Impact of Liposomal Drug Formulations on the RBCs Shape, Transmembrane Potential, and Mechanical Properties
Abstract
1. Introduction
2. Results
2.1. Liposome Characterization: Size Distribution and Encapsulation Efficiency
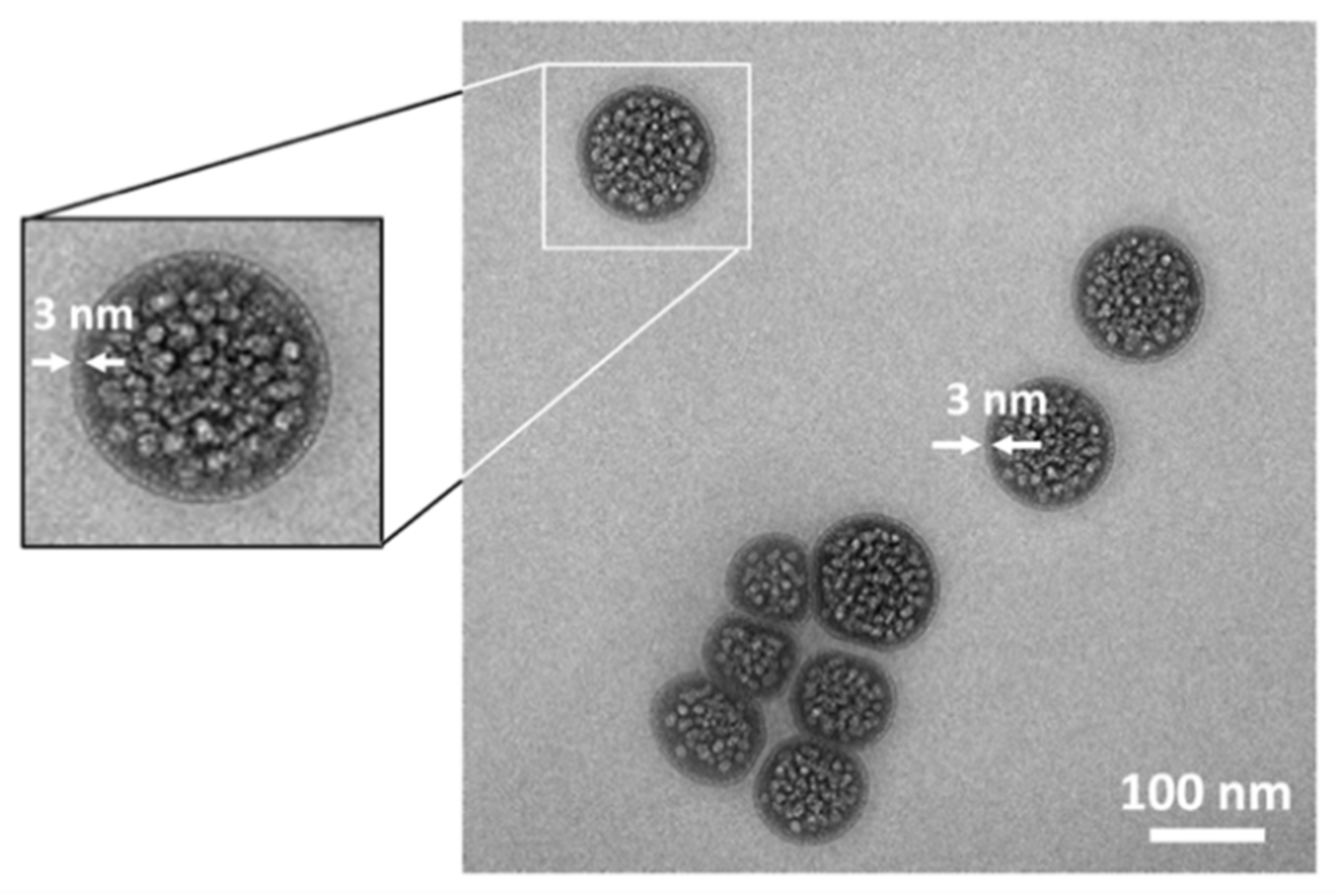
2.2. Electron Microscopy Visualization of Liposomal Formulation
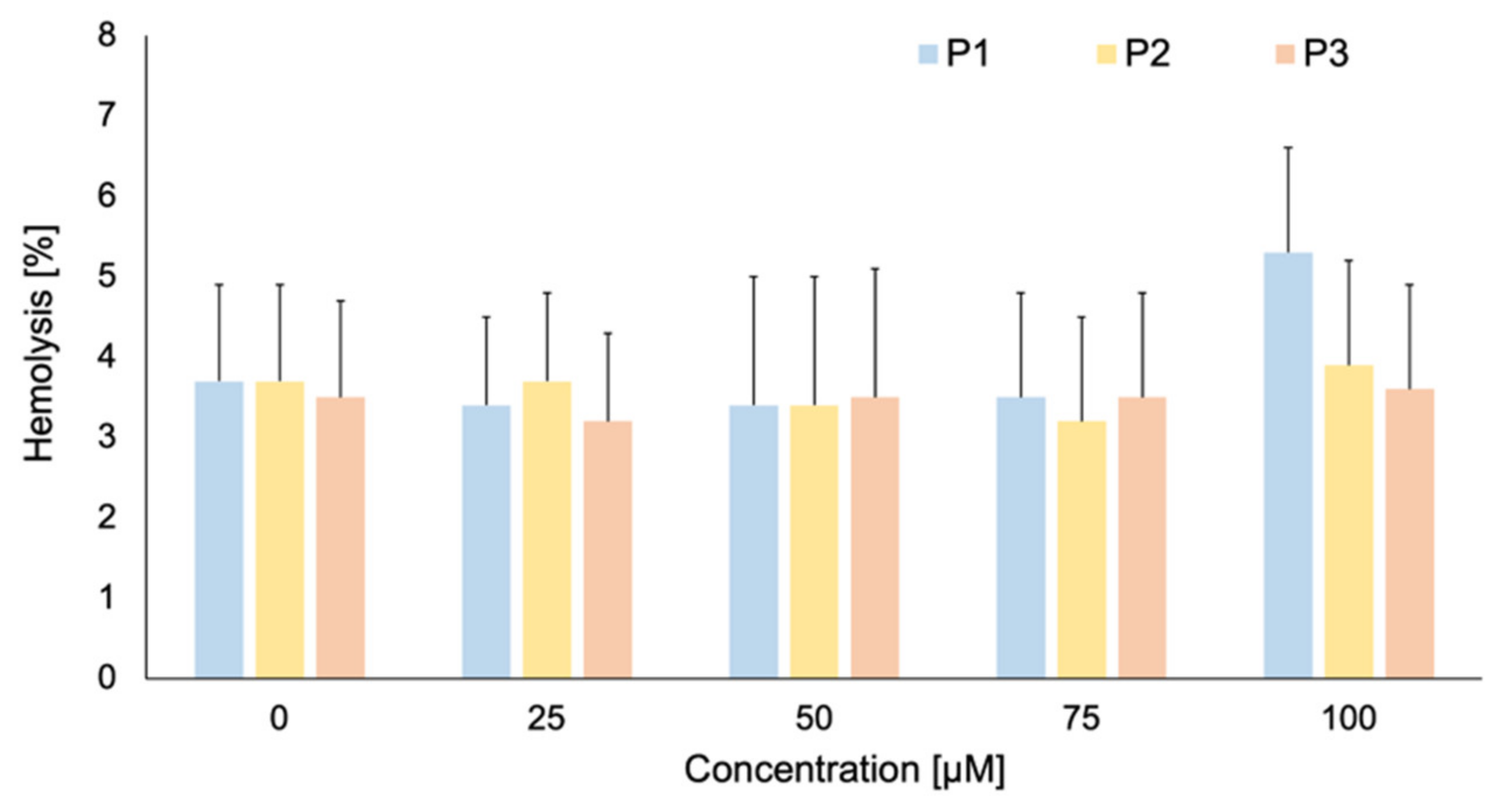
2.3. Photolon Liposomal Formulations Hemolytic Activity
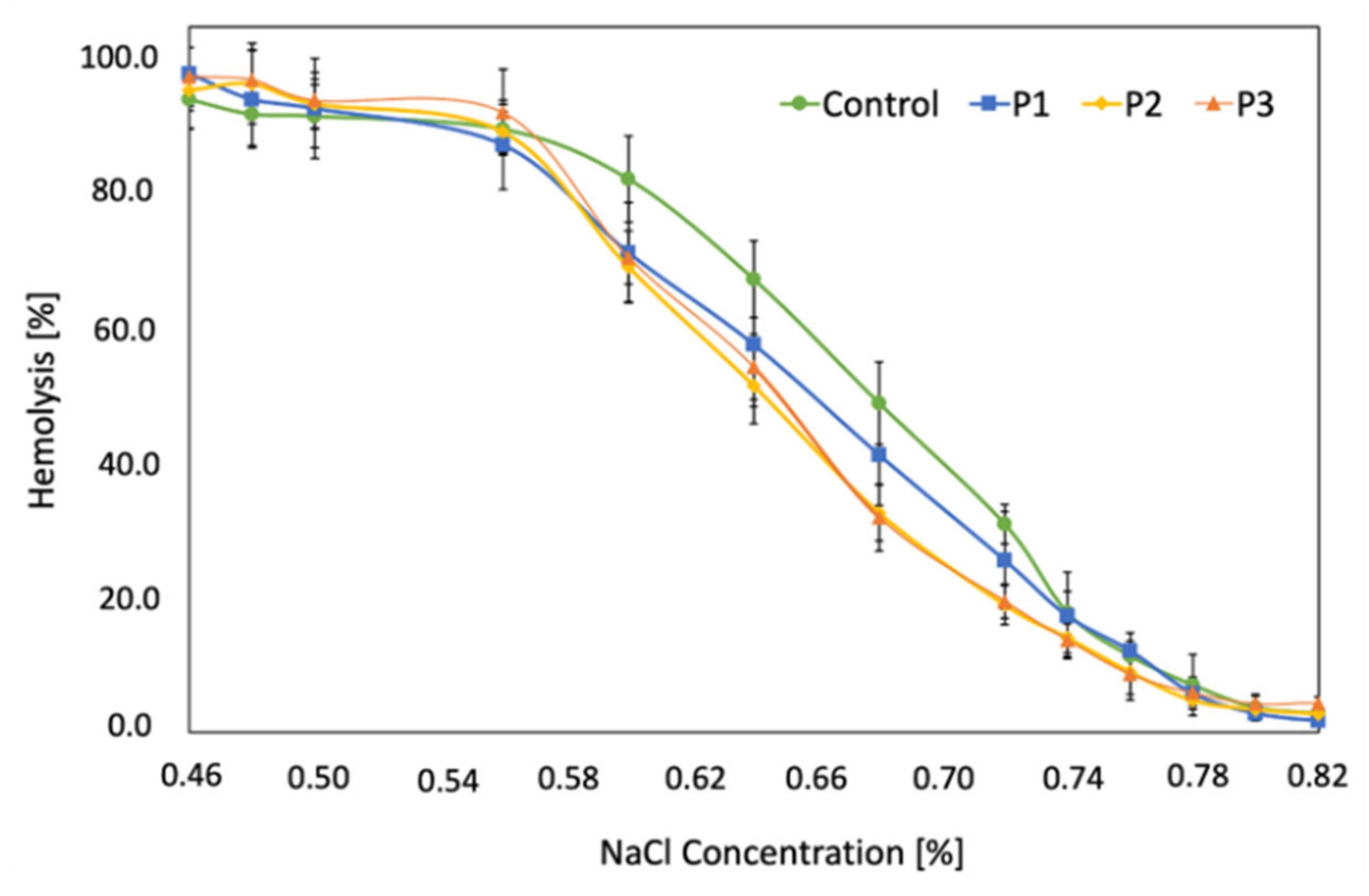
2.4. RBCs Osmotic Resistance Changes under Liposomal Formulation Impact
2.5. RBCs Transmembrane Potential Changes upon LUVs Modification
2.6. Biomechanical Parameters of RBCs upon LUVs Interaction
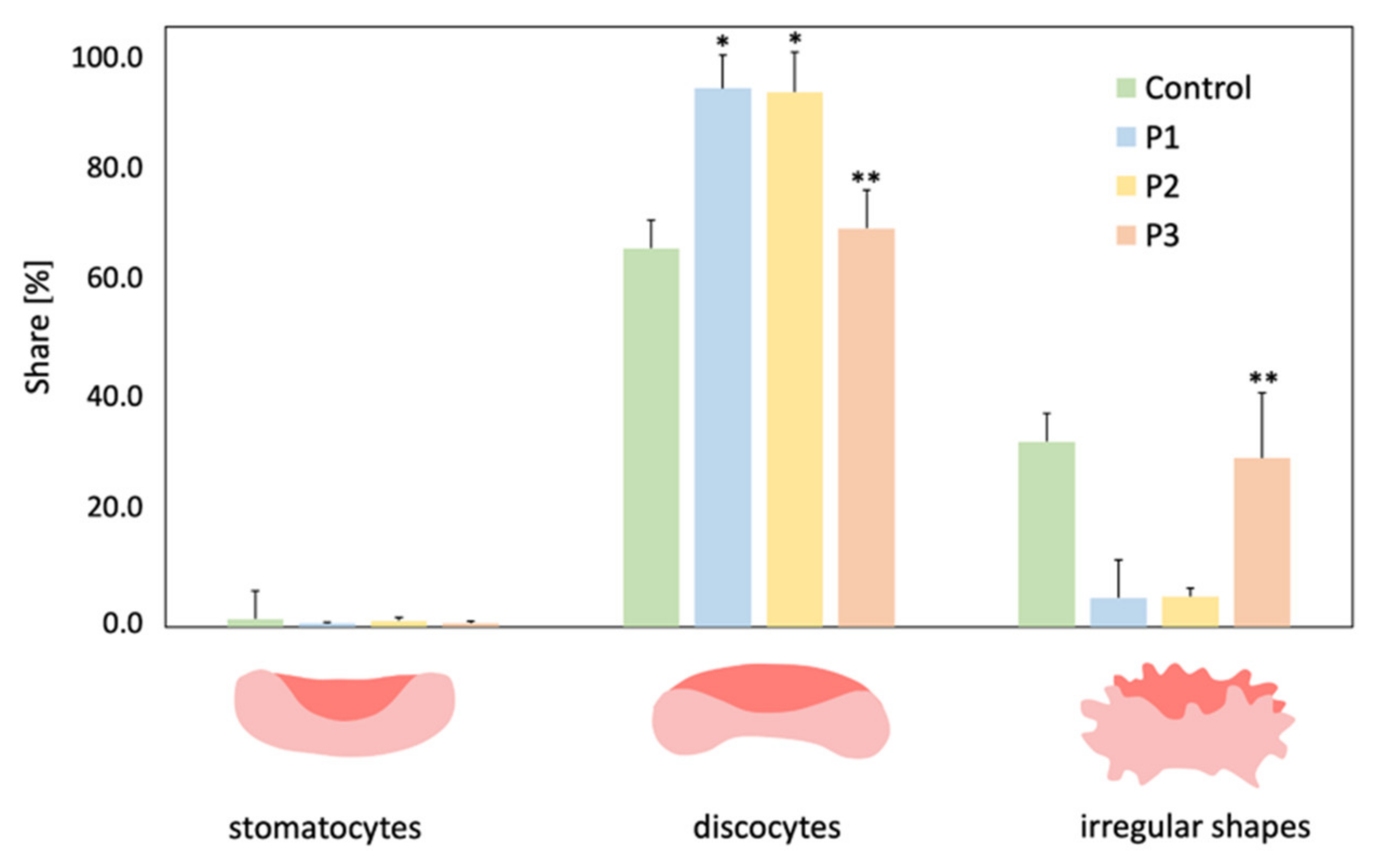
2.7. Microscopic Evaluation of the RBCs Shape
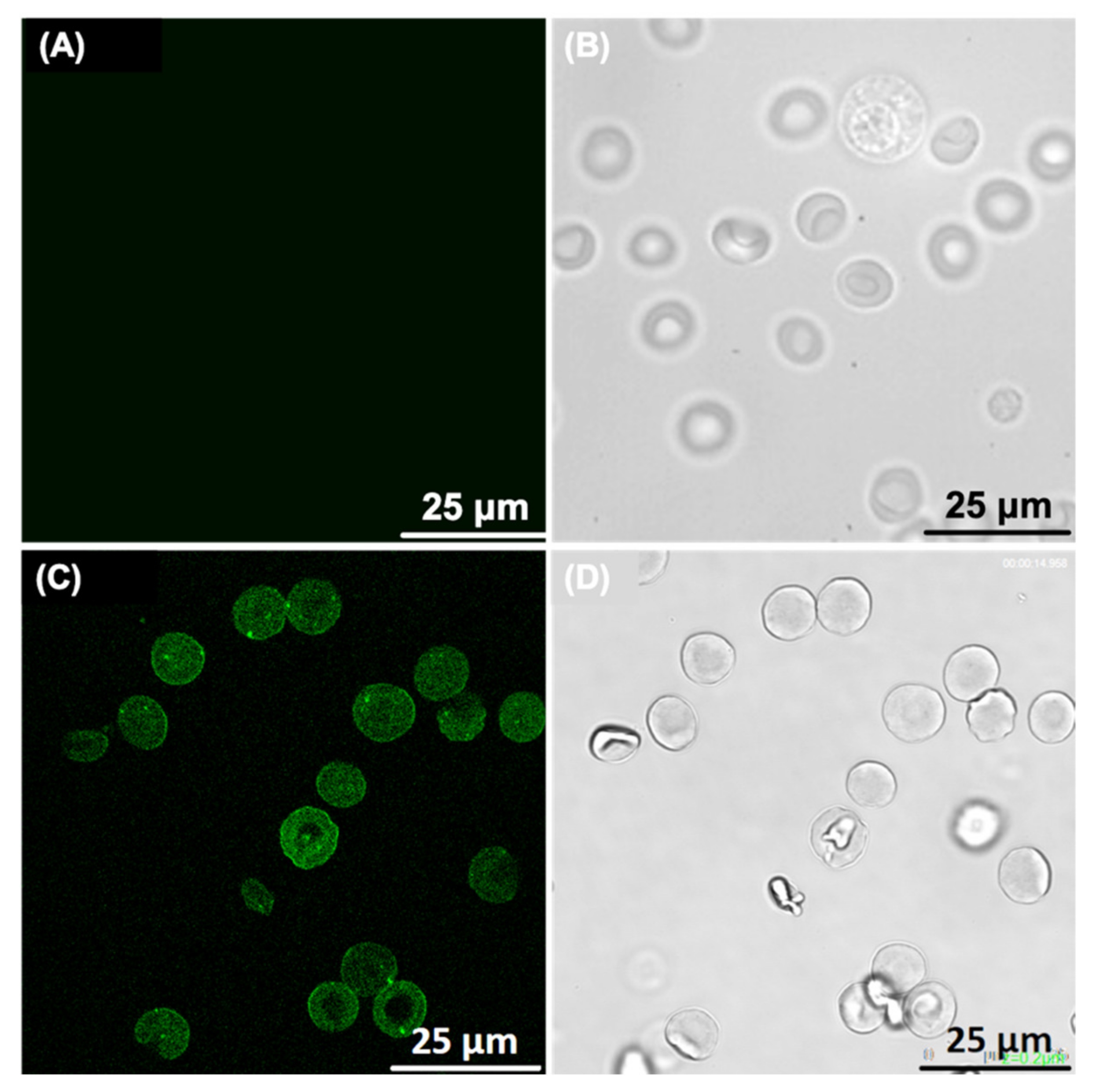
2.8. Confocal Microscopy Study of RBCs-LUVs Photolon Redistribution
2.9. Statistical Analysis
3. Materials and Methods
3.1. Liposome Preparation Protocol
3.2. Size Distribution and Zeta Potential Measurements of the Photoactive Liposomal Formulation
