Voluntary Wheel Running Partially Compensates for the Effects of Global Estrogen Receptor-α Knockout on Cortical Bone in Young Male Mice
Abstract
1. Introduction
2. Results
2.1. Animal Characteristics
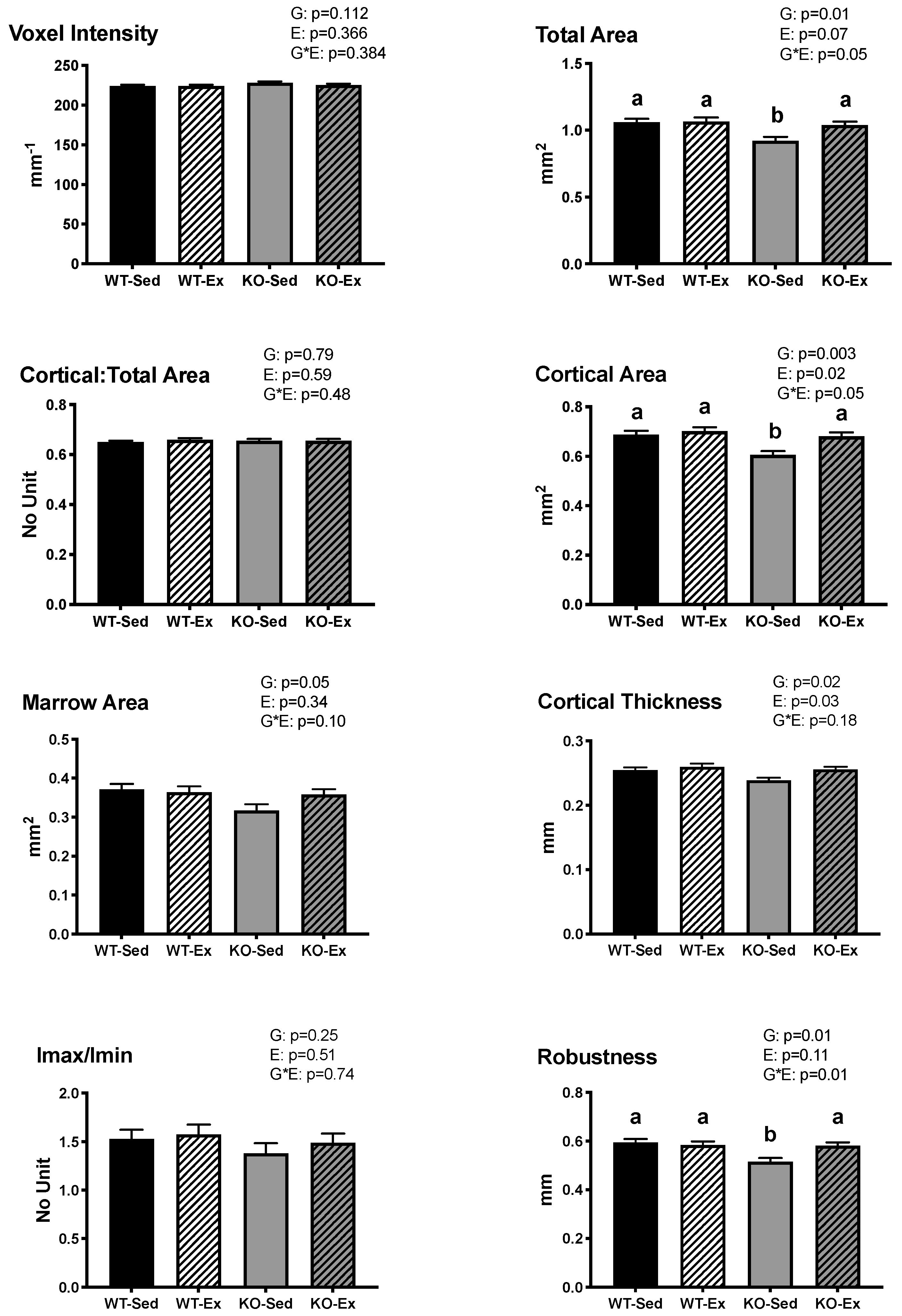
2.2. Tibial Cortical Geometry
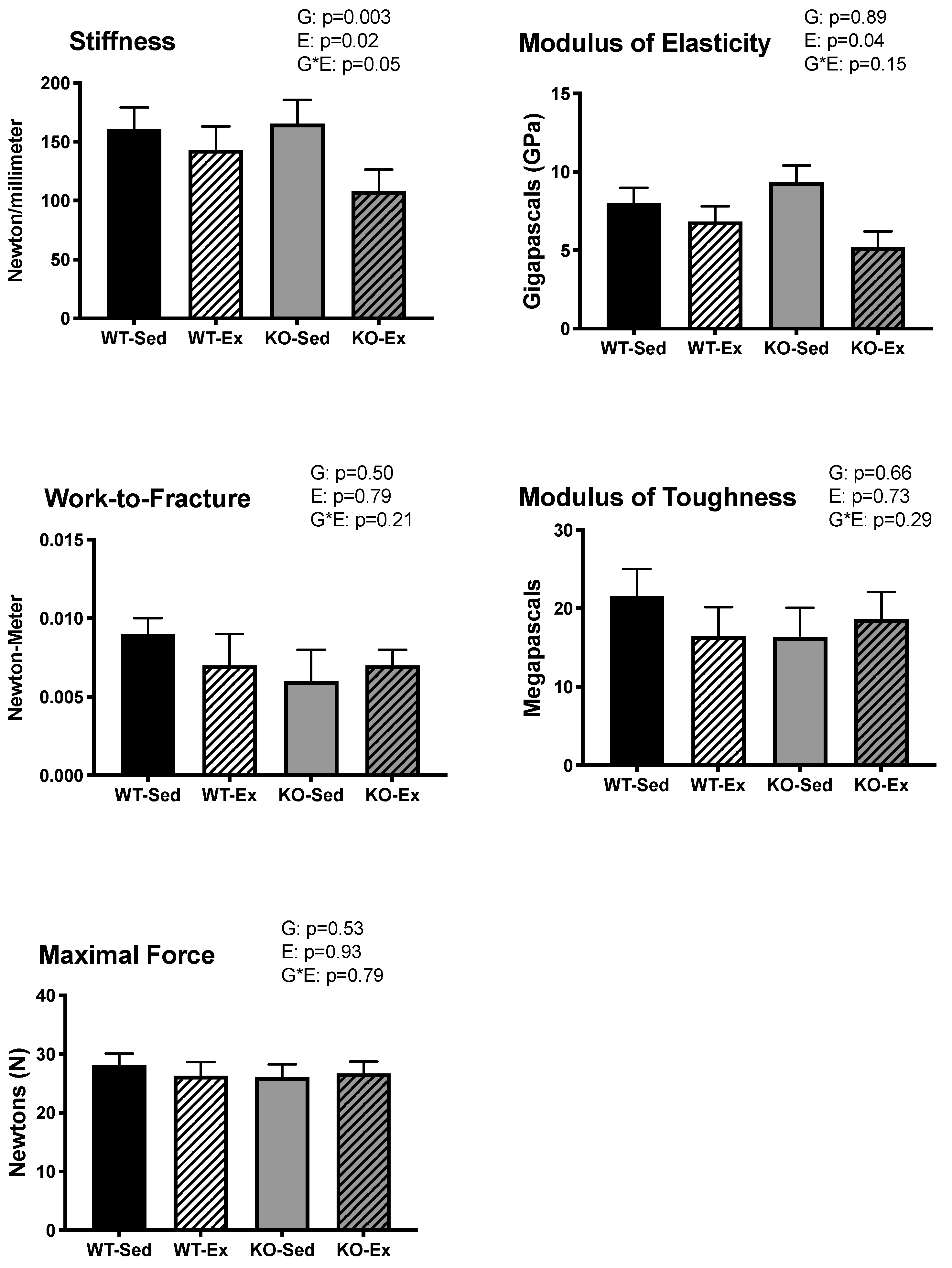
2.3. Tibial Biomechanical Strength
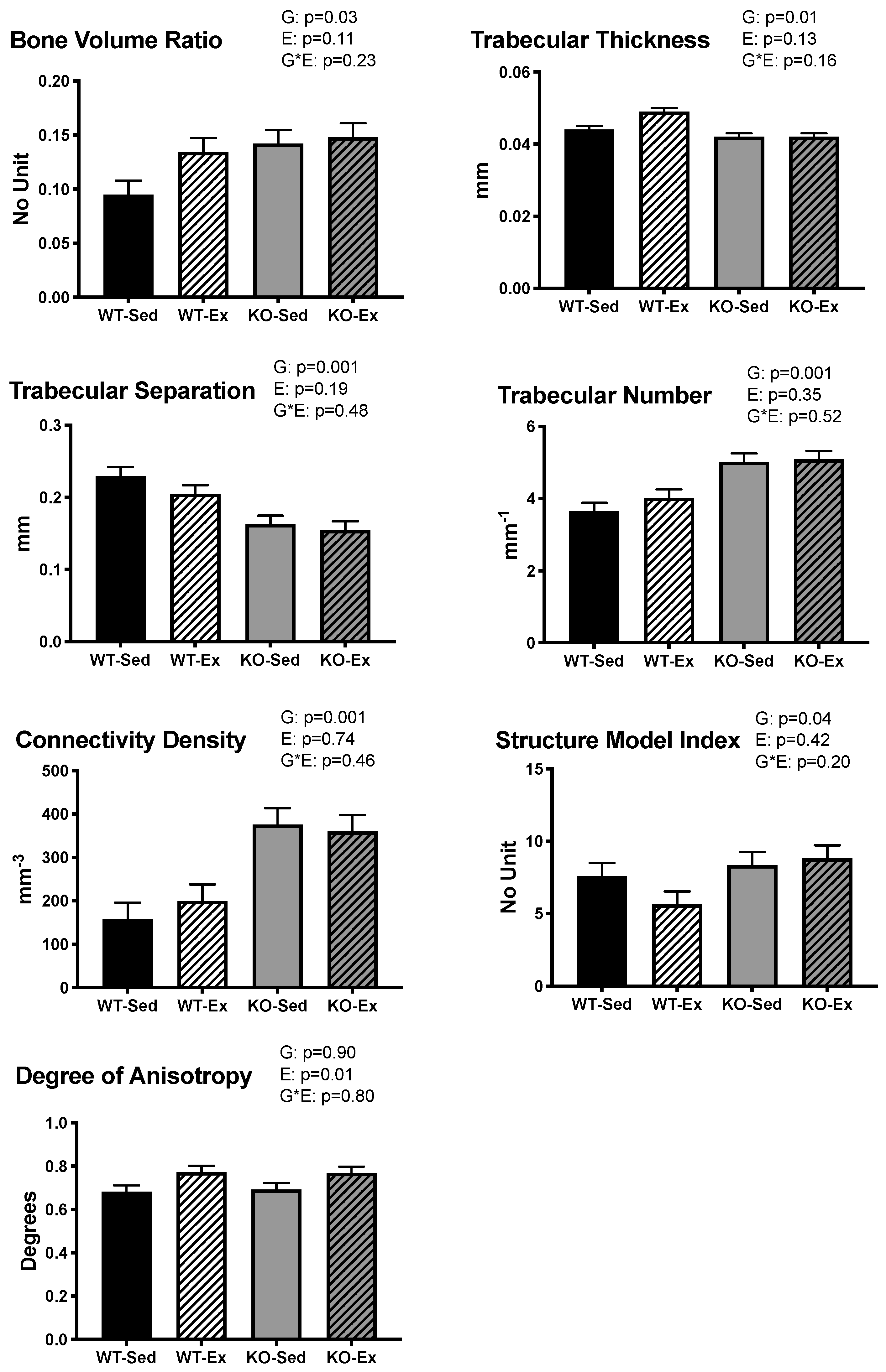
2.4. Tibial Trabecular Microarchitecture
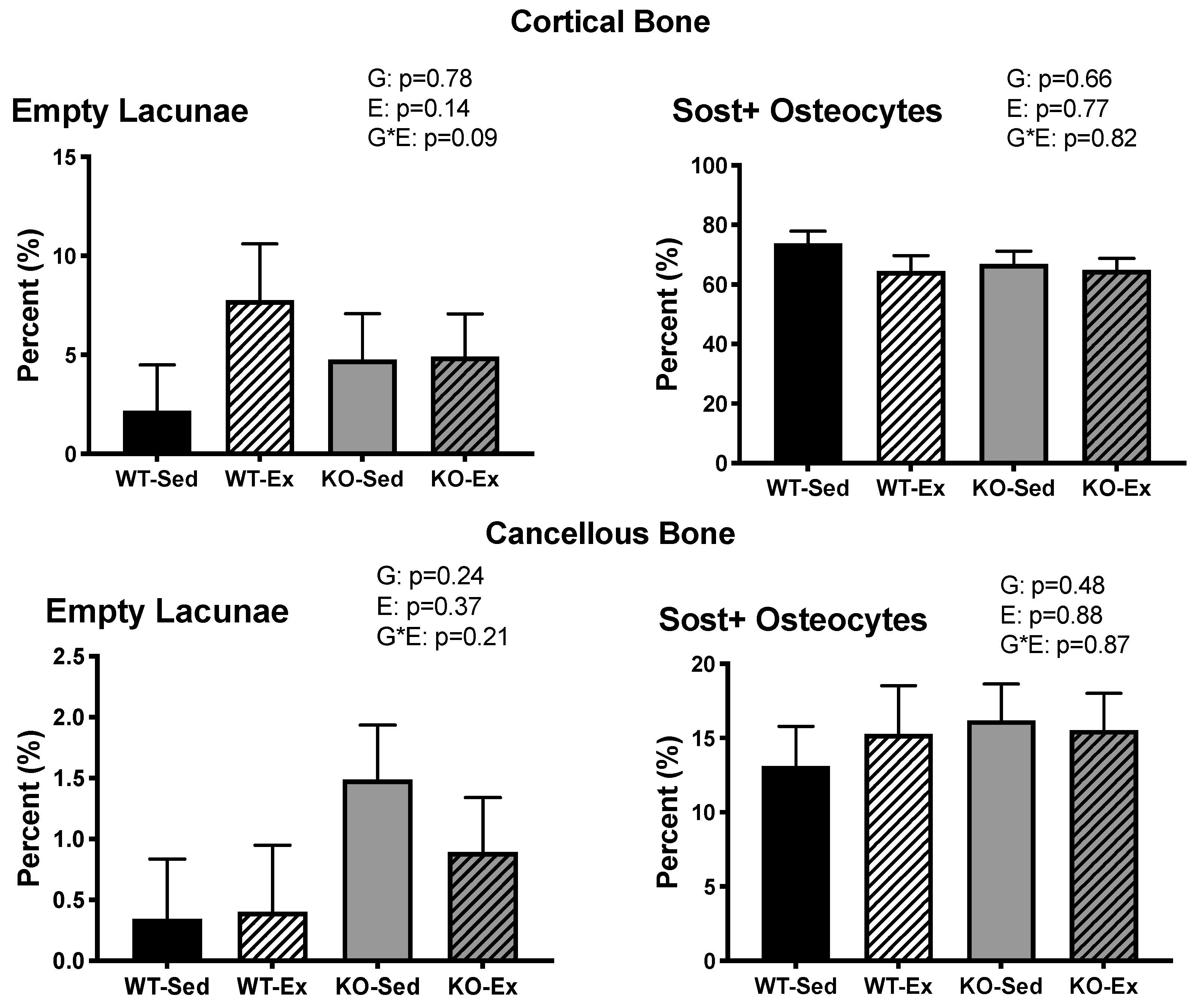
2.5. Femoral Osteocyte Sclerostin Expression
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Tibial Cortical Geometry and Trabecular Microarchitecture
4.3. Tibial Biomechanical Strength
4.4. Femoral Osteocyte Sclerostin Expression
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cutler, G.B. The Role of Estrogen in Bone Growth and Maturation during Childhood and Adolescence. J. Steroid Biochem. Mol. Biol. 1997, 616, 141–144. [Google Scholar] [CrossRef]
- Fortes, C.M.T.; Goldberg, T.B.L.; Kurokawa, C.S.; Silva, C.C.; Moretto, M.R.; Biason, T.P.; Teixeira, A.S.; De Carvalho Nunes, H.R. Relationship between chronological and bone ages and pubertal stage of breasts with bone biomarkers and bone mineral density in adolescents. J. Pediatr. (Rio. J.) 2014, 90, 624–631. [Google Scholar] [CrossRef]
- Yilmaz, D.; Ersoy, B.; Bilgin, E.; Gümüşer, G.; Onur, E.; Pinar, E.D. Bone mineral density in girls and boys at different pubertal stages: Relation with gonadal steroids, bone formation markers, and growth parameters. J. Bone Miner. Metab. 2005, 23, 476–482. [Google Scholar] [CrossRef]
- Khosla, S.; Melton, L.J.; Riggs, B.L. Estrogens and Bone Health in Men. Calcif. Tissue Int. 2001, 69, 189–192. [Google Scholar] [CrossRef]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Onoe, Y.; Miyaura, C.; Ohta, H.; Nozawa, S.; Suda, T. Expression of Estrogen Receptor β in Rat Bone. Endocrinology 1997, 138, 4509–4512. [Google Scholar] [CrossRef] [PubMed]
