A Human Cellular Model for Colorectal Anastomotic Repair: The Effect of Localization and Transforming Growth Factor-β1 Treatment on Collagen Deposition and Biomarkers
Abstract
1. Introduction
2. Results
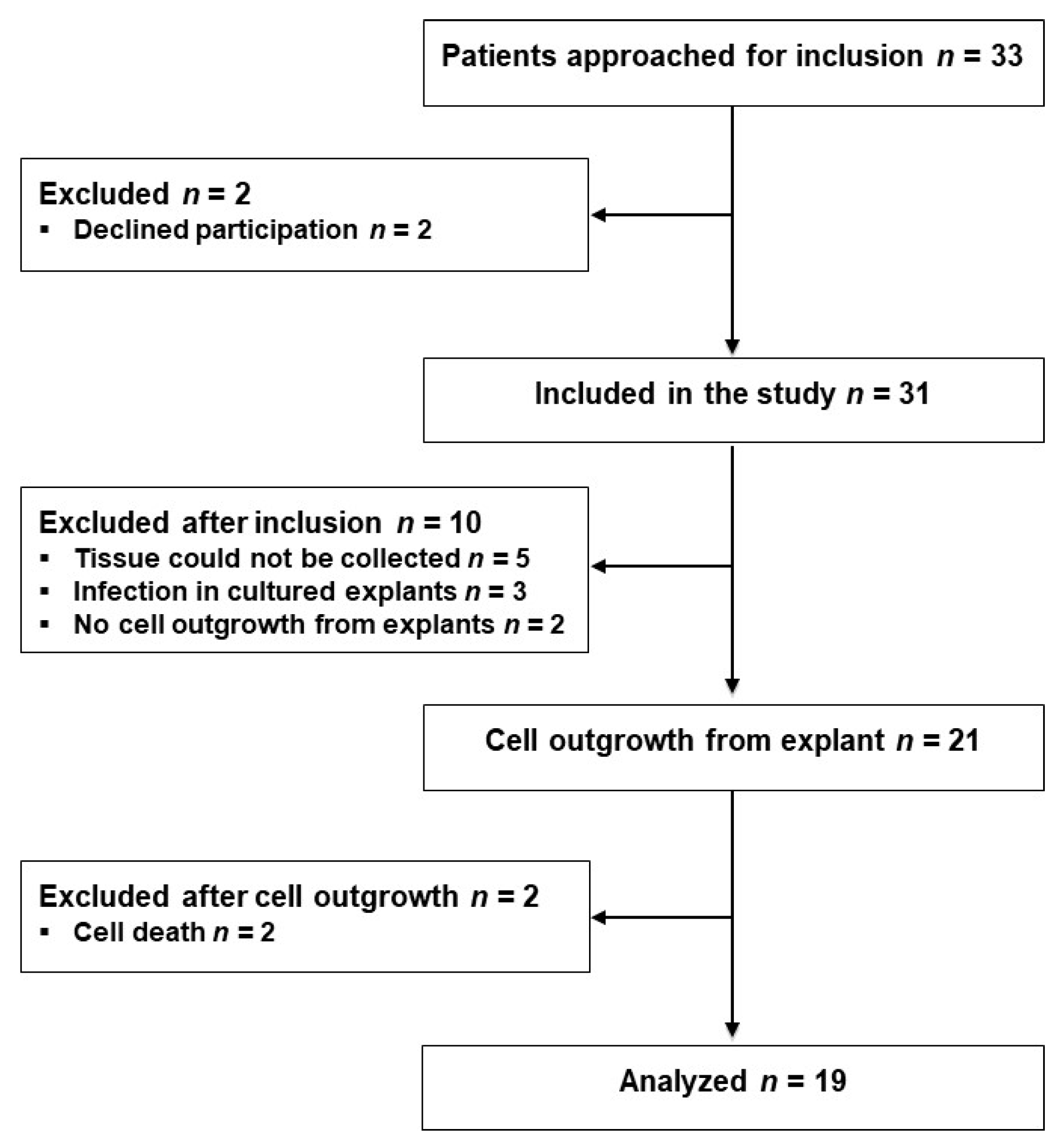
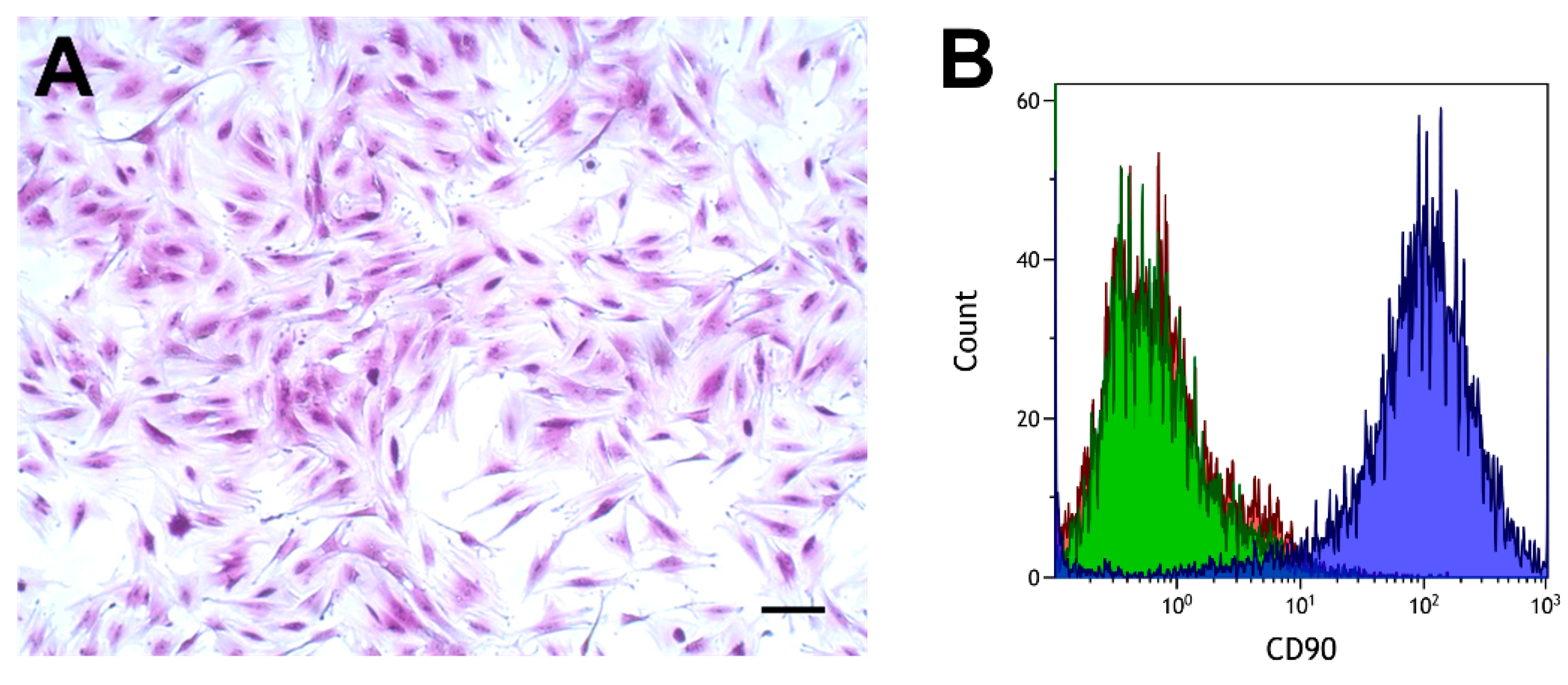
2.1. Characterization of Isolated Cells
2.2. Effect of Anatomic Localization on Collagen Deposition and Collagen Biomarkers
2.3. Effect of Sex on Collagen Deposition and Biomarkers
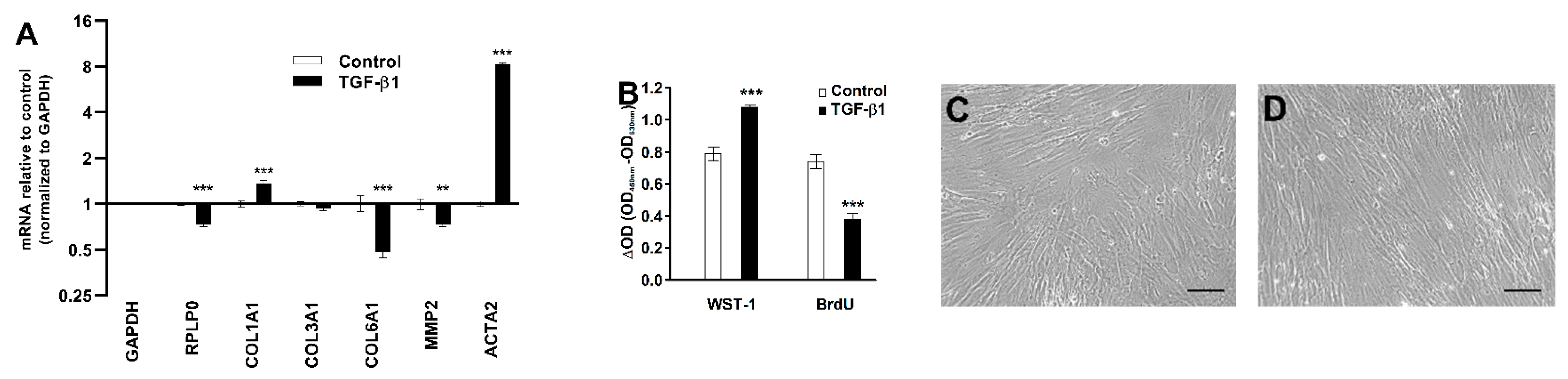
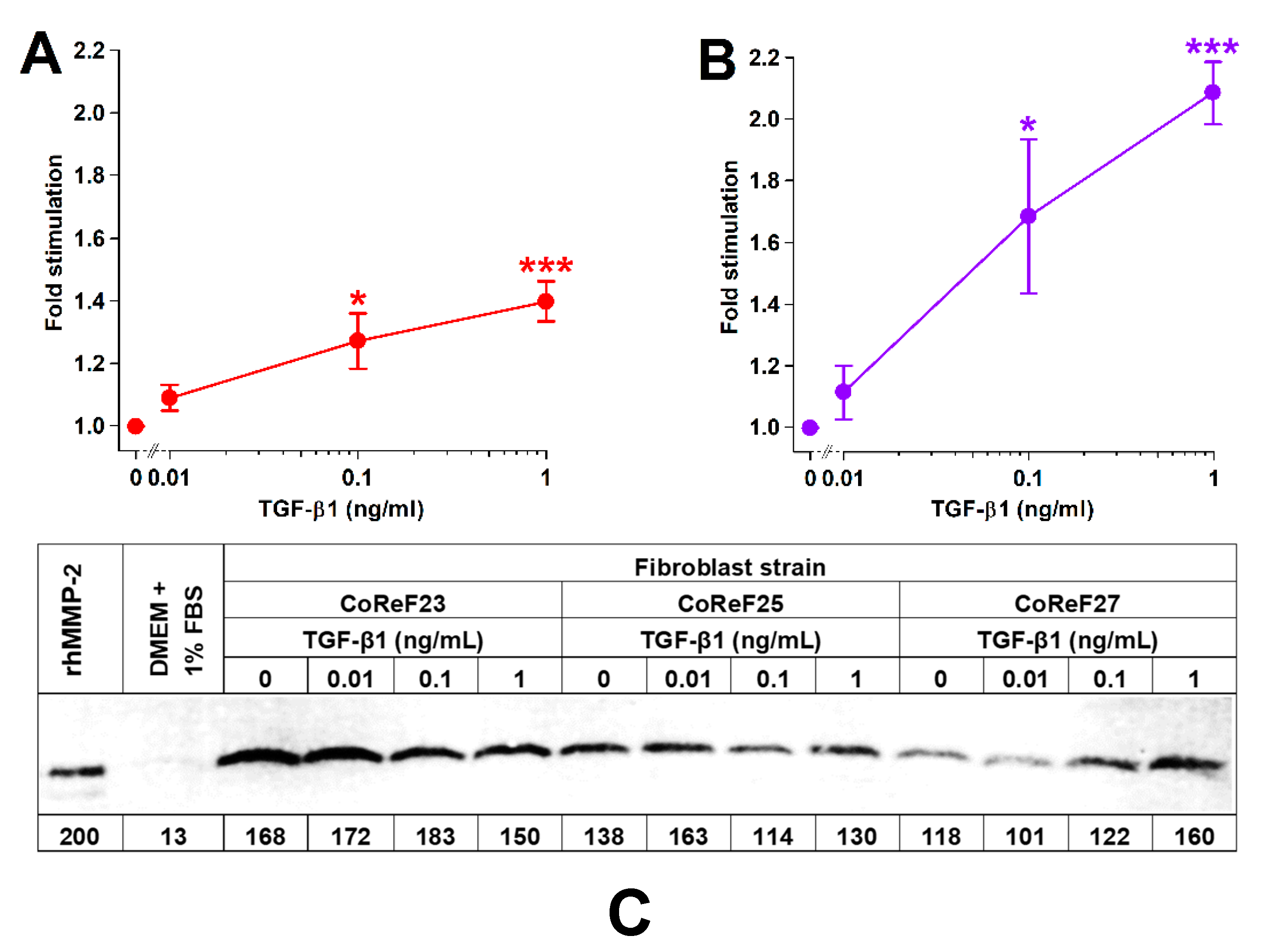
2.4. Effect of TGF-β1 on Collagen Deposition and Cell Density
2.5. Effect of TGF-β1 on Collagen Synthesis and Degradation Biomarkers in Conditioned Media
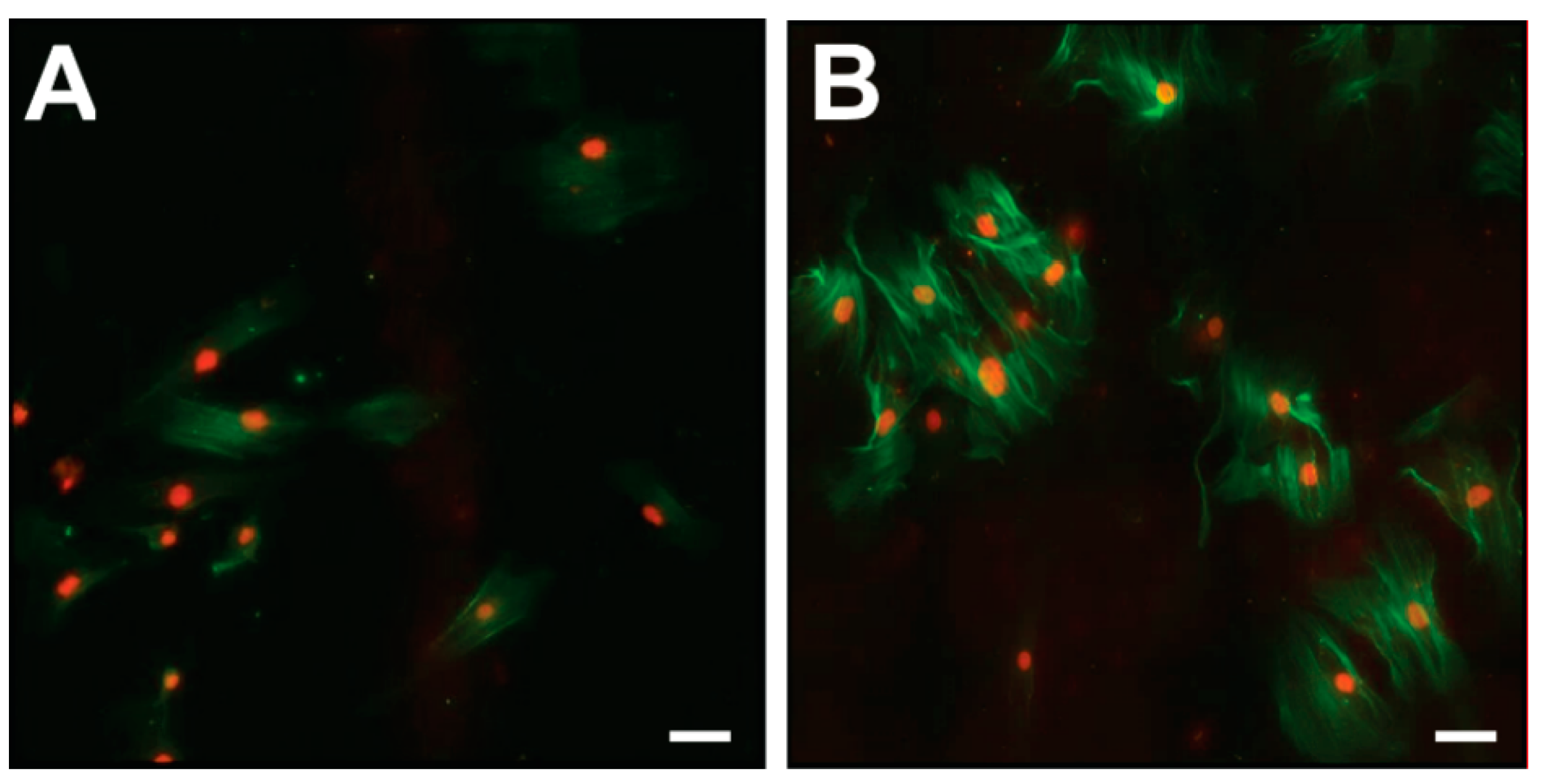
2.6. Effect of TGF-β1 on COL1A1, COL3A1, COL6A1, MMP2, and ACTA2 mRNA Levels, DNA Synthesis, and α-SMA Protein Expression
2.7. Dose-Dependent Effect of TGF-β1 on Collagen Deposition, Cell Growth, and Release of MMP-2 into Conditioned Media
3. Discussion
4. Materials and Methods
4.1. Ethical Statements
4.2. Patients
4.3. Chemicals and Reagents
4.4. Isolation of Primary Fibroblasts, Maintenance, and Flow Cytometry
4.4.1. Subcultivation and Cryopreservation Protocols
4.4.2. Flow Cytometry
4.5. Experimental Design and Execution of the Assay for Collagen Deposition and Cell Density
4.6. Collagen Biomarker Assays
4.7. mRNA Analyses by RT-qPCR
4.8. WST-1 and BrdU Assays
4.9. Immunofluorescence for α-SMA
4.10. MMP-2 in Conditioned Media Analyzed by Gelatin Zymography
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | α-Smooth muscle actin |
| BrdU | Bromodeoxyuridine |
| CoReFs | Colorectal fibroblasts |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| LAR | Low anterior resection |
| PBS | Phosphate-buffered saline |
| RT | Room temperature |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| SR | Sigmoid resection |
| TGF-β1 | Transforming growth factor-β1 |
| TX-100 | Triton X-100 |
References
- Peeters, K.C.; Tollenaar, R.A.; Marijnen, C.A.; Klein Kranenbarg, E.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; van de Velde, C.J. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br. J. Surg. 2005, 92, 211–216. [Google Scholar] [CrossRef]
- Krarup, P.M.; Jorgensen, L.N.; Andreasen, A.H.; Harling, H. A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal. Dis. 2012, 14, e661–e667. [Google Scholar] [CrossRef] [PubMed]
