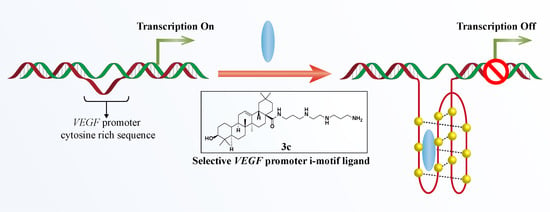
Synthesis and Biological Evaluation of Oleanolic Acid Derivatives as Selective Vascular Endothelial Growth Factor Promoter i-Motif Ligands
Abstract
1. Introduction
2. Results
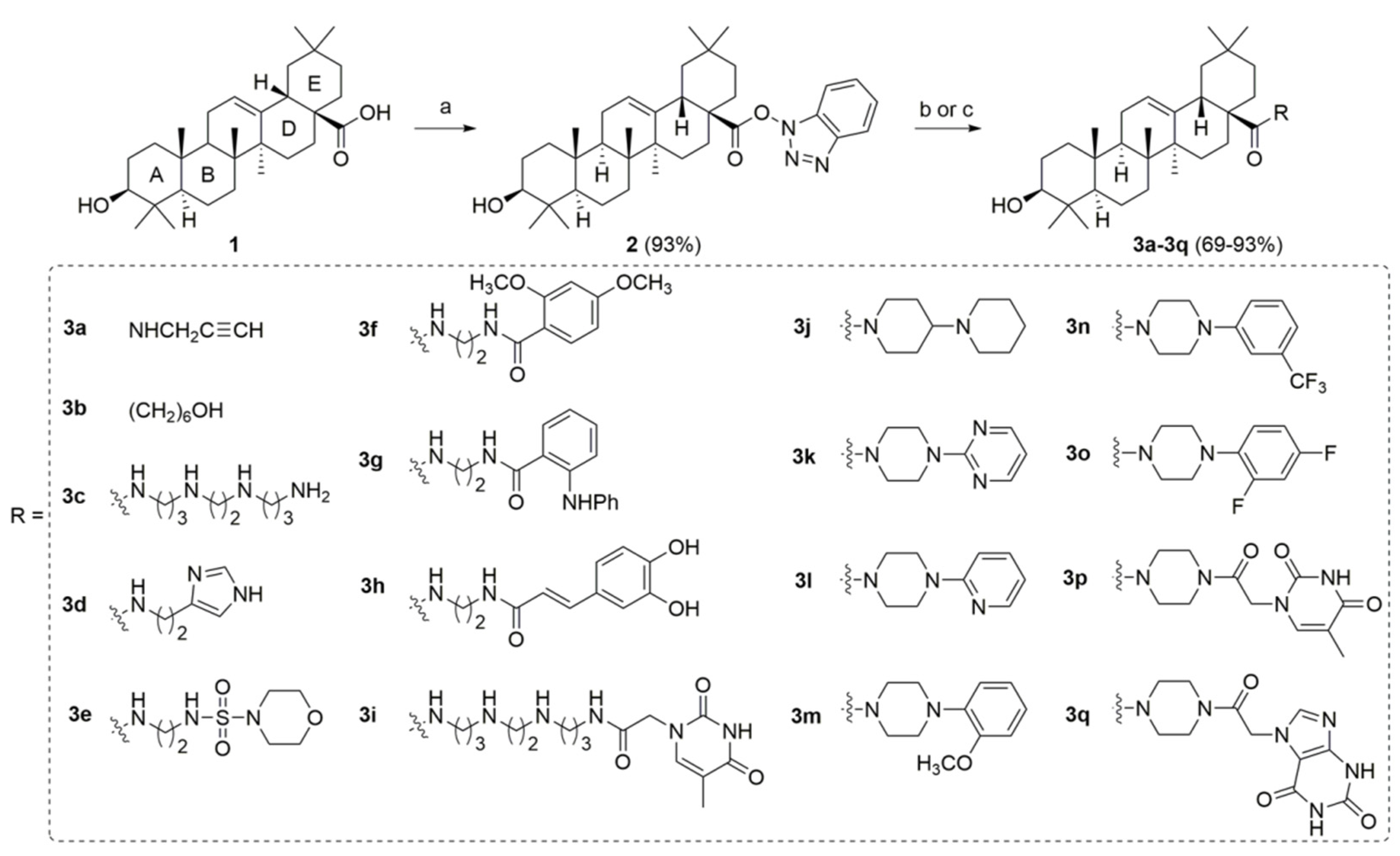
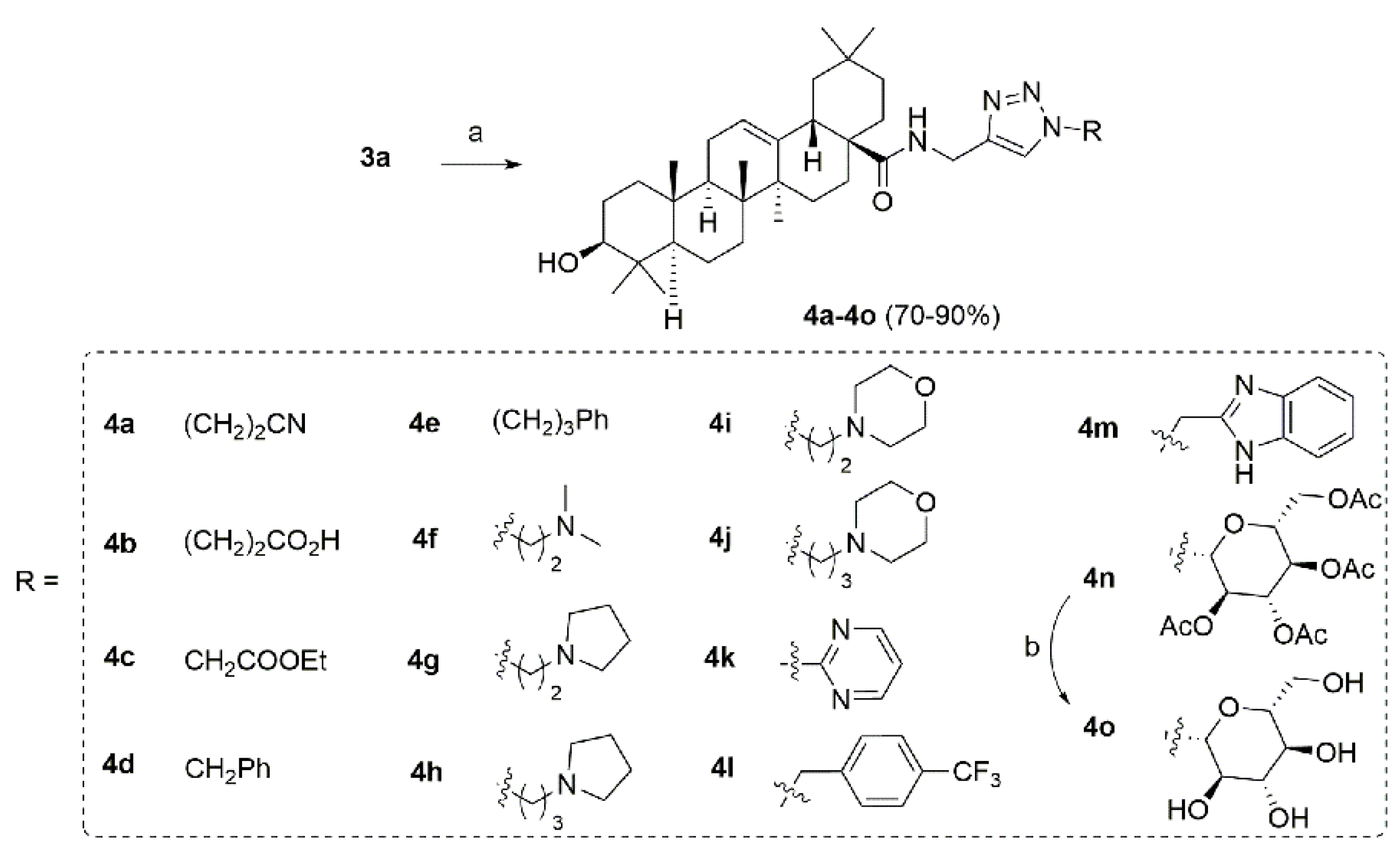
2.1. Chemistry
2.2. Initial Screening
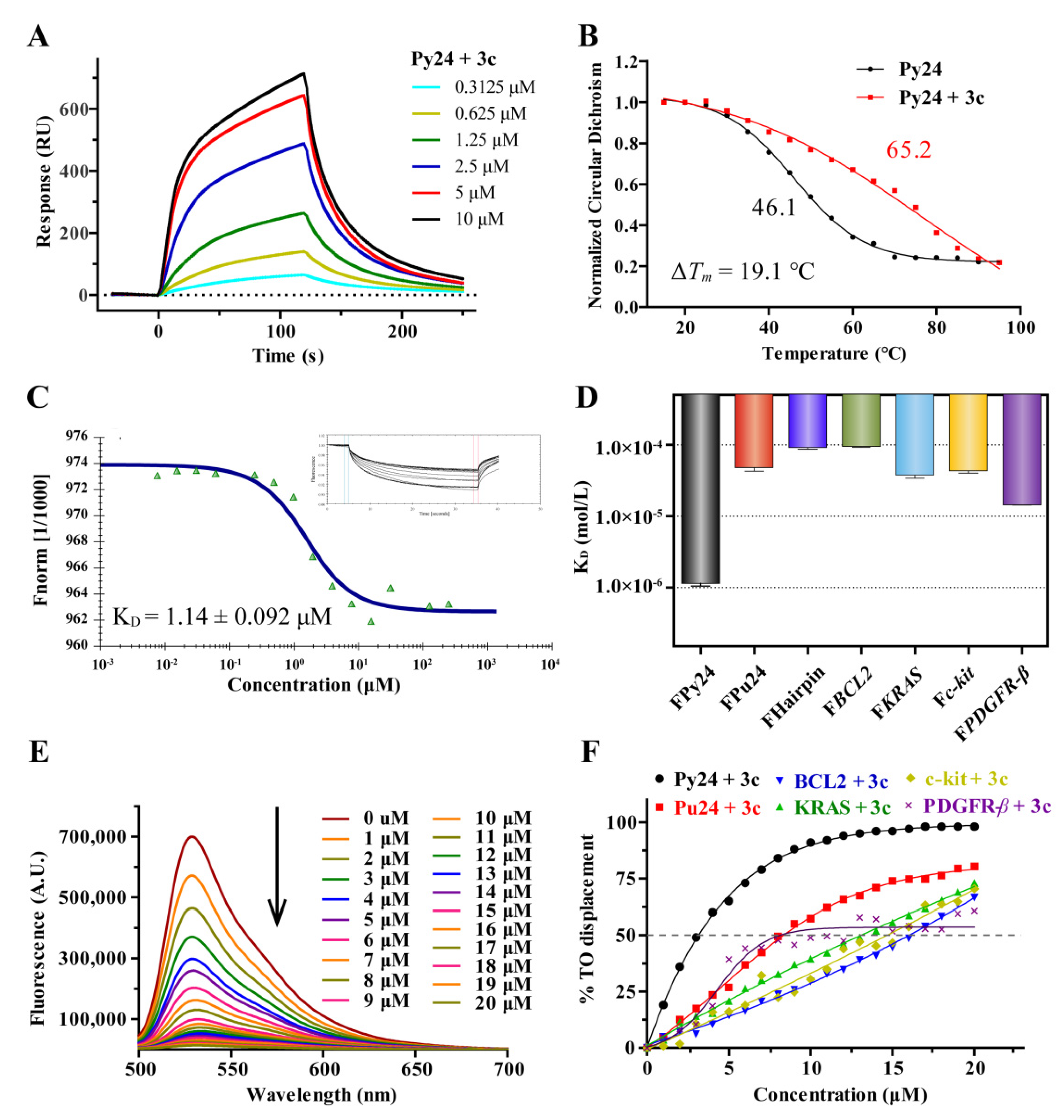
2.3. 3c Could Selectively Bind to VEGF i-Motif Structure
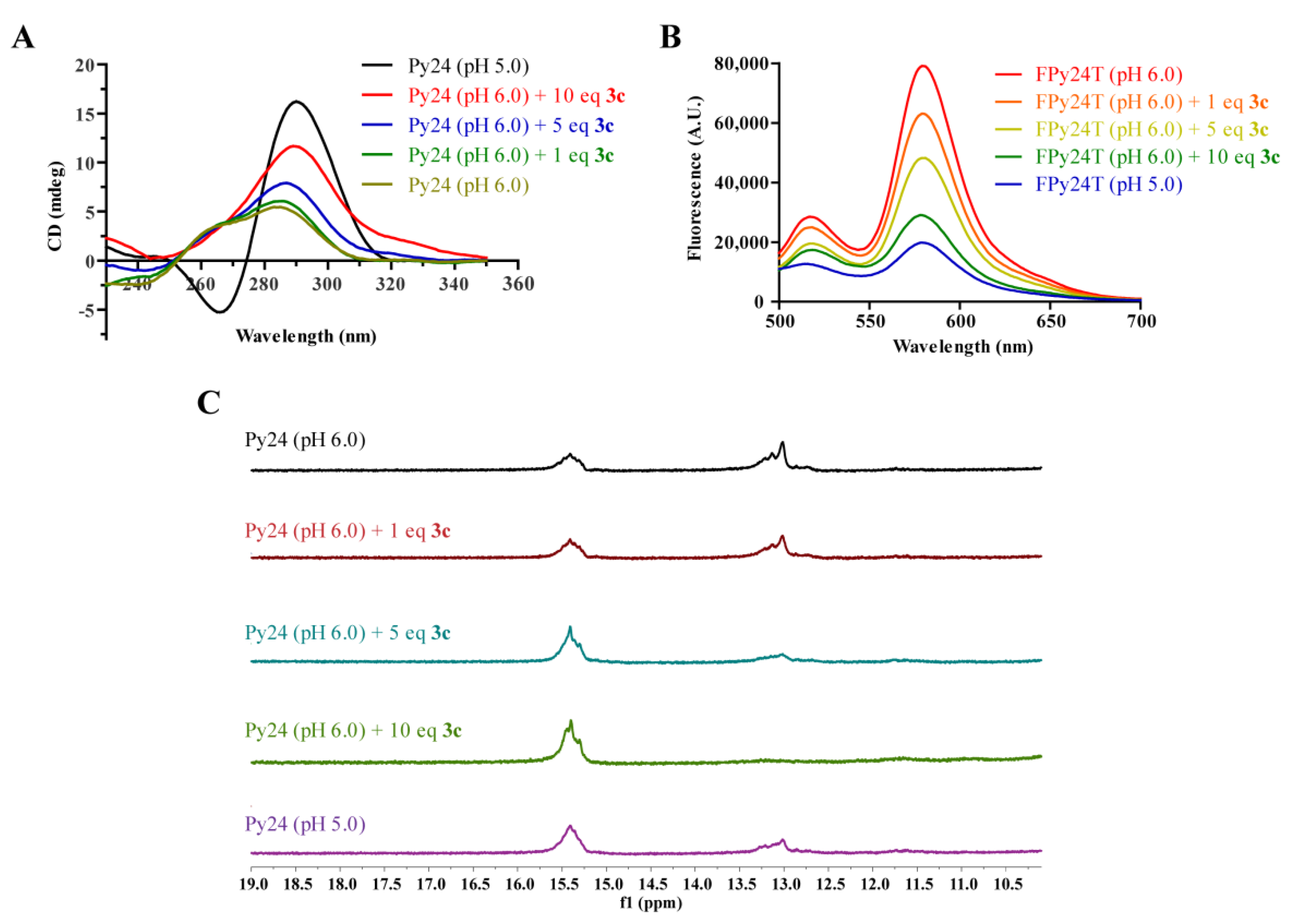
2.4. 3c Could Induce the Formation of VEGF i-Motif
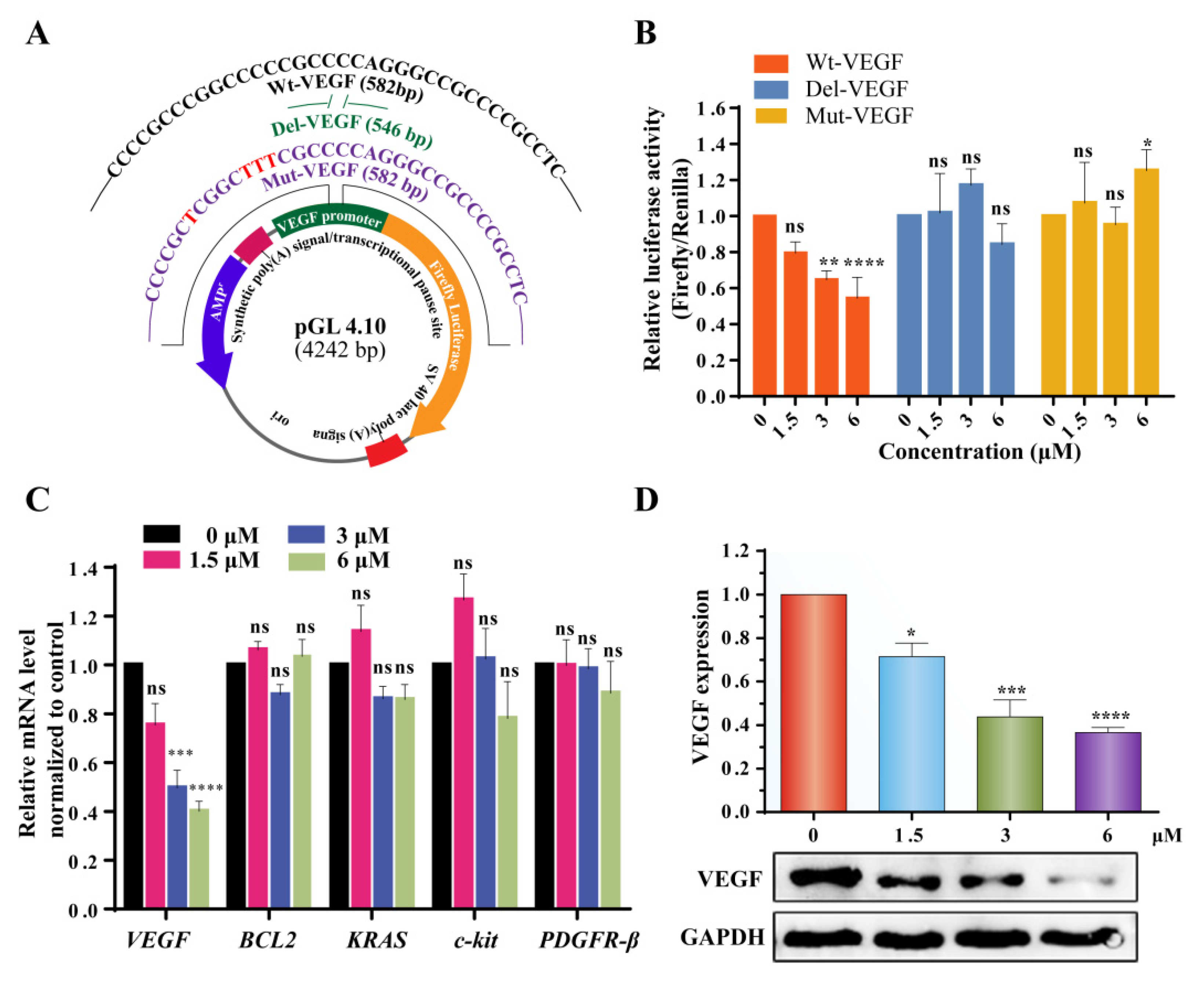
2.5. 3c Could Downregulate VEGF Gene Transcription and Protein Expression
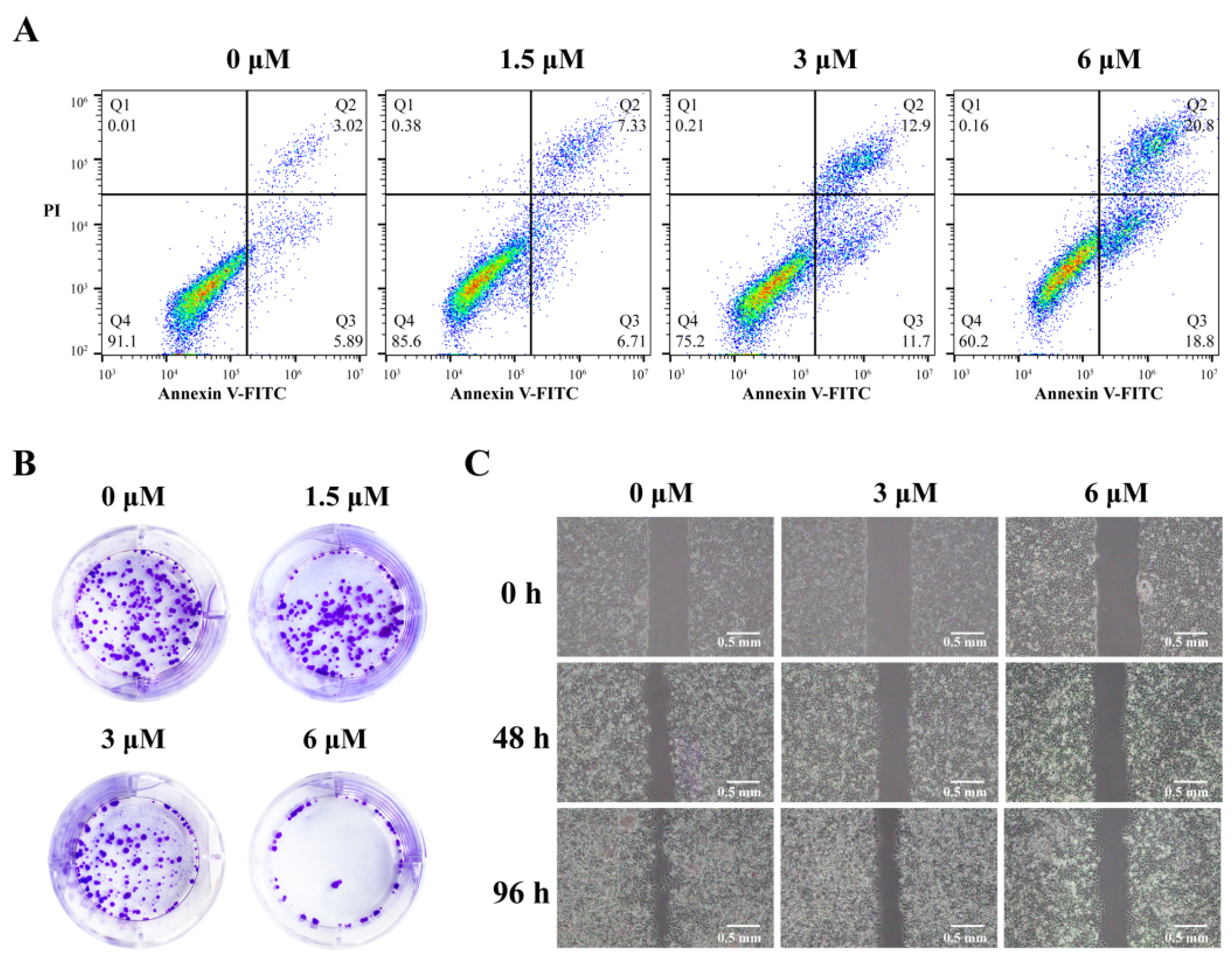
2.6. 3c Could Induce Apoptosis and Inhibit Proliferation and Metastasis of Cancer Cells
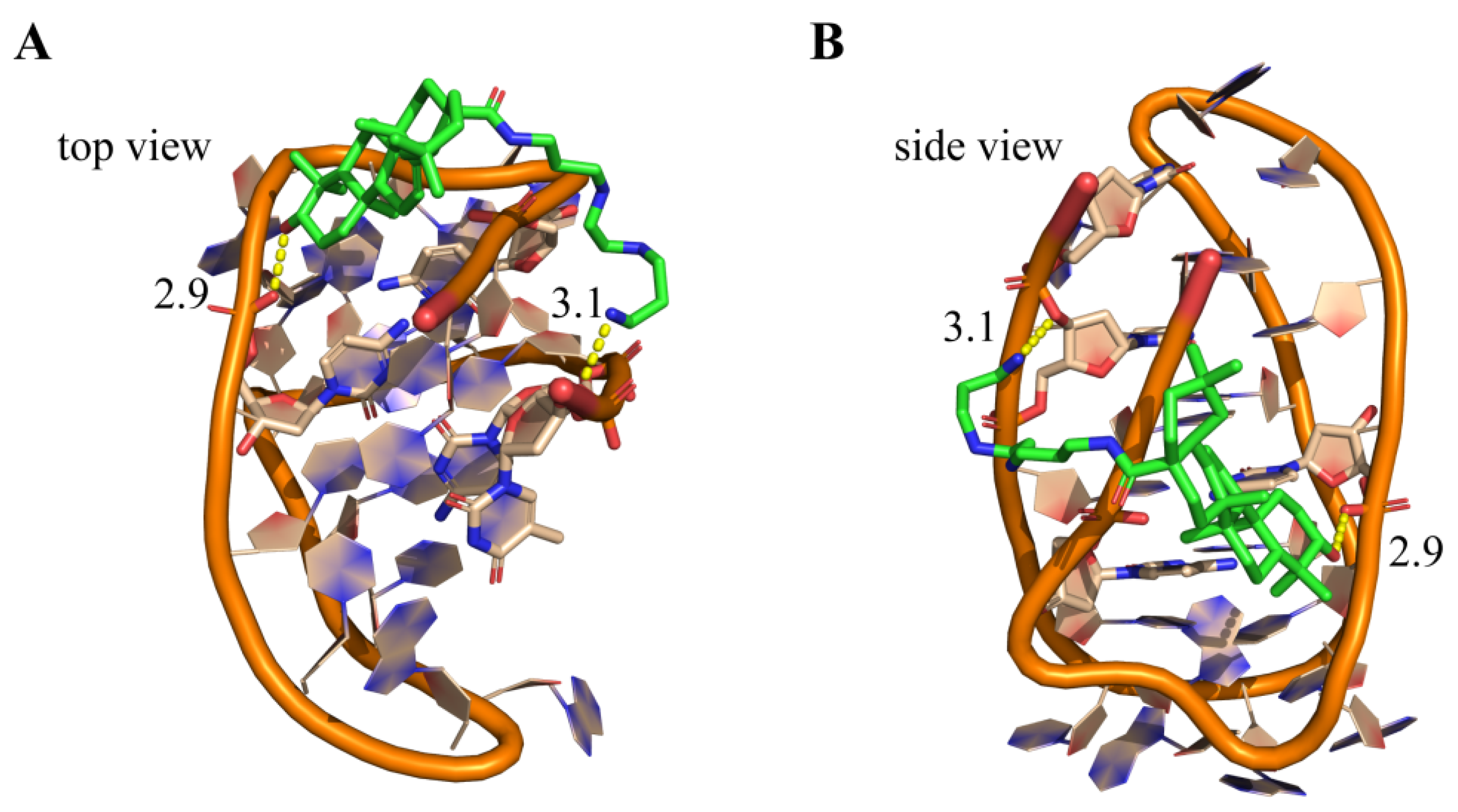
2.7. Molecular Modeling
3. Conclusions
4. Materials and Methods
4.1. General Materials
4.2. Synthesis
4.3. Surface Plasmon Resonance (SPR) Assay
4.4. Microscale Thermophoresis (MST) Assay
4.5. TO Displacement Assay
4.6. ESI-MS Assay
4.7. Isothermal Titration Calorimetry Assay
4.8. Circular Dichroism Spectroscopy
4.9. Fluorescence Resonance Energy Transfer (FRET) Assay
4.10. NMR Titration Experiment
4.11. Cell Culture
4.12. MTT Assay
4.13. Colony Formation Assay
4.14. Cell Scrape Assay
4.15. Flow Cytometric Analysis
4.16. Dual-Luciferase Reporter Assay
4.17. RNA Extraction and Real Time Polymerase Chain Reaction (RT-PCR)
4.18. Western Blot Assay
4.19. Molecular Docking
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Mochizuki, M.; Güç, E.; Park, A.J.; Julier, Z.; Briquez, P.S.; Kuhn, G.A.; Müller, R.; Swartz, M.A.; Hubbell, J.A.; Martino, M.M. Growth factors with enhanced syndecan binding generate tonic signalling and promote tissue healing. Nat. Biomed. Eng. 2020, 4, 463–475. [Google Scholar] [CrossRef]