- Parikka, V.; Peng, Z.; Hentunen, T.; Risteli, J.; Elo, T.; Väänänen, H.K.; Härkönen, P. Estrogen responsiveness of bone formation in vitro and altered bone phenotype in aged estrogen receptor-a-deficient male and female mice. Eur. J. Endocrinol. 2005, 152, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, L.; Ederveen, A.G.H.; Erben, R.G.; Stahr, K.; Swinnen, J.V.; Van Herck, E.; Verstuyf, A.; Boonen, S.; Bouillon, R.; Vanderschueren, D. Testosterone Prevents Orchidectomy-Induced Bone Loss in Estrogen Receptor-α Knockout Mice. Biochem. Biophys. Res. Commun. 2001, 285, 70–76. [Google Scholar] [CrossRef]
- Vidal, O.; Lindberg, M.K.; Hollberg, K.; Baylink, D.J.; Andersson, G.; Lubahn, D.B.; Mohan, S.; Gustafsson, J.A.; Ohlsson, C. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc. Natl. Acad. Sci. USA 2000, 97, 5474–5479. [Google Scholar] [CrossRef]
- Callewaert, F.; Venken, K.; Ophoff, J.; De Gendt, K.; Torcasio, A.; van Lenthe, G.H.; Van Oosterwyck, H.; Boonen, S.; Bouillon, R.; Verhoeven, G.; et al. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J. 2009, 23, 232–240. [Google Scholar] [CrossRef]
- Lindberg, M.K.; Erlandsson, M.; Alatalo, S.L.; Windahl, S.; Andersson, G.; Halleen, J.M.; Carlsten, H.; Gustafsson, J.A.; Ohlsson, C. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the OPG/RANKL (osteoprotegerin/receptor activator of NF-kappa B ligand) ratio and serum interleukin-6 in male mice. J. Endocrinol. 2001, 171, 425–433. [Google Scholar] [CrossRef]
- Galea, G.L.; Price, J.S.; Lanyon, L.E. Estrogen receptors’ roles in the control of mechanically adaptive bone (re)modeling. Bonekey Rep. 2013, 2. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Van Oers, R.F.M.; Bakker, A.D.; Bacabac, R.G. Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J. Biomech. 