- Krarup, P.M.; Nordholm-Carstensen, A.; Jorgensen, L.N.; Harling, H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: A nationwide cohort study. Ann. Surg. 2014, 259, 930–938. [Google Scholar] [CrossRef]
- Schiff, A.; Brady, B.L.; Ghosh, S.K.; Roy, S.; Ruetsch, C.; Fegelman, E. Estimated rate of post-operative anastomotic leak following colorectal resection surgery: A systematic review. J. Surg. Surg. Res. 2016, 2, 60–67. [Google Scholar]
- Øines, M.N.; Krarup, P.M.; Jorgensen, L.N.; Ågren, M.S. Pharmacological interventions for improved colonic anastomotic healing: A meta-analysis. World J. Gastroenterol. 2014, 20, 12637–12648. [Google Scholar] [CrossRef]
- Nerstrøm, M.; Krarup, P.M.; Jorgensen, L.N.; Ågren, M.S. Therapeutic improvement of colonic anastomotic healing under complicated conditions: A systematic review. World J. Gastrointest. Surg. 2016, 8, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Reischl, S.; Wilhelm, D.; Friess, H.; Neumann, P.A. Innovative approaches for induction of gastrointestinal anastomotic healing: An update on experimental and clinical aspects. Langenbecks Arch. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, J.W.; Moossdorff, M.; Al-Taher, M.; van Beek, L.; Derikx, J.P.; Bouvy, N.D. International consensus statement regarding the use of animal models for research on anastomoses in the lower gastrointestinal tract. Int. J. Colorectal. Dis. 2016, 31, 1021–1030. [Google Scholar] [CrossRef]
- Bosmans, J.W.; Jongen, A.C.; Bouvy, N.D.; Derikx, J.P. Colorectal anastomotic healing: Why the biological processes that lead to anastomotic leakage should be revealed prior to conducting intervention studies. BMC Gastroenterol. 2015, 15, 180. [Google Scholar] [CrossRef]
- Ågren, M.S.; Werthén, M. The extracellular matrix in wound healing: A closer look at therapeutics for chronic wounds. Int. J. Low Extrem. Wounds 2007, 6, 82–97. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Nielsen, M.J.; Sand, J.M.; Henriksen, K.; Genovese, F.; Bay-Jensen, A.C.; Smith, V.; Adamkewicz, J.I.; Christiansen, C.; Leeming, D.J. Extracellular matrix remodeling: The common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev. Technol. 2013, 11, 70–92. [Google Scholar] [CrossRef] [PubMed]
- Thomson, H.J.; Busuttil, A.; Eastwood, M.A.; Smith, A.N.; Elton, R.A. The submucosa of the human colon. J. Ultrastruct. Mol. Struct. Res. 1986, 96, 22–30. [Google Scholar] [CrossRef]
