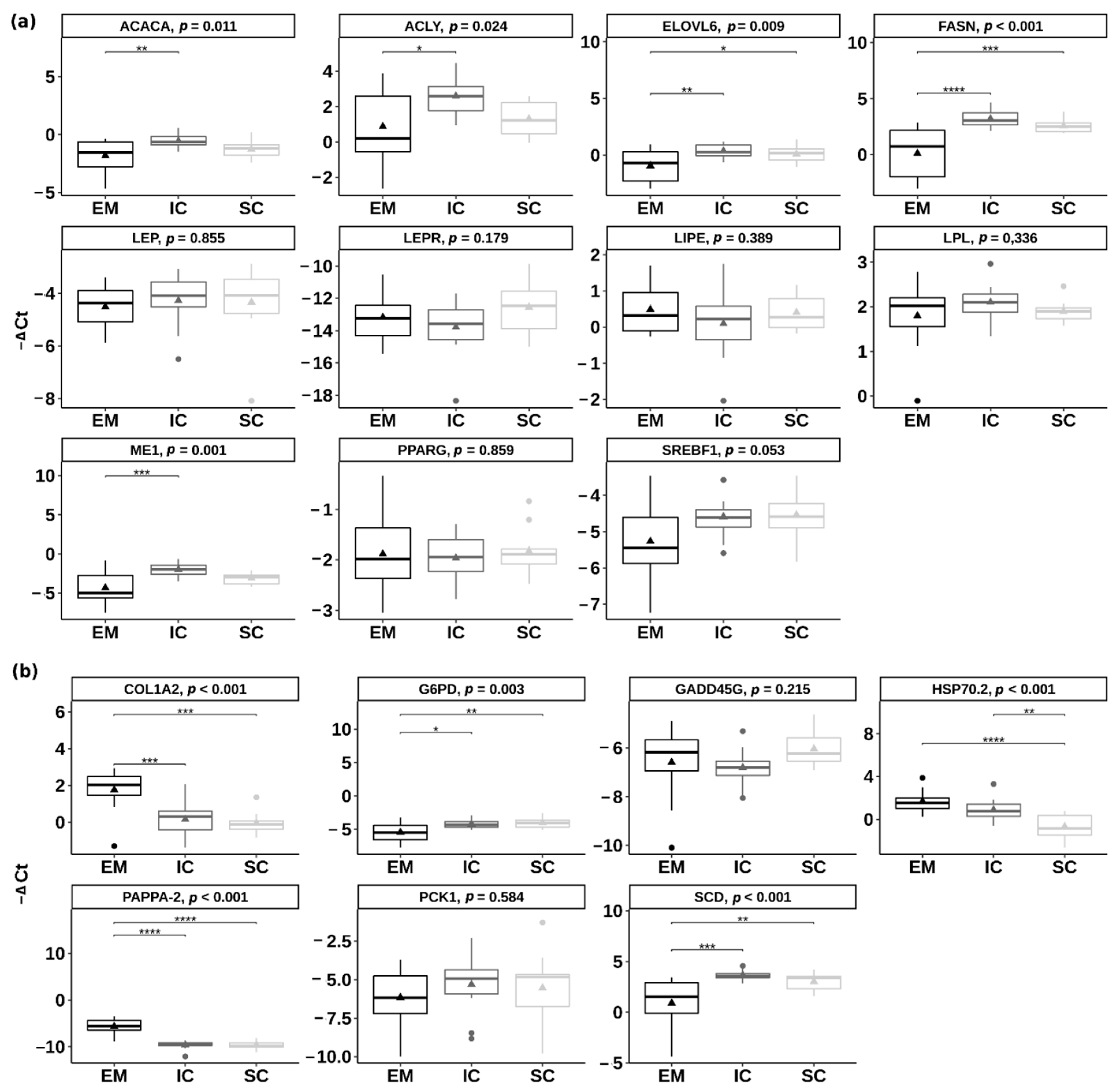
2.2. Gene Expression Using Quantitative PCR Approach
3. Discussion
3.1. RNA-Sequencing Approach
3.2. Candidate Gene Expression Approach
4. Material and Methods
4.1. Animals and Sample Collection
4.2. RNA Extraction and Quality Check
4.3. cDNA Library Construction and RNA-Sequencing
4.4. Bioinformatic Analysis
4.4.1. Quality Control, Mapping and Assembly
4.4.2. Quality Control, Mapping and Assembly
4.4.3. Functional Enrichment Analysis
4.4.4. Candidate Genes Expression Analysis by Quantitative PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EUR-Lex-32008L0120-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008L0120 (accessed on 15 October 2020).