- Yu, P.; Wilhelm, K.; Dubrac, A.; Tung, J.K.; Alves, T.C.; Fang, J.S.; Xie, Y.; Zhu, J.; Chen, Z.; De Smet, F.; et al. FGF-dependent metabolic control of vascular development. Nature 2017, 545, 224–228. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Tamura, R.; Fujioka, M.; Morimoto, Y.; Ohara, K.; Kosugi, K.; Oishi, Y.; Sato, M.; Ueda, R.; Fujiwara, H.; Noji, S.; et al. A VEGF receptor vaccine demonstrates preliminary efficacy in neurofibromatosis type 2. Nat. Commun. 2019, 10, 5758. [Google Scholar] [CrossRef]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 2018, 24, 1941. [Google Scholar] [CrossRef] [PubMed]
- Zeraati, M.; Langley, D.B.; Schofield, P.; Moye, A.L.; Rouet, R.; Hughes, W.E.; Bryan, T.M.; Dinger, M.E.; Christ, D. I-motif DNA structures are formed in the nuclei of human cells. Nat. Chem. 2018, 10, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Abou Assi, H.; Garavis, M.; Gonzalez, C.; Damha, M.J. I-Motif DNA: Structural features and significance to cell biology. Nucleic Acids Res. 2018, 46, 8038–8056. [Google Scholar] [CrossRef]
- Debnath, M.; Fatma, K.; Dash, J. Chemical Regulation of DNA i-Motifs for Nanobiotechnology and Therapeutics. Angew. Chem. Int. Ed. Engl. 2019, 58, 2942–2957. [Google Scholar] [CrossRef] [PubMed]
- Day, H.A.; Pavlou, P.; Waller, Z.A.E. i-Motif DNA: Structure, stability and targeting with ligands. Biorg. Med. Chem. 2014, 22, 4407–4418. [Google Scholar] [CrossRef]