- Siri, S.; Zhao, Y.; Maier, F.; Pierce, D.M.; Feng, B. The macro- and micro-mechanics of the colon and rectum I: Experimental evidence. Bioengineering 2020, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, M.; Klinge, U.; Wilms, A.; Zabrocki, R.; Rosch, R.; Junge, K.; Krones, C.; Schumpelick, V. Changes of the extracellular matrix as a risk factor for anastomotic leakage after large bowel surgery. Surgery 2005, 137, 229–234. [Google Scholar] [CrossRef]
- Rijcken, E.; Sachs, L.; Fuchs, T.; Spiegel, H.U.; Neumann, P.A. Growth factors and gastrointestinal anastomotic healing. J. Surg. Res. 2014, 187, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M.S.; Andersen, T.L.; Mirastschijski, U.; Syk, I.; Schiødt, C.B.; Surve, V.; Lindebjerg, J.; Delaissé, J.M. Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: An immunohistochemical and biochemical study. Surgery 2006, 140, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M.S.; auf dem Keller, U. Matrix metalloproteinases: How much can they do? Int. J. Mol. Sci. 2020, 21, 2678. [Google Scholar] [CrossRef]
- Braskén, P.; Lehto, M.; Renvall, S. Changes in the connective tissue composition of the submucosal layer of colonic anastomosis. An immunohistologic study in rats. Acta Chir. Scand. 1989, 155, 413–419. [Google Scholar]
- Braskén, P.; Renvall, S.; Sandberg, M. Fibronectin and collagen gene expression in healing experimental colonic anastomoses. Br. J. Surg. 1991, 78, 1048–1052. [Google Scholar] [CrossRef]
- Keene, D.R.; Engvall, E.; Glanville, R.W. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J. Cell. Biol. 1988, 107, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.J.; Maslen, C.L.; Keene, D.R.; Glanville, R.W. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J. Biol. Chem. 1997, 272, 26522–26529. [Google Scholar] [CrossRef] [PubMed]
- Minamitani, T.; Ikuta, T.; Saito, Y.; Takebe, G.; Sato, M.; Sawa, H.; Nishimura, T.; Nakamura, F.; Takahashi, K.; Ariga, H.; et al. Modulation of collagen fibrillogenesis by tenascin-X and type VI collagen. Exp. Cell. Res. 2004, 298, 305–315. [Google Scholar] [CrossRef]
- Theocharidis, G.; Drymoussi, Z.; Kao, A.P.; Barber, A.H.; Lee, D.A.; Braun, K.M.; Connelly, J.T. Type VI collagen regulates dermal matrix assembly and fibroblast motility. J. Investig. Dermatol. 2016, 136, 74–83. [Google Scholar] [CrossRef]
- Knight, J.M.; Kim, E.; Ivanov, I.; Davidson, L.A.; Goldsby, J.S.; Hullar, M.A.; Randolph, T.W.; Kaz, A.M.; Levy, L.; Lampe, J.W.; et al. Comprehensive site-specific whole genome profiling of stromal and epithelial colonic gene signatures in human sigmoid colon and rectal tissue. Physiol. Genom. 2016, 48, 651–659. [Google Scholar] [CrossRef]