- Prunier, A.; Bonneau, M.; Morton, D.; Tuyttens, F.; Velarde, A. A review of the welfare consequences of surgical castration in piglets and the evaluation of non-surgical methods. Anim. Welf. 2006, 15, 277–289. [Google Scholar]
- Von Borell, E.; Baumgartner, J.; Giersing, M.; Jäggin, N.; Prunier, A.; Tuyttens, F.A.M.; Edwards, S.A. Animal welfare implications of surgical castration and its alternatives in pigs. Animal 2009, 3, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Weiler, U. Pros and Cons of Alternatives to Piglet Castration: Welfare, Boar Taint, and Other Meat Quality Traits. Animal 2019, 9, 884. [Google Scholar] [CrossRef]
- Kress, K.; Millet, S.; Labussière, É.; Weiler, U.; Stefanski, V. Sustainability of Pork Production with Immunocastration in Europe. Sustainability 2019, 11, 3335. [Google Scholar] [CrossRef]
- Kouba, M.; Sellier, P. A review of the factors influencing the development of intermuscular adipose tissue in the growing pig. Meat Sci. 2011, 88, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.; Squires, E.J.; Mandell, I.B.; De Lange, C.F.M. Age at castration (surgical or immunological) impacts carcass characteristics and meat quality of male pigs. Animal 2018, 12, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Lundström, K.; Matthews, K.R.; Haugen, J.-E. Pig meat quality from entire males. Animal 2009, 3, 1497–1507. [Google Scholar] [CrossRef]
- Rydhmer, L.; Zamaratskaia, G.; Andersson, H.K.; Algers, B.; Guillemet, R.; Lundström, K. Aggressive and sexual behaviour of growing and finishing pigs reared in groups, without castration. Acta Agric. Scand. Sect. A Anim. Sci. 2006, 56, 109–119. [Google Scholar] [CrossRef]
- Claus, R.; Lacorn, M.; Danowski, K.; Pearce, M.C.; Bauer, A. Short-term endocrine and metabolic reactions before and after second immunization against GnRH in boars. Vaccine 2007, 25, 4689–4696. [Google Scholar] [CrossRef]
- Brunius, C.; Zamaratskaia, G.; Andersson, K.; Chen, G.; Norrby, M.; Madej, A.; Lundström, K. Early immunocastration of male pigs with Improvac®–Effect on boar taint, hormones and reproductive organs. Vaccine 2011, 29, 9514–9520. [Google Scholar] [CrossRef] [PubMed]
- Batorek-Lukač, N.; Dubois, S.; Noblet, J.; Čandek-Potokar, M.; Labussière, E. Effect of high dietary fat content on heat production and lipid and protein deposition in growing immunocastrated male pigs. Animal 2016, 10, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Weiler, U.; Götz, M.; Schmidt, A.; Otto, M.; Müller, S. Influence of sex and immunocastration on feed intake behavior, skatole and indole concentrations in adipose tissue of pigs. Animal 2013, 7, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Batorek, N.; Čandek-Potokar, M.; Bonneau, M.; Van Milgen, J. Meta-analysis of the effect of immunocastration on production performance, reproductive organs and boar taint compounds in pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Lealiifano, A.K.; Pluske, J.R.; Nicholls, R.R.; Dunshea, F.R.; Campbell, R.G.; Hennessy, D.P.; Miller, D.W.; Hansen, C.F.; Mullan, B.P. Reducing the length of time between slaughter and the secondary gonadotropin-releasing factor immunization improves growth performance and clears boar taint compounds in male finishing pigs1. J. Anim. Sci. 2011, 89, 2782–2792. [Google Scholar] [CrossRef] [PubMed]
- Poklukar, K.; Čandek-Potokar, M.; Vrecl, M.; Batorek-Lukač, N.; Fazarinc, G.; Kress, K.; Weiler, U.; Stefanski, V.; Škrlep, M. The effect of immunocastration on adipose tissue deposition and composition in pigs. Animal 2020, 100118. [Google Scholar] [CrossRef]