3.3. Encapsulation Efficiency of the Photoactive Liposomal Formulation
3.4. Electron Microscopy Visualization of Liposomal Formulation
3.5. Animals and Blood Samples Collection
3.6. Photolon Liposomal Formulations Hemolytic Activity Examination
3.7. Assessment of the Osmotic Resistance of RBCs Exposed to Liposomal Formulations
3.8. Erythrocytes Transmembrane Potential Measurement
3.9. Biomechanical Parameters of RBCs
3.10. Shape of RBCs Assessment
3.11. Confocal Microscopy of Erythrocytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal. Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Kałas, W.; Wysokińska, E.; Przybyło, M.; Langner, M.; Ulatowska-Jarża, A.; Biały, D.; Zioło, E.; Gil, W.; Trzeciak, A.M.; Podbielska, H.; et al. Photoactive liposomal formulation of PVP-conjugated chlorin e6 for photodynamic reduction of atherosclerotic plaque. Int. J. Mol. Sci. 2019, 20, 3852. [Google Scholar] [CrossRef] [PubMed]
- Plourde, K.; Derbali, R.M.; Desrosiers, A.; Dubath, C.; Vallee-Belisle, A.; Leblond, J. Aptamer-based liposomes improve specific drug loading and release. J. Control. Rel. 