Lactones in the Synthesis of Prostaglandins and Prostaglandin Analogs
Abstract
1. Introduction
2. Lactones in the Synthesis of Prostaglandins and Prostaglandin Analogs
2.1. Synthesis of Prostaglandin Lactones
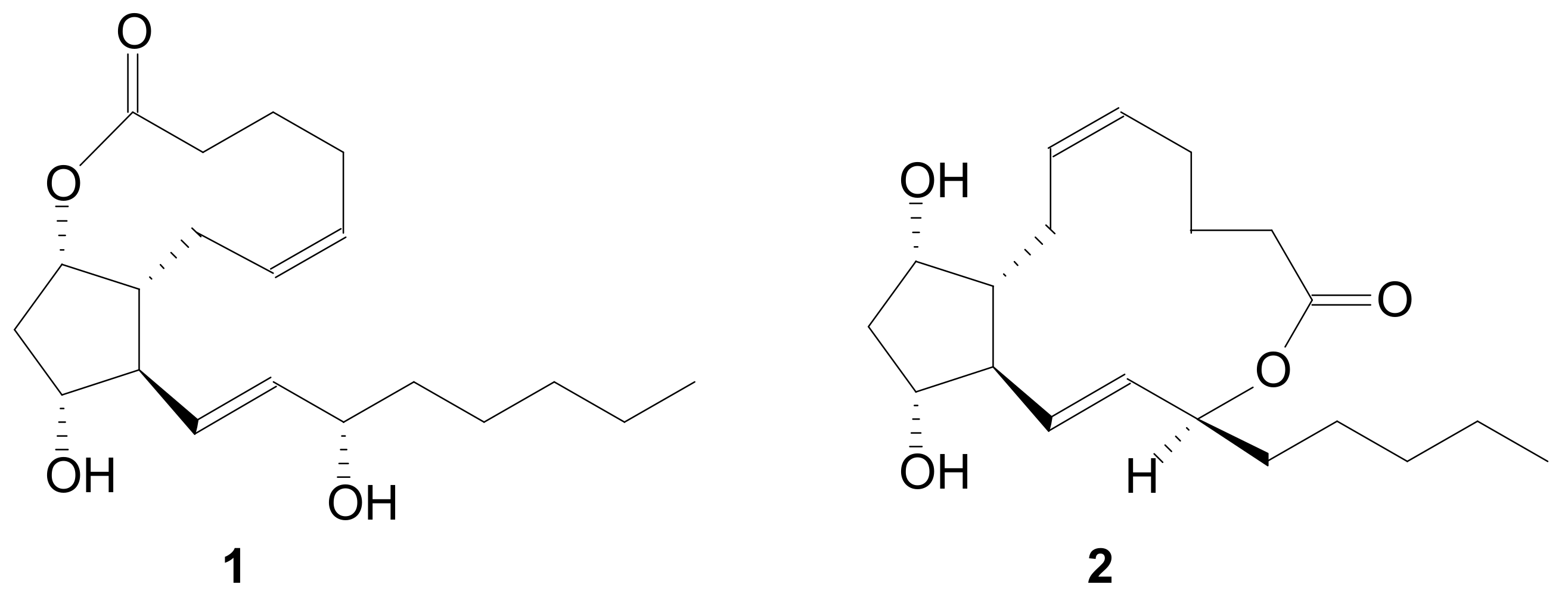
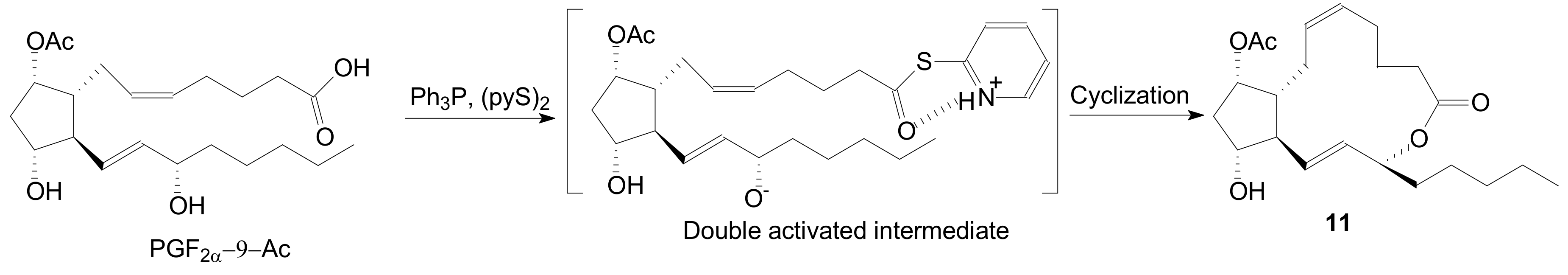
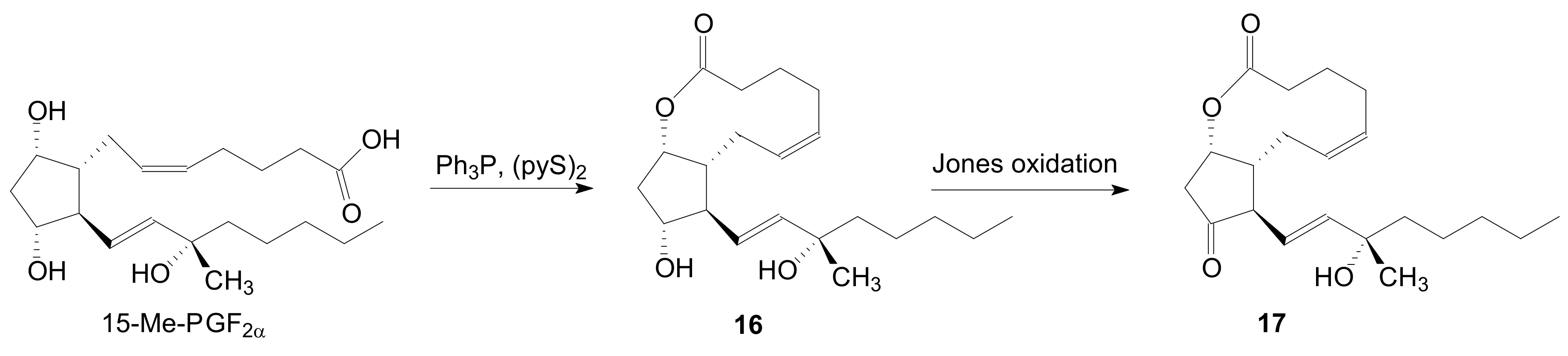
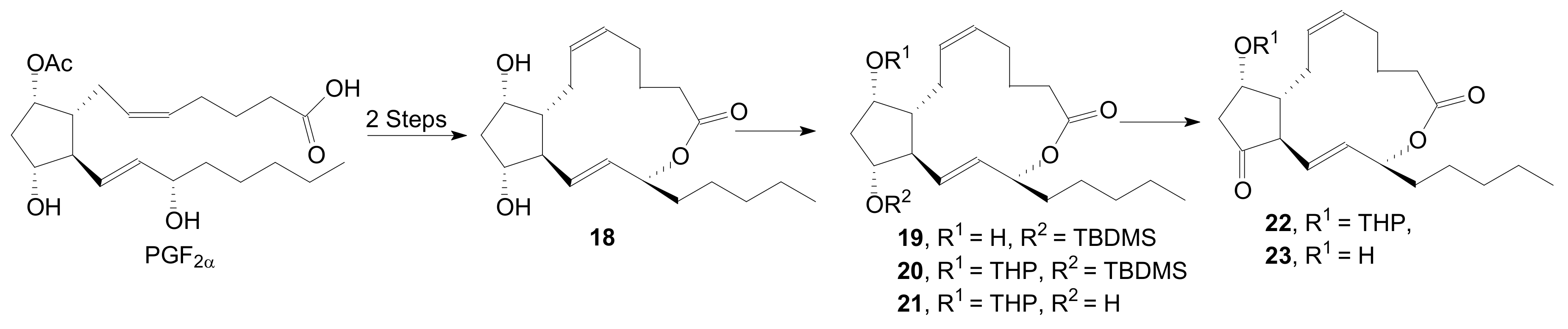
2.1.1. Internal Lactonization of an Acid with a Hydroxyl Group of a Prostaglandin by the Corey-Nicolaou Procedure
2.1.2. Internal Lactonization of an Acid with a Hydroxyl Group by the Mixed Anhydride (Yamaguchi and Coworkers) Procedure
2.1.3. Internal Lactonization of an Acid with a Hydroxyl Group by the Shiina Procedure
2.1.4. Internal Lactonization of an Acid with a Hydroxyl Group by the Mukaiyama Procedure