2015, 48, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.L.; Meakin, L.B.; Sugiyama, T.; Zebda, N.; Sunters, A.; Taipaleenmaki, H.; Stein, G.S.; Van Wijnen, A.J.; Lanyon, L.E.; Price, J.S. Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J. Biol. Chem. 2013, 288, 9035–9048. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, V.J.; Muzylak, M.; Sunters, A.; Zaman, G.; Saxon, L.K.; Price, J.S.; Lanyon, L.E. Wnt/Beta-Catenin Signaling Is a Component of Osteoblastic Bone Cell Early Responses to Load-bearing and Requires Estrogen Receptor-alpha. J. Biol. Chem. 2007, 282, 20715–20727. [Google Scholar] [CrossRef] [PubMed]
- Jessop, H.L.; Sjöberg, M.; Cheng, M.Z.; Zaman, G.; Wheeler-Jones, C.P.D.; Lanyon, L.E. Mechanical Strain and Estrogen Activate Estrogen Receptor α in Bone Cells. J. Bone Miner. Res. 2001, 16, 1045–1055. [Google Scholar] [CrossRef]
- Okazaki, R.; Inoue, D.; Shibata, M.; Saika, M.; Kido, S.; Ooka, H.; Tomiyama, H.; Sakamoto, Y.; Matsumoto, T. Estrogen Promotes Early Osteoblast Differentiation and Inhibits Adipocyte Differentiation in Mouse Bone Marrow Stromal Cell Lines that Express Estrogen Receptor (ER) α or β. Endocrinology 2002, 143, 2349–2356. [Google Scholar] [CrossRef]
- Windahl, S.H.; Saxon, L.; Börjesson, A.E.; Lagerquist, M.K.; Frenkel, B.; Henning, P.; Lerner, U.H.; Galea, G.L.; Meakin, L.B.; Engdahl, C.; et al. Estrogen receptor-α is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2. J. Bone Miner. Res. 2013, 28, 291–301. [Google Scholar] [CrossRef]
- Saxon, L.K.; Galea, G.; Meakin, L.; Price, J.; Lanyon, L.E. Estrogen Receptors Alpha and Beta Have Different Gender-Dependent Effects on the Adaptive Responses to Load Bearing in Cancellous and Cortical Bone. Endocrinology 2012, 153, 2254–2266. [Google Scholar] [CrossRef]
- Melville, K.M.; Kelly, N.H.; Surita, G.; Buchalter, D.B.; Schimenti, J.C.; Main, R.P.; Ross, F.P.; van der Meulen, M.C. Effects of Deletion of ERα in Osteoblast-Lineage Cells on Bone Mass and Adaptation to Mechanical Loading Differ in Female and Male Mice. J. Bone Miner. Res. 2015, 30, 1468–1480. [Google Scholar] [CrossRef]