- Brown, R.V.; Wang, T.; Chappeta, V.R.; Wu, G.; Onel, B.; Chawla, R.; Quijada, H.; Camp, S.M.; Chiang, E.T.; Lassiter, Q.R.; et al. The Consequences of Overlapping G-Quadruplexes and i-Motifs in the Platelet-Derived Growth Factor Receptor β Core Promoter Nuclease Hypersensitive Element Can Explain the Unexpected Effects of Mutations and Provide Opportunities for Selective Targeting of Both Structures by Small Molecules To Downregulate Gene Expression. J. Am. Chem. Soc. 2017, 139, 7456–7475. [Google Scholar]
- Kendrick, S.; Kang, H.-J.; Alam, M.P.; Madathil, M.M.; Agrawal, P.; Gokhale, V.; Yang, D.; Hecht, S.M.; Hurley, L.H. Correction to “The Dynamic Character of the BCL2 Promoter i-Motif Provides a Mechanism for Modulation of Gene Expression by Compounds That Bind Selectively to the Alternative DNA Hairpin Structure”. J. Am. Chem. Soc. 2016, 138, 11408. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.E.; Van Ert, N.A.; Agrawal, P.; Chawla, R.; Yang, D.; Hurley, L.H. Insight into the Complexity of the i-Motif and G-Quadruplex DNA Structures Formed in the KRAS Promoter and Subsequent Drug-Induced Gene Repression. J. Am. Chem. Soc. 2017, 139, 8522–8536. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Cao, J.; Kuang, G.; Qiu, J.; Zhang, M.; Zhang, Y.; Wang, M.; Li, X.; Kang, S.; Ou, T.-M.; et al. Syntheses and evaluation of new acridone derivatives for selective binding of oncogene c-myc promoter i-motifs in gene transcriptional regulation. Chem. Commun. 2018, 54, 2036–2039. [Google Scholar] [CrossRef]
- Debnath, M.; Ghosh, S.; Chauhan, A.; Paul, R.; Bhattacharyya, K.; Dash, J. Preferential targeting of i-motifs and G-quadruplexes by small molecules. Chem. Sci. 2017, 8, 7448–7456. [Google Scholar] [CrossRef] [PubMed]