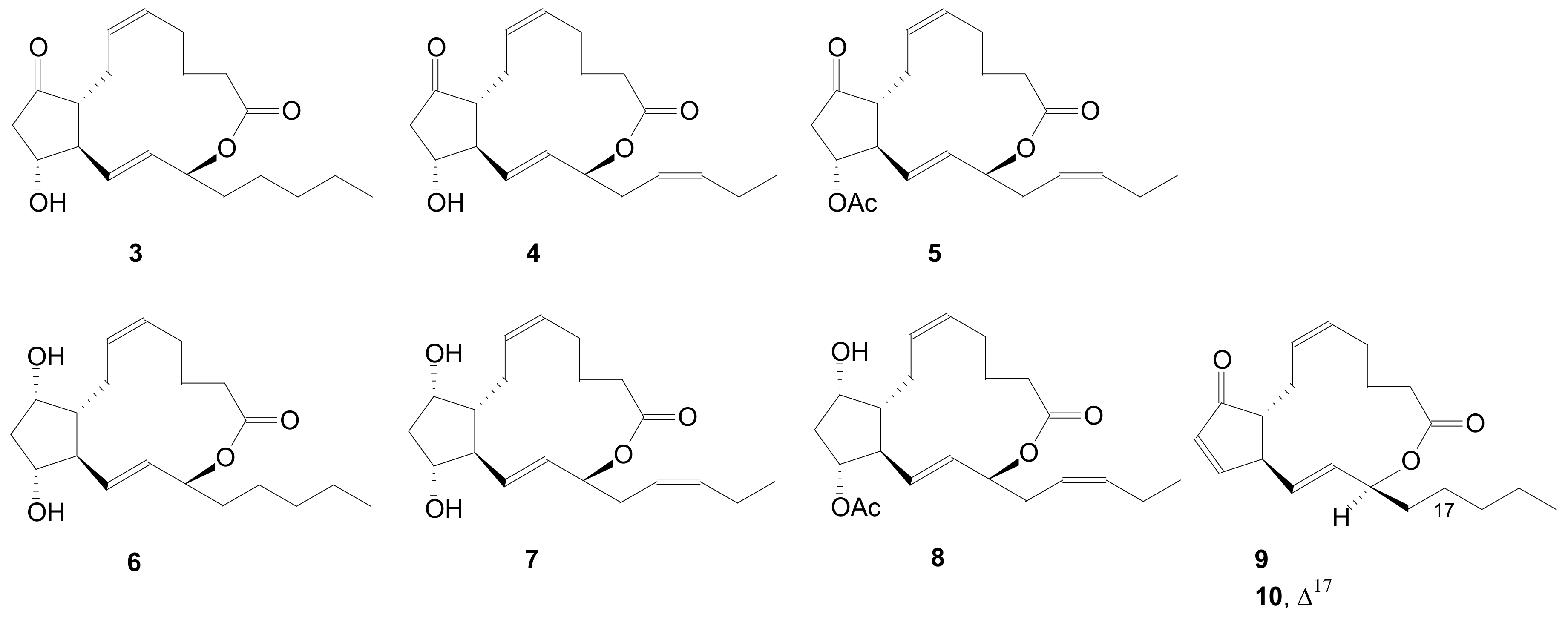
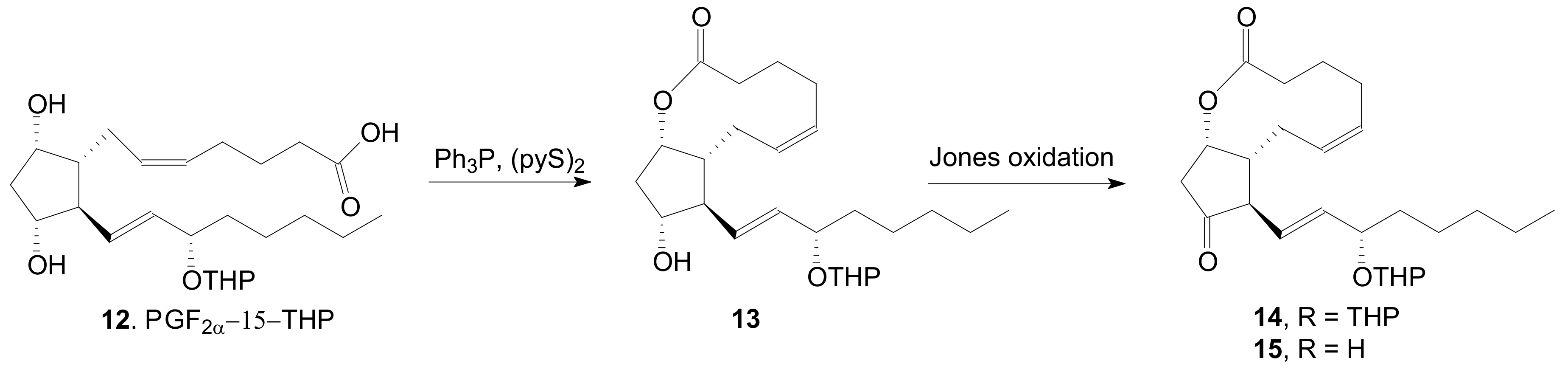
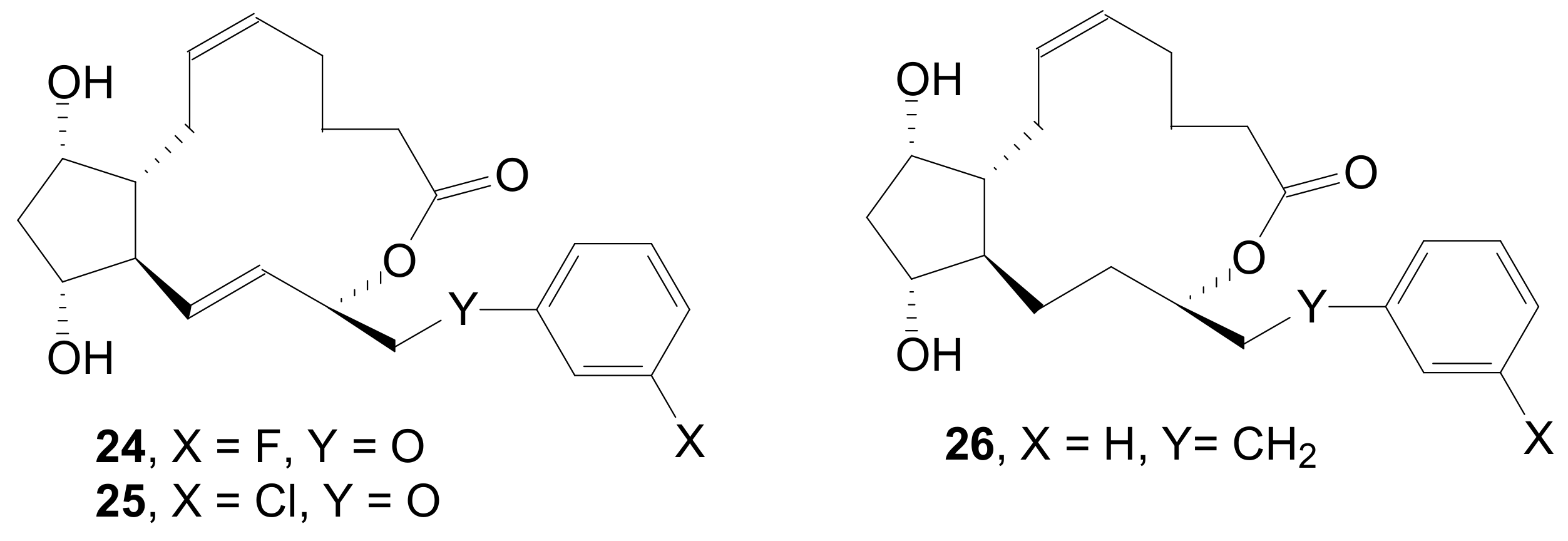
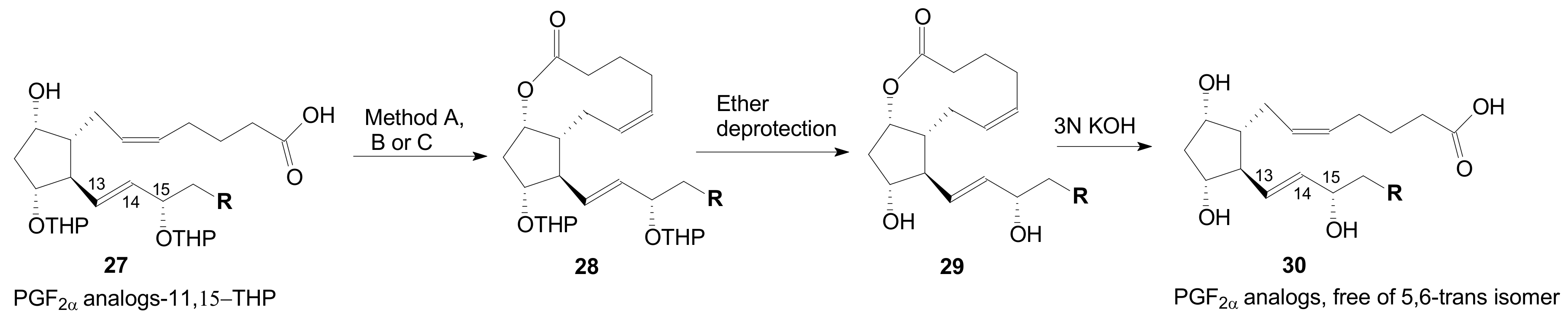
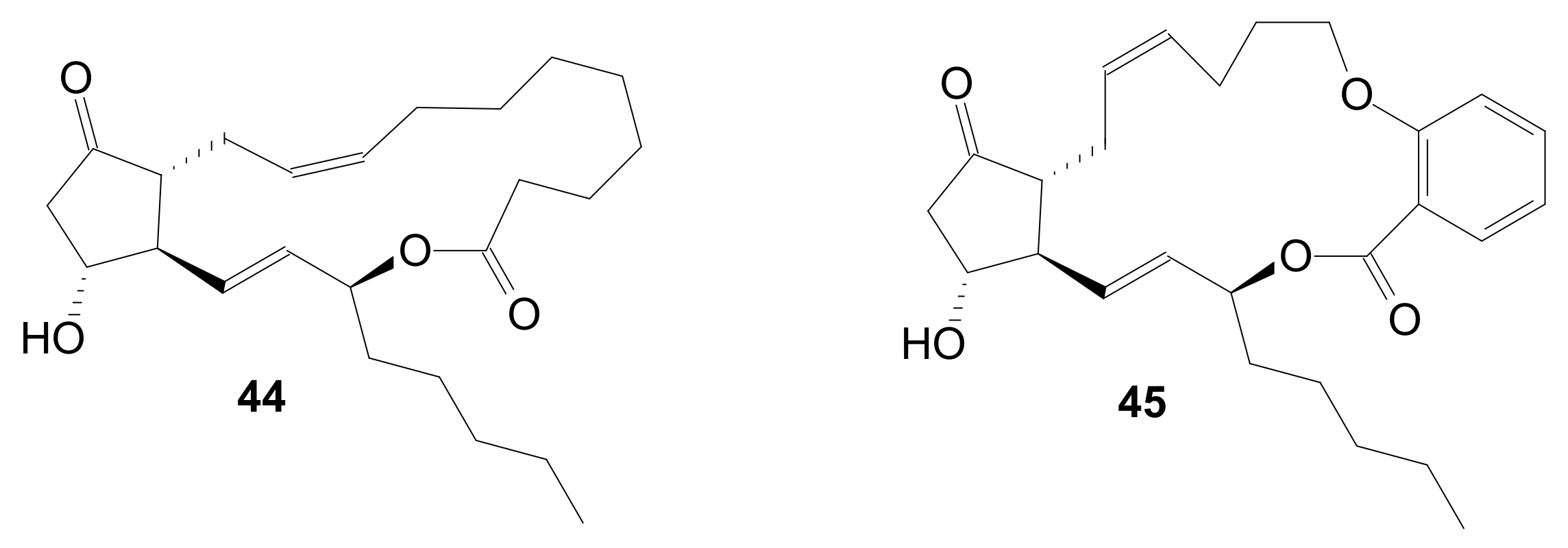
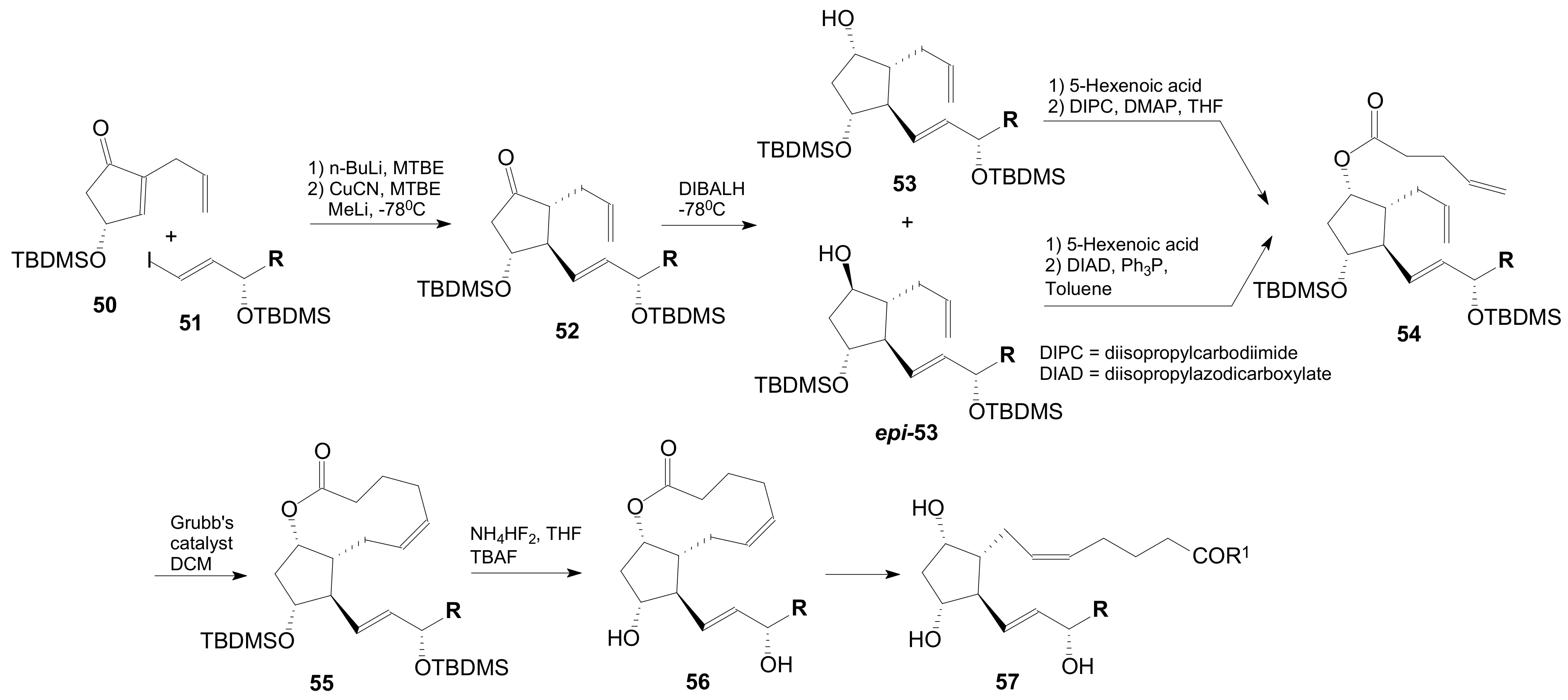
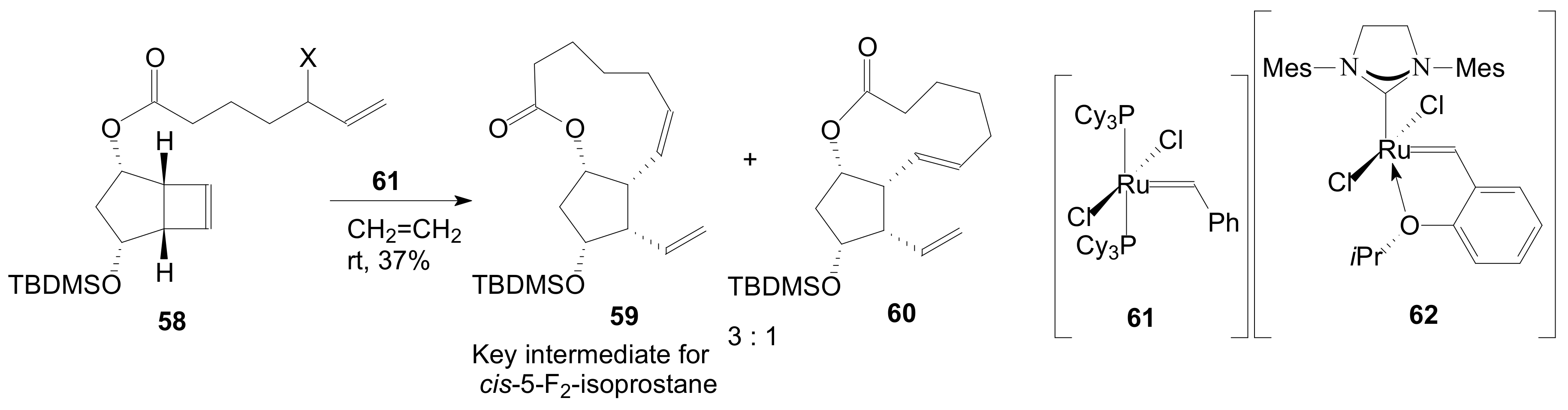
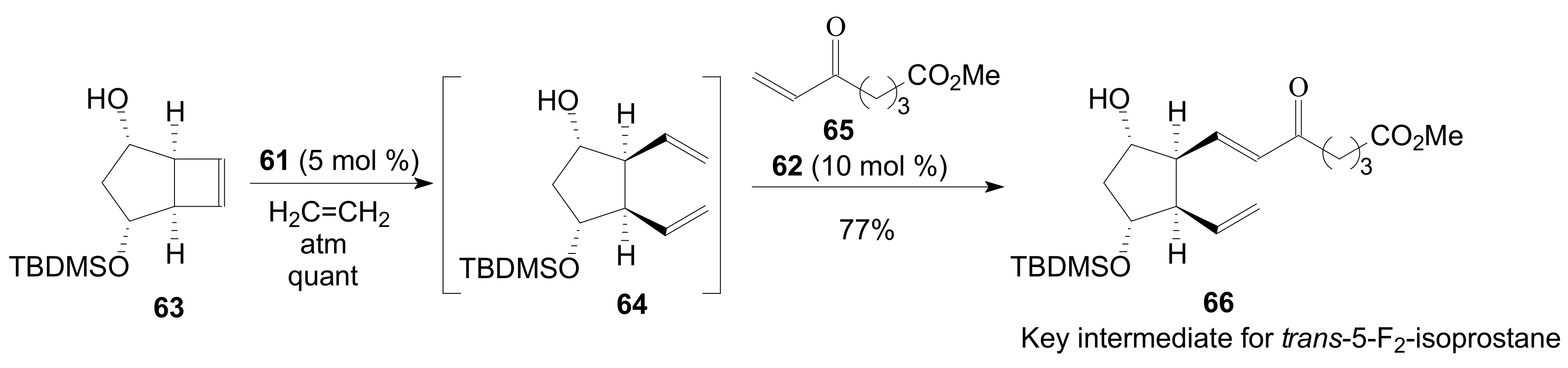
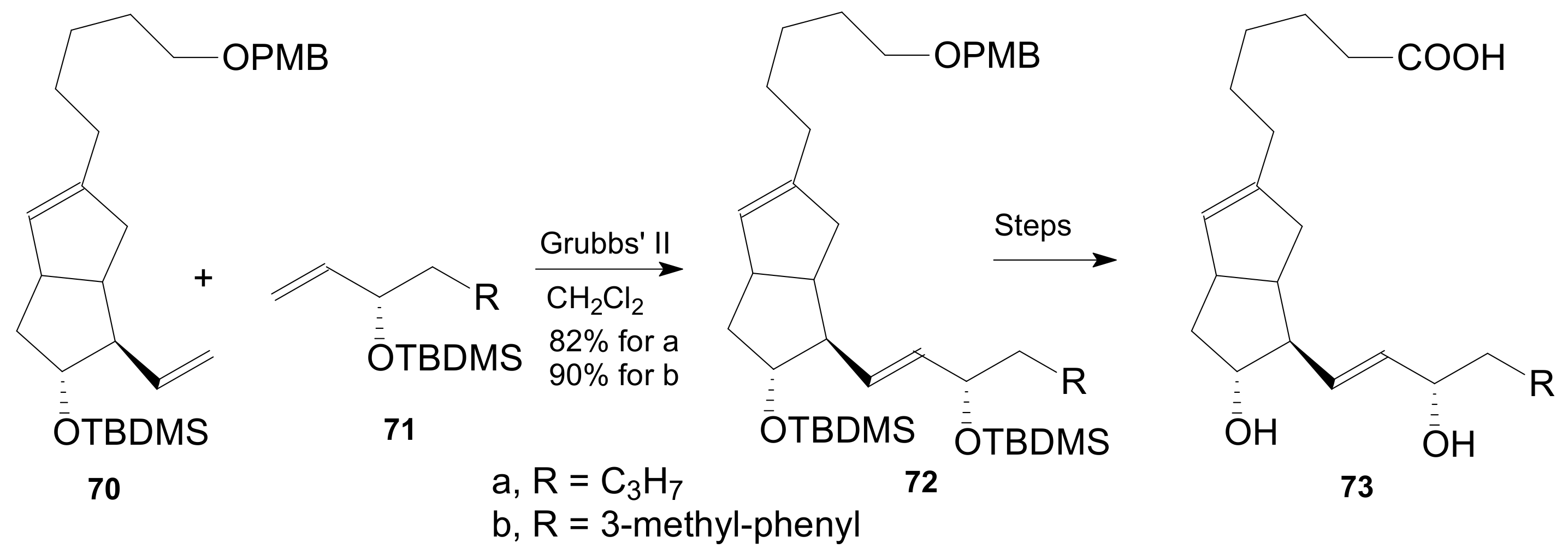
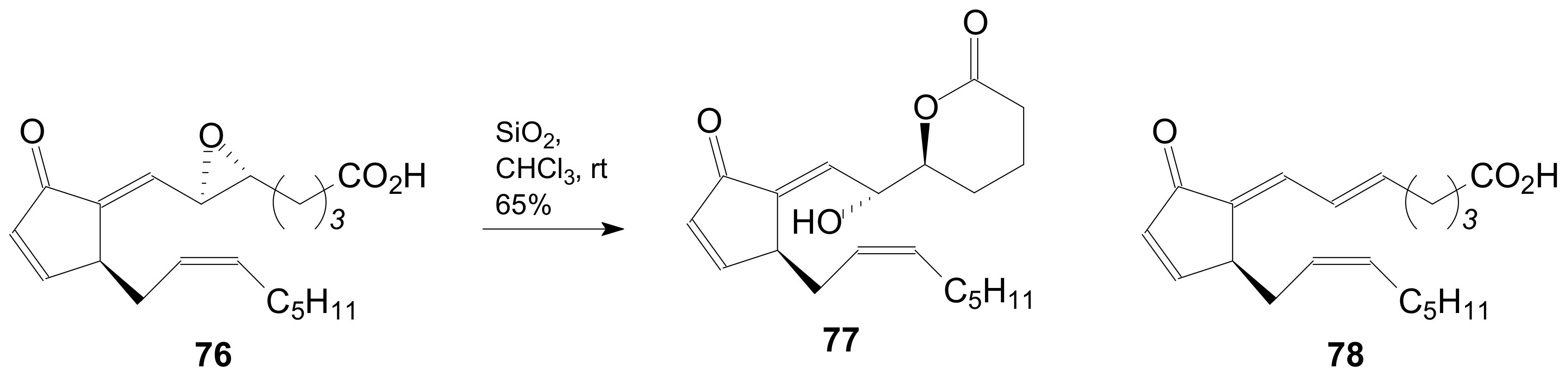
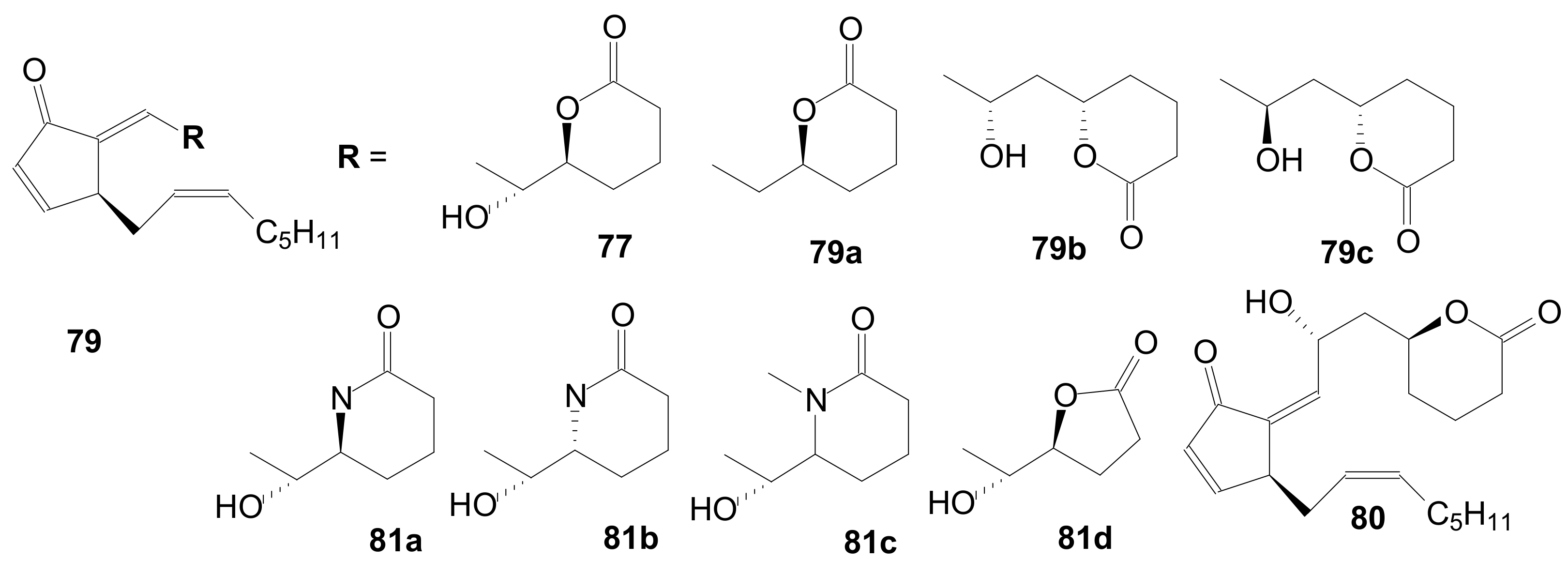
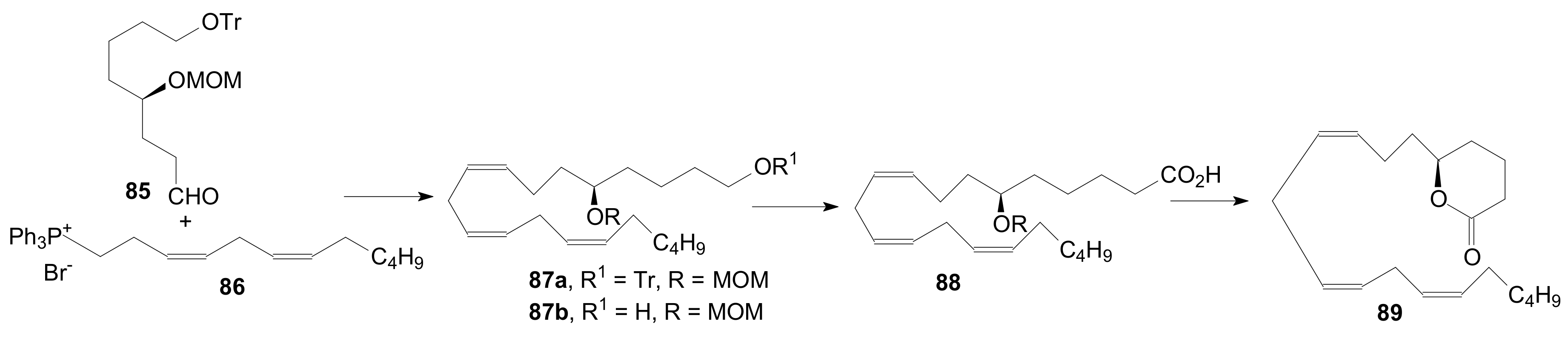
2.1.5. Internal Lactonization by Ring-Closing Metathesis
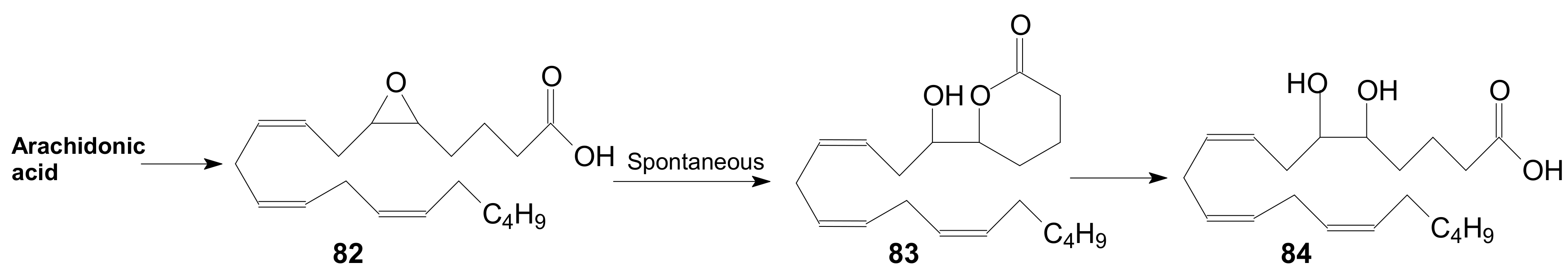
2.1.6. Other Methods for Building Prostaglandin or Eicosanoid Lactones
2.2. Synthesis of γ-Lactone Intermediates in the Sequence for Obtaining Prostaglandin Analogues