- Callewaert, F.; Sinnesael, M.; Gielen, E.; Boonen, S.; Vanderschueren, D. Skeletal sexual dimorphism: Relative contribution of sex steroids, GH-IGF1, and mechanical loading. J. Endocrinol. 2010, 207, 127–134. [Google Scholar] [CrossRef]
- Lerner, U.H.; Ohlsson, C. The WNT system: Background and its role in bone. J. Intern. Med. 2015, 277, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Burgers, T.A.; Williams, B.O.; Bonewald, L.F.; Johnson, M.E.; Kneissel, M. Regulation of Wnt/β-catenin signaling within and from osteocytes. Bone 2013, 54, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.L.; Lanyon, L.E.; Price, J.S. Sclerostin’s role in bone’s adaptive response to mechanical loading. Bone 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Mödder, U.I.; Hoey, K.A.; Amin, S.; McCready, L.K.; Achenbach, S.J.; Riggs, B.L.; Melton, L.J.; Khosla, S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J. Bone Miner. Res. 2011, 26, 373–379. [Google Scholar] [CrossRef]
- Kim, W.; Chung, Y.; Kim, S.H.; Park, S.; Bae, J.H.; Kim, G.; Lee, S.J.; Kim, J.E.; Park, B.-W.; Lim, S.-K.; et al. Increased Sclerostin Levels after Further Ablation of Remnant Estrogen by Aromatase Inhibitors. Endocrinol. Metab. 2015, 30, 58–64. [Google Scholar] [CrossRef]
- Mirza, F.S.; Padhi, I.D.; Raisz, L.G.; Lorenzo, J.A. Serum Sclerostin Levels Negatively Correlate with Parathyroid Hormone Levels and Free Estrogen Index in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2010, 95, 1991–1997. [Google Scholar] [CrossRef]
- Fujita, K.; Roforth, M.M.; Demaray, S.; Mcgregor, U.; Kirmani, S.; Mccready, L.K.; Peterson, J.M.; Drake, M.T.; Monroe, D.G.; Khosla, S. Effects of Estrogen on Bone mRNA Levels of Sclerostin and Other Genes Relevant to Bone Metabolism in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014, 99, E81–E88. [Google Scholar] [CrossRef]
