Phosphofructokinases A and B from Mycobacterium tuberculosis Display Different Catalytic Properties and Allosteric Regulation
Abstract
1. Introduction
2. Results
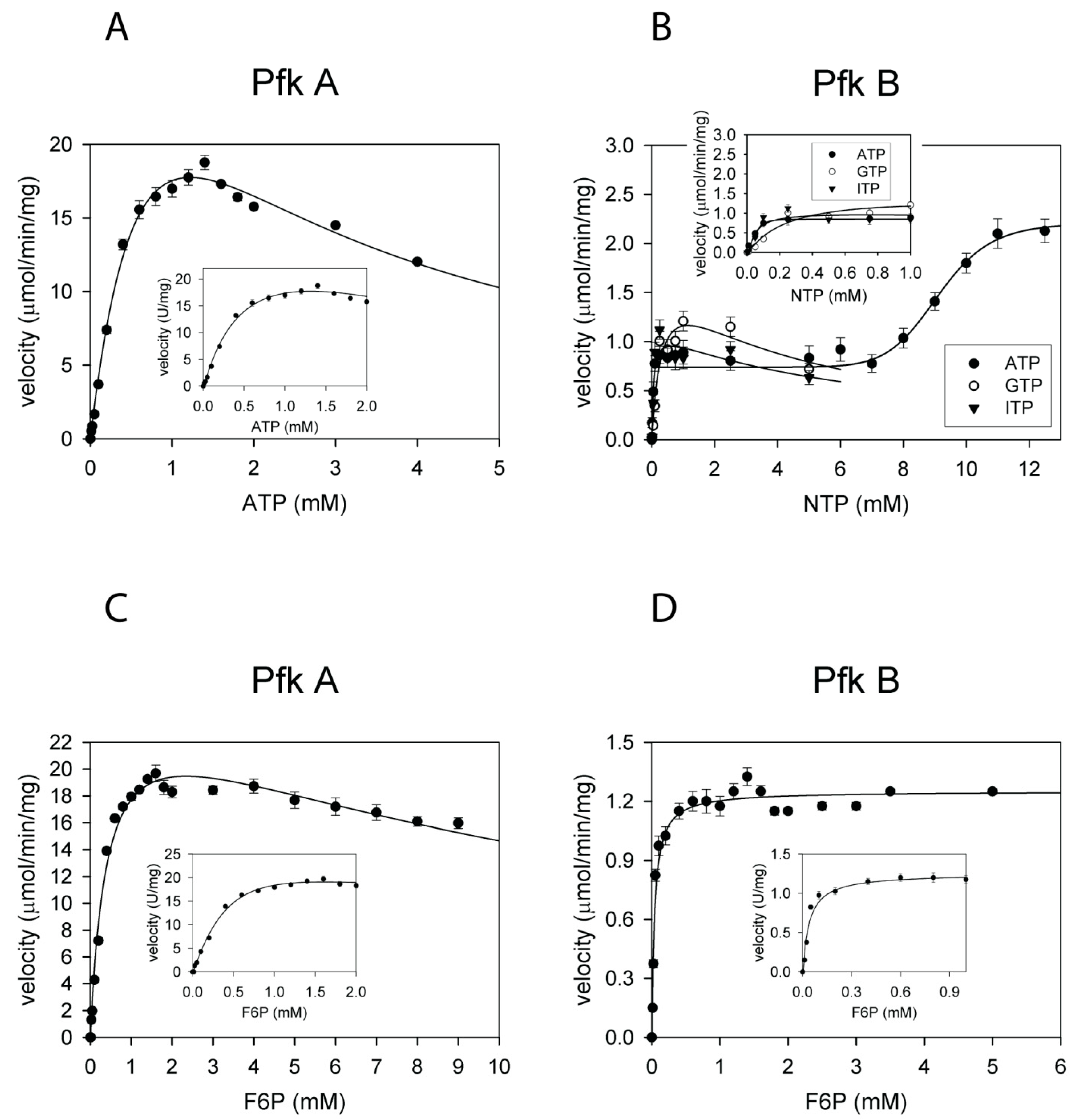
2.1. The Kinetics of Glycolytic Reactions Catalyzed by Pfk A and Pfk B Differ Significantly
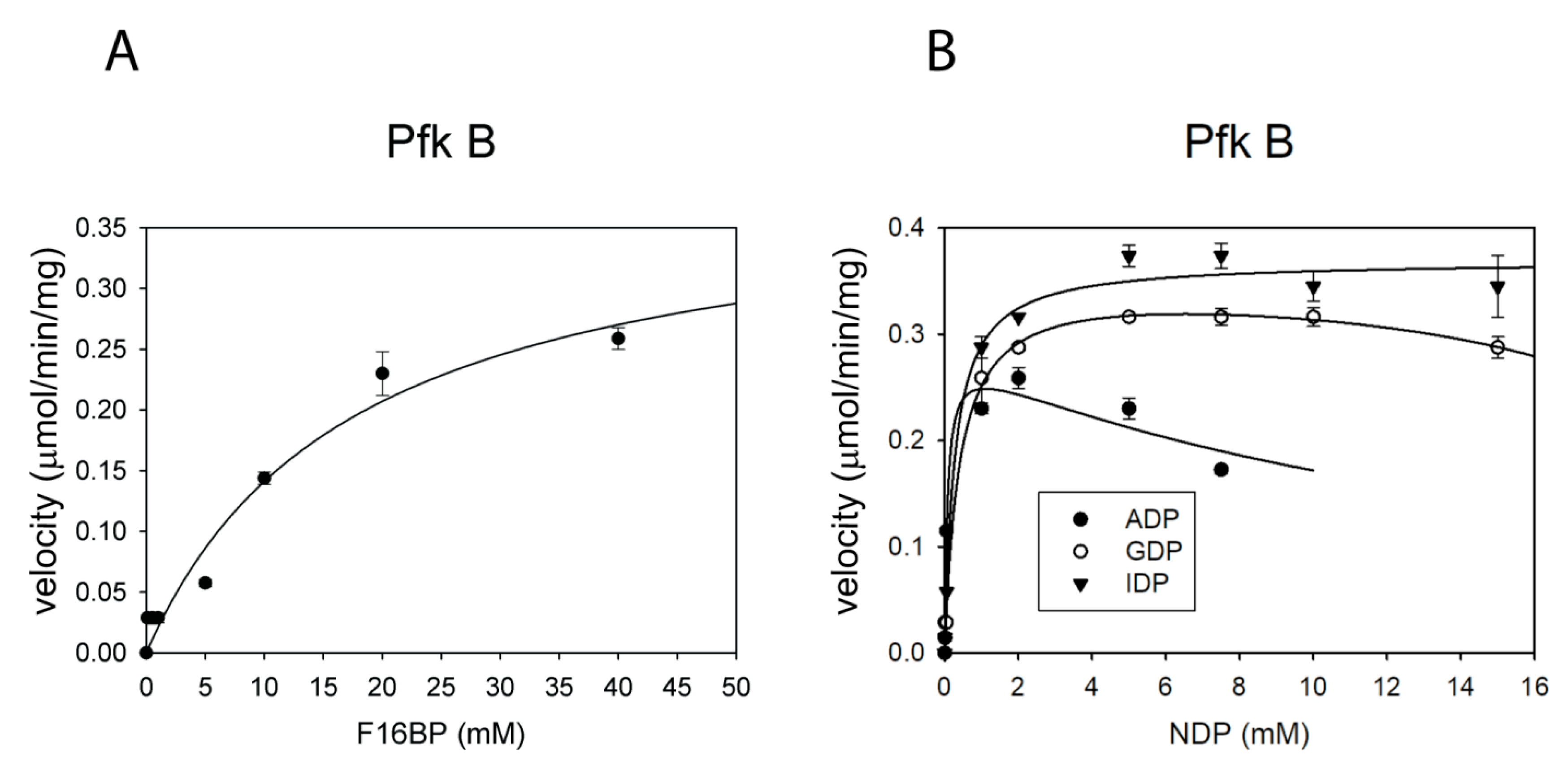
2.2. Pfk B, but Not Pfk A, Detectably Catalyzes the Gluconeogenic Reaction
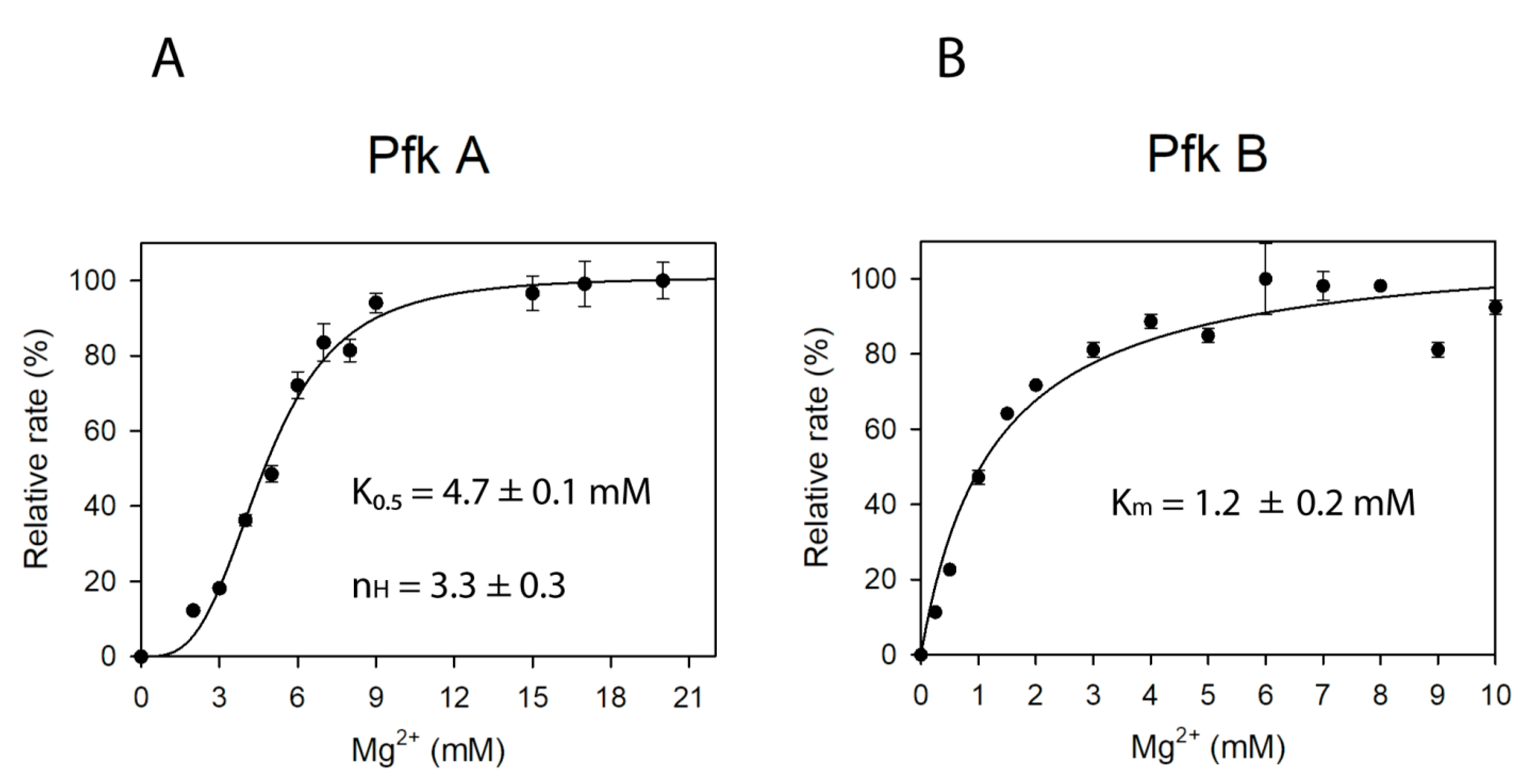
2.3. Magnesium Is Essential for Pfk A and B Catalysis but Affects Their Activities with Different Mechanism