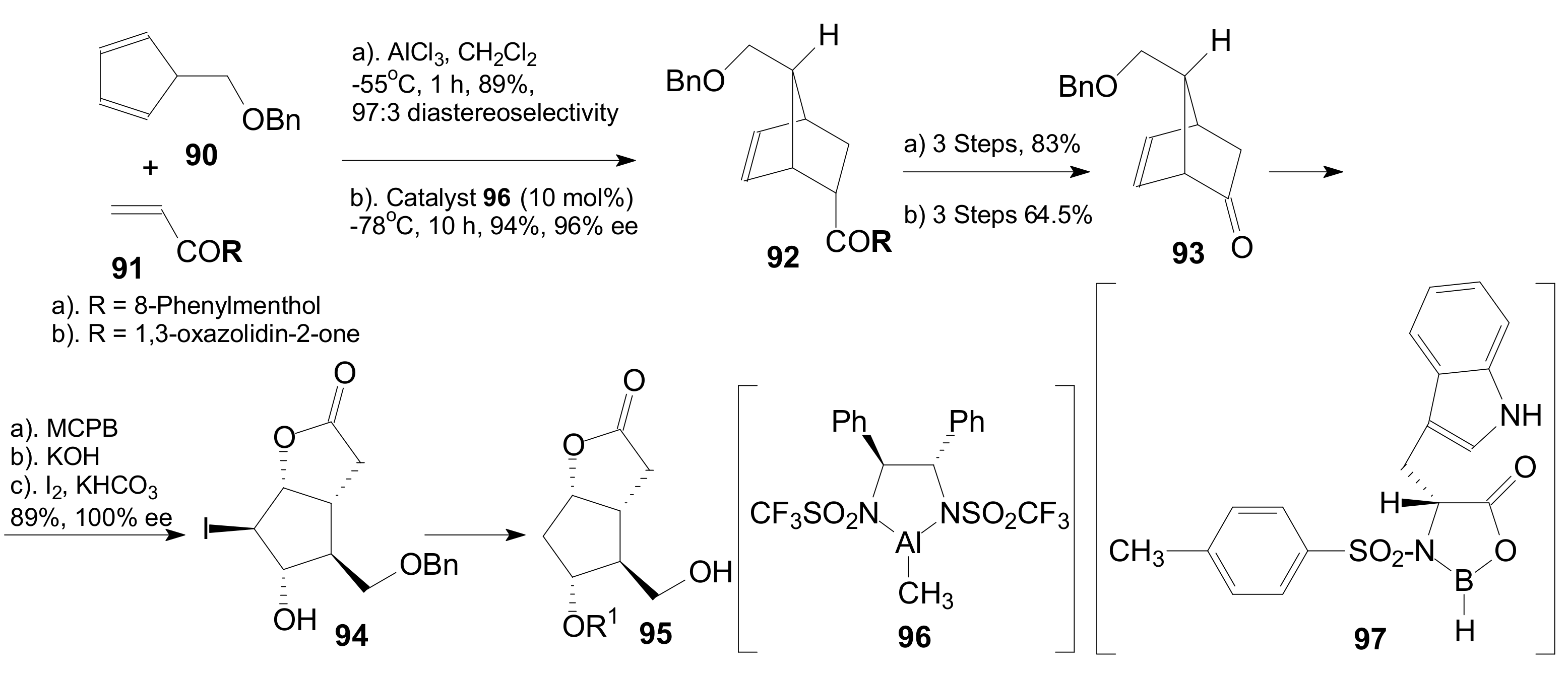
2.2.1. Synthesis of Corey γ-Lactone by the Most Used Methods
2.2.2. The Improvements in the Synthesis of Corey Lactone
2.2.3. Synthesis of the Corey Lactone by Other Promising Methods
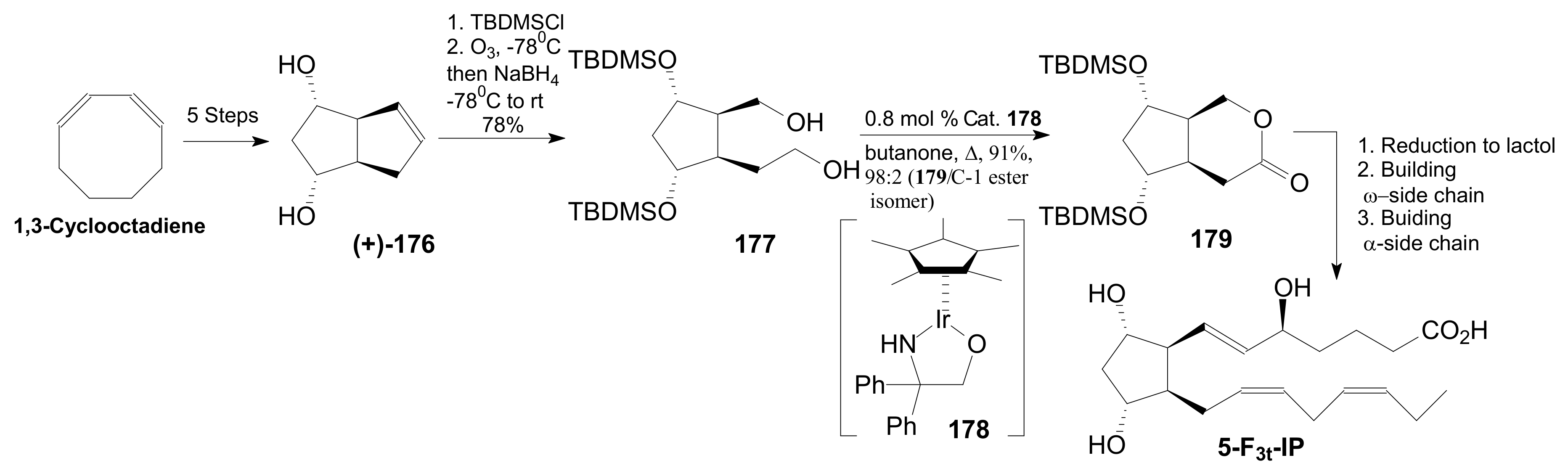
2.2.4. Corey Lactones for the Synthesis of Isoprostanes
- −
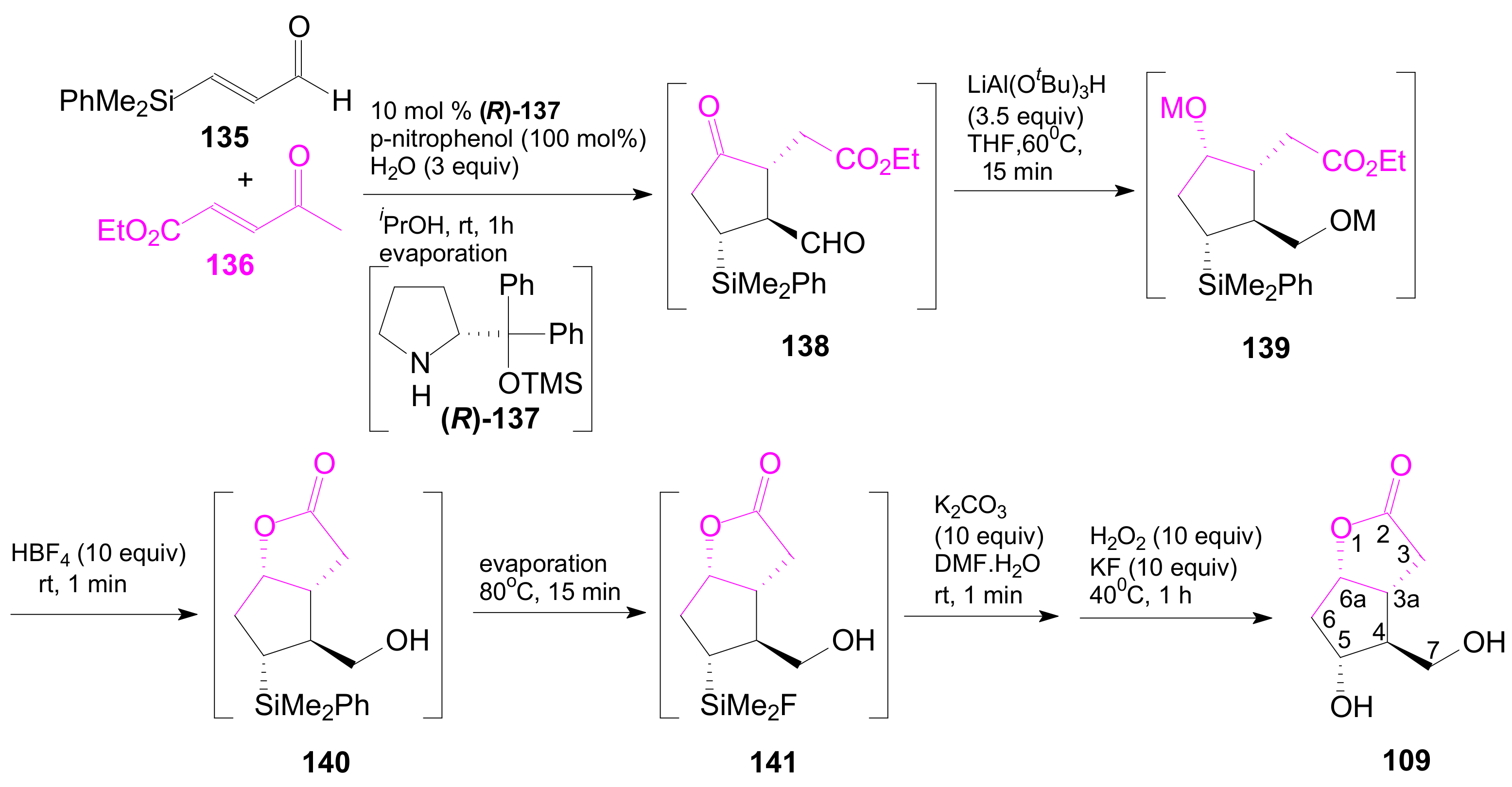
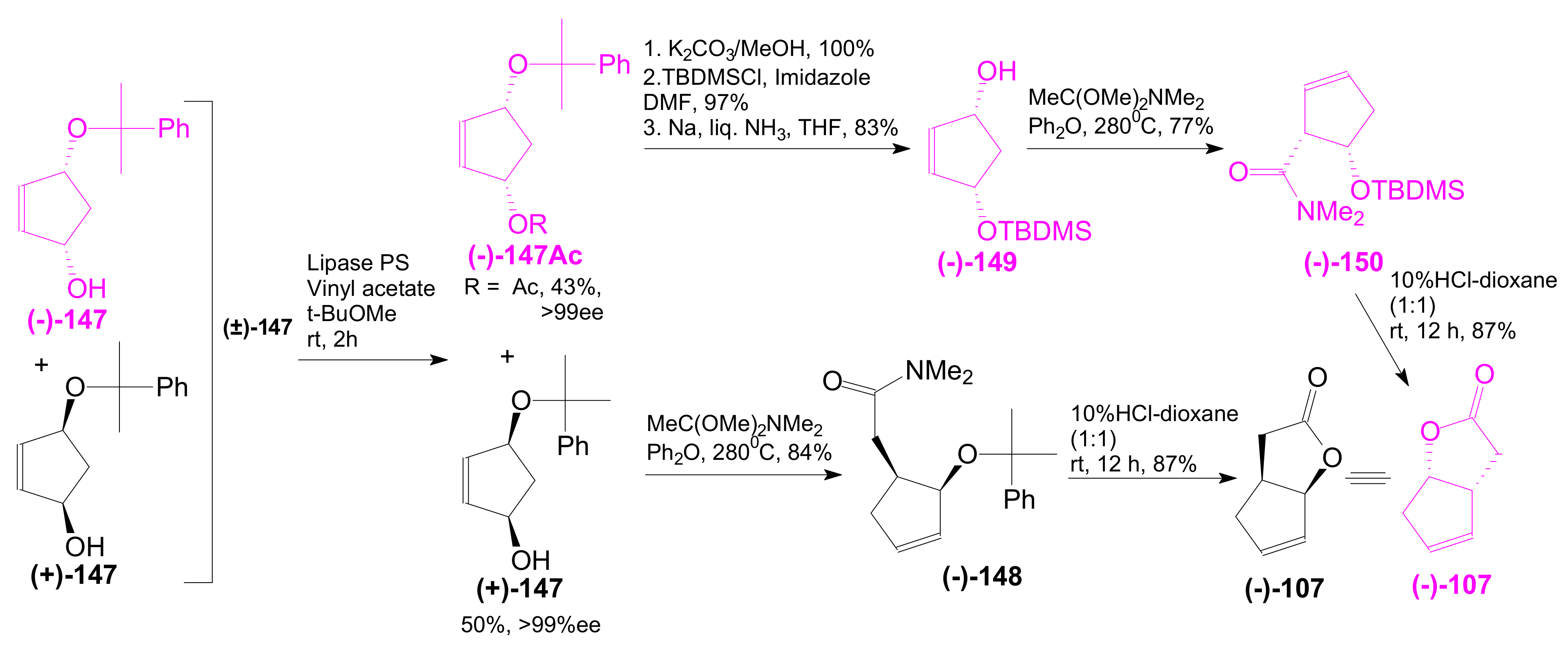
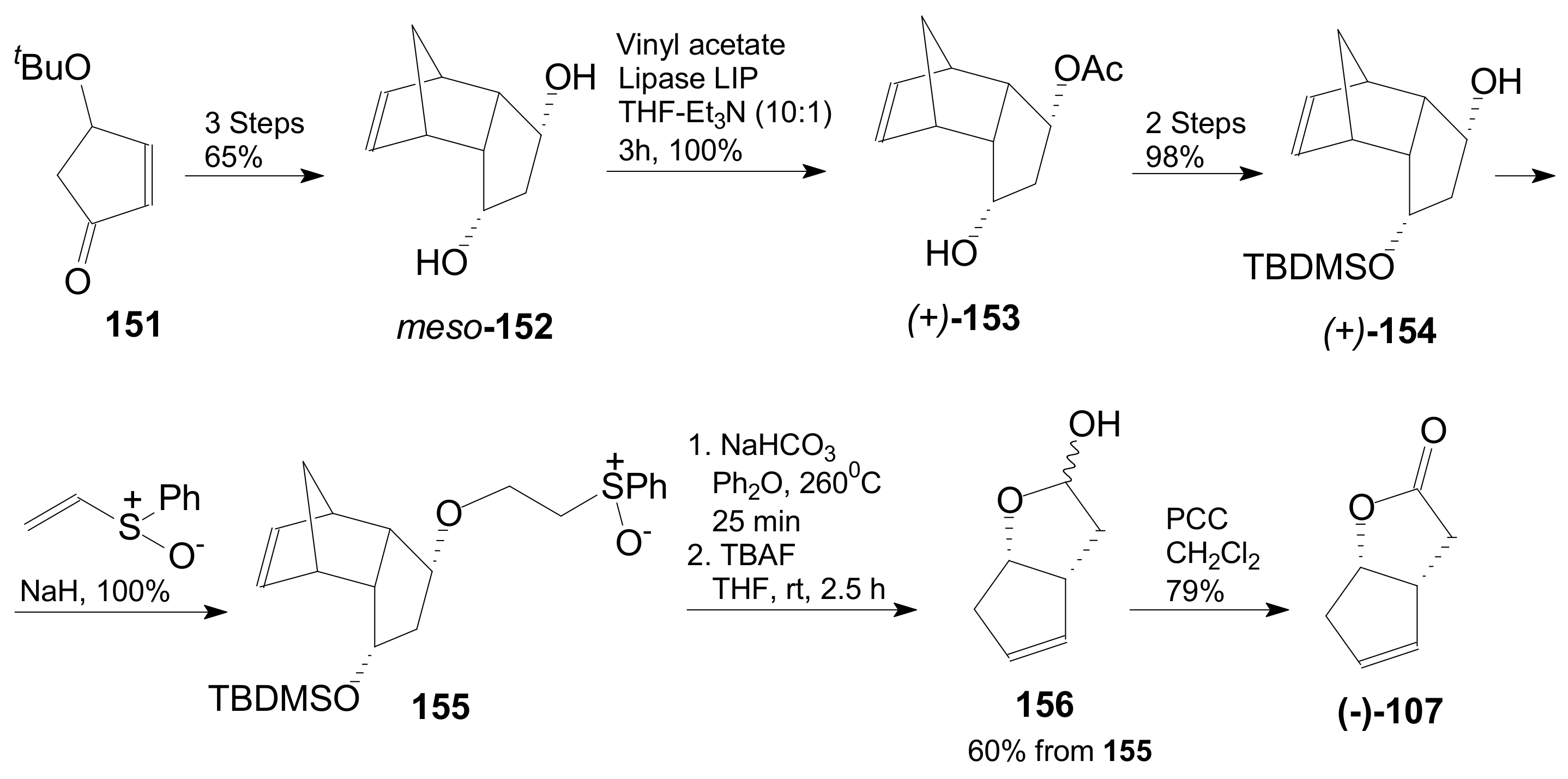
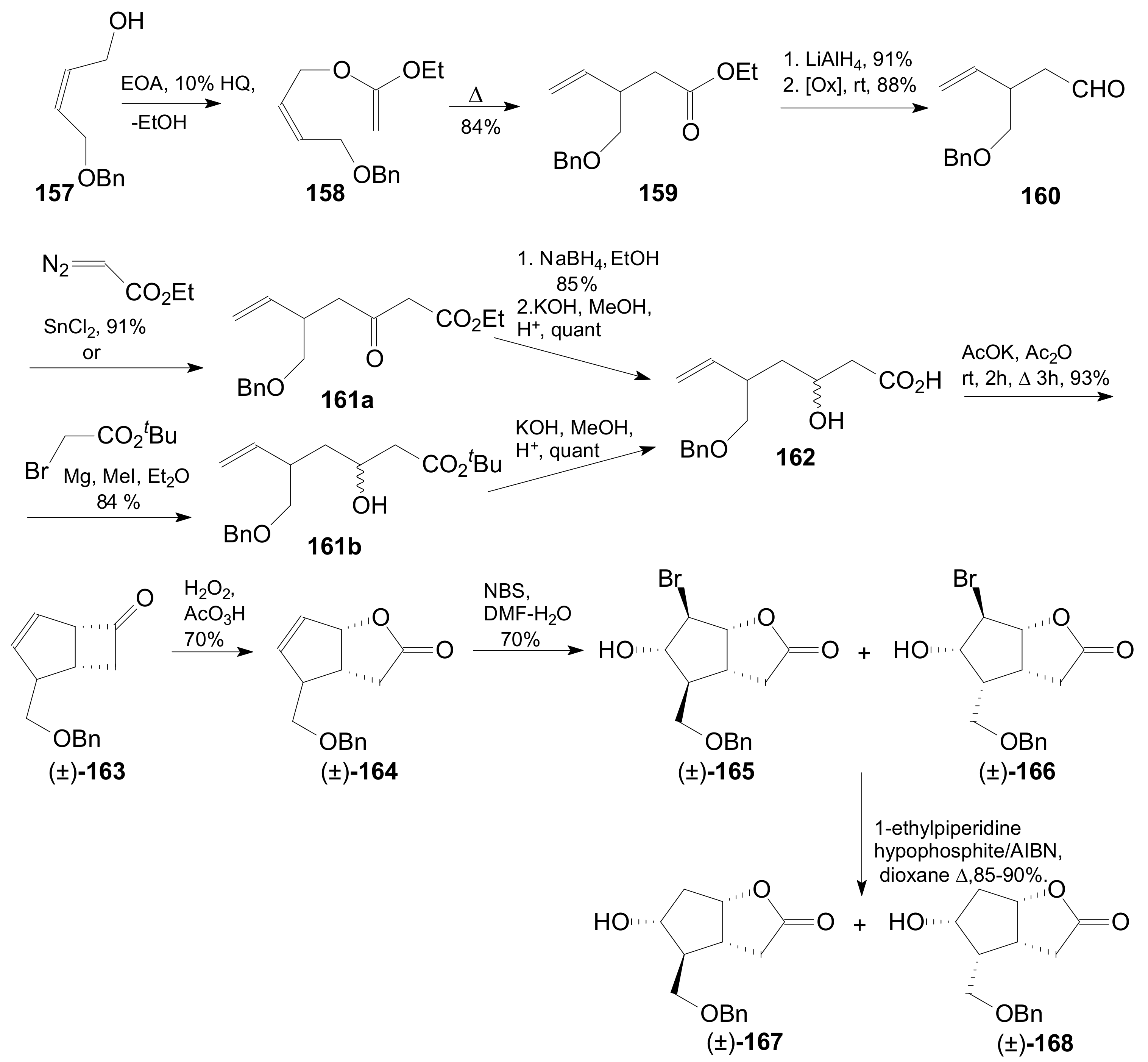
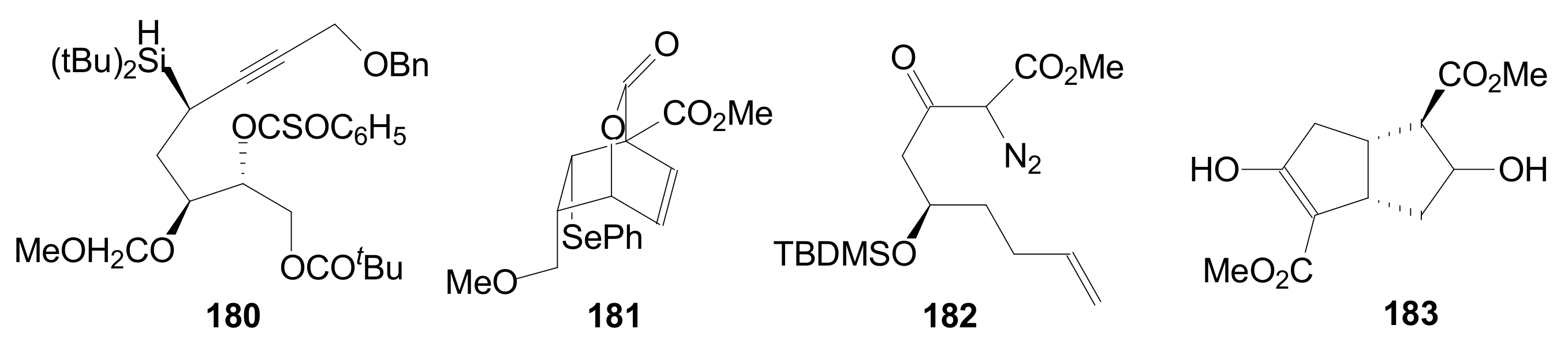
- An optically active compound 180, (obtained in 14.6% from 2-deoxy-D-ribose, in a nine-step reaction) was used to give the optically active ent-Corey lactone in four steps (~37%) [115].