- Kim, B.-J.; Bae, S.J.; Lee, S.-Y.; Lee, Y.-S.; Baek, J.-E.; Park, S.-Y.; Lee, S.H.; Koh, J.-M.; Kim, G.S. TNF-alpha mediates the stimulation of sclerostin expression in an estrogen-deficient condition. Biochem. Biophys. Res. Commun. 2012, 424, 170–175. [Google Scholar] [CrossRef]
- Jia, H.B.; Ma, J.X.; Ma, X.L.; Yu, J.T.; Feng, R.; Xu, L.Y.; Wang, J.; Xing, D.; Zhu, S.W.; Wang, Y. Estrogen alone or in combination with parathyroid hormone can decrease vertebral MEF2 and sclerostin expression and increase vertebral bone mass in ovariectomized rats. Osteoporos. Int. 2014, 25, 2743–2754. [Google Scholar] [CrossRef] [PubMed]
- Mödder, U.I.L.; Clowes, J.A.; Hoey, K.; Peterson, J.M.; McCready, L.; Oursler, M.J.; Riggs, B.L.; Khosla, S. Regulation of circulating sclerostin levels by sex steroids in women and in men. J. Bone Miner. Res. 2011, 26, 27–34. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sañudo, C.; Bolado, A.; Fernández, A.F.; Arozamena, J.; Pascual-Carra, M.A.; Rodriguez-Rey, J.C.; Fraga, M.F.; Bonewald, L.; Riancho, J.A. DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J. Bone Miner. Res. 2012, 27, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Winn, N.C.; Jurrissen, T.J.; Grunewald, Z.I.; Cunningham, R.P.; Woodford, M.L.; Kanaley, J.A.; Lubahn, D.B.; Manrique-Acevedo, C.; Rector, R.S.; Vieira-Potter, V.J.; et al. Estrogen receptor alpha signaling maintains immunometabolic function in males and is obligatory for exercise-induced amelioration of nonalcoholic fatty liver. Am. J. Physiol. Metab. 2018, 16, E156–E167. [Google Scholar] [CrossRef]