2017, 251, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wang, C.Z.; Fan, H.J.; Zhang, C.J.; Zhang, H.W.; Lv, M.H.; Cui, S.D. A dual-targeting liposome conjugated with transferrin and arginine-glycane-aspartic acid peptide for glioma-targeting therapy. Oncol. Lett. 2014, 8, 20006. [Google Scholar] [CrossRef]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.D. Combination Therapy using Co-encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in vivo. Sci Rep. 2016, 7, 22390. [Google Scholar] [CrossRef] [PubMed]
- Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G.L. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 110–116. [Google Scholar] [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Børresen, B.; Hansen, A.E.; Fliedner, F.P.; Henriksen, J.R.; Elema, D.R.; Brandt-Larsen, M.; Kristensen, L.K.; Kristensen, A.T.; Andresen, T.L.; Kjær, A. Noninvasive molecular imaging of the enhanced permeability and retention effect by 64Cu-liposomes: In vivo correlations with 68Ga-RGD, fluid pressure, diffusivity and 18F-FDG. Inter. J. Nanomed. 2020, 15, 8571–8581. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Morib, Y.; Feng, H.; Phan, K.Q.; Kishimura, A.; Kang, J.H.; Mori, T.; Katayama, Y. Rapid and continuous accumulation of nitric oxide-releasing liposomes in tumors to augment the enhanced permeability and retention (EPR) effect. Int. J. Pharm. 2019, 565, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, M.; Pastorino, F.; Di Paolo, D.; Perri, P.; Brignole, C. Targeting macrophages as a potential therapeutic intervention: Impact on inflammatory diseases and cancer. Int J. Mol. Sci. 2018, 19, 1953. [Google Scholar] [CrossRef] [PubMed]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and toxicological consideration for the design of liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak, Z.; Szostak-Paluch, K.; Przybyło, M.; Langner, M.; Witkiewicz, W.; Jędruchniewicz, N.; Dąbrowolska, K. Endocytosis in cellular uptake of drug delivery vectors: Molecular aspects in drug development. Bioorg. Med. Chem. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Bessis, M. Erythrocyte form and deformability for normal blood and some hereditary hemolytic anemias. Nouv. Rev. Fr. Hematol. 1997, 18, 75–94. [Google Scholar] [PubMed]