- −
- An adduct of 3-carbomethoxy-2-pyrone (3-CMP) with vinylselenide, 181, was used in a six-step reaction (41%) to give (±)-Corey lactone substituted as Me ether at the primary alcohol and acetyl at the secondary one [116].
- −
- An optically active diazo compound 182 was used to give Corey lactone (−)-95 (R1 = p-PhBz) in six steps (~19%) [117].
- −
- The optically active compound 183 (obtained in three steps from dimethyl 3-oxoglutarate and glyoxal, followed by enzymatic demethoxycarbonylation) gave (−)-95 (R1 = Ac) in eight steps [118].
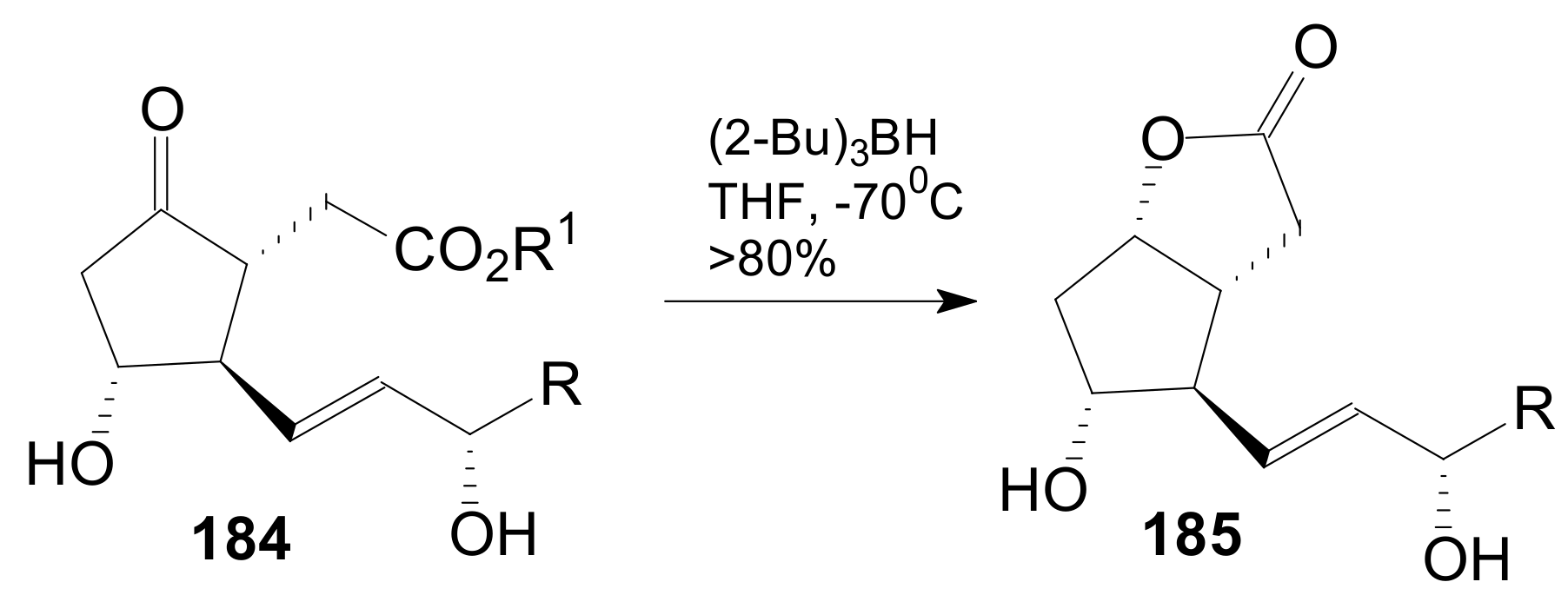
2.2.5. Other Lactonizations for Obtaining Corey γ-Lactone Intermediates of Type 185
2.2.6. δ-Lactones in the Synthesis of Prostaglandins
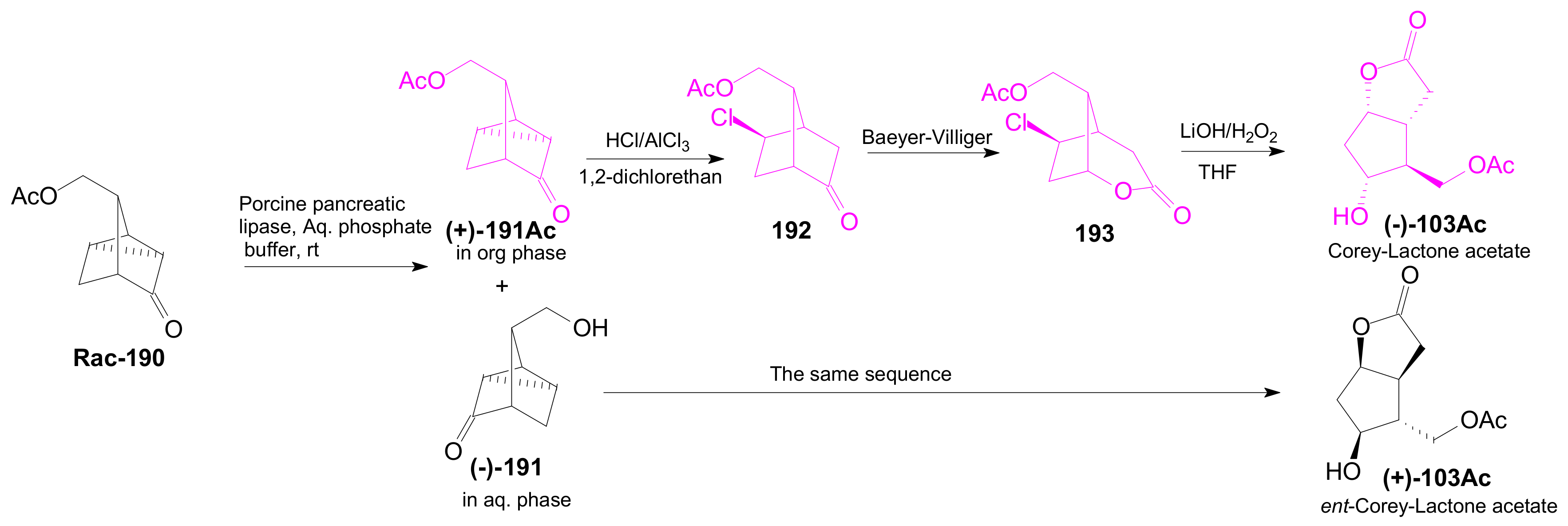
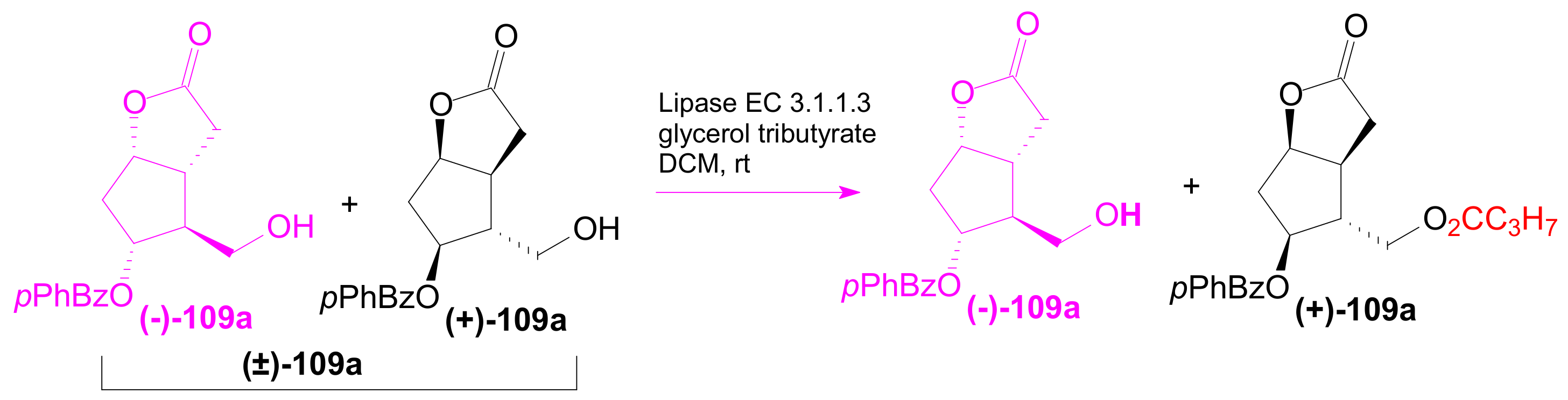
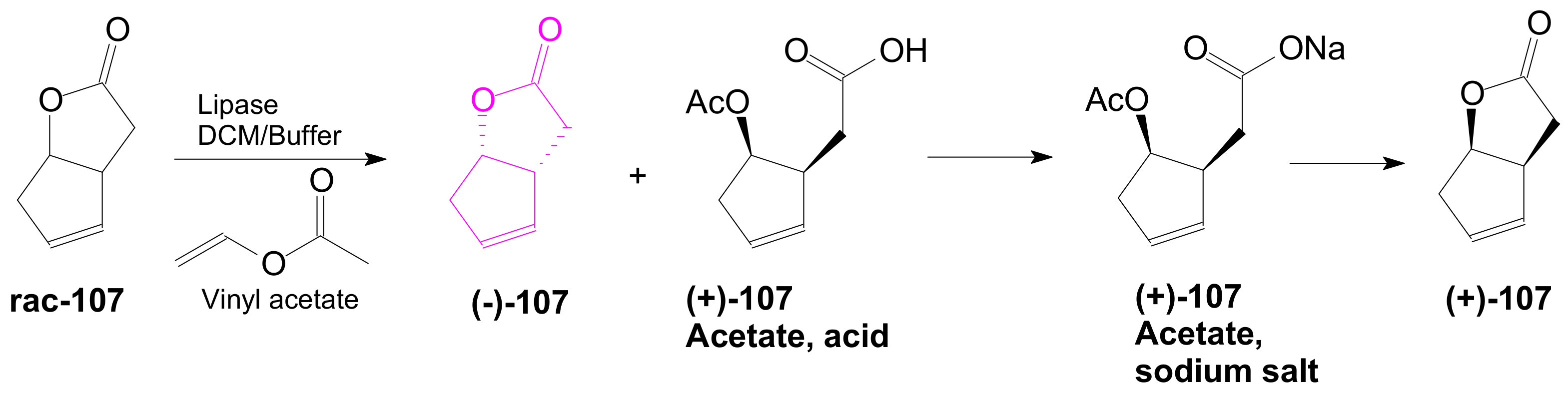
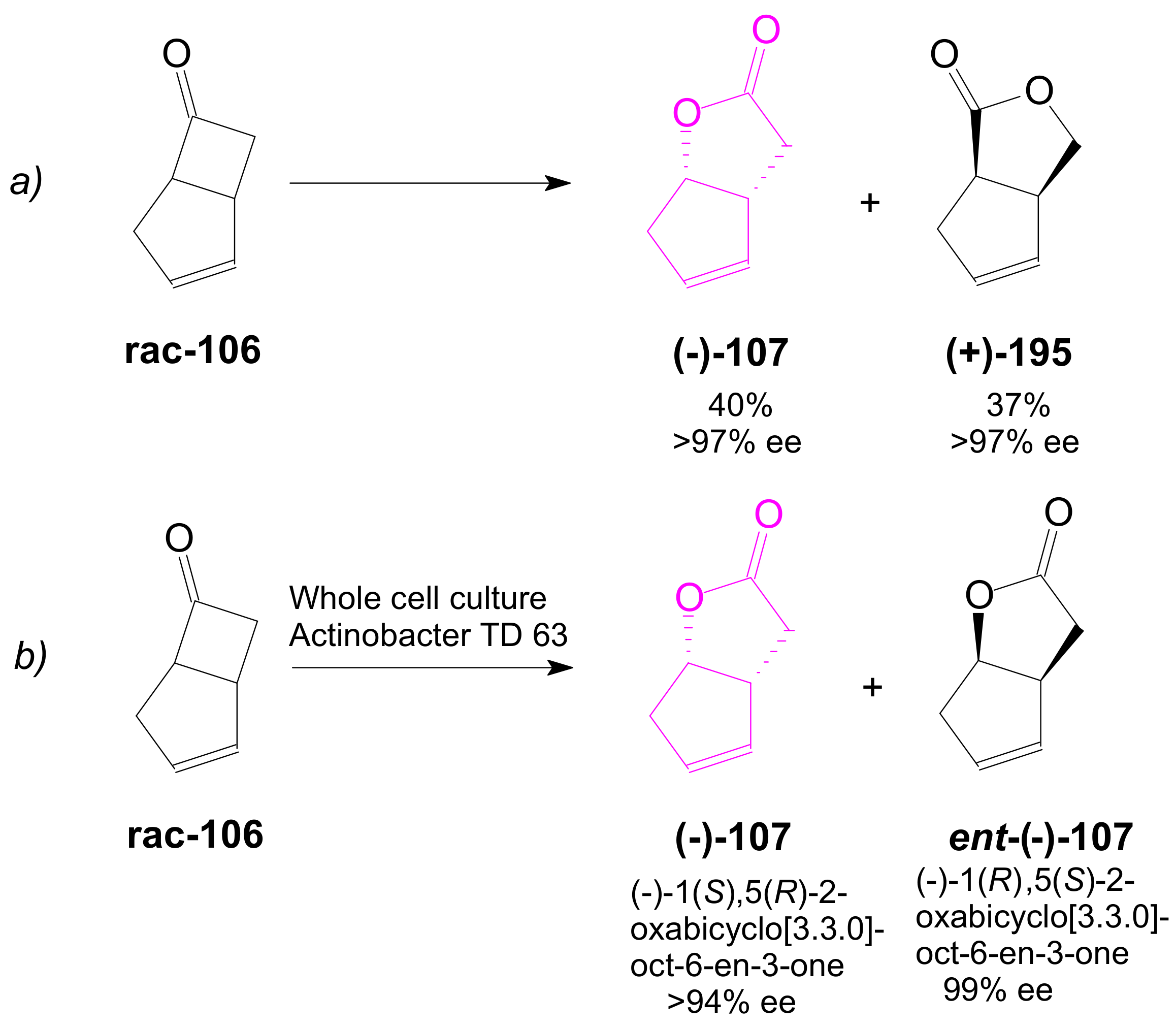
2.3. The Use of Enzymes for Obtaining Enantiomerically Pure Key δ-Lactone and γ-Lactone Intermediates for the Synthesis of Prostaglandins
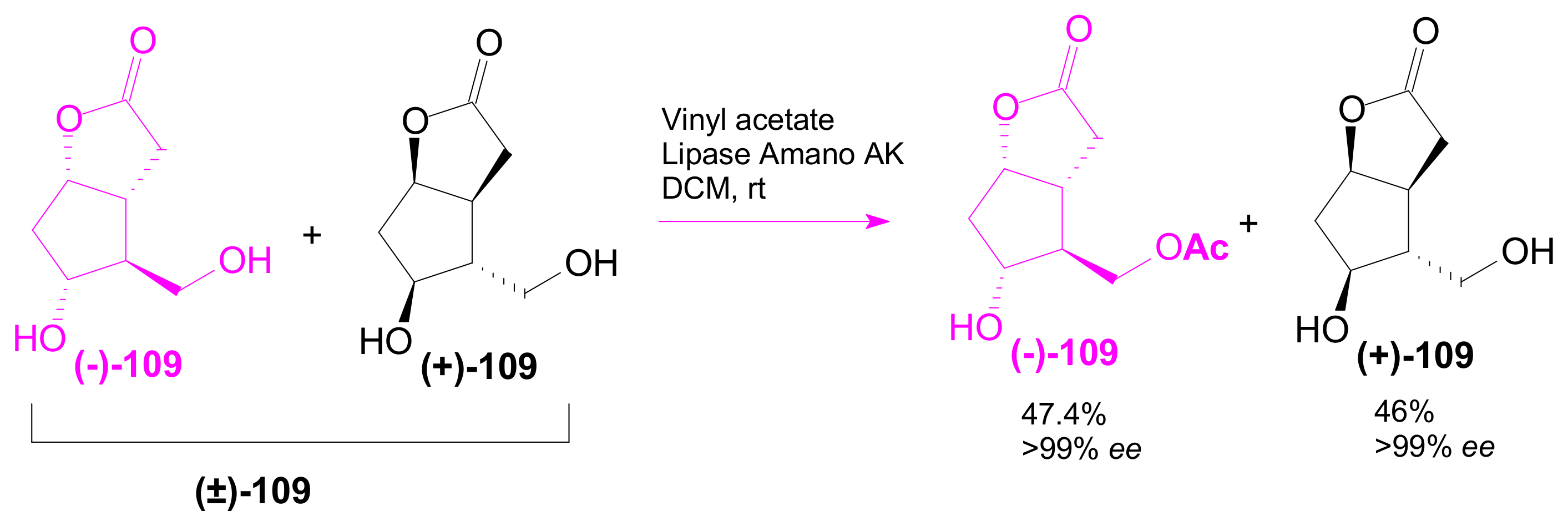
2.3.1. Selective Enzyme de-Acetalization (Acetate Hydrolysis)
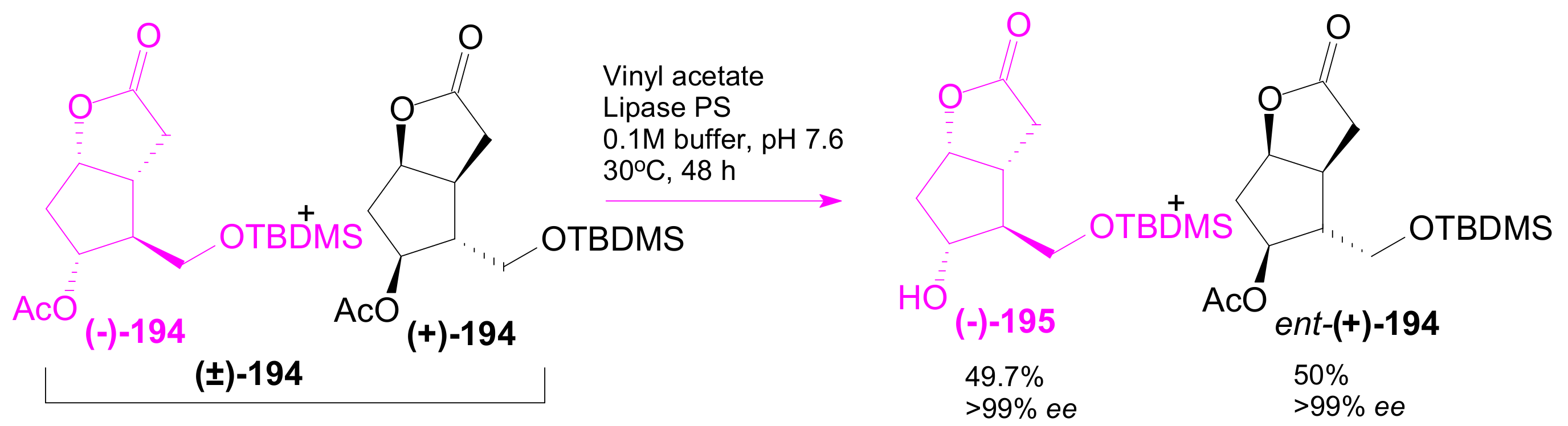
2.3.2. Selective Enzyme Acetalization
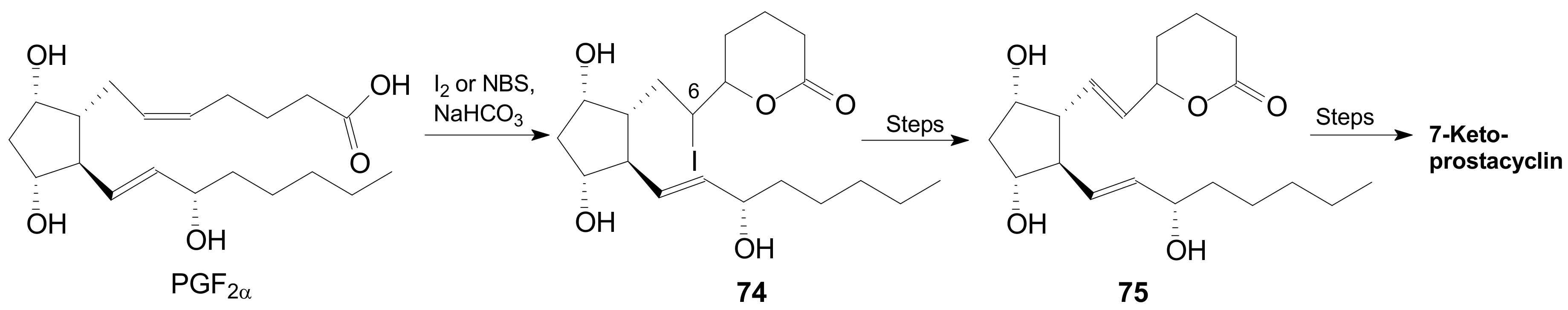
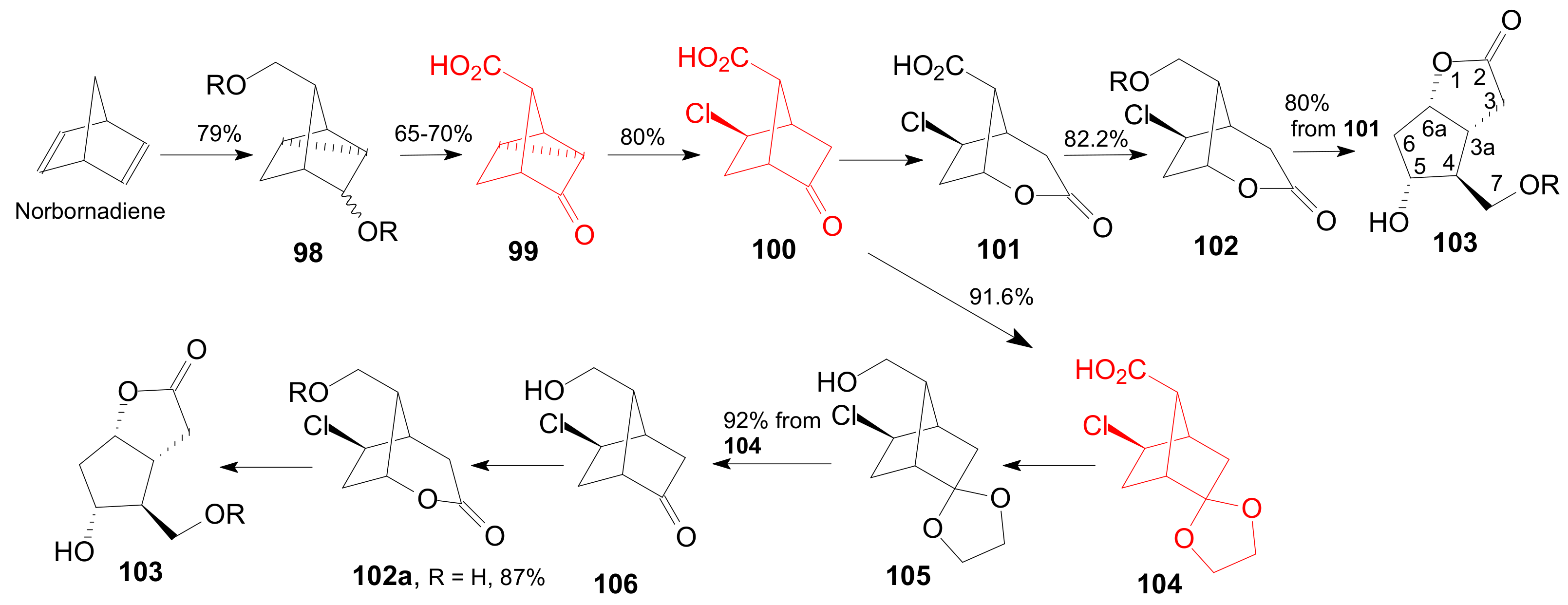
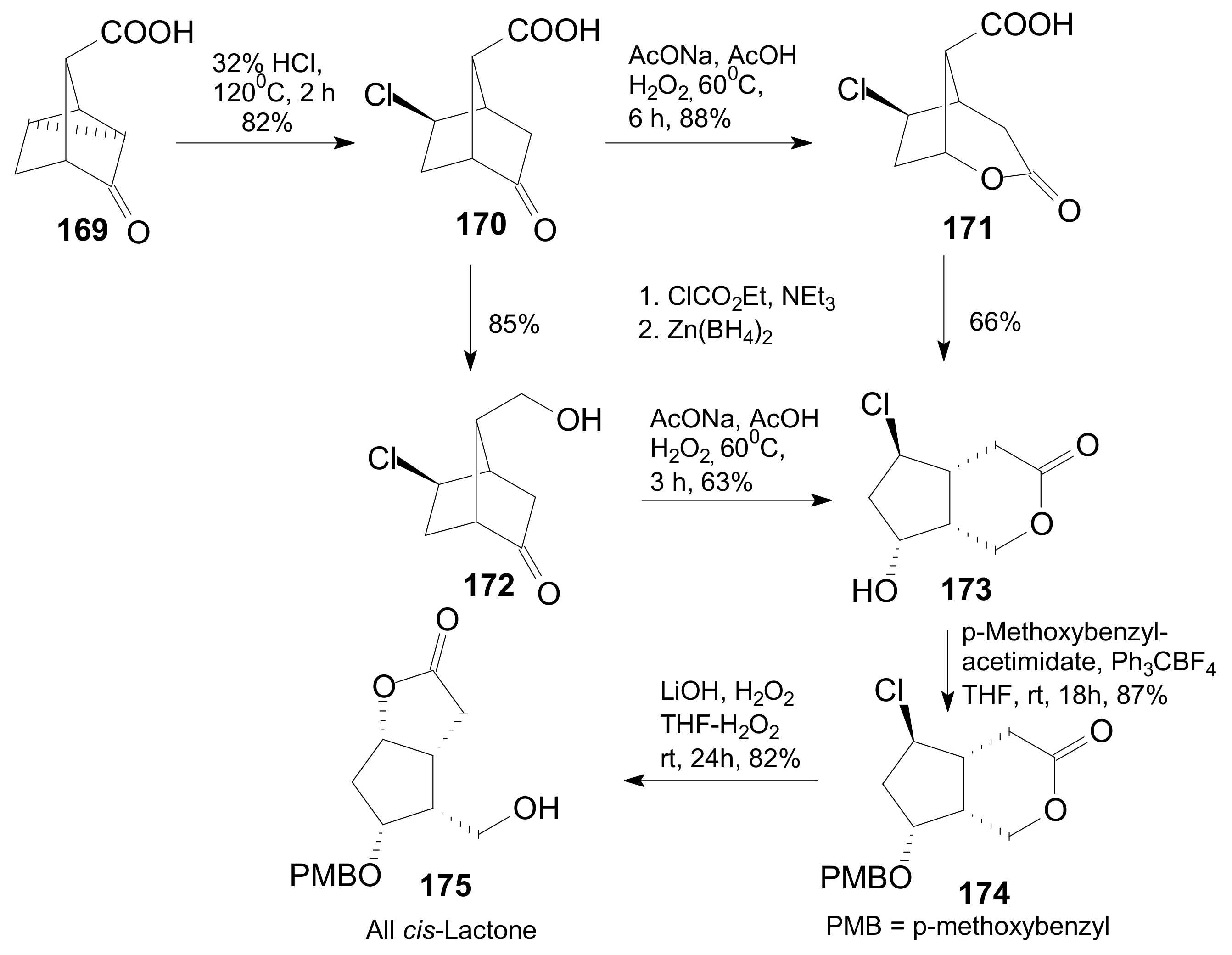
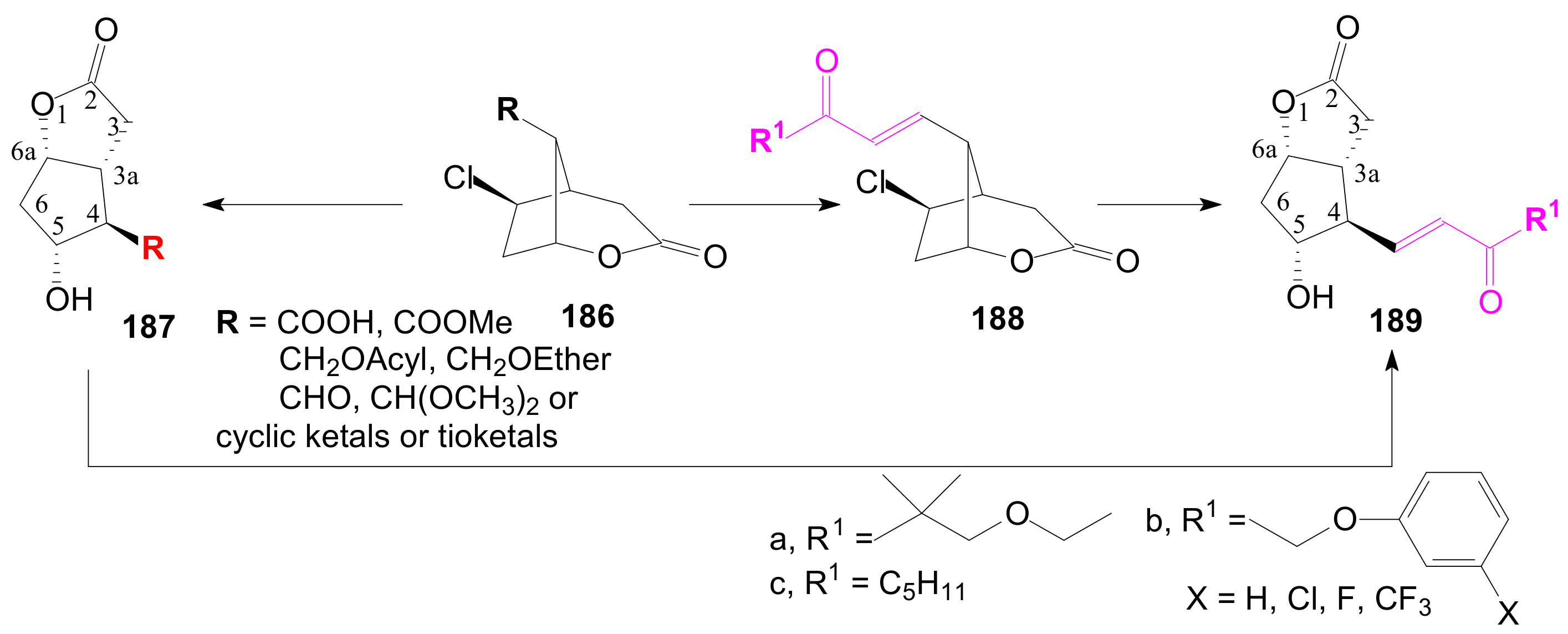
2.4. The Use of δ-Lactones for Obtaining 9β-Halo-Prostaglandins and 9β-Halogenocyclopentane Intermediates
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gil, S.; Parra, M.; Rodriguez, P.; Segura, J. Recent Development in γ-lactone synthesis. Mini-Rev. Org. Chem. 2009, 6, 345–358. [Google Scholar] [CrossRef]
- Van der Schaft, P.H.; ter Burg, N.; van den Bosch, S.; and Cohen, A.M. Microbial production of natural δ-decalactone and δ-dodecalactone from the corresponding α,β-unsaturated lactones in Massoi bark oil. Appl. Microbiol. Biotechnol. 1992, 36, 712–716. [Google Scholar] [CrossRef]
- Liu, Q.-A.; Zheng, J.-J.; Gu, Y.-C.; Wang, C.-Y.; Shao, C.-L. The Chemistry and Bioactivity of Macrolides from Marine Microorganisms. Stud. Nat. Products Chem. 2015, 353–401. [Google Scholar] [CrossRef]
- Garratt, P.J. 1.7—Alkylations of Alkynyl Carbanions. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Compr. Org. Synth. 1991, 3, 271–292. [Google Scholar]
- Parenty, A.; Moreau, X.; Campagne, J.-M. Macrolactonizations in the Total Synthesis of Natural Products. Chem. Rev. 2006, 106, 911–939. [Google Scholar] [CrossRef] [PubMed]
- Lohsen, S.; Stephens, D.S. Current Macrolide Antibiotics and Their Mechanisms of Actions. In Antibiotic Drug Resistance; Capelo-Martinez, J.-L., Igrejas, G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 97–117. [Google Scholar] [CrossRef]
- Mazur, M.; Włoch, A.; Bahri, F.; Pruchnik, H.; Pawlak, A.; Obmińska-Mrukowicz, B.; Maciejewska, G.; Gładkowski, W. Chemoenzymatic Synthesis of Enantiomeric, Bicyclic δ-Halo-γ-lactones with a Cyclohexane Ring, Their Biological Activity and Interaction with Biological Membranes. Biomolecules 2020, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, F.; Di Dato, V.; Ianora, A.; Romano, G. Prostaglandins in Marine Organisms: A Review. Mar. Drugs 2019, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Cimino, G.; Spinella, A.; Sodano, G. Naturally occurring prostaglandin-1,15-lactone. Tetrahedron Lett. 1989, 30, 3589–3592. [Google Scholar] [CrossRef]
- Corey, E.J.; Nicolaou, K.C.; Melvin, L.S. Letter: Synthesis of novel macrocyclic lactones in the prostaglandin and polyether antibiotic series. J. Am. Chem. Soc. 1975, 97, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Cimino, G.; Crispino, A.; Di Marzo, V.; Sodano, G.; Spinella, A.; Villani, G. A marine mollusc provides the first example of in vivo storage of prostaglandins: Prostaglandin-l,15-1actones. Experientia 1991, 47, 56–60. [Google Scholar] [CrossRef]
- Cimino, G.; Crispino, A.; Di Marzo, V.; Spinella, A.; Sodano, G. Prostaglandin 1,15-lactones of the F series from the nudibranch mollusk Tethys fimbria. J. Organ. Chem. 1991, 56, 2907–2911. [Google Scholar] [CrossRef]
- Di Marzo, V.; Cimino, G.; Crispino, A.; Minardi, C.; Sodano, G.; Spinella, A. A novel multifunctional metabolic pathway in a marine mollusc leads to unprecedented prostaglandin derivatives (prostaglandin 1,15-lactones). Biochem. J. 1991, 273, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Minardi, C.; Vardaro, R.R.; Mollo, E.; Cimino, G. Prostaglandin F-1,15-lactone fatty acyl esters: Aprostaglandin lactone pathway branch developed during the reproduction and early larval stages of a marine mollusc. Comp. Biochem. Physiol. 1992, 101B, 99–104. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Di Marzo, V. Aquatic intervertebrates open up new perspectivess in eicosanoid research: Biosynthesis and Bioactivity. Prostaglandins Leukotrienes Essential Fatty Acids 1994, 51, 215–219. [Google Scholar] [CrossRef]
- Stoner, E.J.; Negrón, G.; Gaviño, R. 2,2′-Dipyridyl Disulfide. Encycl. Reagents Org. Synth. 2005, 1–3. [Google Scholar] [CrossRef]
- Bundy, G.L.; Peterson, D.C.; Cornette, J.C.; Miller, W.L.; Spilman, C.H.; Wilks, J.W. Synthesis and biological activity of prostaglandin lactones. J. Med. Chem. 1983, 26, 1089–1099. [Google Scholar] [CrossRef]