- Kuang, G.; Zhang, M.; Kang, S.; Hu, D.; Li, X.; Wei, Z.; Gong, X.; An, L.-K.; Huang, Z.-S.; Shu, B.; et al. Syntheses and Evaluation of New Bisacridine Derivatives for Dual Binding of G-Quadruplex and i-Motif in Regulating Oncogene c-myc Expression. J. Med. Chem. 2020, 63, 9136–9153. [Google Scholar] [CrossRef]
- Guo, K.; Gokhale, V.; Hurley, L.H.; Sun, D. Intramolecularly folded G-quadruplex and i-motif structures in the proximal promoter of the vascular endothelial growth factor gene. Nucleic Acids Res. 2008, 36, 4598–4608. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zan, L.P.; Wang, X.D.; Lu, Y.J.; Ou, T.M.; Lin, J.; Huang, Z.S.; Gu, L.Q. Stabilization of VEGF G-quadruplex and inhibition of angiogenesis by quindoline derivatives. Biochim. Biophys. Acta 2014, 1840, 2970–2977. [Google Scholar] [CrossRef]
- Jana, J.; Mondal, S.; Bhattacharjee, P.; Sengupta, P.; Roychowdhury, T.; Saha, P.; Kundu, P.; Chatterjee, S. Chelerythrine down regulates expression of VEGFA, BCL2 and KRAS by arresting G-Quadruplex structures at their promoter regions. Sci. Rep. 2017, 7, 40706. [Google Scholar] [CrossRef] [PubMed]
- Asamitsu, S.; Obata, S.; Yu, Z.; Bando, T.; Sugiyama, H. Recent Progress of Targeted G-Quadruplex-Preferred Ligands Toward Cancer Therapy. Molecules 2019, 24, 429. [Google Scholar] [CrossRef] [PubMed]
- Neidle, S. Quadruplex Nucleic Acids as Novel Therapeutic Targets. J. Med. Chem. 2016, 59, 5987–6011. [Google Scholar] [CrossRef]
- Che, T.; Chen, S.B.; Tu, J.L.; Wang, B.; Wang, Y.Q.; Zhang, Y.; Wang, J.; Wang, Z.Q.; Zhang, Z.P.; Ou, T.M.; et al. Discovery of Novel Schizocommunin Derivatives as Telomeric G-Quadruplex Ligands That Trigger Telomere Dysfunction and the Deoxyribonucleic Acid (DNA) Damage Response. J. Med. Chem. 2018, 61, 3436–3453. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.-Y.; Kuang, G.