- Finnson, K.W.; McLean, S.; Di Guglielmo, G.M.; Philip, A. Dynamics of transforming growth factor beta signaling in wound healing and scarring. Adv. Wound Care 2013, 2, 195–214. [Google Scholar] [CrossRef]
- Ignotz, R.A.; Massague, J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 1986, 261, 4337–4345. [Google Scholar] [CrossRef]
- Simmons, J.G.; Pucilowska, J.B.; Keku, T.O.; Lund, P.K. IGF-I and TGF-beta1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am. J. Physiol. Gastrointest. Liver. Physiol. 2002, 283, G809–G818. [Google Scholar] [CrossRef]
- Evans, R.A.; Tian, Y.C.; Steadman, R.; Phillips, A.O. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp. Cell. Res. 2003, 282, 90–100. [Google Scholar] [CrossRef]
- Trackman, P.C.; Saxena, D.; Bais, M.V. TGF-beta1- and CCN2-stimulated Sirius red assay for collagen accumulation in cultured cells. Methods Mol. Biol. 2017, 1489, 481–485. [Google Scholar] [PubMed]
- Moulin, V.; Castilloux, G.; Auger, F.A.; Garrel, D.; O’Connor-McCourt, M.D.; Germain, L. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp. Cell. Res. 1998, 238, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, M.; Leng, C.; Blokzijl, T.; Jansen, B.H.; Dijkstra, G.; Faber, K.N. Pirfenidone inhibits cell proliferation and collagen I production of primary human intestinal fibroblasts. Cells 2020, 9, 775. [Google Scholar] [CrossRef]
- Buckmire, M.A.; Parquet, G.; Greenway, S.; Rolandelli, R.H. Temporal expression of TGF-beta1, EGF, and PDGF-BB in a model of colonic wound healing. J. Surg. Res. 1998, 80, 52–57. [Google Scholar] [CrossRef]
- Kjaer, M.; Kristjansdottir, H.; Andersen, L.; Heegaard, A.M.; Ågren, M.S.; Jorgensen, L.N. The effect of gender on early colonic anastomotic wound healing. Int. J. Colorectal. Dis. 2018, 33, 1269–1276. [Google Scholar] [CrossRef]
- Migaly, J.; Lieberman, J.; Long, W.; Fisher, C.; Rolandelli, R.H. Effect of adenoviral-mediated transfer of transforming growth factor-beta1 on colonic anastomotic healing. Dis. Colon. Rectum. 2004, 47, 1699–1705. [Google Scholar] [CrossRef]
- Buch, A.S.; Schjerling, P.; Kjaer, M.; Jorgensen, L.N.; Krarup, P.M.; Ågren, M.S. Impaired collagen synthesis in the rectum may be a molecular target in anastomotic leakage prophylaxis. Wound Repair Regen. 2017, 25, 532–535. [Google Scholar] [CrossRef]