- Yao, Y.-C.; Cai, Z.-W.; Zhao, C.-J.; Wu, K.-L.; Wu, C.-X.; Han, W.-P.; Xu, N.-Y. Influence of Castration-induced Sex Hormone Deficiency on Serum Lipid Levels and the Genes Expression in Male Pigs. Horm. Metab. Res. 2011, 43, 674–680. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, L.; Chen, M.; Jiang, X.; Xu, N. Castration-induced changes in microRNA expression profiles in subcutaneous adipose tissue of male pigs. J. Appl. Genet. 2014, 55, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ma, H.; Wu, K.; Shao, Y.; Han, W.; Cai, Z.; Xu, N.; Qi, M.; Zhao, C.; Wu, C. Body composition, serum lipid levels, and transcriptomic characterization in the adipose tissue of male pigs in response to sex hormone deficiency. Gene 2018, 646, 74–82. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef]
- Nold, R.A.; Romans, J.R.; Costello, W.J.; Libal, G.W. Characterization of muscles from boars, barrows, and gilts slaughtered at 100 or 110 kilograms: Differences in fat, moisture, color, water-holding capacity, and collagen. J. Anim. Sci. 1999, 77, 1746–1754. [Google Scholar] [CrossRef]
- Škrlep, M.; Poklukar, K.; Kress, K.; Vrecl, M.; Fazarinc, G.; Lukač, N.B.; Weiler, U.; Stefanski, V.; Čandek-Potokar, M. Effect of immunocastration and housing conditions on pig carcass and meat quality traits1. Transl. Anim. Sci. 2020, 4, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Ibrahim, M.; Kim, M.J.; So, K.; Jeong, Y.D.; Park, S.; Kim, M.; Lee, H.-J. Comparisons of extracellular matrix-related gene expression levels in different adipose tissues from Korean cattle. Livest. Sci. 2017, 198, 138–146. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zan, L.-S.; Wang, H.-B.; Qing, L.; Wu, K.-X.; Quan, S.-A.; Li, C.-Q.; Zhong, X.; Wang, C.-J. Differentially expressed genes in skeletal muscle tissues from castrated Qinchuan cattle males compared with those from intact males. Livest. Sci. 2011, 135, 76–83. [Google Scholar] [CrossRef]
- Gjaltema, R.A.F.; Bank, R.A. Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit. Rev. Biochem. Mol. Biol. 2016, 52, 74–95. [Google Scholar] [CrossRef]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted Disruption of Decorin Leads to Abnormal Collagen Fibril Morphology and Skin Fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef]
- Gubbiotti, M.A.; Vallet, S.D.; Ricard-Blum, S.; Iozzo, R.V. Decorin interacting network: A comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biol. 2016, 55, 7–21. [Google Scholar] [CrossRef]
- Font, B.; Eichenberger, D.; Rosenberg, L.; Van Der Rest, M. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 1996, 15, 341–348. [Google Scholar] [CrossRef]
- Norris, R.A.; Damon, B.; Mironov, V.; Kasyanov, V.; Ramamurthi, A.; Moreno-Rodriguez, R.; Trusk, T.; Potts, J.D.; Goodwin, R.L.; Davis, J.; et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell. Biochem. 2007, 101, 695–711. [Google Scholar] [CrossRef]
- Schwanekamp, J.A.; Lorts, A.; Vagnozzi, R.J.; Vanhoutte, D.; Molkentin, J.D. Deletion of Periostin Protects Against Atherosclerosis in Mice by Altering Inflammation and Extracellular Matrix Remodeling. Arter. Thromb. Vasc. Biol. 2016, 36, 60–68. [Google Scholar] [CrossRef]
- Nakazeki, F.; Nishiga, M.; Horie, T.; Nishi, H.; Nakashima, Y.; Baba, O.; Kuwabara, Y.; Nishino, T.; Nakao, T.; Ide, Y.; et al. Loss of periostin ameliorates adipose tissue inflammation and fibrosis in vivo. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Christians, J.K.; Bath, A.K.; Amiri, N. Pappa2 deletion alters IGFBPs but has little effect on glucose disposal or adiposity. Growth Horm. IGF Res. 2015, 25, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Forbes, B.E. Two years in IGF research. Growth Horm. IGF Res. 2016, 30–31, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Pathcards. Available online: https://pathcards.genecards.org/card/regulation_of_insulin-like_growth_factor_(igf)_transport_and_uptake_by_insulin-like_growth_factor_binding_proteins_(igfbps) (accessed on 30 January 2021).