-T.; Wang, S.-K.; Peng, W.; Lin, S.-L.; Zhang, Q.; Su, X.-X.; Hu, M.-H.; Wang, H.; Tan, J.-H.; et al. Discovery of Novel 11-Triazole Substituted Benzofuro[3,2-b]quinolone Derivatives as c-myc G-Quadruplex Specific Stabilizers via Click Chemistry. J. Med. Chem. 2017, 60, 5407–5423. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Sun, Z.-Y.; Zhang, Q.; Cheng, S.-Q.; Wang, S.-K.; Wang, X.-N.; Kuang, G.-T.; Su, X.-X.; Tan, J.-H.; Huang, Z.-S.; et al. Design, Synthesis, and Evaluation of Novel p-(Methylthio)styryl Substituted Quindoline Derivatives as Neuroblastoma RAS (NRAS) Repressors via Specific Stabilizing the RNA G-Quadruplex. J. Med. Chem. 2018, 61, 6629–6646. [Google Scholar] [CrossRef] [PubMed]
- Sparapani, S.; Haider, S.M.; Doria, F.; Gunaratnam, M.; Neidle, S. Rational Design of Acridine-Based Ligands with Selectivity for Human Telomeric Quadruplexes. J. Am. Chem. Soc. 2010, 132, 12263–12272. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Meng, L.K.; Su, Y.Q.; Yang, F.; Xiao, S.L.; Yin, Z.L.; Liu, J.X.; Zhong, J.D.; Zhou, D.M.; Yu, F. Design, synthesis and biological evaluation of amino acids-oleanolic acid conjugates as influenza virus inhibitors. Biorg. Med. Chem. 2019, 27, 115147. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Shi, Y.; Si, L.; Jiao, P.; Fan, Z.; Han, X.; Wu, X.; Zhou, X.; Yu, F.; et al. Design, synthesis and biological evaluation of novel L-ascorbic acid-conjugated pentacyclic triterpene derivatives as potential influenza virus entry inhibitors. Eur. J. Med. Chem. 2016, 110, 376–388. [Google Scholar] [CrossRef]
- Su, Y.Q.; Meng, L.K.; Sun, J.Q.; Li, W.J.; Shao, L.; Chen, K.X.; Zhou, D.M.; Yang, F.; Yu, F. Design, synthesis of oleanolic acid-saccharide conjugates using click chemistry methodology and study of their anti-influenza activity. Eur. J. Med. Chem. 2019, 182, 111–622. [Google Scholar] [CrossRef]