- Bundy, G.L. 1,9-Lactone von prostaglandinderivaten und verfahren zu ihrer herstellung. DE Patent Application No. 2,627,675 A1, 21 June 1976. [Google Scholar]
- Bundy, G.L. Prostaglandin artige 1,11-lactone und ein verfahren zu ihrer herstellung. DE Patent Application No. 2,627,674 A1, 21 June 1976. [Google Scholar]
- Bundy, G.L. Prostaglandin artige 1,15-lactone sowie verfahren zu deren herstellung. DE Patent Application No. 2,627,673 A1, 21 January 1976. See also DE Patent 2,627,671, 13 January 1977 for 1,15-, 1,11- and 1,9-Prostaglandin Lactones. [Google Scholar]
- Peterson, D.C. 9-Deoxy-PGD-type, 1,15-lactones. U.S. Patent 4,049, 678, 20 September 1977. [Google Scholar]
- Maxey, K.M.; Stanton, M.L. Preparation of Macrocyclic 1,15-lactones of Fluprostenol and Related Prostaglandin F2a Analogs for Use in the Treatment of Glaucoma and Intraocular Hypertension. International Patent Application No. PCT/WO2001057015 A1, 9 August 2001. [Google Scholar]
- Maxey, K.M.; Stanton, M.L. Internal 1,15-lactones of Fluprostenol and Related Prosraglandin F2α Analogs and their Use in the Treatment of Glaucoma and Intraocular Hypertension. U.S. Patent 7,674,921 B2, 9 March 2010. [Google Scholar]
- Maxey, K.M.; Johnson, J. Prostaglandin F2α Analogs and their use in Combination with Antimicrobial Proteins for the Treatment of Glaucoma and Intraocular Hypertension. International Patent Application No. PCT/WO2003/079997, 2 October 2003. [Google Scholar]
- Gluchowski, C. Preparation of Intraocular Pressure-Reducing 1,11-lactone Prostaglandins. U.S. Patent 4,927,846, 22 May 1990. [Google Scholar]
- Wei, S.-Y.; Yeh, Y.-C.; Hsu, M.-K.; Kao, L.-T. Processes and Intermediates for the Preparations of Isomer Free Prostaglandins. U.S. Patent 9,115,109 B2, 25 August 2015. [Google Scholar]
- Corey, E.J.; Brunelle, D.J. New reagents for the conversion of hydroxy acids to macrolactones by the double activation method. Tetrahedron Lett. 1976, 17, 3409–3412. [Google Scholar] [CrossRef]
- Inanaga, J.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. A rapid esterification by means of mixed anhydride and its application to large-ring lactonization. Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993. [Google Scholar] [CrossRef]
- Nicolaou, K.C. Synthesis of Macrolides. Tetrahedron 1977, 33, 695, 683–710. [Google Scholar] [CrossRef]
- Shiina, I.; Ibuka, R.; Kubota, M. A New Condensation Reaction for the Synthesis of Carboxylic Esters from Nearly Equimolar Amounts of Carboxylic Acids and Alcohols Using 2-Methyl-6-nitrobenzoic Anhydride. Chem. Lett. 2002, 31, 286–287. [Google Scholar] [CrossRef]
- Shiina, I.; Kubota, M.; Ibuka, R. A novel and efficient macrolactonization ofω-hydroxycarboxylic acids using 2-methyl-6-nitrobenzoic anhydride (MNBA). Tetrehedron Lett. 2002, 43, 7535–7539. [Google Scholar] [CrossRef]
- Shiina, I.; Kubota, M.; Oshiumi, H.; Hashizume, M. An Effective Use of Benzoic Anhydride and Its Derivatives for the Synthesis of Carboxylic Esters and Lactones: A Powerful and Convenient Mixed Anhydride Method Promoted by Basic Catalysts. J. Org. Chem. 2004, 69, 1822–1830. [Google Scholar] [CrossRef]
- Shiina, I. An Adventurous Synthetic Journey with MNBA from Its Reaction Chemistry to the Total Synthesisof Natural Products. Bull. Chem. Soc. Jpn. 2014, 87, 196–233. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pulukuri, K.K.; Rigol, S.; Heretsch, P.; Yu, R.; Grove, C.I.; Hale, C.R.H.; El Marrouni, A.; Fetz, V.; Brönstrup, M.; et al. Synthesis and Biological Investigation of Δ12-Prostaglandin J3 (Δ12-PGJ3) Analogues and Related Compounds. J. Am. Chem. Soc. 2016, 138, 6550–6560. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pulukuri, K.K.; Rigol, S.; Peitsinis, Z.; Yu, R.; Kishigami, S.; Cen, N.; Aujay, M.; Sandoval, J.; Zepeda, N.; et al. Short Total Synthesis of Δ12-Prostaglandin J2 and Related Prostaglandins. Design, Synthesis, and Biological Evaluation of Macrocyclic Δ12-Prostaglandin J2 Analogues. J. Org. Chem. 2019, 84, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Narasaka, K.; Maruyama, K.; Mukaiyama, T. A useful method for synthesis of macrocyclic lactone. Chem. Lett. 1978, 8, 885–888. [Google Scholar] [CrossRef]
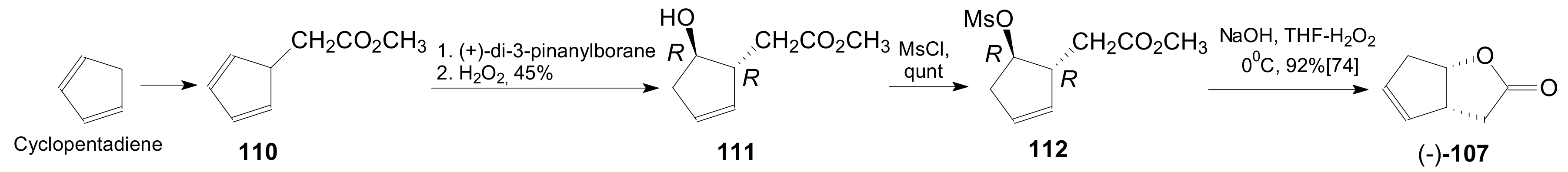
- Meng, Q.; Hesse, M. Ring Closure Methods in the Synthesis of Macrocyclic Natural Products. Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 1991; Volume 161, pp. 107–176. [Google Scholar] [CrossRef]
- Ohba, Y.; Takatsuji, M.; Nakahara, K.; Fujioka, H.; Kita, Y. A Highly Efficient Macrolactonization Method via Ethoxyvinyl Ester. Chem. Eur. J. 2009, 15, 3526–3537. [Google Scholar] [CrossRef]
- Justus, K.; Steglich, W. First Synthesis of a Strained 14-Membered Biaryl Ether Lactone by Macrolactonization. Tetrahedron Lett. 1991, 32, 5781–5784. [Google Scholar] [CrossRef]
- Kasar, R.A. Chapter I, Macrolactonization in the Synthesis of Biologically Active Macrocyclic Lactones Methodologies and Reagents: A Review. In Total Synthesis of Macrolides Rminus_plus_minusPatulolide A and Monocillins. Ph.D. Thesis, Savitribai Phule Pune University, Pune, India, 1991. Available online: http://hdl.handle.net/10603/149716 (accessed on 7 August 2020).