- Stoll, C.; Holovati, J.L.; Acker, J.P.; Wolkers, W.F. Liposomes composed of unsaturated lipids for membrane modification of human erythrocytes. Mol. Membr. Biol. 2011, 28, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Holovati, J.L.; Gyongyossy-Issa, M.I.C.; Acker, J.P. Effect of liposome charge and composition on the delivery of trehalose into red blood cells. Cell Preserv. Technol. 2008, 6, 3207–3218. [Google Scholar] [CrossRef]
- Da Silveira Cavalcante, L.; Acker, J.P.; Holovati, J.L. Effect of liposome treatment on hemorheology and metabolic profile of human red blood cells during hypothermic storage. Biopreserv. Biobank 2018, 16, 304–311. [Google Scholar] [CrossRef]
- Holovati, J.L.; Acker, J.P. Emerging role for use of liposomes in the biopreservation of red blood cells. Transfus. Med. Hemother. 2011, 38, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mourtas, S.; Michanetzis, G.P.; Missirlis, Y.F.; Antimisiaris, S.G. Haemolytic activity of liposomes: Effect of vesicle size, lipid concentration and polyethylene glycol-lipid or arsonolipid incorporation. J. Biomed. Nanotechnol. 2009, 5, 409–415. [Google Scholar] [CrossRef]
- Russo, R.; Witos, J.; Rantamaki, A.H.; Wiedmer, S.K. Cholesterol affects the interaction between an ionic liquid and phospholipid vesicles. A study by differential scanning calorimetry and nanoplasmonic sensing. Biochim. Biophys. Acta Biomembr. 2017, 12, 2361–2372. [Google Scholar] [CrossRef]
- Cui, Z.K.; Edwards, K.; Orellana, A.N.; Bastiat, G.; Benoit, J.P.; Lafleur, M. Imapct of interfacial cholesterol-anchored polyethylene glycol on sterol-rich non-phospholipid liposomes. J. Coll. Interface Sci. 2014, 428, 111–120. [Google Scholar] [CrossRef]
- Rawicz, W.; Olbrich, K.C.; McIntosh, T.; Needham, D.; Evans, E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef]
- Zavodnik, I.B.; Piasecka, A.; Szosland, K.; Bryszewska, M. Human red blood cell membrane potential and fluidity in glucose solutions. Scand J. Clin. Lab. Investig. 1997, 57, 59–63. [Google Scholar] [CrossRef] [PubMed]