- Del Prete, D.; Taglialatela-Scafati, O.; Minassi, A.; Sirignano, C.; Cruz, C.; Bellido, M.L.; Munoz, E.; Appendino, G. Electrophilic Triterpenoid Enones: A Comparative Thiol-Trapping and Bioactivity Study. J. Nat. Prod. 2017, 80, 2276–2283. [Google Scholar] [CrossRef]
- Farina, C.; Pinza, M. Synthesis of new 12-oxo derivatives of oleanolic acid with 13α configuration. Gazz. Chim. Ital. 1987, 117, 561–562. [Google Scholar]
- Martinez, A.; Perojil, A.; Rivas, F.; Parra, A.; Garcia-Granados, A.; Fernandez-Vivas, A. Biotransformation of oleanolic and maslinic methyl esters by Rhizomucor miehei CECT 2749. Phytochemistry 2015, 117, 500–508. [Google Scholar] [CrossRef]
- Li, W.; Yang, F.; Meng, L.; Sun, J.; Su, Y.; Shao, L.; Zhou, D.; Yu, F. Synthesis, structure activity relationship and anti-influenza A virus evaluation of oleanolic acid-linear amino derivatives. Chem. Pharm. Bull. 2019, 67, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.M.; Liddell, S.C.; Wadkins, R.M. Effects of Polyamine Binding on the Stability of DNA i-Motif Structures. Acs Omega 2019, 4, 8967–8973. [Google Scholar] [CrossRef]
- Kumar, N.; Basundra, R.; Maiti, S. Elevated polyamines induce c-MYC overexpression by perturbing quadruplex-WC duplex equilibrium. Nucleic Acids Res. 2009, 37, 3321–3331. [Google Scholar] [CrossRef]
- Phan, A.T.; Gueron, M.; Leroy, J.L. The solution structure and internal motions of a fragment of the cytidine-rich strand of the human telomere. J. Mol. Biol. 2000, 299, 123–144. [Google Scholar] [CrossRef]