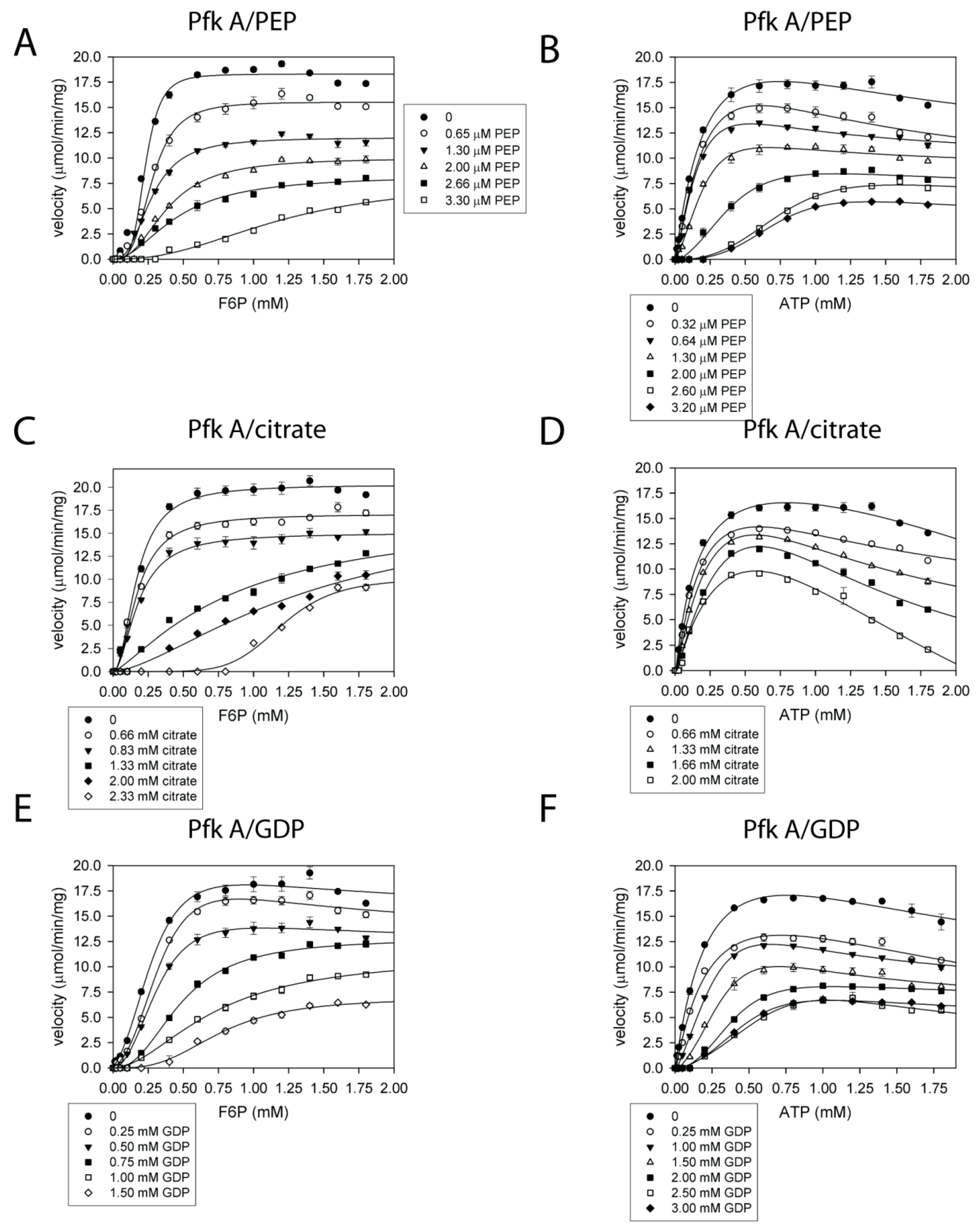
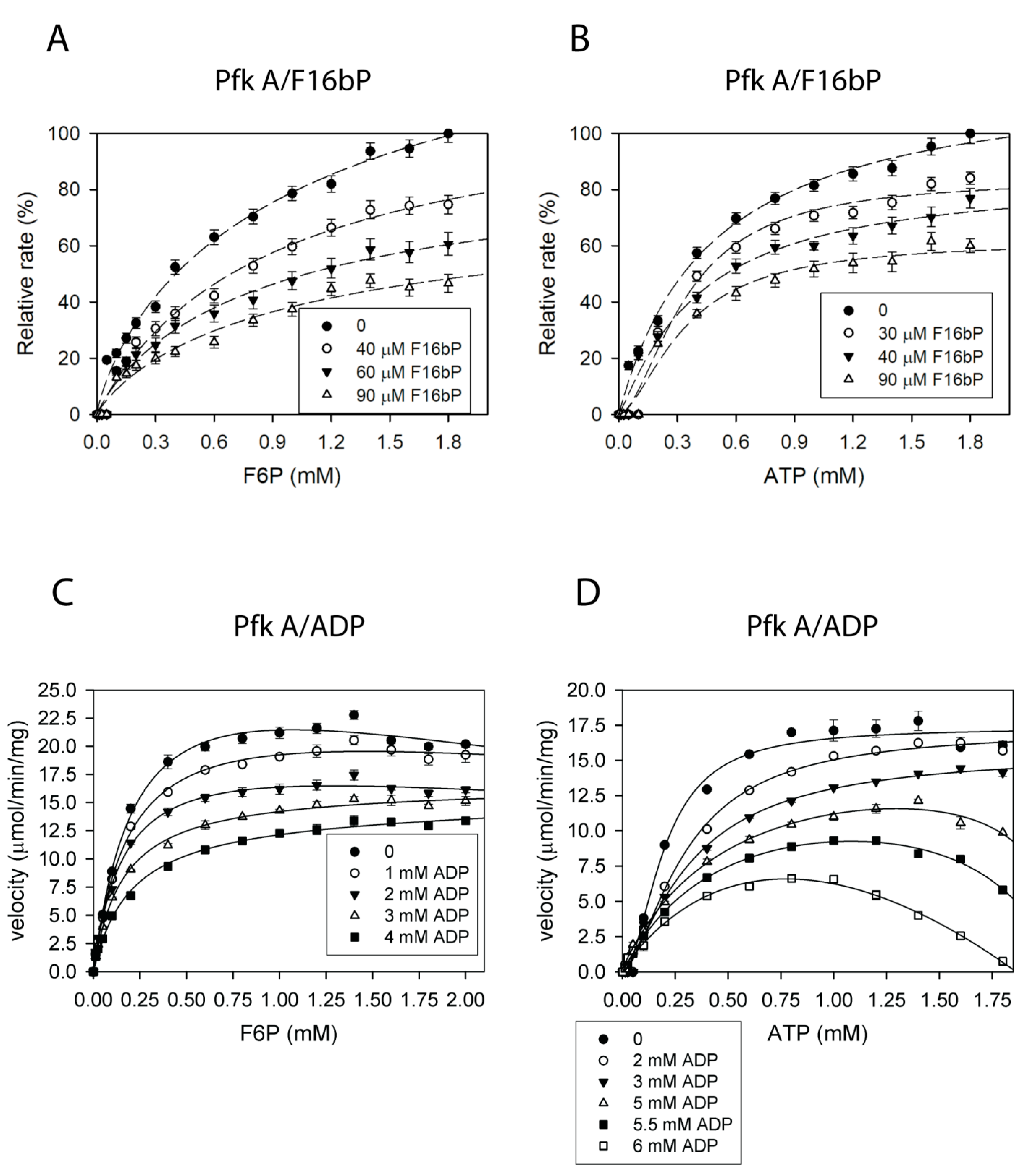
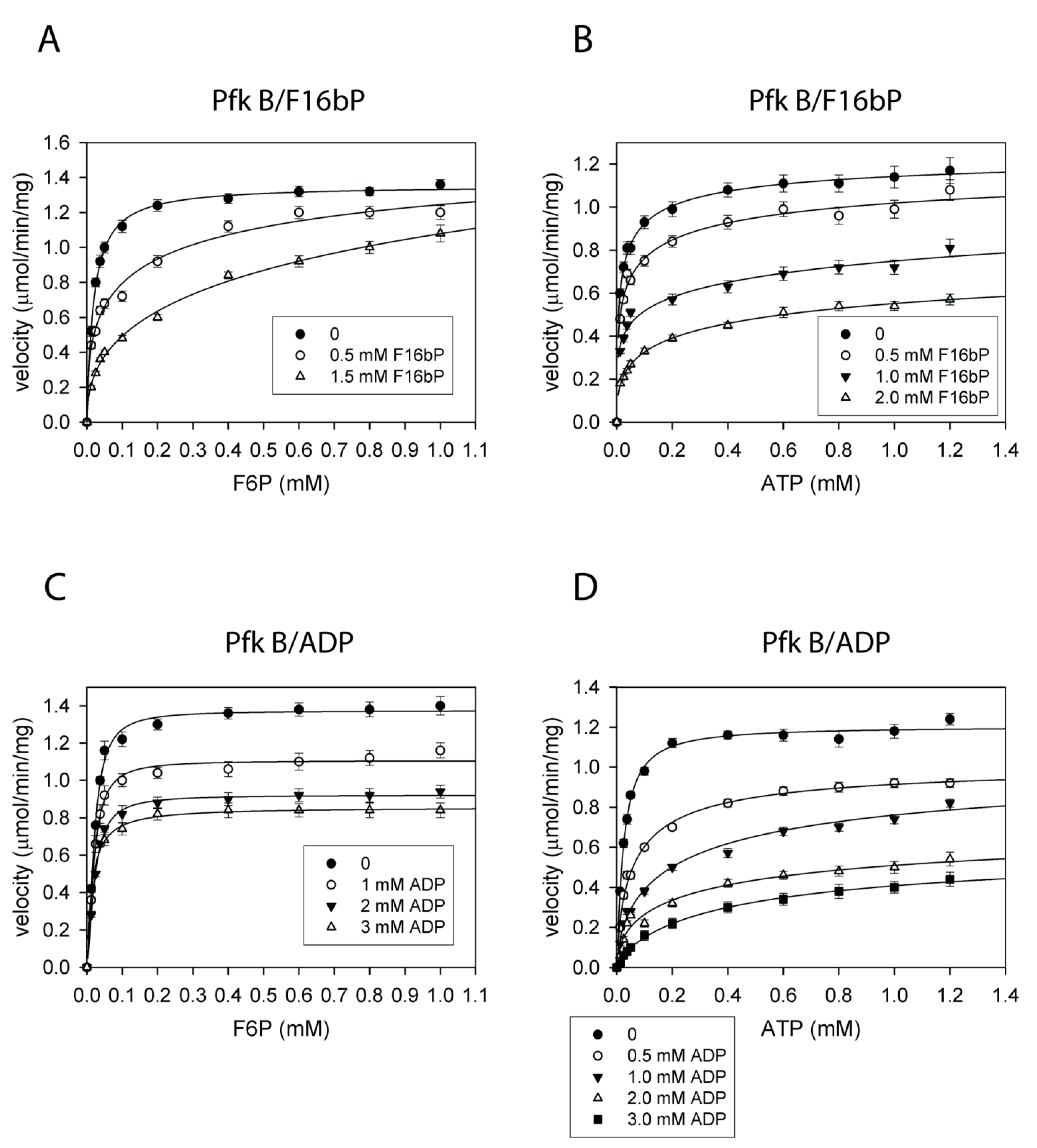
2.4. Pfk A, but Not Pfk B Activity Is Allosterically Regulated by Metabolites
2.5. Effect of Fructose-2,6-Bisphosphate on Pfk Isoenzymes
2.6. Pfk A and Pfk B Activities Are Controlled by Negative Feedback by Both Reaction Products but with Different Efficiency
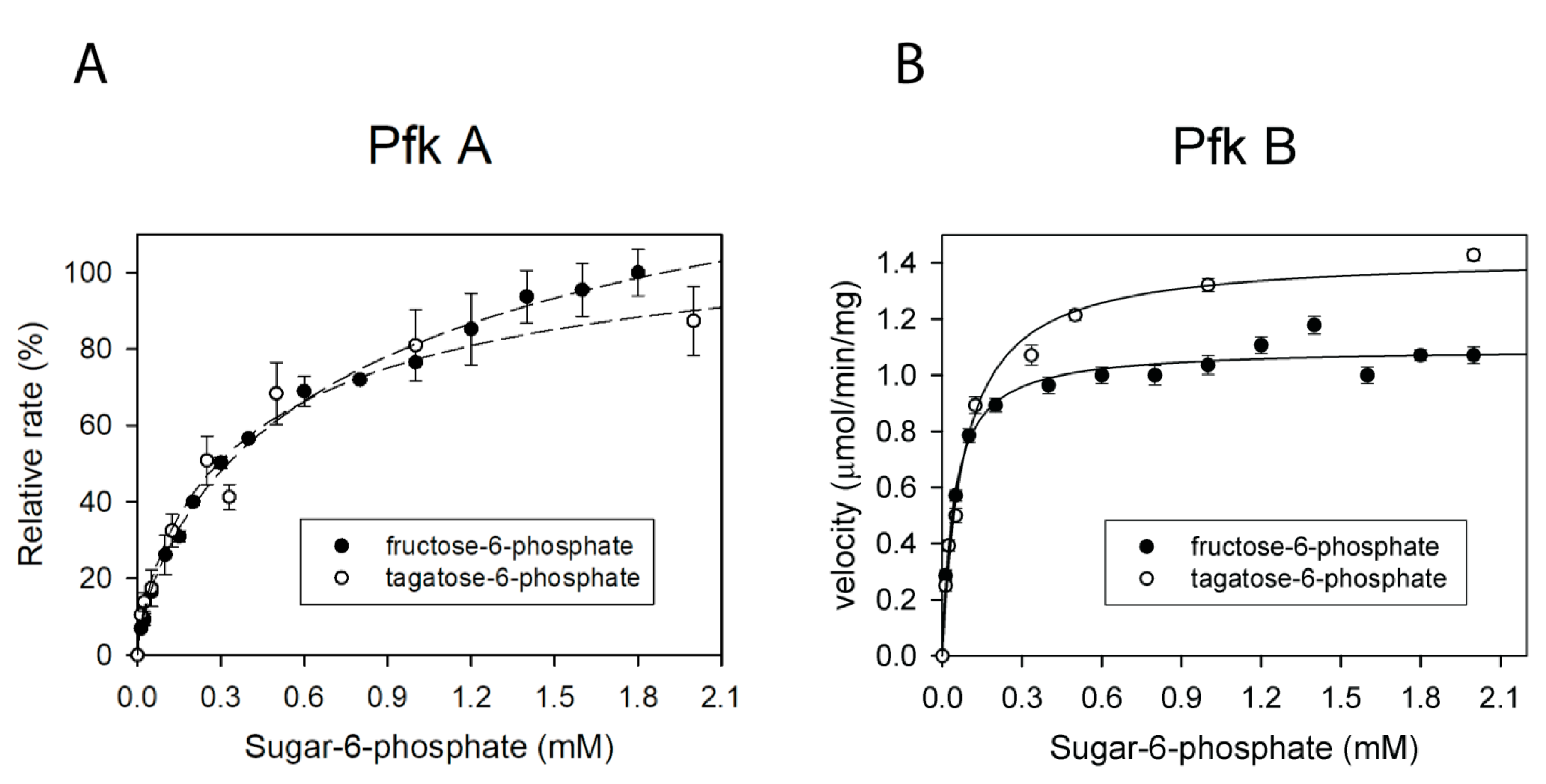
2.7. Pfk A and B Can Convert Fructose-6-Phosphate and Tagatose-6-Phosphate
2.8. Pfk B Is Not a Member of PPi-Pfk Family
3. Discussion