- Hausman, D.B.; DiGirolamo, M.; Bartness, T.J.; Hausman, G.J.; Martin, R.J. The biology of white adipocyte proliferation. Obes. Rev. 2001, 2, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.; Claus, R. Active immunization of boars against GnRH does not affect growth hormone but lowers IGF-I in plasma. Livest. Prod. Sci. 2003, 81, 129–137. [Google Scholar] [CrossRef]
- Lee, M.-J.; Fried, S.K. Sex-dependent Depot Differences in Adipose Tissue Development and Function; Role of Sex Steroids. J. Obes. Metab. Syndr. 2017, 26, 172–180. [Google Scholar] [CrossRef]
- Baek, J.-H.; Kim, S.-J.; Kang, H.G.; Lee, H.-W.; Kim, J.-H.; Hwang, K.-A.; Song, J.; Chun, K.-H. Galectin-3 Activates PPARγ and Supports White Adipose Tissue Formation and High-Fat Diet-Induced Obesity. Endocrinology 2015, 156, 147–156. [Google Scholar] [CrossRef]
- Zizola, C.F.; Frey, S.K.; Jitngarmkusol, S.; Kadereit, B.; Yan, N.; Vogel, S. Cellular Retinol-Binding Protein Type I (CRBP-I) Regulates Adipogenesis. Mol. Cell. Biol. 2010, 30, 3412–3420. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Hanson, R. Glyceroneogenesis revisited. Biochimie 2003, 85, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Benítez, R.; Trakooljul, N.; Núñez, Y.; Isabel, B.; Murani, E.; De Mercado, E.; Gómez-Izquierdo, E.; García-Casco, J.; López-Bote, C.; Wimmers, K.; et al. Breed, Diet, and Interaction Effects on Adipose Tissue Transcriptome in Iberian and Duroc Pigs Fed Different Energy Sources. Genes 2019, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Olswang, Y.; Cohen, H.; Papo, O.; Cassuto, H.; Croniger, C.M.; Hakimi, P.; Tilghman, S.M.; Hanson, R.W.; Reshef, L. A mutation in the peroxisome proliferator-activated receptor -binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Franckhauser, S.; Muñoz, S.; Pujol, A.; Casellas, A.; Riu, E.; Otaegui, P.; Su, B.; Bosch, F. Increased Fatty Acid Re-esterification by PEPCK Overexpression in Adipose Tissue Leads to Obesity Without Insulin Resistance. Diabetes 2002, 51, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Latorre, P.; Burgos, C.; Hidalgo, J.; Varona, L.; Carrodeguas, J.A.; López-Buesa, P. c.A2456C-substitution in Pck1 changes the enzyme kinetic and functional properties modifying fat distribution in pigs. Sci. Rep. 2016, 6, 19617. [Google Scholar] [CrossRef]
- Huang, J.; Feng, X.; Zhu, R.; Guo, D.; Wei, Y.; Cao, X.; Ma, Y.; Shi, D. Comparative transcriptome analysis reveals that PCK1 is a potential gene affecting IMF deposition in buffalo. BMC Genom. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Liang, X.; Wu, X.; Liu, J.; Yang, S.; Tao, C.; Zhang, J.; Tian, J.; Zhao, J.; et al. Stearoyl-CoA Desaturase Is Essential for Porcine Adipocyte Differentiation. Int. J. Mol. Sci. 2020, 21, 2446. [Google Scholar] [CrossRef]
- Mauvoisin, D.; Mounier, C. Hormonal and nutritional regulation of SCD1 gene expression. Biochimie 2011, 93, 78–86. [Google Scholar] [CrossRef]
- Le Floc’H, N.; Furbeyre, H.; Prunier, A.; Louveau, I. Effect of surgical or immune castration on postprandial nutrient profiles in male pigs. Arch. Anim. Nutr. 2019, 73, 255–270. [Google Scholar] [CrossRef]
- Wei, X.; Liu, X.; Tan, C.; Mo, L.; Wang, H.; Peng, X.; Deng, F.; Chen, L. Expression and Function of Zinc-α2-Glycoprotein. Neurosci. Bull. 2019, 35, 540–550. [Google Scholar] [CrossRef]
- Balaz, M.; Ukropcova, B.; Kurdiova, T.; Gajdošechova, L.; Vlcek, M.; Janakova, Z.; Fedeleš, J.; Pura, M.; Gasperikova, D.; Smith, S.R.; et al. Adipokine zinc-α2-glycoprotein regulated by growth hormone and linked to insulin sensitivity. Obesity 2014, 23, 322–328. [Google Scholar] [CrossRef]