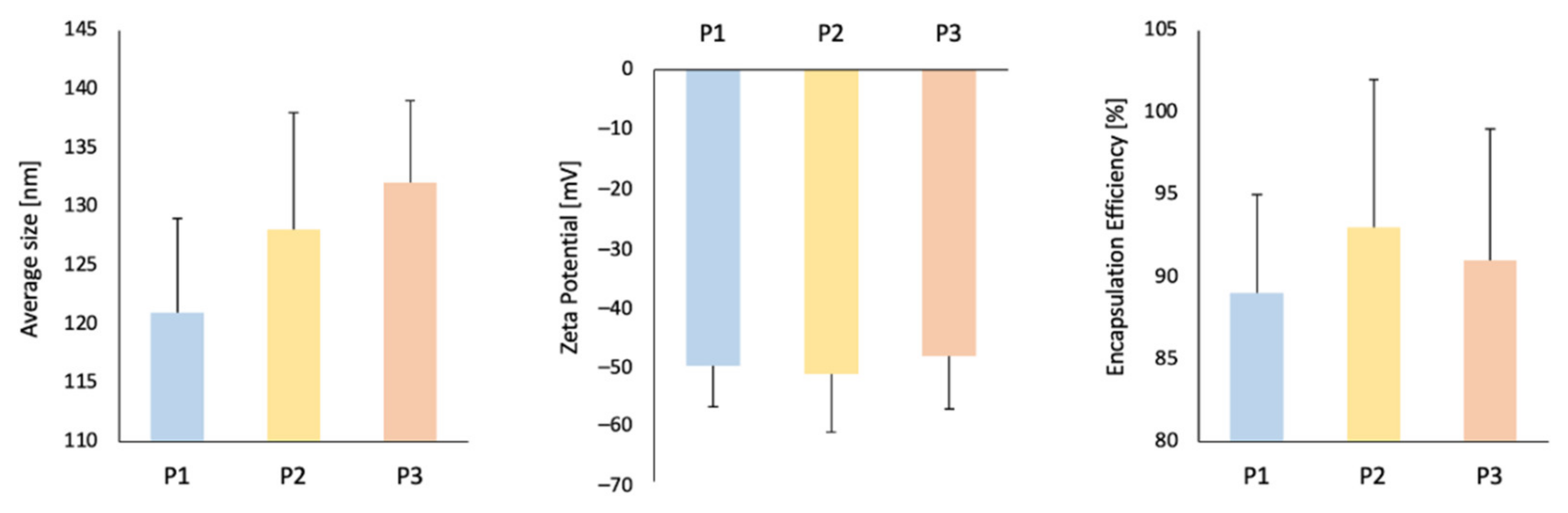
| Average Size [nm] | PDI [–] | Zeta Potential [mV] | EE [%] | |
|---|---|---|---|---|
| P1 | 121 ± 8 | 0.15 | −50.3 ± 7 | 89 ± 6 |
| P2 | 128 ± 10 | 0.18 | −51.7 ± 10 | 93 ± 9 |
| P3 | 132 ± 7 | 0.17 | −48.7 ± 9 | 91 ± 8 |
| Transmembrane Potential of RBCs [mV] | |
|---|---|
| control | −15.6 ± 2.6 |
| P1 | −19.6 ± 1.9 * |
| P2 | −20.9 ± 2.1 * |
| P3 | −16.0 ± 1.9 |
| RBCs Stiffness [pN/μm] | |
|---|---|
| control | 32 ± 3 |
| P1 | 21 ± 4 * |
| P2 | 20 ± 4 * |
| P3 | 26 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyboran-Mikołajczyk, S.; Sareło, P.; Pasławski, R.; Pasławska, U.; Przybyło, M.; Nowak, K.; Płóciennik, M.; Podbielska, H.; Kopaczyńska, M.; Wawrzyńska, M. Impact of Liposomal Drug Formulations on the RBCs Shape, Transmembrane Potential, and Mechanical Properties. Int. J. Mol. Sci. 2021, 22, 1710. https://doi.org/10.3390/ijms22041710