- Noyori, R.; Suzuki, M. Prostaglandin Syntheses by Three-Component Coupling. New Synthetic Methods. Angew. Chem. Int. Ed. 1984, 23, 847–876. [Google Scholar] [CrossRef]
- Fürstner, A. Olefin metathesis and beyond. Angew. Chem. Int. Ed. 2000, 39, 3012–3043. [Google Scholar] [CrossRef]
- Li, J.; Shmed, T.S.; Xu, C.; Stolz, B.M.; Grubbs, R.H. Concise Synthesis of ∆12-Prostaglandin J natural products via stereoretentive metathesis. J. Am. Chem. Soc. 2019, 141, 154–158. [Google Scholar]
- Fürstner, A.; Grela, K. Ring-closing alkyne metathesis: Application to the stereoselective total synthesis of prostaglandin E2-1,15-Lactone. Angew. Chem. Int. Ed. 2000, 39, 1234–1236. [Google Scholar] [CrossRef]
- Fürstner, A.; Grela, K.; Mathes, C.; Lehmann, C.W. Novel and Flexible Entries into Prostaglandins and Analogues Based on Ring Closing Alkyne Metathesis or Alkyne Cross Metathesis. J. Am. Chem. Soc. 2000, 122, 11799–11805. [Google Scholar] [CrossRef]
- Yiannikouros, G.P.; Kalaritis, P.; Gamage, C.P.; Abernathy, S.B. Synthesis of Prostanoids via a Ring-Closing Metathesis with Grubb’s Catalyst. International Patent Application No. PCT/WO2011008756A1, 20 January 2011. ibid. U.S. patent 8,476,471 B2, 2 July 2013. [Google Scholar]
- Pandya, B.; Snapper, M.L. A Cross-Metathesis Route to the5-F2-Isoprostanes. J. Org. Chem. 2008, 73, 3754–3758. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shen, X.; Hoveyda, A.H. In Situ Methylene Capping: A General Strategy for Efficient Stereoselective Catalytic Olefin Metathesis. The Concept, Methodological Implications, and Applications to Synthesis of Biologically Active Compounds. J. Am. Chem. Soc. 2017, 139, 10919–10928. [Google Scholar] [CrossRef]
- Hoveyda, A.H.; Xu, C.; Shen, X. Method of Making a Cross Metathesis Product. International Patent Application No. PCT/WO 2019/018773 A1, 24 January 2019. ibid. U.S. Patent Application 2020/0123197 A1, 23 April 2020. [Google Scholar]
- Sheddan, N.A.; Mulzer, J. Cross Metathesis as a General Strategy for the Synthesis of Prostacyclin and Prostaglandin Analogues. Org. Lett. 2006, 8, 3101–3104. [Google Scholar] [CrossRef]
- Trnka, T.M.; Morgan, J.P.; Sanford, M.S.; Wilhelm, T.E.; Scholl, M.; Choi, T.-L.; Ding, S.; Day, M.W.; Grubbs, R.H. Synthesis and Activityof Ruthenium Alkylidene Complexes Coordinated with Phosphine and M-Heterocyclic Carbene Ligands. J. Am. Chem. Soc. 2003, 125, 2546–2558. [Google Scholar] [CrossRef] [PubMed]
- Ivanics, J.; Riss, I.; Galambos, G.; Dorman, G.; Kanai, K.; Kovacs, G.; Ori, J. 5-Hydroxy-PGF1α-1,5-lacton-Derivate, Verfahren zu deren Herstellung sowie diese Verbindung anthaltende pharmazeutische Praparate. DE Patent 3,742,437, 23 June 1988. 5-Hydroxy-PGF1alfa-1,5-lactones. GB Patent 2,198,730, 22 June 1988. [Google Scholar]
- Acharya, H.P.; Kobayashi, Y. Total Synthesis of 2-(5,6-Epoxyisoprostane A2) phosphorylcholine and Elucidation of the Relative Configuration of the Isoprostane Moiety. Angew. Chem. Int. Ed. 2005, 44, 3481–3484. [Google Scholar] [CrossRef] [PubMed]
- Egger, J.; Bretscher, P.; Freigang, S.; Kopf, M.; Carreira, E.M. Discovery of a Highly Potent Anti-inflammatory Epoxyisoprostane Derived Lactone. J. Am. Chem. Soc. 2014, 136, 17382–17385. [Google Scholar] [CrossRef] [PubMed]
- Wolleb, H.; Ogawa, S.; Schneider, M.; Shemet, A.; Muri, J.; Kopf, M.; Carreira, E.M. Synthesis and Structure−Activity Relationship Studies of AntiInflammatory Epoxyisoprostane Analogues. Org. Lett. 2018, 20, 3014–3016. [Google Scholar] [CrossRef]
- Teiber, J.F.; Xiao, J.; Kramer, G.L.; Ogawa, S.; Ebner, C.; Wolleb, H.; Carreira, E.M.; Shih, D.M.; Haley, R.W. Identification of biologically actived-lactone eicosanoids as paraoxonase substrates. Biochem. Biophys. Res. Commun. 2018, 505, 87–92. [Google Scholar] [CrossRef]
- Yamauchi, S.; Takeda, K.; Ganaha, M.; Kinoshito, Y. Synthesis of (R)-6,7-dihydro-5-HETE lactone and(S)- 6,7-dihydro-5-HETE lactone by using novel yeast reduction as a key reaction. J. Chem. Soc. Perkin Trans I 2002, 2156–2160. [Google Scholar] [CrossRef]
- Corey, E.J.; Weinschenker, N.M.; Schaaf, T.K.; Huber, W. Stereo-controlled synthesis of dl-prostaglandin F2alfa and E2. J. Am. Chem. Soc. 1969, 91, 5675–5677. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Ensley, H.E. Preparation of an Optically Active Prostaglandin Intermediate via Asymmetric Induction. J. Am. Chem. Soc. 1975, 97, 6908–6909. [Google Scholar] [CrossRef]
- Corey, E.J.; Imai, N.; Pikul, S. Catalytic Enantioselective Synthesis of a Key Intermediate for the Synthesis of Prostanoids. Tetrahedron Lett. 1991, 32, 7517–7520. [Google Scholar] [CrossRef]
- Miyaji, K.; Ohara, Y.; Takahashi, Y.; Tsuruda, T.; Arai, K. Synthesis of Corey Lactone Via Highly Stereoselective Asymmetric Diels-Alder Reaction. Tetrahedron Lett. 1991, 32, 4557–4560. [Google Scholar] [CrossRef]
- Corey, E.J. Catalytic Enantioselective Diels ± Alder Reactions: Methods, Mechanistic Fundamentals, Pathways, and Applications. Angew. Chem. Int. Ed. 2002, 41, 1650–1667. [Google Scholar] [CrossRef]
- Corey, E.J.; Loh, T.-P. First Application of Attractive Intramolecular Interactions to the Design of Chiral Catalysts for Highly Enantioselective Diels-Alder Reactions. J. Am. Chem. Soc. 1991, 113, 8966–8967. [Google Scholar] [CrossRef]
- Corey, E.J.; William Suggs, J. A Method for Catalytic Dehalogenations via Trialkyltin Hydrides. J. Org. Chem. 1975, 40, 2554–2555. [Google Scholar] [CrossRef]
- Bindra, J.S.; Grodski, A.; Schaaf, T.K.; Corey, E.J. New Extensions of the Bicyclo[2.2.l]heptane Route to Prostaglandins. J. Am. Chem. Soc. 1973, 95, 7522–7523. [Google Scholar] [CrossRef] [PubMed]
- Beeley, N.R.A.; Peel, R.; Sutherland, J.K.; Holohan, J.J.; Malllon, K.B.; Sependa, G.J. Syntheses of PGF2α and Cloprostenol. Tetrahedron 1981, 37 (Suppl. 1), 411–420. [Google Scholar] [CrossRef]
- Peel, R.; Sutherland, J.K. An Alternative Synthesis of the Corey Prostaglandin Aldehyde. J.C.S. Chem. Commun. 1974, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Bindra, J.S.; Schaaf, T.K. Novel Prostaglandin Intermediates. U.S. Patent 3,992,438, 9 March 1976. [Google Scholar]
- Faustini, F.; Andreoni, A.; Fava, W.; Di Somma, M.; Gandolfi, C. Racemic or Optically Active 5β-halo-2β-hydroxymethylcyclopentane-3α-hydroxy-1α-acetic acid (1′ → 3) δ-lactone. DE Patent 2,702,807, 9 March 1977. [Google Scholar]
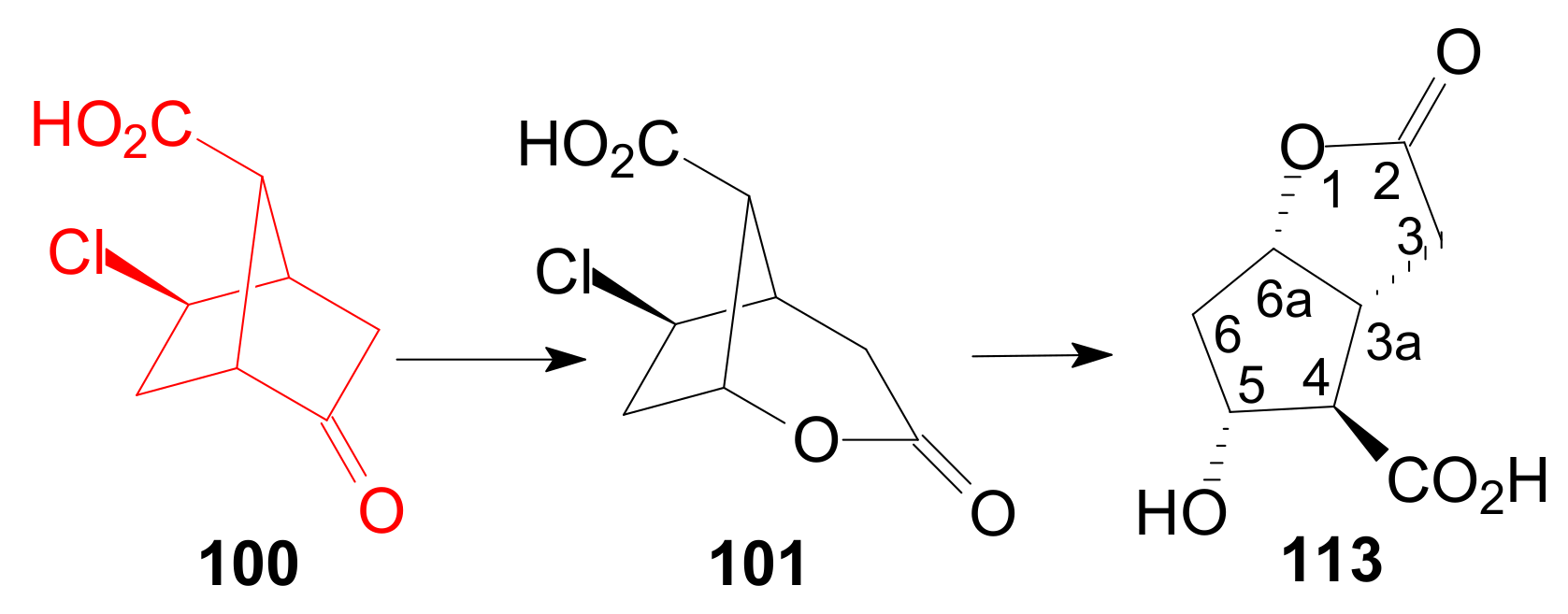
- Du, N. Method for Preparing Optically Pure 2-oxy tricyclic[2.2.1.0]heptane-7-carboxylic Acid. CN Patent 107573228, 12 January 2018. [Google Scholar]
- Loza, V.V.; Vostrikov, N.S.; Miftakhov, M.S. Method of Producing Corey Lactone Enantiomeric Derivatives. RU Patent 2,501,793, 20 December 2013. [Google Scholar]
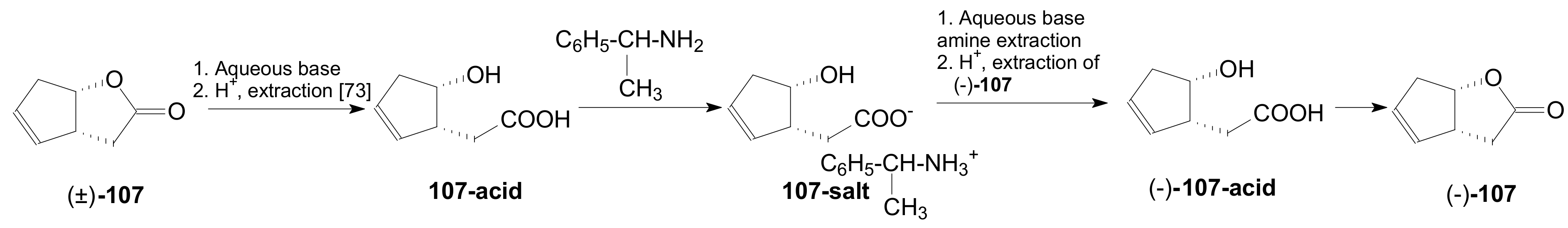
- Zak, B.; Vesely, I.; Neumitka, K.; Palecek, J. Preparation of optically pure enantiomers of Corey lactone by resolution of the racemate. Coll. Czech. Chem. Commun. 1991, 56, 1690–1700. [Google Scholar] [CrossRef]
- Grieco, P. Cyclopentenones. An Efficient Synthesis of cis-Jasmone. J. Org. Chem. 1976, 37, 2363–2364. [Google Scholar] [CrossRef]
- Stevens, H.C.; Reich, D.A.; Brandt, D.R.; Fountain, K.R.; Gaughan, E.J. A New Tropolone Synthesis via Dichloroketene. J. Am. Chem. Soc. 1965, 87, 5257–5259. [Google Scholar] [CrossRef]
- Tömösközi, I.; Gruber, L.; Baitz-Gacs, E. Prins Reaction of 2-Oxabicyclo[3.3.0]oct-6-en-3-one and Related Derivatives. Tetrahedron 1992, 48, 10345–10352. [Google Scholar] [CrossRef]
- Tömösközi, I.; Gruber, L.; Kovács, G.; Székely, I.; Simonidesz, V. Regiospecific Prins Reaction, a New Way to Prostanoids. Tetrahedron Lett. 1976, 50, 4639–4642. [Google Scholar] [CrossRef]
- Corey, E.J.; Mann, J. A New Stereocontrolled Synthesis of Prostaglandins via Prostaglandin A2. J. Am. Chem. Soc. 1973, 95, 6832–6833. [Google Scholar] [CrossRef]
- Partridge, J.J.; Chadha, N.K.; Uskokovik, M.R. Asymmetric Synthesis of Prostaglandin Intermediates. J. Am. Chem. Soc. 1973, 95, 7171–7172. [Google Scholar] [CrossRef]
- Zheng, F.; Li, G.; Zhang, J.; Lai, Y.; Wang, W.; Zhang, L.; Chen, S.; Huang, Y.; Gao, W.; Cai, J. A simple and Efficient Synthesis Process of (-)-Corey’s Lactone Diol. CN Patent 108546258 B, 26 February 2019. [Google Scholar]