- Kregel, K.C. Invited Review: Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186. [Google Scholar] [CrossRef]
- Cronin, G.; Dunshea, F.; Butler, K.; McCauley, I.; Barnett, J.; Hemsworth, P. The effects of immuno- and surgical-castration on the behaviour and consequently growth of group-housed, male finisher pigs. Appl. Anim. Behav. Sci. 2003, 81, 111–126. [Google Scholar] [CrossRef]
- Carroll, J.A.; Berg, E.L.; Strauch, T.A.; Roberts, M.P.; Kattesh, H.G. Hormonal profiles, behavioral responses, and short-term growth performance after castration of pigs at three, six, nine, or twelve days of age1,2. J. Anim. Sci. 2006, 84, 1271–1278. [Google Scholar] [CrossRef]
- Škrlep, M.; Tomažin, U.; Lukač, N.B.; Poklukar, K.; Čandek-Potokar, M. Proteomic Profiles of the Longissimus Muscles of Entire Male and Castrated Pigs as Related to Meat Quality. Animals 2019, 9, 74. [Google Scholar] [CrossRef]
- Fàbrega, E.; Velarde, A.; Cros, J.; Gispert, M.; Suárez, P.; Tibau, J.; Soler, J. Effect of vaccination against gonadotrophin-releasing hormone, using Improvac®, on growth performance, body composition, behaviour and acute phase proteins. Livest. Sci. 2010, 132, 53–59. [Google Scholar] [CrossRef]
- Zambonelli, P.; Gaffo, E.; Zappaterra, M.; Bortoluzzi, S.; Davoli, R. Transcriptional profiling of subcutaneous adipose tissue in Italian Large White pigs divergent for backfat thickness. Anim. Genet. 2016, 47, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Park, B.Y.; Park, J.C.; Hwang, I.H.; Lee, S.S.; Yeon, S.H.; Kim, C.D.; Cho, C.Y.; Kim, Y.K.; Min, K.S.; et al. The Effects of Stress Related Genes on Carcass Traits and Meat Quality in Pigs. Asian-Australas. J. Anim. Sci. 2005, 19, 280–285. [Google Scholar] [CrossRef]
- Bazin, R.; Ferré, P. Assays of Lipogenic Enzymes. In Adipose Tissue Protocols; Humana Press: Totowa, NJ, USA, 2003; Volume 155, pp. 121–127. [Google Scholar]
- Mackay, J.; Pearce, M.C.; Thevasagayam, S.; Doran, O. Fatty acid composition and lipogenic enzyme protein expression in subcutaneous adipose tissue of male pigs vaccinated against boar taint, barrows, and entire boars. J. Anim. Sci. 2013, 91, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Mammi, C.; Calanchini, M.; Antelmi, A.; Cinti, F.; Rosano, G.M.C.; Lenzi, A.; Caprio, M.; Fabbri, A. Androgens and Adipose Tissue in Males: A Complex and Reciprocal Interplay. Int. J. Endocrinol. 2011, 2012, 1–8. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H. Elovl6: A new player in fatty acid metabolism and insulin sensitivity. J. Mol. Med. 2009, 87, 379–384. [Google Scholar] [CrossRef]
- Costa, C.; Giménez-Capitán, A.; Karachaliou, N.; Rosell, R. Comprehensive molecular screening: From the RT-PCR to the RNA-seq. Transl. Lung Cancer Res. 2013, 2, 87–91. [Google Scholar]
- Kress, K.; Weiler, U.; Schmucker, S.; Čandek-Potokar, M.; Vrecl, M.; Fazarinc, G.; Škrlep, M.; Batorek-Lukač, N.; Stefanski, V. Influence of Housing Conditions on Reliability of Immunocastration and Consequences for Growth Performance of Male Pigs. Animal 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Anders, S. HTSeq: Analysing High-Throughput Sequencing Data with Phyton. Available online: https://htseq.readthedocs.io/en/master/overview.html (accessed on 9 February 2021).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2009, 26, 136–138. [Google Scholar] [CrossRef]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 9 February 2021).