- Agrawal, P.; Hatzakis, E.; Guo, K.; Carver, M.; Yang, D. Solution structure of the major G-quadruplex formed in the human VEGF promoter in K+: Insights into loop interactions of the parallel G-quadruplexes. Nucleic Acids Res. 2013, 41, 10584–10592. [Google Scholar] [CrossRef] [PubMed]

| Comp. | KD (μM) 1 | TO (%) 2 | ΔTm (°C) 3 | Comp. | KD (μM) | TO (%) | ΔTm (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Py24 | Pu24 | Duplex | Py24 | Py24 | Py24 | Pu24 | Duplex | Py24 | Py24 | ||
| OA-His | 13.3 | >50 4 | >50 | n.d. 5 | 6.2 | 4g | >50 | >50 | >50 | n.d. | n.d. |
| 3b | 11.4 | >50 | >50 | 27% | 1.0 | 4h | >50 | >50 | >50 | n.d. | n.d. |
| 3c | 1.95 | >50 | >50 | 67% | 19.1 | 4i | >50 | >50 | >50 | n.d. | n.d. |
| 3d | 12.1 | >50 | >50 | 13%. | −1.6 | 4j | >50 | >50 | >50 | n.d. | n.d. |
| 3e | 13.8 | >50 | >50 | 20% | 0.6 | 4k | 12.3 | >50 | >50 | 15% | 3.3 |
| 3f | >50 | >50 | >50 | n.d. | n.d. | 4l | >50 | >50 | >50 | n.d. | n.d. |
| 3g | >50 | >50 | >50 | n.d. | n.d. | 4m | 10.6 | >50 | >50 | 24% | 1.8 |
| 3h | 3.09 | >50 | >50 | 37% | 0.5 | 4n | >50 | >50 | >50 | n.d. | n.d. |
| 3i | 5.01 | >50 | >50 | 51% | 7.0 | 4o | 12.0 | >50 | >50 | 25% | 0.1 |
| 3j | >50 | >50 | >50 | n.d. | n.d. | 8a | >50 | >50 | >50 | n.d. | n.d. |
| 3k | >50 | >50 | >50 | n.d. | n.d. | 8b | 31.3 | >50 | >50 | 10% | −0.3 |
| 3l | >50 | >50 | >50 | n.d. | n.d. | 8c | 21.1 | >50 | >50 | 5% | 0.2 |
| 3m | >50 | >50 | >50 | n.d. | n.d. | 8d | >50 | >50 | >50 | n.d. | n.d. |
| 3n | >50 | >50 | >50 | n.d. | n.d. | 8e | >50 | >50 | >50 | n.d. | n.d. |
| 3o | >50 | >50 | >50 | n.d. | n.d. | 8f | >50 | >50 | >50 | n.d. | n.d. |
| 3p | >50 | >50 | >50 | n.d. | n.d. | 8g | >50 | >50 | >50 | n.d. | n.d. |
| 3q | >50 | >50 | >50 | n.d. | n.d. | 8h | >50 | >50 | >50 | n.d. | n.d. |