Cyboran-Mikołajczyk S, Sareło P, Pasławski R, Pasławska U, Przybyło M, Nowak K, Płóciennik M, Podbielska H, Kopaczyńska M, Wawrzyńska M. Impact of Liposomal Drug Formulations on the RBCs Shape, Transmembrane Potential, and Mechanical Properties. International Journal of Molecular Sciences. 2021; 22(4):1710. https://doi.org/10.3390/ijms22041710
Chicago/Turabian StyleCyboran-Mikołajczyk, Sylwia, Przemysław Sareło, Robert Pasławski, Urszula Pasławska, Magdalena Przybyło, Kacper Nowak, Michał Płóciennik, Halina Podbielska, Marta Kopaczyńska, and Magdalena Wawrzyńska. 2021. "Impact of Liposomal Drug Formulations on the RBCs Shape, Transmembrane Potential, and Mechanical Properties" International Journal of Molecular Sciences 22, no. 4: 1710. https://doi.org/10.3390/ijms22041710
APA StyleCyboran-Mikołajczyk, S., Sareło, P., Pasławski, R., Pasławska, U., Przybyło, M., Nowak, K., Płóciennik, M., Podbielska, H., Kopaczyńska, M., & Wawrzyńska, M. (2021). Impact of Liposomal Drug Formulations on the RBCs Shape, Transmembrane Potential, and Mechanical Properties. International Journal of Molecular Sciences, 22(4), 1710. https://doi.org/10.3390/ijms22041710