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Benítez, R.; Fernández, A.; Isabel, B.; Núñez, Y.; De Mercado, E.; Gómez-Izquierdo, E.; García-Casco, J.; López-Bote, C.; Óvilo, C. Modulatory Effects of Breed, Feeding Status, and Diet on Adipogenic, Lipogenic, and Lipolytic Gene Expression in Growing Iberian and Duroc Pigs. Int. J. Mol. Sci. 2017, 19, 22. [Google Scholar] [CrossRef]
- Miao, Z.; Zhu, F.; Zhang, H.; Chang, X.; Xie, H.; Zhang, J.; Xu, Z. Developmental patterns of FASN and LIPE mRNA expression in adipose tissue of growing Jinhua and Landrace gilts. Czech. J. Anim. Sci. 2010, 55, 557–564. [Google Scholar] [CrossRef]
- Spurlock, M.E.; Ji, S.Q.; Godat, R.L.; Kuske, J.L.; Willis, G.M.; Frank, G.; Cornelius, S.G. Changes in the expression of uncoupling proteins and lipases in porcine adipose tissue and skeletal muscle during feed deprivation☆11☆ Purdue Univ. Agric. Res. Programs journal paper no. 16265. This study was presented in part at the 1999 meeting of the Midwestern Section, American Society of Animal Science, Des Moines, IA USA. J. Nutr. Biochem. 2001, 12, 81–87. [Google Scholar] [CrossRef]
- Batorek, N.; Škrlep, M.; Prunier, A.; Louveau, I.; Noblet, J.; Bonneau, M.; Čandek-Potokar, M. Effect of feed restriction on hormones, performance, carcass traits, and meat quality in immunocastrated pigs1. J. Anim. Sci. 2012, 90, 4593–4603. [Google Scholar] [CrossRef]
- Wylie, A.R.G. Leptin in farm animals: Where are we and where can we go? Animal 2011, 5, 246–267. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cinar, M.U.; Tesfaye, D.; Looft, C.; Tholen, E.; Schellander, K. Age-related changes in relative expression stability of commonly used housekeeping genes in selected porcine tissues. BMC Res. Notes 2011, 4, 441. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Kwon, S.G.; Hwang, J.H.; Park, D.H.; Kim, T.W.; Kim, C.W. Selection of appropriate reference genes for RT-qPCR analysis in Berkshire, Duroc, Landrace, and Yorkshire pigs. Gene 2015, 558, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, M.; Zhang, K.; Chen, L.; Jiang, A.; Wang, J.; Li, X. Evaluation of endogenous control genes for gene expression studies across multiple tissues and in the specific sets of fat- and muscle-type samples of the pig. J. Anim. Breed. Genet. 2011, 128, 319–325. [Google Scholar] [CrossRef] [PubMed]
| Metabolic Function | Full Gene Name | Gene Symbol | Primer Sequence 1 or Taq-Man Assay ID 2 | Amplicon Length 3 | RNA-Seq or Reference 4 |
|---|---|---|---|---|---|
| Lipogenesis | Fatty acid synthase | FASN | Ss03386194_u1 | 95 | [17] |
| Glucose-6-phosphate dehydrogenase | G6PD | F: GCTTTCCATCAGTCGGATACACATA R: GAACAGCCACCACAGGGT P: CAAGTCGCCCGATGCT | 96 | RNA-seq | |
| Malic enzyme 1 | ME1 | Ss03374853_m1 | 92 | [16] | |