- Dirkes, R.K.; Winn, N.C.; Jurrissen, T.J.; Lubahn, D.B.; Vieira-Potter, V.J.; Padilla, J.; Hinton, P.S. Global estrogen receptor-α knockout has differential effects on cortical and cancellous bone in aged male mice. FACETS 2020, 5, 328–348. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Syed, F.; Khosla, S. Mechanisms of sex steroid effects on bone. Biochem. Biophys. Res. Commun. 2005, 328, 688–696. [Google Scholar] [CrossRef]
- Shevde, N.K.; Bendixen, A.C.; Dienger, K.M.; Pike, J.W.; Deluca, H.F. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc. Natl. Acad. Sci. USA 2000, 97, 7829–7834. [Google Scholar] [CrossRef]
- Bord, S.; Ireland, D.; Beavan, S.; Compston, J. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 2003, 32, 136–141. [Google Scholar] [CrossRef]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef]
- Almeida, M.; Iyer, S.; Martin-Millan, M.; Bartell, S.M.; Han, L.; Ambrogini, E.; Onal, M.; Xiong, J.; Weinstein, R.S.; Jilka, R.L.; et al. Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J. Clin. Investig. 2013, 123, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Windahl, S.H.; Börjesson, A.E.; Farman, H.H.; Engdahl, C.; Movérare-Skrtic, S.; Sjögren, K.; Lagerquist, M.K.; Kindblom, J.M.; Koskela, A.; Tuukkanen, J.; et al. Estrogen receptor-α in osteocytes is important for trabecular bone formation in male mice. Proc. Natl. Acad. Sci. USA 2013, 110, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Somerville, J.M.; Aspden, R.M.; Armour, K.E.; Armour, K.J.; Reid, D.M. Growth of C57Bl/6 Mice and the Material and Mechanical Properties of Cortical Bone from the Tibia. Calcif. Tissue Int. 2004, 74, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Takano, Y.; Owan, I. Aging changes mechanical loading thresholds for bone formation in rats. J. Bone Miner. Res. 2009, 10, 1544–1549. [Google Scholar] [CrossRef]
- Hughes, J.M.; Petit, M.A. Biological underpinnings of frost’s mechanostat thresholds: The important role of osteocytes. J. Musculoskelet. Neuronal Interact. 2010, 10, 128–135. [Google Scholar]