4. Materials and Methods
4.1. Cloning, Expression and Purification of Mtb Pfk A and Pfk B
4.2. Synthesis of Fructose-2,6-Bisphosphate (F26bP)
4.2.1. Cyclization
4.2.2. Alkaline Hydrolysis
4.3. Measurement of Pfk Glycolytic Activity
4.4. Determination of Pfks Substrate Specificity
4.4.1. Sugar Substrate Specificity of Pfk A
4.4.2. Sugar Substrate Specificity of Pfk B
4.5. Measurement of Pfk Gluconeogenic Activity
4.6. Dependence of Pfk A and Pfk B activities on Mg2+ Concentration
4.7. Testing Metabolites as Allosteric Modulators and Feedback Inhibitors
- (1)
- The effect of F26bP on Pfk A or Pfk B with fixed concentration of one substrate and varied concentration of the second substrate: 190 µL of reaction mix (50 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 5 mM DTT) containing 1 mM F6P or ATP, 1 U/mL aldolase, 1 U/mL triosephosphate isomerase, 1 U/mL glycerol-3-phosphate dehydrogenase, 300 µM NADH, 0–100 µM F26bP and 30 nM Pfk A or 300 nM Pfk B was pipetted into microtiter plate wells containing 10 µL of 0.25, 0.5, 1, 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40 mM F6P or 1, 2, 4, 8, 12, 16, 20, 24, 28, 32, 40, 50, 60, 70, 75, 80 mM ATP.
- (2)
- The effect of F26bP on the inhibition of Pfk A by citrate or phosphoenolpyruvate: 190 µL of reaction mix (50 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 5 mM DTT) containing 1 mM F6P, 1 U/mL aldolase, 1 U/mL triosephosphate isomerase, 1 U/mL glycerol-3-phosphate dehydrogenase, 300 µM NADH, 1 mM citrate or 1 µM PEP, 0 or 50 µM F26bP and 30 nM Pfk A or 300 nM Pfk B was pipetted into microtiter plate wells containing 10 µL of 1, 2, 4, 8, 12, 16, 20, 24, 28, 32 mM ATP.