| 4a | 19.3 | >50 | >50 | 19% | 1.8 | 9 | >50 | >50 | >50 | n.d. | n.d. |
| 4b | 13.0 | >50 | >50 | 16% | 1.9 | 10a | >50 | >50 | >50 | n.d. | n.d. |
| 4c | >50 | >50 | >50 | n.d. | n.d. | 10b | >50 | >50 | >50 | n.d. | n.d. |
| 4d | >50 | >50 | >50 | n.d. | n.d. | 10c | >50 | >50 | >50 | n.d. | n.d. |
| 4e | >50 | >50 | >50 | n.d. | n.d. | 10d | >50 | >50 | >50 | n.d. | n.d. |
| 4f | >50 | >50 | >50 | n.d. | n.d. | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Kang, S.; Zhang, Y.; Liu, K.; Yu, Q.; Li, D.; An, L.-K. Synthesis and Biological Evaluation of Oleanolic Acid Derivatives as Selective Vascular Endothelial Growth Factor Promoter i-Motif Ligands. Int. J. Mol. Sci. 2021, 22, 1711. https://doi.org/10.3390/ijms22041711
Zeng H, Kang S, Zhang Y, Liu K, Yu Q, Li D, An L-K. Synthesis and Biological Evaluation of Oleanolic Acid Derivatives as Selective Vascular Endothelial Growth Factor Promoter i-Motif Ligands. International Journal of Molecular Sciences. 2021; 22(4):1711. https://doi.org/10.3390/ijms22041711
Chicago/Turabian StyleZeng, Huang, Shuangshuang Kang, Yu Zhang, Ke Liu, Qian Yu, Ding Li, and Lin-Kun An. 2021. "Synthesis and Biological Evaluation of Oleanolic Acid Derivatives as Selective Vascular Endothelial Growth Factor Promoter i-Motif Ligands" International Journal of Molecular Sciences 22, no. 4: 1711. https://doi.org/10.3390/ijms22041711
APA StyleZeng, H., Kang, S., Zhang, Y., Liu, K., Yu, Q., Li, D., & An, L.-K. (2021). Synthesis and Biological Evaluation of Oleanolic Acid Derivatives as Selective Vascular Endothelial Growth Factor Promoter i-Motif Ligands. International Journal of Molecular Sciences, 22(4), 1711. https://doi.org/10.3390/ijms22041711