- Du, N. Process for Preparation of Corey Lactone Diol. CN Patent 107573310, 12 January 2018. [Google Scholar]
- Corey, E.J.; Cheng, X.M. The Logic of Chemical Synthesis; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Collins, P.W.; Djuric, S.W. Synthesis of therapeutically useful prostaglandin analogs. Chem. Rev. 1993, 93, 1533–1564. [Google Scholar] [CrossRef]
- Das, S.; Chandrasekhar, S.; Yadav, J.S.; Grée, R. Recent developments in the synthesis of prostaglandin analogues. Chem. Rev. 2007, 107, 3286–3337. [Google Scholar] [CrossRef]
- Peng, H.; Chen, F.-E. Recent advances in asymmetric total synthesis of prostaglandins. Org. Biomol. Chem. 2017, 15, 6281–6301. [Google Scholar] [CrossRef]
- Ajahn, E.; Durand, T.; Galano, J.-M.; Jahn, U. Recent Approaches to the Total Synthesis of Phytoprostanes, Isoprostanes and Neuroprostanes as Important Products of Lipid Oxidative Stress and Biomarkers of Disease. Chem. Listy 2014, 108, 301–319. [Google Scholar]
- Sun, H.; Gong, Y.; Hou, Y.; He, G.A. Process for One-Pot Synthesis of Corey Lactone. International Patent Application No. PCT/WO2010083722, 29 July 2010. [Google Scholar]
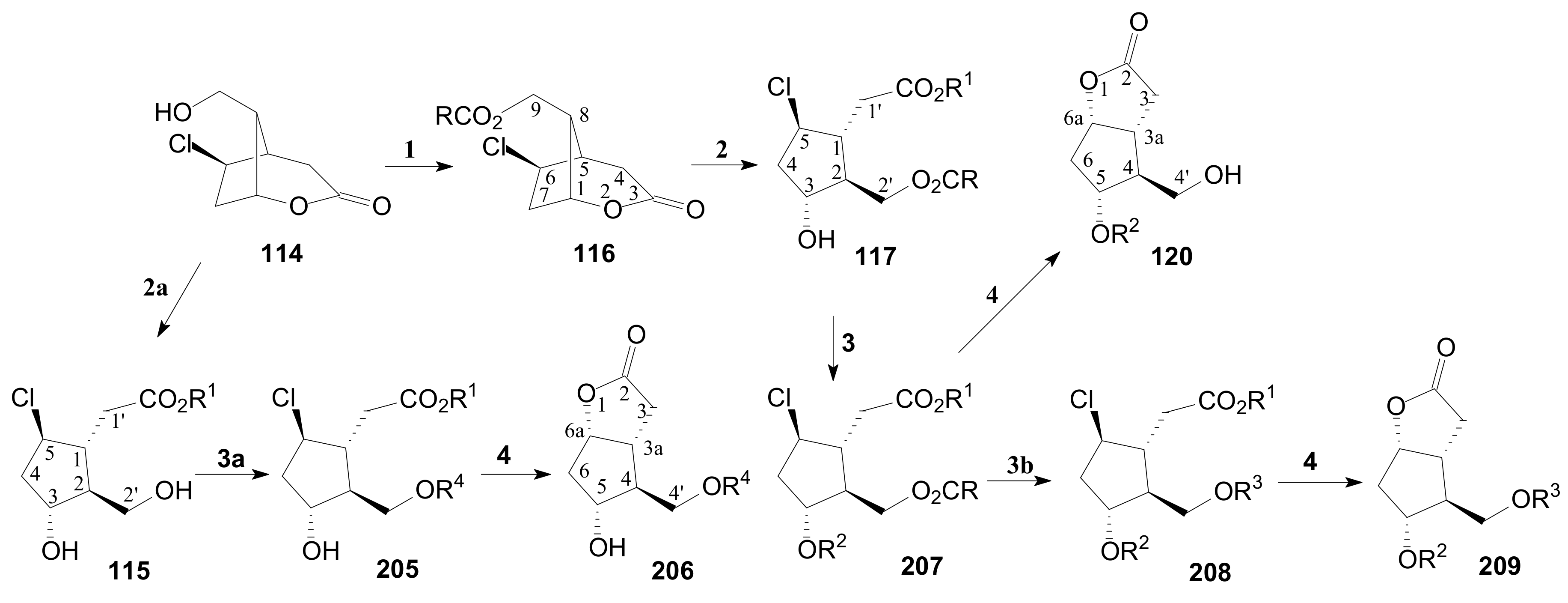
- Tănase, C.; Drăghici, C. Transformation of δ-Lactone in γ-Lactone in the Corey Route for Synthesis of Prostaglandins. Rev. Roum. Chim. 2014, 59, 845–853. [Google Scholar]
- Tănase, C.; Cocu, F.; Căproiu, M.T.; Drăghici, C. γ-Lactone oxabicyclo[3.3.0] Octane Polyfunctional Cyclopentanic Chloroester Key Compounds Prepared by Stereoselective Transformation of γ-Lactone Intermediates and Processes for Preparing the Same. RO Patent 129083B1, 19 February 2015. [Google Scholar]
- Zhu, K.; Hu, S.; Liu, M.; Peng, H.; Chen, F.-E. Access to a Key Building Block for the Prostaglandin Family via Stereocontrolled Organocatalytic Baeyer–Villiger Oxidation. Angew.Chem. Int. Ed. 2019, 58, 9923–9927. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Hu, S.; Liu, M.; Peng, H.; Chen, F.-E. Collective Total Synthesis of the Prostaglandins Family via Stereocontrolled Organocatalytic Baeyer-Villiger Oxidation. Angew. Chem. Int. Ed. 2019, 58. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.A.; Amburgey, J.S., Jr.; Woss, J.A.; De, B.; Soper, D.L. C11 Oxymyl and Hydroxylamino Prostaglanding Useful as Medicaments. U.S. Patent 641,078,0B1, 25 June 2002. [Google Scholar]
- Tănase, C.; Cocu, F.; Căproiu, M.T.; Drăghici, C. Corey-Type-γ-Lactonic Aldehydic Intermediates Protected as Cyclic Acetals or Thioacetals. RO Patent Application No. 131,617 A2, 28 July 2015. [Google Scholar]
- Prévost, S.; Thai, K.; Schützenmeister, N.; Coulthard, G.; Erb, W.; Aggarwal, V.K. Synthesis of Prostaglandin Analogues, Latanoprost and Bimatoprost, Using Organocatalysis via a Key Bicyclic Enal Intermediate. Org. Lett. 2015, 17, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Pelss, A.; Gandhamsetty, N.; Smith, J.R.; Mailhol, D.; Silvi, M.; Watson, A.; Perez-Powell, I.; Prevost, S.; Schützenmeister, N.; Moore, P.; et al. Reoptimization of the Organocatalyzed Double Aldol Domino Process to a Key Enal Intermediate and Its Application to the Total Synthesis of ∆12-Prostaglandin J3. Chem. Eur. J. 2018, 24, 9542–9545. [Google Scholar] [CrossRef] [PubMed]
- Baars, H.; Classen, M.J.; Aggarwal, V.K. Synthesis of Alfaprostol and PGF2α Through 1,4-Addition of an Alkyne to an Enal Intermediate as the Key Step. Org. Lett. 2017, 19, 6008–6011. [Google Scholar] [CrossRef] [PubMed]
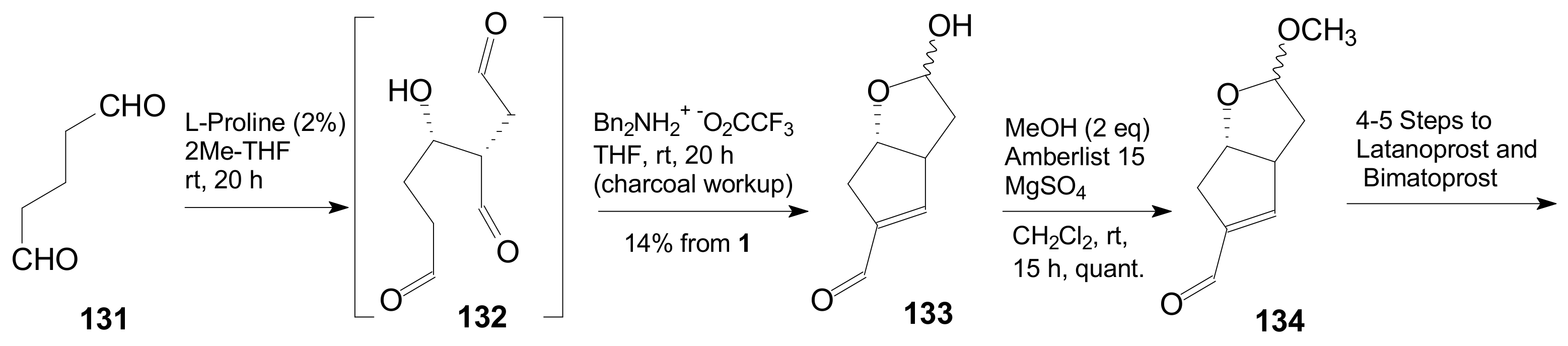
- Umekubo, N.; Suga, Y.; Hayashi, Y. Pot and time economies in the total synthesis of Corey lactone. Chem. Sci. 2020, 11, 1205–1209. [Google Scholar] [CrossRef]
- Umekubo, N.; Hayashi, Y. Asymmetric synthesis of Corey lactone and latanoprost. Eur. J. Org. Chem. 2020. [Google Scholar] [CrossRef]
- Doyle, M.P.; Catino, A.J. A short stereoselective synthesis of (+)- and(−)-2-oxabicyclo[3.3.0]oct-6-en-3-one by intramolecular carbon–hydrogen insertion catalyzed by chiral dirhodium(II) carboxamidates. Tetrahedron Asymmetry 2003, 14, 925–928. [Google Scholar] [CrossRef]
- Nakashima, H.; Sato, M.; Taniguchi, T.; Ogasawara, K. Lipase-mediated Resolution of cis-4-Cumyloxy-2-cyclopenten-1-ol and its Utilization for Enantioconvergent Preparation of(–)-Oxabicyclo[3.3.0]-oct-6-en-3-one. Synlett 1999, 11, 1754–1756. [Google Scholar] [CrossRef]
- Gais, H.-J.; Bondarev, O.; Hetzer, R. Palladium-catalyzed asymmetric synthesis of allylic alcohols from unsymmetrical ans symmetrical racemic allylic carbonates featuring C-O bond formation and dynamic kinetic resolution. Tetrahedron Lett. 2005, 46, 6279–6283. [Google Scholar] [CrossRef]
- Asami, M.; Takahashi, J.; Inoue, S. Reconfirmation of high enantiomeric excesses in the enantioselective transformation of meso-epoxides. Tetrahedron 1995, 51, 11725–11730. [Google Scholar] [CrossRef]
- Bhuniya, D.; DattaGupta, A.; Singh, V.K. Design, Synthesis, and Application of Chiral Nonracemic Lithium Amide Bases in Enantioselective Deprotonation of Epoxides. J. Org. Chem. 1996, 61, 6108–6113. [Google Scholar] [CrossRef] [PubMed]
- Kumaraguru, T.; Babita, P.; Sheelu, G.; Lavanya, K.; Fadnavis, N.W. Synthesis of Enantiomerically Pure 4-Hydroxy-2-cyclopentenones. Org. Proc. Res. Develop. 2013, 17, 1526–1530. [Google Scholar] [CrossRef]
- Chang, C.-T.; Jacobo, S.H.; Powell, W.S.; Lawson, J.A.; Fitzgerald, G.A.; Pratico, D.; Rokach, J. A new synthetic approach for 4(S)-hydroxycyclopent-2-enone: A precursor to prostanoid synthesis. Tetrahedron Lett. 2005, 46, 6325–6328. [Google Scholar] [CrossRef]
- Sugahara, T.; Ogasawara, K. An Expedient Route to (-)-cis-2-Oxabicyclo[3.3.0]oct-6-en-3-one via a meso-Asymmetrization. Synlett 1996, 1996, 319–320. [Google Scholar] [CrossRef]
- Marotta, E.; Righi, P.; Rosini, G. A Bicyclo[3.2.0]hept-3-en-6-one Approach to Prostaglandin Intermediates. Org. Lett. 2000, 2, 4145–4148. [Google Scholar] [CrossRef]
- Marotta, E.; Medici, M.; Righi, P.; Rosini, G. The Conversion of 3-Hydroxy-6-heptenoic Acids into Bicyclo[3.2.0]hept-3-en-6-ones. J. Org Chem. 1994, 59, 7529–7531. [Google Scholar] [CrossRef]
- Galano, J.-M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C.-Y. Review. Isoprostanes, neuroprostanes and phytoprostanes: An overview of 25 years of research in chemistry and biology. Progress Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Elsner, P.; Jetter, P.; Brödner, K.; Helmchen, G. Stereoselective Synthesis of acis-1,2-Dialkylcyclopentane Building Block and Its Application in Isoprostane Synthesis(5-ent-F2c-IsoP). Eur. J. Org. Chem. 2008, 2008, 2551–2563. [Google Scholar] [CrossRef]
- Helmchen, G.; Goeke, A.; Lauer, G.; Urmann, M.; Fries, J. Building Blocks for the Synthesis of Enantiomerically Pure Jasmonoids: Synthesis of (+)-Methyl Epijasmonate. Angew. Chem. Int. Ed. 1990, 29, 1024–1025. [Google Scholar] [CrossRef]
- Oger, C.; Brinkmann, Y.; Bouazzaoui, S.; Durand, T.; Galano, J.-M. Stereocontrolled Accesss to Isoprostaned via a Bicyclo[3.3.0]octene Framework. Org. Lett. 2008, 10, 5087–5090. [Google Scholar] [CrossRef]
- Suzuki, T.; Morita, K.; Tsuchida, M.; Hiroi, K. Mild and Selective Synthesis of Lactones from Diols Using a Novel Metal-Ligand Bifunctional Catalyst. Org. Lett. 2002, 4, 2361–2363. [Google Scholar] [CrossRef]
- Oger, C.; Marton, Z.; Brinkmann, Y.; Bultel-Poncé, V.; Durand, T.; Graber, M.; Galano, J.-M. Lipase-Catalyzed Regioselective Monoacetylation of Unsymmetrical 1,5-Primary Diols. J. Org. Chem. 2010, 75, 1892–1897. [Google Scholar] [CrossRef]
- Oger, C.; Bultel-Poncé, V.; Guy, A.; Durand, T.; Galano, J.-M. Total Synthesis of Isoprostanes Derived from Adrenic Acid and EPA. Eur. J. Org. Chem. 2012, 2012, 2621–2634. [Google Scholar] [CrossRef]
- Sannigrahi, M.; Mayhew, D.L.; Clive, D.L. Applications of 5-Endo-trigonal Cyclization: Construction of Compounds Relevant to the Synthesis of Prostaglandins and Methyl epi-Jasmonate. J. Org. Chem. 1999, 64, 2776–2788. [Google Scholar] [CrossRef]
- Augustyns, B.; Maulide, N.; Marko, I.E. Skeletal rearrangements of bicyclo[2.2.2]lactones: A short and efficient route towards Coreys lactone. Tetrahedron Lett. 2005, 46, 3895–3899. [Google Scholar] [CrossRef]