- (3)
- To test the effect of F26bP on the inhibition of Pfk A by an excess of ATP: 190 µL of reaction mix (50 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 5 mM DTT) containing 1 mM F6P, 1 U/mL aldolase, 1 U/mL triosephosphate isomerase, 1 U/mL glycerol-3-phosphate dehydrogenase, 300 µM NADH, 0 or 50 µM F26bP and 30 nM Pfk A was pipetted into microtiter plate wells containing 10 µL of 0.2, 1, 2, 5, 10, 15, 20, 50, 100, 120, 140, 160, 180, 200 mM ATP.
4.8. Enzyme Kinetics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020.
- Sharma, S.K.; Mohan, A.; Kadhiravan, T. HIV-TB co-infection: Epidemiology, diagnosis&management. Indian J. Med. Res. 2005, 121, 550–567. [Google Scholar] [PubMed]
- Pizzol, D.; Di Gennaro, F.; Chhaganlal, K.D.; Fabrizio, C.; Monno, L.; Putoto, G.; Saracino, A. Prevalence of diabetes mellitus in newly diagnosed pulmonary tuberculosis in Beira, Mozambique. Afr. Health Sci. 2017, 17, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, F.; Vittozzi, P.; Gualano, G.; Musso, M.; Mosti, S.; Mencarini, P.; Pareo, C.; Di Caro, A.; Schininà, V.; Girardi, E.; et al. Active Pulmonary Tuberculosis in Elderly Patients: A 2016–2019 Retrospective Analysis from an Italian Referral Hospital. Antibiotics 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.P.S.; Fischer, S.M.; Marrero, J.; Nathan, C.; Ehrt, S.; Rhee, K.Y. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem. Biol. 2010, 17, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Ehrt, S.; Schnappinger, D.; Rhee, K.Y. Metabolic principles of persistence and pathogenicity in Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, B.; Ramachandra, P.; Suryanarayana, M.; Venkitasubramanian, T. Pathways of carbohydrate metabolism in Mycobacterium tuberculosis H37Rv1. Can. J. Microbiol. 1975, 21, 1688–1691. [Google Scholar] [CrossRef]
- Voskuil, M.I.; Schnappinger, D.; Visconti, K.C.; Harrell, M.I.; Dolganov, G.M.; Sherman, D.R.; Schoolnik, G.K. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 2003, 198, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sohaskey, C.D.; Pfeiffer, C.; Datta, P.; Parks, M.; McFadden, J.; North, R.J.; Gennaro, M.L. Carbon flux rerouting during Mycobacterium tuberculosis growth arrest. Mol. Microbiol. 2010, 78, 1199–1215. [Google Scholar] [CrossRef]
- Schubert, O.T.; Ludwig, C.; Kogadeeva, M.; Zimmermann, M.; Rosenberger, G.; Gengenbacher, M.; Gillet, L.C.; Collins, B.C.; Röst, H.L.; Kaufmann, S.H.E.; et al. Absolute proteome composition and dynamics during dormancy and resuscitation of Mycobacterium tuberculosis. Cell Host Microbe 2015, 18, 96–108. [Google Scholar] [CrossRef]
- Cabrera, R.; Ambrosio, A.L.B.; Garratt, R.C.; Guixé, V.; Babul, J. Crystallographic structure of phosphofructokinase-2 from Escherichia coli in complex with two ATP molecules. Implications for substrate inhibition. J. Mol. Biol. 2008, 383, 588–602. [Google Scholar] [CrossRef]
- Shirakihara, Y.; Evans, P.R. Crystal structure of the complex of phosphofructokinase from Escherichia coli with its reaction products. J. Mol. Biol. 1988, 204, 973–994. [Google Scholar] [CrossRef]
- Ganapathy, U.; Marrero, J.; Calhoun, S.; Eoh, H.; Sorio de Carvalho, L.P.; Rhee, K.; Ehrt, S. Two enzymes with redundant fructose bisphosphatase activity sustain gluconeogenesis and virulence in Mycobacterium tuberculosis. Nat. Commun. 2015, 6, 7912. [Google Scholar] [CrossRef] [PubMed]
- Hellinga, H.W.; Evans, P.R. Mutations in the active site of Escherichia coli phosphofructokinase. Nature 1987, 327, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, M.; Zhu, X.; Younathan, E.S.; Chang, S.H. Kinetic characteristics of phosphofructokinase from Bacillus stearothermophilus: MgATP nonallosterically inhibits the enzyme. Biochemistry 1994, 33, 3424–3431. [Google Scholar] [CrossRef]
- McKinney, J.D.; Höner zu Bentrup, K.; Muñoz-Elías, E.J.; Miczak, A.; Chen, B.; Chan, W.T.; Swenson, D.; Sacchettini, J.C.; Jacobs, W.R.; Russell, D. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 2000, 406, 735–738. [Google Scholar] [CrossRef]
- Marrero, J.; Rhee, K.Y.; Schnappinger, D.; Pethe, K.; Ehrt, S. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. USA 2010, 107, 9819–9824. [Google Scholar] [CrossRef]
- Boshoff, H.I.; Barry, C.E. Tuberculosis-metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 2005, 3, 70–80. [Google Scholar] [CrossRef]
- Phong, W.Y.; Lin, W.; Rao, S.P.S.; Dick, T.; Alonso, S.; Pethe, K. Characterization of phosphofructokinase activity in Mycobacterium tuberculosis reveals that a functional glycolytic carbon flow is necessary to limit the accumulation of toxic metabolic intermediates under hypoxia. PLoS ONE 2013, 8, e56037. [Google Scholar] [CrossRef]
- Uyeda, K.; Furuya, E.; Sherry, D. The Structure of “Activation Factor” for Phosphofructokinase. J. Biol. Chem. 1981, 256, 8679–8684. [Google Scholar] [CrossRef]
- Arts, E.; Kubicek, C.P.; Rohr, M. Regulation of phosphofructokinase from Aspergillus niger: Effect of fructose-2,6-bisphosphate on the action of citrate, ammonium ions and AMP. J. Gen. Microbiol. 1987, 133, 1195–1199. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. The Glycolytic Pathway Is Tightly Controlled. In Biochemistry, 5th ed.; Section 16.2; Freeman, W.H., Ed.; Publisher: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK22395/ (accessed on 20 January 2021).