- Leeming, D.J.; Larsen, D.V.; Zhang, C.; Hi, Y.; Veidal, S.S.; Nielsen, R.H.; Henriksen, K.; Zheng, Q.; Barkholt, V.; Riis, B.J.; et al. Enzyme-linked immunosorbent serum assays (ELISAs) for rat and human N-terminal pro-peptide of collagen type I (PINP)—Assessment of corresponding epitopes. Clin. Biochem. 2010, 43, 1249–1256. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Nedergaard, A.F.; Sun, S.; Veidal, S.S.; Larsen, L.; Zheng, Q.; Suetta, C.; Henriksen, K.; Christiansen, C.; Karsdal, M.A.; et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 2013, 5, 303–315. [Google Scholar] [PubMed]
- Sun, S.; Henriksen, K.; Karsdal, M.A.; Byrjalsen, I.; Rittweger, J.; Armbrecht, G.; Belavy, D.L.; Felsenberg, D.; Nedergaard, A.F. Collagen type III and VI turnover in response to long-term immobilization. PLoS ONE 2015, 10, e0144525. [Google Scholar] [CrossRef] [PubMed]
- Barascuk, N.; Veidal, S.S.; Larsen, L.; Larsen, D.V.; Larsen, M.R.; Wang, J.; Zheng, Q.; Xing, R.; Cao, Y.; Rasmussen, L.M.; et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin. Biochem. 2010, 43, 899–904. [Google Scholar] [CrossRef]
- Ågren, M.S.; Steenfos, H.H.; Dabelsteen, S.; Hansen, J.B.; Dabelsteen, E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J. Investig. Dermatol. 1999, 112, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Zhu, Y.; Andersen, V.; Sciaky, D.; Cao, H.J.; Meekins, H.; Smith, T.J.; Lance, P. Cytokine-mediated PGE2 expression in human colonic fibroblasts. Am. J. Physiol. 1998, 275, C988–C994. [Google Scholar] [CrossRef]
- Getliffe, K.M.; Martin Ruiz, C.; Passos, J.F.; von Zglinicki, T.; Nwokolo, C.U. Extended lifespan and long telomeres in rectal fibroblasts from late-onset ulcerative colitis patients. Eur. J. Gastroenterol. Hepatol. 2006, 18, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Saada, J.I.; Pinchuk, I.V.; Barrera, C.A.; Adegboyega, P.A.; Suarez, G.; Mifflin, R.C.; Di Mari, J.F.; Reyes, V.E.; Powell, D.W. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J. Immunol. 2006, 177, 5968–5979. [Google Scholar] [CrossRef]
- Leeb, S.N.; Vogl, D.; Gunckel, M.; Kiessling, S.; Falk, W.; Goke, M.; Scholmerich, J.; Gelbmann, C.M.; Rogler, G. Reduced migration of fibroblasts in inflammatory bowel disease: Role of inflammatory mediators and focal adhesion kinase. Gastroenterology 2003, 125, 1341–1354. [Google Scholar] [CrossRef]
- Saalbach, A.; Aneregg, U.; Bruns, M.; Schnabel, E.; Herrmann, K.; Haustein, U.F. Novel fibroblast-specific monoclonal antibodies: Properties and specificities. J. Investig. Dermatol. 1996, 106, 1314–1319. [Google Scholar] [CrossRef]