- Schriefer, J.L.; Robling, A.G.; Warden, S.J.; Fournier, A.J.; Mason, J.J.; Turner, C.H. A comparison of mechanical properties derived from multiple skeletal sites in mice. J. Biomech. 2005, 38, 467–475. [Google Scholar] [CrossRef]
- Ammann, P.; Rizzoli, A.R. Bone strength and its determinants. Osteoporos. Int. 2003, 14, 13–18. [Google Scholar] [CrossRef]
- Lubahn, D.B.; Moyer, J.S.; Golding, T.S.; Couse, J.F.; KoRach, K.S.; Smithies, O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Genetics 1993, 90, 11162–11166. Available online: http://www.pnas.org/content/90/23/11162.full.pdf (accessed on 2 August 2017). [CrossRef]
- Eddy, E.M.; Washburn, T.F.; Bunch, D.O.; Goulding, E.H.; Gladen, B.C.; Lubahn, D.B.; Korach, K.S. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 1996, 137, 4796–4805. [Google Scholar] [CrossRef]
- Rubanyi, G.M.; Freay, A.D.; Kauser, K.; Sukovich, D.; Burton, G.; Lubahn, D.B.; Couse, J.F.; Curtis, S.W.; Korach, K.S. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J. Clin. Investig. 1997, 99, 2429–2437. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Ortinau, L.C.; Linden, M.A.; Dirkes, R.K.; Rector, R.S.; Hinton, P.S. Exercise initiated after the onset of insulin resistance improves trabecular microarchitecture and cortical bone biomechanics of the tibia in hyperphagic Otsuka Long Evans Tokushima Fatty rats. Bone 2017, 103, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Ortinau, L.C.; Linden, M.A.; Dirkes, R.; Rector, R.S.; Hinton, P.S. Obesity and type 2 diabetes, not a diet high in fat, sucrose, and cholesterol, negatively impacts bone outcomes in the hyperphagic Otsuka Long Evans Tokushima Fatty rat. Bone 2017, 105, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Bruker-microCT, Morphometric parameters measured by Skyscan™ CT—Analyser Software. Ref. Man. 2012, 1, 1–49. Available online: http://bruker-microct.com/next/CTAn03.pdf (accessed on 17 March 2019).