- Mertens, E.; van Schaftingen, E.; Muller, M. Presence of a fructose-2,6-bisphosphate-insensitive pyrophosphate: Fructose-6-phosphate phosphotransferase in the anaerobic protozoa Trichomonas foetus, Trichomonas vaginalis and Isotricha prostoma. Mol. Biochem. Parasitol. 1989, 37, 183–190. [Google Scholar] [CrossRef]
- Reshetnikov, A.S.; Rozova, O.N.; Khmelenina, V.N.; Mustakhimov, I.I.; Beschastny, A.P.; Murrell, J.C.; Trotsenko, Y.A. Characterization of the pyrophosphate-dependent 6-phosphofructokinase from Methylococcus capsulatus Bath. FEMS Microbiol. Lett. 2008, 288, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Machová, I.; Snášel, J.; Zimmermann, M.; Laubitz, D.; Plocinski, P.; Oehlmann, W.; Singh, M.; Dostál, J.; Sauer, U.; Pichová, I. Mycobacterium tuberculosis phosphoenolpyruvate carboxykinase is regulated by redox mechanisms and interaction with thioredoxin. J. Biol. Chem. 2014, 289, 13066–13078. [Google Scholar] [CrossRef] [PubMed]
- Snášel, J.; Pichová, I. Allosteric regulation of pyruvate kinase from Mycobacterium tuberculosis by metabolites. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.W.; Reinhart, G.D. Mechanism of substrate inhibition in Escherichia coli phosphofructokinase. Biochemistry 2003, 42, 12676–12681. [Google Scholar] [CrossRef]
- Fideu, M.D.; Pérez, M.L.; Herranz, M.J.; Ruiz-Amil, M. Regulation of glycolysis in sea bass liver: Phosphofructokinase isoenzymes. Rev. Esp. Fisiol. 1989, 45, 179–186. [Google Scholar]
- Babul, J. Phosphofructokinases from Escherichia coli. Purification and characterization of the nonallosteric isoenzyme. J. Biol. Chem. 1978, 253, 4350–4355. [Google Scholar] [CrossRef]
- Reeves, R.E.; South, D.J.; Blytt, H.J.; Warren, L.G. Pyrophosphate: D-fructose-6-phosphate 1-phosphotransferase. A new enzyme with the glycolytic function of 6-phosphofructokinase. J. Biol. Chem. 1974, 249, 7737–7741. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Kinkead, J.; McNae, I.W.; Bringaud, F.; Michels, P.A.M.; Walkinshaw, M.D. The kinetic characteristics of human and trypanosomid phosphofructokinases for the reverse reaction. Biochem. J. 2019, 476, 179–191. [Google Scholar] [CrossRef]
- Orchard, L.M.D.; Kornberg, H.L. Sequence similarities between the gene specifying 1-phosphofructokinase (fruK), genes specifying other kinases in Escherichia coli K12, and lacC of Staphylococcus aureus. Proc. R. Soc. B Biol. Sci. 1990, 242, 87–90. [Google Scholar] [CrossRef]
- Buschmeier, B.; Hengstenberg, W.; Deutscher, J. Purification and properties of 1-phosphofructokinase from Escherichia coli. FEMS Microbiol. Lett. 1985, 29, 231–235. [Google Scholar] [CrossRef]
- Miallau, L.; Hunter, W.N.; McSweeney, S.M.; Leonard, G. Structures of Staphylococcus aureus D-tagatose-6-phosphate kinase implicate domain motions in specificity and mechanism. J. Biol. Chem. 2007, 282, 19948–19957. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Anderson, R.L. Lactose and D-galactose metabolism in group of N-Streptococci: Presence of enzymes for both the D-galactose-1-phosphate and D-tagatose-6-phosphate pathways. J. Bacteriol. 1974, 117, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Sugden, P.H.; Williams, T. Effect of citrate on the activities of 6-phosphofructokinase from nervous and muscle tissues from different animals and its relationship to the regulation of glycolysis. Biochem. J. 1977, 166, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kemp, R.G.; Gunasekera, D. Evolution of the allosteric ligand sites of mammalian phosphofructo-1-kinase. Biochemistry 2002, 41, 9426–9430. [Google Scholar] [CrossRef]
- Fenton, A.W.; Reinhart, G.D. Disentagling the web of allosteric communication in a homotetramer: Heterotropic inhibition in phosphofructokinase from Escherichia coli. Biochemistry 2009, 48, 12323–12328. [Google Scholar] [CrossRef]
- Bartrons, R.; van Schaftingen, E.; Vissers, S.; Hers, H.-G. The stimulation of yeast phosphofructokinase by fructose 2,6-bisphosphate. FEBS Lett. 1981, 102, 985–991. [Google Scholar] [CrossRef]
- van Laere, A.J. Stimulation of phosphofructokinase from Phycomyces blakesleeanus and some other fungi by micromolar concentrations of fructose 2,6-bisphosphate. J. Gen. Microbiol. 1983, 129, 3281–3285. [Google Scholar] [CrossRef][Green Version]
- Heylen, A.; van Schaftingen, E.; Hers, H.-G. The stimulation of phosphofructokinase from human erythrocytes by fructose 2,6 bisphosphate. FEBS Lett. 1982, 143, 141–143. [Google Scholar] [CrossRef]