- Hsia, L.T.; Ashley, N.; Ouaret, D.; Wang, L.M.; Wilding, J.; Bodmer, W.F. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc. Natl. Acad. Sci. USA 2016, 113, E2162–E2171. [Google Scholar] [CrossRef]
- Tullberg-Reinert, H.; Jundt, G. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: Effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem. Cell. Biol. 1999, 112, 271–276. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Miller, S.N.; Hofmann, C.; Vogl, D.; Falk, W.; Scholmerich, J.; Rogler, G. Transforming growth factor-beta 1 induces intestinal myofibroblast differentiation and modulates their migration. World J. Gastroenterol. 2009, 15, 1431–1442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clark, R.A.; McCoy, G.A.; Folkvord, J.M.; McPherson, J.M. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: A fibronectin matrix-dependent event. J. Cell. Physiol. 1997, 170, 69–80. [Google Scholar] [CrossRef]
- Juhl, P.; Bondesen, S.; Hawkins, C.L.; Karsdal, M.A.; Bay-Jensen, A.C.; Davies, M.J.; Siebuhr, A.S. Dermal fibroblasts have different extracellular matrix profiles induced by TGF-beta, PDGF and IL-6 in a model for skin fibrosis. Sci. Repair 2020, 10, 17300. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.F.; Huyben, C.M.; Hendriks, T. Collagen synthesis in fibroblasts from human colon: Regulatory aspects and differences with skin fibroblasts. Gut 1992, 33, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M.S.; Rasmussen, K.; Pakkenberg, B.; Jørgensen, B. Growth factor and proteinase profile of Vivostat(R) platelet-rich fibrin linked to tissue repair. Vox Sang 2014, 107, 37–43. [Google Scholar] [CrossRef]
- Ågren, M.S.; Jorgensen, L.N.; Andersen, M.; Viljanto, J.; Gottrup, F. Matrix metalloproteinase 9 level predicts optimal collagen deposition during early wound repair in humans. Br. J. Surg. 1998, 85, 68–71. [Google Scholar] [CrossRef]
- Overall, C.M.; Wrana, J.L.; Sodek, J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J. Biol. Chem. 1991, 266, 14064–14071. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hattori, S.; Shinkai, H. Matrix metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm. Venereol. 2003, 83, 105–107. [Google Scholar] [CrossRef]
- Philips, N.; Keller, T.; Gonzalez, S. TGF beta-like regulation of matrix metalloproteinases by anti-transforming growth factor-beta, and anti-transforming growth factor-beta 1 antibodies in dermal fibroblasts: Implications for wound healing. Wound Repair Regen. 2004, 12, 53–59. [Google Scholar] [CrossRef]