- Jepsen, K.J.; Silva, M.J.; Vashishth, D.; Guo, X.E.; Van Der Meulen, M.C.H. Establishing biomechanical mechanisms in mouse models: Practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J. Bone Miner. Res. 2015, 30, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Burr, D.B. Experimental Techniques for Bone Mechanics. In Bone Mechanics; Taylor and Francis Group: Boca Raton, FL, USA, 2001; pp. 7.1–7.35. Available online: https://doc-04-24-docs.googleusercontent.com/docs/securesc/5bsd23h7nijs0o191tafmm7te1qj95pc/qkrv573nk7i06ql51smbun5sj4uhp21l/1560981600000/03597488174621737607/07265225710666323276/1wa2m7nwhy2gB7Vo0uVu7SAArC8F6NO1E?nonce=e9c26ime80ukc&user=072652257106663 (accessed on 19 June 2019).
- Pereira, M.; Gohin, S.; Lund, N.; Hvid, A.; Smitham, P.J.; Oddy, M.J.; Reichert, I.; Farlay, D.; Roux, J.P.; Cleasby, M.E.; et al. Sclerostin does not play a major role in the pathogenesis of skeletal complications in type 2 diabetes mellitus. Osteoporos. Int. 2017, 28, 309–320. [Google Scholar] [CrossRef]
| WT | ERKO | ||||
|---|---|---|---|---|---|
| Outcome | SED | EX | SED | EX | p-Value |
| Body Mass (g) | 39.63 ± 1.70 | 33.06 ± 1.77 * | 41.52 ± 1.70 | 36.39 ± 1.58 * | G: 0.129 E: 0.001 G*E: 0.670 |
| Body Fat (%) | 32.28 ± 2.33 | 22.96 ± 2.64 * | 33.22 ± 2.33 | 27.56 ± 2.21 * | G: 0.254 E: 0.004 G*E: 0.450 |
| Average Food Intake (g/week) | 9.09 ± 0.21 | 10.20 ± 0.24 * | 8.83 ± 0.21 | 9.60 ± 0.20 * | G: 0.054 E: 0.001 G*E: 0.432 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dirkes, R.K.; Winn, N.C.; Jurrissen, T.J.; Lubahn, D.B.; Vieira-Potter, V.J.; Padilla, J.; Hinton, P.S. Voluntary Wheel Running Partially Compensates for the Effects of Global Estrogen Receptor-α Knockout on Cortical Bone in Young Male Mice. Int. J. Mol. Sci. 2021, 22, 1734. https://doi.org/10.3390/ijms22041734
Dirkes RK, Winn NC, Jurrissen TJ, Lubahn DB, Vieira-Potter VJ, Padilla J, Hinton PS. Voluntary Wheel Running Partially Compensates for the Effects of Global Estrogen Receptor-α Knockout on Cortical Bone in Young Male Mice. International Journal of Molecular Sciences. 2021; 22(4):1734. https://doi.org/10.3390/ijms22041734
Chicago/Turabian StyleDirkes, Rebecca K., Nathan C. Winn, Thomas J. Jurrissen, Dennis B. Lubahn, Victoria J. Vieira-Potter, Jaume Padilla, and Pamela S. Hinton. 2021. "Voluntary Wheel Running Partially Compensates for the Effects of Global Estrogen Receptor-α Knockout on Cortical Bone in Young Male Mice" International Journal of Molecular Sciences 22, no. 4: 1734. https://doi.org/10.3390/ijms22041734
APA StyleDirkes, R. K., Winn, N. C., Jurrissen, T. J., Lubahn, D. B., Vieira-Potter, V. J., Padilla, J., & Hinton, P. S. (2021). Voluntary Wheel Running Partially Compensates for the Effects of Global Estrogen Receptor-α Knockout on Cortical Bone in Young Male Mice. International Journal of Molecular Sciences, 22(4), 1734. https://doi.org/10.3390/ijms22041734