- Tlapak-Simmons, V.L.; Reinhart, G.D. Comparison of the inhibition by phospho (enol)pyruvate and phosphoglycolate of phosphofructokinase from B. stearothermophilus. Arch. Biochem. Biophys. 1994, 308, 226–230. [Google Scholar] [CrossRef]
- Johnson, J.L.; Reinhart, G.D. Influence of MgADP on phosphofructokinase from Escherichia coli. Elucidation of coupling interactions with both substrates. Biochemistry 1994, 33, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Reinhart, G.D. MgATP and fructose-6-phosphate interactions with phosphofructokinase from Escherichia coli. Biochemistry 1992, 31, 11510–11518. [Google Scholar] [CrossRef] [PubMed]
- Novak, B.; Tyson, J.J. Design principles of biochemical oscillators. Nat. Rev. Mol. Cell. Biol. 2008, 9, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Noy, T.; Vergnolle, O.; Hartman, T.E.; Rhee, K.Y.; Jacobs, W.R.; Berney, M. Central role of pyruvate kinase in carbon co-catabolism of Mycobacterium tuberculosis. J. Biol. Chem. 2016, 291, 7060–7069. [Google Scholar] [CrossRef]
- Mertens, E. Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett. 1991, 285, 1–5. [Google Scholar] [CrossRef]
- Bapteste, E.; Moreira, D.; Philippe, H. Rampant horizontal gene transfer and phospho-donor change in the evolution of phosphofructokinase. Gene 2003, 318, 185–191. [Google Scholar] [CrossRef]
- Mertens, E.; de Jonckheere, J.; van Schaftingen, E. Pyrophosphate-dependent phosphofructokinase from the amoeba Naegleria fowleri, an AMP-sensitive enzyme. Biochem. J. 1993, 292, 797–803. [Google Scholar] [CrossRef]
- van Schaftingen, E.; Hers, H.-G. Formation of Fructose 2,6-Bisphosphate from Fructose 1,6-Bisphosphate by Intramolecular Cyclization followed by Alkaline Hydrolysis. Eur. J. Biochem. 1981, 117, 319–323. [Google Scholar] [CrossRef]
- Voll, R.J.; Ramaprasad, S.; Vargas, D.; Younathan, E.S. Two-dimensional 1H-, 13C-, and 31P-nuclear magnetic resonance and molecular-mechanics investigation of D-fructose 2,6-bisphosphate. Carbohydr. Res. 1990, 203, 173–182. [Google Scholar] [CrossRef]
| Glycolytic Reaction | |||||
|---|---|---|---|---|---|
| Isoenzyme | Substrate | Vmax (µmol/min/mg) | K1/2 (Km) (mM) | nH | Vmax/K1/2 (L/min/mg) |
| Pfk A | ATP | 19 ± 0.5 | 1.0 ± 0.2 Ki,ATP = 1.5 ± 0.3 | 1.6 ± 0.1 | 0.019 ± 0.004 |
| Pfk A | F6P | 20 ± 1 | 0.4 ± 0.1 Ki,F6P = 13.2 ± 2.0 | 1.6 ± 0.2 | 0.05 ± 0.01 |
| Pfk B | ATP | 0.8 ± 0.1 * 2.1 ± 0.1 ** | 0.052 ± 0.005 * 9.2 ± 0.1 ** | 1 | 0.015 ± 0.002 |
| Pfk B | F6P | 1.2 ± 0.1 | 0.04 ± 0.01 | 1 | 0.030 ± 0.008 |
| Gluconeogenic Reaction | |||
|---|---|---|---|
| Substrate | Vmax (µmol/min/mg) | Km (mM) | Vmax/Km (L/min/mg) |
| F16bP | 0.39 ± 0.05 | 17.5 ± 1.5 | 2.2 × 10−5 ± 3 × 10−6 |
| ADP | 3.6 ± 0.2 | 12.9 ± 3.0 | 1.4 × 10−4 ± 4.9 × 10−5 |
| IDP | 0.37 ± 0.02 | 0.27 ± 0.05 | 1.4 × 10−3 ± 2.6 × 10−4 |
| GDP | 0.32 ± 0.07 | 0.28 ± 0.03 | 1.1 × 10−3 ± 2.8 × 10−4 |
| K1/2 (mM) | Vmax (µmol/min/mg) | Vmax/K1/2 (L/min/mg) | |
|---|---|---|---|
| PfkA (F6P) | 0.30 ± 0.05 | 22 ± 3 | 0.073 ± 0.015 |
| Pfk A (T6P) | 0.43 ± 0.05 | 30 ± 4 | 0.070 ± 0.012 |
| Pfk B (F6P) | 0.040 ± 0.005 | 1.2 ± 0.1 | 0.030 ± 0.005 |
| Pfk B (T6P) | 0.090 ± 0.005 | 1.5 ± 0.2 | 0.017 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snášel, J.; Machová, I.; Šolínová, V.; Kašička, V.; Krečmerová, M.; Pichová, I. Phosphofructokinases A and B from Mycobacterium tuberculosis Display Different Catalytic Properties and Allosteric Regulation. Int. J. Mol. Sci. 2021, 22, 1483. https://doi.org/10.3390/ijms22031483
Snášel J, Machová I, Šolínová V, Kašička V, Krečmerová M, Pichová I. Phosphofructokinases A and B from Mycobacterium tuberculosis Display Different Catalytic Properties and Allosteric Regulation. International Journal of Molecular Sciences. 2021; 22(3):1483. https://doi.org/10.3390/ijms22031483
Chicago/Turabian StyleSnášel, Jan, Iva Machová, Veronika Šolínová, Václav Kašička, Marcela Krečmerová, and Iva Pichová. 2021. "Phosphofructokinases A and B from Mycobacterium tuberculosis Display Different Catalytic Properties and Allosteric Regulation" International Journal of Molecular Sciences 22, no. 3: 1483. https://doi.org/10.3390/ijms22031483
APA StyleSnášel, J., Machová, I., Šolínová, V., Kašička, V., Krečmerová, M., & Pichová, I. (2021). Phosphofructokinases A and B from Mycobacterium tuberculosis Display Different Catalytic Properties and Allosteric Regulation. International Journal of Molecular Sciences, 22(3), 1483. https://doi.org/10.3390/ijms22031483