- van Praagh, J.B.; de Goffau, M.C.; Bakker, I.S.; van Goor, H.; Harmsen, H.J.M.; Olinga, P.; Havenga, K. Mucus microbiome of anastomotic tissue during surgery has predictive value for colorectal anastomotic leakage. Ann. Surg. 2019, 269, 911–916. [Google Scholar] [CrossRef]
- Lam, A.; Fleischer, B.; Alverdy, J. The biology of anastomotic healing-the unknown overwhelms the known. J. Gastrointest. Surg. 2020, 24, 2160–2166. [Google Scholar] [CrossRef]
- Gaines, S.; Shao, C.; Hyman, N.; Alverdy, J.C. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br. J. Surg. 2018, 105, e131–e141. [Google Scholar] [CrossRef]
- Seltana, A.; Basora, N.; Beaulieu, J.F. Intestinal epithelial wound healing assay in an epithelial-mesenchymal co-culture system. Wound Repair Regen. 2010, 18, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A review of ASA physical status—Historical perspectives and modern developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Roulis, M.; Flavell, R.A. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 2016, 92, 116–131. [Google Scholar] [CrossRef]
- Smith-Clerc, J.; Hinz, B. Immunofluorescence detection of the cytoskeleton and extracellular matrix in tissue and cultured cells. Methods Mol. Biol. 2010, 611, 43–57. [Google Scholar] [PubMed]
- Henriksen, N.A.; Sørensen, L.T.; Jorgensen, L.N.; Ågren, M.S. Circulating levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with incisional hernia. Wound Repair Regen. 2013, 21, 661–666. [Google Scholar] [CrossRef] [PubMed]




| Collagen Biomarker | Proximal | Distal | p-Value 2 |
|---|---|---|---|
| PINP | 1580 (855–2290) | 2620 (655–4190) | 0.0854 |
| PRO-C3 | 3.7 (2.0–4.9) | 3.7 (1.5–4.3) | 0.184 |
| PRO-C6 | 4.2 (2.3–6.2) | 6.7 (3.2–14.2) | 0.00497 |
| C3M | 2.0 (1.7–2.8) | 2.9 (1.4–3.9) | 0.125 |
| Collagen Biomarker | Males | Females | p-Value 2 |
|---|---|---|---|
| PINP | 1470 (716–3460) | 2650 (2090–3620) | 0.206 |
| PRO-C3 | 4.0 (2.8–4.9) | 3.0 (1.8–4.4) | 0.206 |
| PRO-C6 | 5.0 (4.1–6.0) | 6.7 (2.4–11.4) | 0.778 |
| C3M | 2.2 (1.6–2.6) | 2.9 (2.3–3.5) | 0.174 |
| Collagen Biomarker | Control | TGF-β1 | p-Value 2 |
|---|---|---|---|
| PINP | 2520 (2040–3340) | 4550 (3310–6660) | 0.00222 |
| PRO-C3 | 3.4 (2.6–4.6) | 3.9 (2.2–4.8) | 0.666 |
| PRO-C6 | 5.4 (3.8–10.3) | 9.3 (6.6–12.1) | 0.00221 |
| C3M | 2.3 (1.7–2.9) | 2.3 (1.6–2.8) | 0.593 |
| Included | Analyzed | |
|---|---|---|
| Number of patients | 31 | 19 |
| Males/females | 18/13 | 11/8 |