| ATP citrate lyase | ACLY | Ss03386194_u1 | 69 | [16] | |
| Acetyl-CoA carboxylase | ACACA | Ss03389963_m1 | 61 | [17] | |
| Stearoyl-CoA desaturase | SCD | Ss03392313_m1 | 65 | RNA-seq | |
| ELOVL fatty acid elongase 6 | ELOVL6 | Ss06879466_m1 | 90 | [77] | |
| Lipid and carbohydrate metabolism | Phosphoenolpyruvate carboxykinase 1 | PCK1 | Ss03390599_g1 | 65 | RNA-seq |
| Adipogenesis | Peroxisome proliferator activated receptor gamma | PPARγ | Ss03394829_m1 | 72 | [77] |
| Sterol regulatory element binding transcription factor 1 | SREBF1 | Ss03382914_u1 | 102 | [77] | |
| Lipolysis | Lipoprotein lipase | LPL | Ss03394612_m1 | 66 | [78] |
| Lipase E | LIPE | Ss04955671_mH | 65 | [79] | |
| Energy homeostasis | Leptin | LEP | Ss03392404_m1 | 68 | [80] |
| Leptin receptor | LEPR | Ss03379257_u1 | 142 | [81] | |
| Collagen synthesis | Collagen type I alpha 2 chain | COL1A2 | Ss03375009_u1 | 76 | RNA-seq |
| Response to stress | Growth arrest and DNA damage inducible gamma | GADD45G | Ss04246860_g1 | 54 | RNA-seq |
| Protein protection from the oxidative stress | Heat shock protein family A member 1B | HSP70.2 | Ss03392270_g1 | 69 | RNA-seq |
| Proteolysis against IGFBPs | Pappalysin 2 | PAPPA-2 | F: CGGAGGGAGGACAGAACAG R: TCACTGATTGTGTGGGAGCAA P: ACACACCTGCAATGAT | 70 | RNA-seq |
| Endogenous control | Beta-2 microglobulin | B2M | Ss03391154_m1 | 60 | [82] |
| Peptidylprolyl isomerase A | PPIA | Ss03394782_g1 | 91 | [83] | |
| DNA Topoisomerase II Beta | TOP2B | Ss04953704_m1 | 61 | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poklukar, K.; Čandek-Potokar, M.; Vrecl, M.; Batorek-Lukač, N.; Fazarinc, G.; Kress, K.; Stefanski, V.; Škrlep, M. Adipose Tissue Gene Expression of Entire Male, Immunocastrated and Surgically Castrated Pigs. Int. J. Mol. Sci. 2021, 22, 1768. https://doi.org/10.3390/ijms22041768
Poklukar K, Čandek-Potokar M, Vrecl M, Batorek-Lukač N, Fazarinc G, Kress K, Stefanski V, Škrlep M. Adipose Tissue Gene Expression of Entire Male, Immunocastrated and Surgically Castrated Pigs. International Journal of Molecular Sciences. 2021; 22(4):1768. https://doi.org/10.3390/ijms22041768
Chicago/Turabian StylePoklukar, Klavdija, Marjeta Čandek-Potokar, Milka Vrecl, Nina Batorek-Lukač, Gregor Fazarinc, Kevin Kress, Volker Stefanski, and Martin Škrlep. 2021. "Adipose Tissue Gene Expression of Entire Male, Immunocastrated and Surgically Castrated Pigs" International Journal of Molecular Sciences 22, no. 4: 1768. https://doi.org/10.3390/ijms22041768
APA StylePoklukar, K., Čandek-Potokar, M., Vrecl, M., Batorek-Lukač, N., Fazarinc, G., Kress, K., Stefanski, V., & Škrlep, M. (2021). Adipose Tissue Gene Expression of Entire Male, Immunocastrated and Surgically Castrated Pigs. International Journal of Molecular Sciences, 22(4), 1768. https://doi.org/10.3390/ijms22041768