| Age (years) | 64.5 ± 2.5 | 62.6 ± 2.4 |
| Body mass index (kg/m2) | 26.3 ± 1.0 | 26.7 ± 1.4 |
| Diabetes mellitus | 5 | 4 |
| Smoker | 7 | 4 |
| ASA physical status classification of 2014 [61] 2 | ||
| I | 13 | 7 |
| II | 15 | 9 |
| III | 3 | 3 |
| Type of resection 3 | ||
| SR | 20 | 13 |
| LAR | 11 | 6 |
| mRNA | GenBank® ID | Sense (Forward) | Antisense (Reverse) |
|---|---|---|---|
| COL1A1 | NM_000088.3 | GGCAACAGCCGCTTCACCTAC | GCGGGAGGTCTTGGTGGTTTT |
| COL3A1 | NM_000090.3 | CACGGAAACACTGGTGGACAGATT | ATGCCAGCTGCACATCAAGGAC |
| COL6A1 | NM_001848.2 | CACACCGCTCAACGTGCTCTG | GCTGGTCTGAGCCTGGGATGAA |
| MMP2 | NM_004530.5 | CCGCCTTTAACTGGAGCAAAAACA | TTGGGGAAGCCAGGATCCATTT |
| ACTA2 | NM_001613.4 | AGAAGGAGATCACGGCCCTAGCA | CCCGGCTTCATCGTATTCCTGT |
| GAPDH | NM_002046.4 | CCTCCTGCACCACCAACTGCTT | GAGGGGCCATCCACAGTCTTCT |
| RPLP0 | NM_053275.3 | GGAAACTCTGCATTCTCGCTTCCT | CCAGGACTCGTTTGTACCCGTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türlü, C.; Willumsen, N.; Marando, D.; Schjerling, P.; Biskup, E.; Hannibal, J.; Jorgensen, L.N.; Ågren, M.S. A Human Cellular Model for Colorectal Anastomotic Repair: The Effect of Localization and Transforming Growth Factor-β1 Treatment on Collagen Deposition and Biomarkers. Int. J. Mol. Sci. 2021, 22, 1616. https://doi.org/10.3390/ijms22041616
Türlü C, Willumsen N, Marando D, Schjerling P, Biskup E, Hannibal J, Jorgensen LN, Ågren MS. A Human Cellular Model for Colorectal Anastomotic Repair: The Effect of Localization and Transforming Growth Factor-β1 Treatment on Collagen Deposition and Biomarkers. International Journal of Molecular Sciences. 2021; 22(4):1616. https://doi.org/10.3390/ijms22041616
Chicago/Turabian StyleTürlü, Ceylan, Nicholas Willumsen, Debora Marando, Peter Schjerling, Edyta Biskup, Jens Hannibal, Lars N. Jorgensen, and Magnus S. Ågren. 2021. "A Human Cellular Model for Colorectal Anastomotic Repair: The Effect of Localization and Transforming Growth Factor-β1 Treatment on Collagen Deposition and Biomarkers" International Journal of Molecular Sciences 22, no. 4: 1616. https://doi.org/10.3390/ijms22041616
APA StyleTürlü, C., Willumsen, N., Marando, D., Schjerling, P., Biskup, E., Hannibal, J., Jorgensen, L. N., & Ågren, M. S. (2021). A Human Cellular Model for Colorectal Anastomotic Repair: The Effect of Localization and Transforming Growth Factor-β1 Treatment on Collagen Deposition and Biomarkers. International Journal of Molecular Sciences, 22(4), 1616. https://doi.org/10.3390/ijms22041616