- Yakura, T.; Yamada, S.; Azuma, M.; Ueki, A.; Ikeda, M. A short synthesis of optically active Corey lactone by means of the dirhodium(II)-catalyzed intramolecular C-H insertion reaction of an a-diazo ketone. Synthesis 1998, 7, 973–974. [Google Scholar] [CrossRef]
- Node, M.; Nakamura, D.; Nishide, K.; Inoue, T. A facile asymmetric synthesis of Corey lactone utilizing C2-symmetric dimethyl 3,7-dihydroxy-cisbicyclo[3.3.0]octan-2,6-diene-2,6-dicarboxylate. Heterocycles 1997, 46, 535–540. [Google Scholar] [CrossRef]
- Wei, S.-Y.; Yeh, Y.-C. Process and Intermediates for the Preparation of Prostaglandins. U.S. Patent 7,511,168B2, 31 March 2006. [Google Scholar]
- Beck, G. Dimoxaprost. Drugs Fut. 1987, 12, 1101. [Google Scholar] [CrossRef]
- Bartman, W.; Beck, G.; Jahne, G.; Wess, G. Synthese eines biologisch aktiven Analogons des Prostaglandins E2 (Racemate und reine Enantiomere). Liebigs Ann. Chem 1987, 321–326. [Google Scholar] [CrossRef]
- Francesca, C.; Molinari, F.; Gandolfi, R.; Romano, D. Process for the Preparation of D-Cloprostenol. IT Patent 1,349,182 B1, 20 November 2008. [Google Scholar]
- Tănase, C.I.; Cocu, F.; Drăghici, C.; Hanganu, A.; Pintilie, L.; Maganu, M.; Munteanu, C.V.A.; Shova, S. Secondary compounds in the catalytic hydrogenation of enone and allylic alcohol prostaglandin intermediates: Isolation, characterization, and X-raycrystallography. New J. Chem. 2019, 43, 7582–7599. [Google Scholar] [CrossRef]
- Zak, B.; Koran, J.; Pavelkova, F.; Urie, M. Process for Preparing Optically Active p-phenylbenzoyl Corey Alcohol and Optically Active Benzoyl Corey Alcohol. CZ Patent 287,685 B6, 17 January 2001. [Google Scholar]
- Sugahara, T.; Satoh, I.; Yamada, O.; Takano, S. Efficient enzymic preparation of (+)- and (-)-Corey lactone derivatives. Chem. Pharm. Bull. 1991, 39, 2758–2760. [Google Scholar] [CrossRef][Green Version]
- Takano, S.; Sugahara, T. Enzymic Resolution of Corey Lactone Diols. European Patent No. 501310 B1, 20 February 1992. [Google Scholar]
- Vesely, I.; Palecek, J.; Stibor, I. Resolution of a racemic Corey lactone via enantioselective esterification. Collect. Czech. Chem. Comm. 1992, 57, 357–361. [Google Scholar] [CrossRef]
- Vesely, I.; Zak, B.; Spacek, M.; Mostecky, J.; Palecek, J.; Kubelka, V.; Eichler, P. Resolution of a Racemic Corey Lactone via Enantioselective Esterification. CZ Patent 261,797 B1, 15 June 1989. [Google Scholar]
- Zheng, F.; Li, G.; Zhao, H.; Cai, J.; Chen, S.; Wan, M.; Zhang, L.; Wang, P.; Gao, W. L-Corey Lactone Diol (CLA) Intermediate, its Preparation Method and Medicinal Application. CN Patent 109,161,577, 8 January 2019. [Google Scholar]
- Alphand, V.; Archelas, A.; Furstoss, R. Microbial Transformations 16. One-step synthesis of a pivotal prostaglandin chiral synthon via a highly enantioselective microbiological Baeyer-Villiger type reaction. Tetrahedron Lett. 1989, 30, 3663–3664. [Google Scholar] [CrossRef]
- Alphand, V.; Furstoss, R. Microbial transformations 22. Microbiologically mediated Bayer-Villiger reactions: A unique route to several bicyclic γ–lactones in high enantiomeric purity. J. Org. Chem. 1992, 57, 1306–1309. [Google Scholar] [CrossRef]
- Alphand, V.; Carrea, G.; Wohlgemuth, R.; Furstoss, R.; Woodley, J.M. Towards large-scale synthetic applications of Baeyer-Villiger monooxygenases. TRENDS Biotechnol. 2003, 21, 318–323. [Google Scholar] [CrossRef]
- Balke, K.; Bäumgen, M.; Bornscheuer, U.T. Controlling the Regioselectivity of Baeyer-Villiger Monooxygenases by Mutation of Active Site Residues. ChemBioChem 2017, 18, 1627–1638. [Google Scholar] [CrossRef]
- Baldwin, C.V.F.; Wohlgemuth, R.; Woodley, J.M. Reactor Operation and Scale-Up of Whole Cell Baeyer-Villiger Catalyzed Lactone Synthesis. Org. Process. Res. Dev. 2008, 12, 660–665. [Google Scholar] [CrossRef]
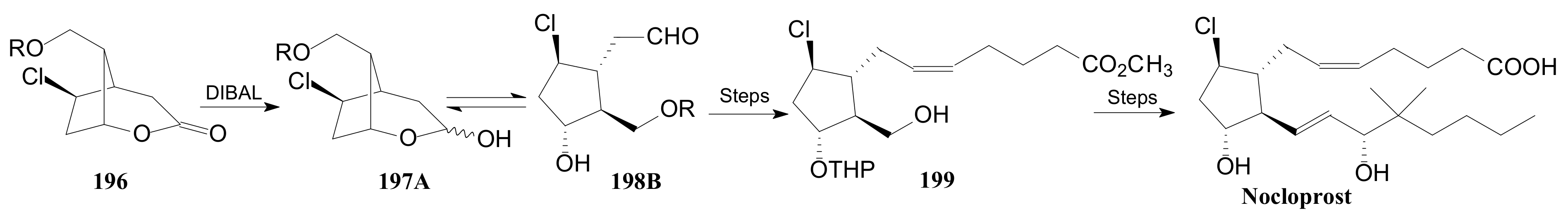
- Konturek, S.J.; Brzozowski, T.; Drozdowicz, D.; Krzyzek, E.; Garlicki, J.; Majka, J.; Dembinski, A.; Stachura, J.; Amon, I. Nocloprost, a unique prostaglandin E2 analog with local gastroprotective and ulcer-healing activity. Eur. J. Pharmacol. 1991, 195, 347–357. [Google Scholar] [CrossRef]
- Skuballa, W.; Raduchel, B.; Vorbruggen, H.; Elger, W.; Loge, O.; Schillinger, E. 9-Haloprostaglandin Clathrates and their Use as Medicines. U.S. Patent 5,204,371, 20 April 1992. [Google Scholar]
- Okamuro, S.; Katayama, S.; Ono, N.; Sato, F. A New Efficient Synthesis of the Biologically Potent PGD2-Analogue ZK118182. Tetrahedron Asimmetry 1992, 3, 1525–1528. [Google Scholar]
- Thierauch, K.H.; Sturzebecher, C.; Schillinger, E.; Rewinkel, H.; Raduchel, B.; Skuballa, W.; Vorbruggen, H. Stable 9β- or 11α-Halogen-15-Cyclohexyl-Prostaglandins with High Affinity to the PGD2-Receptor. Prostaglandins 1988, 35, 855–868. [Google Scholar] [CrossRef]
- Arroniz, C.E.; Gallina, J.; Martinez, E.; Muchowski, J.; Velarde, E.; Rooks, W.H. Synthesis of Ring Halogenated Prostaglandins. Prostaglandins 1978, 16, 47–65. [Google Scholar] [CrossRef]
- Buchmann, B.; Faulds, D.; Guilford, W.; Langer, G.; Lindenthal, B.; Skuballa, W.; Toschi, L. 9-Chloro-15-Deoxyprostaglandin Derivatives, Process for their Preparation and their Use as Medicaments. U.S. Patent 7,563,924 B2, 21 July 2009. [Google Scholar]
- Ito, S.; Okuda, E.; Sugama, K.; Negishi, M.; Hayashi, O.; Hayashi, O. Evaluation of ZK1 10841 and AH6809, an agonist and an antagonist of prostaglandin DP-receptors on human platelets, with a PGD2-responsive cell line from bovine embryonic trachea. Br. J. Pharmacol. 1990, 99, 13–14. [Google Scholar] [CrossRef]
- Ohuchida, S.; Tani, K. Omega-cycloalkyl-prostaglandin E1 Derivatives. European Patent No 0,974,580 B1, 20 July 1999. [Google Scholar]
- Raduchel, B.; Skuballa, W.; Vorbruggen, H. Process for the Production of9Beta-Chloroprostaglandins. U.S. Patent 4,806,668, 21 February 1989. [Google Scholar]
- Skuballa, W.; Raduchel, B.; Schwarz, N.; Vorbruggen, H.; Elger, W.; Raduechel, B.; Vorbrueggen, H. 9-Bromo-prostaglandin derivs.-prepd. by bromination of 9-hydroxy cpd. with tetrabromo-methane and triphenylphosphine. U.S. Patent 4,487,953, 11 December 1984. [Google Scholar]
- Skuballa, W.; Raduchel, B.; Vorbruggen, H.; Elger, W.; Loge, O. 9-Halogenated-2-Prostaglandin Derivatives, Processes for the Preparation thereof and the Use thereof as Medicinal Agents. U.S. Patent 4,699,920, 13 October 1987. [Google Scholar]
- Skuballa, W.; Raduechel, B.; Schwarz, N.; Elger, W.; Town, M.H.; Vorbrueggen, H. 9-Fluoroprostaglandin Derivatives, their Preparation and Use as Medcnal Agents. U.S. Patent 4,789,685, 6 December 1988. [Google Scholar]
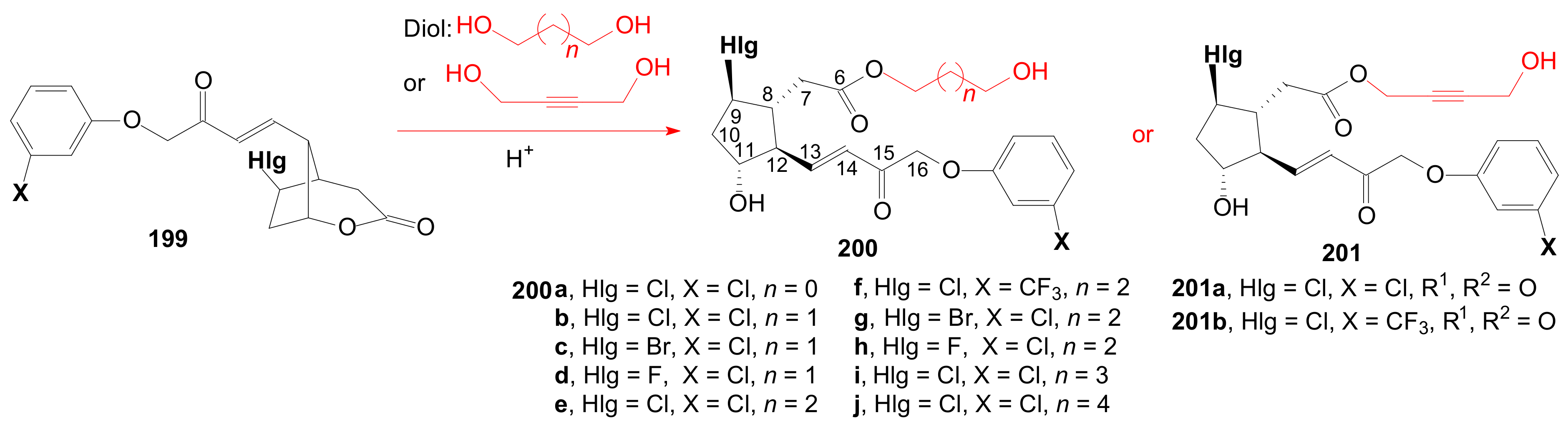
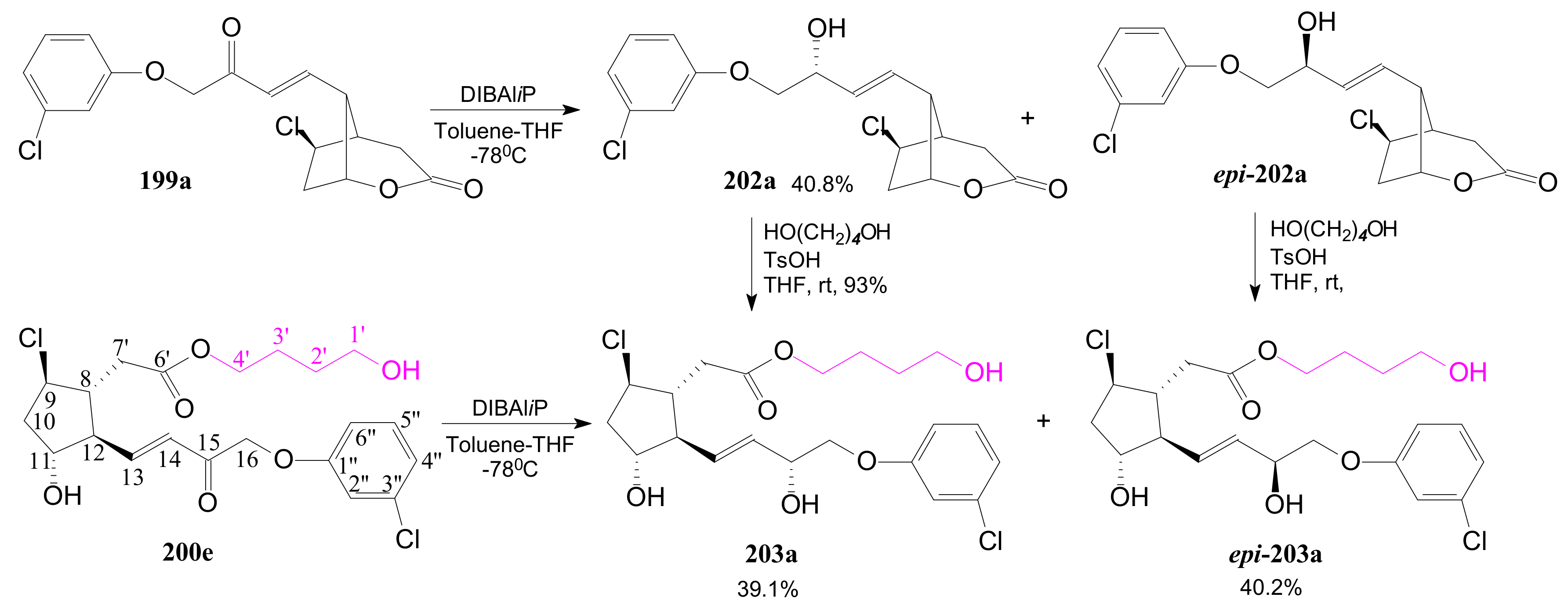
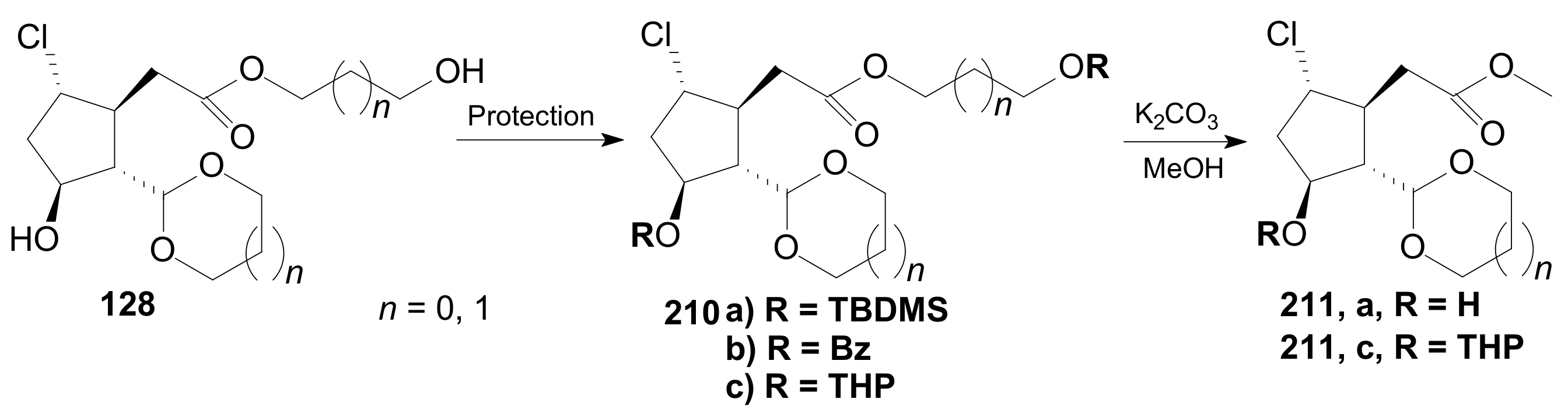
- Tănase, C.; Cocu, F.; Drăghici, C.; Căproiu, M.T.; Maganu, M. New β-halogenated prostaglandine analogues with an ester group at C-6 of the α-side chain. Rev. Chim. 2016, 67, 468–475. [Google Scholar]
- Tănase, C.; Cocu, F.; Căproiu, M.T.; Drăghici, C.; Maganu, M. 9 β-halogenated Prostaglandine Compounds and Processes for Preparing the Same. RO Patent 129,720 B1, 29 August 2014. [Google Scholar]
- Tănase, C.I.; Pintilie, L.; Mihai, E. A Molecular Docking of New 9β-Halogenated Prostaglandin Analogs with an Ester Group at C-6 Atom of the α-Side Chain. Rev. Chim. 2020, 71, 101–110. [Google Scholar] [CrossRef]
- Konishi, Y.; Kawamura, M. Novel 5-hetero-6-exo-PGE Derivatives. European Patent No. 0,386,901 A1, 19 February 1990. [Google Scholar]
- Tănase, C.; Drăghici, C.; Căproiu, M.T.; Cojocaru, A. Transeterification of the intermediate chloro-ester compounds in the sequence for obtaining Corey aldehyde protected as cyclic acetals. Rev. Chim. 2017, 68, 260–264. [Google Scholar] [CrossRef]
- Wilson, N.S.; Keay, B.A. Mild base mediated desilylation of various phenols. Tetrahedron Lett. 1997, 38, 187–190. [Google Scholar] [CrossRef]
- Derwing, C.; Hoppe, D. Synthesis of Enantioenriched Tertiary Benzylic alcohols via Stereospecific Lithiation of Secondary Benzyl Carbamates-Design of Dialkylcarbamates, Cleavable under Basic, Mild Conditions. Synthesis 1996, 1996, 149–154. [Google Scholar] [CrossRef]
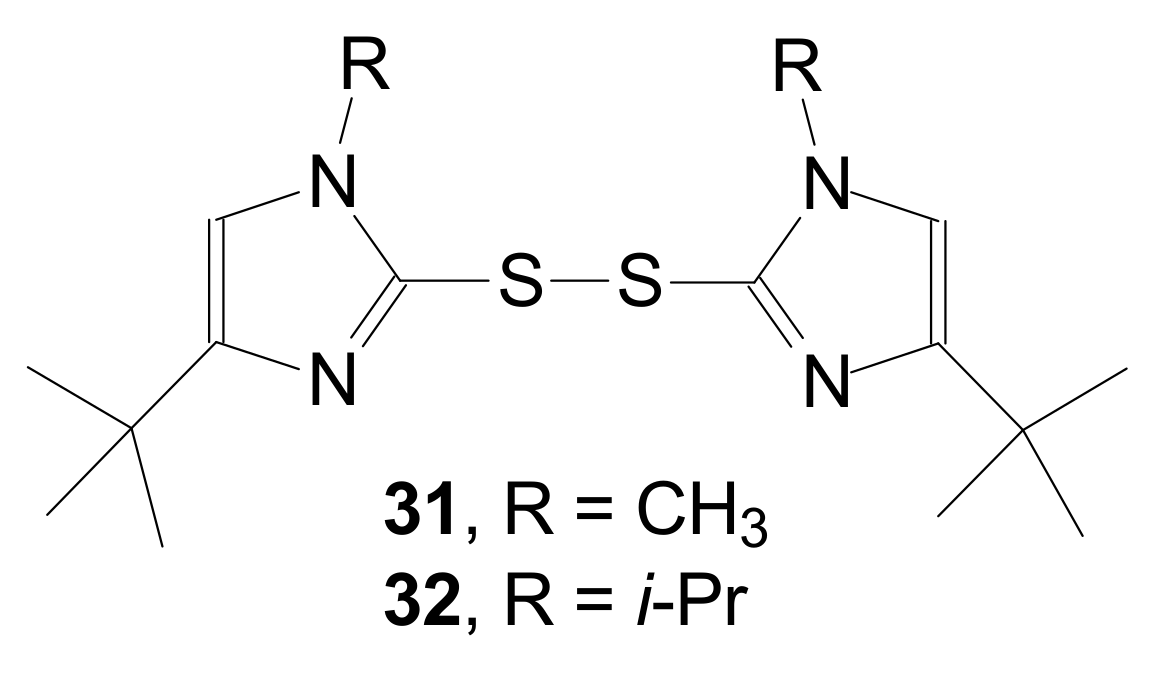
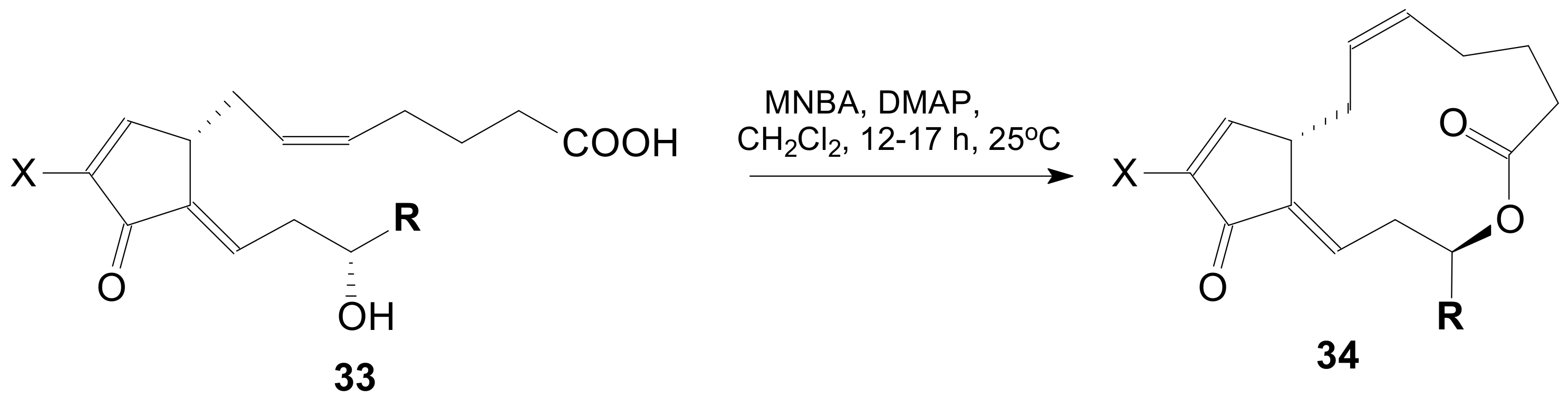
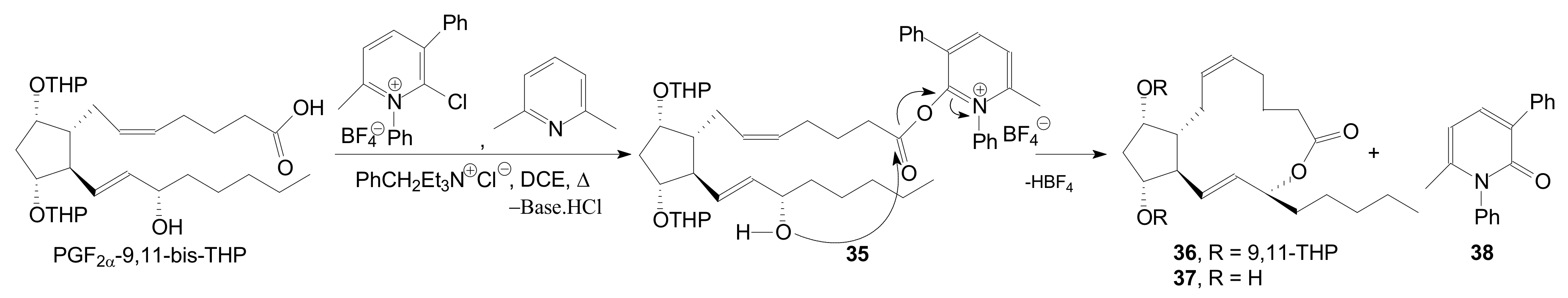
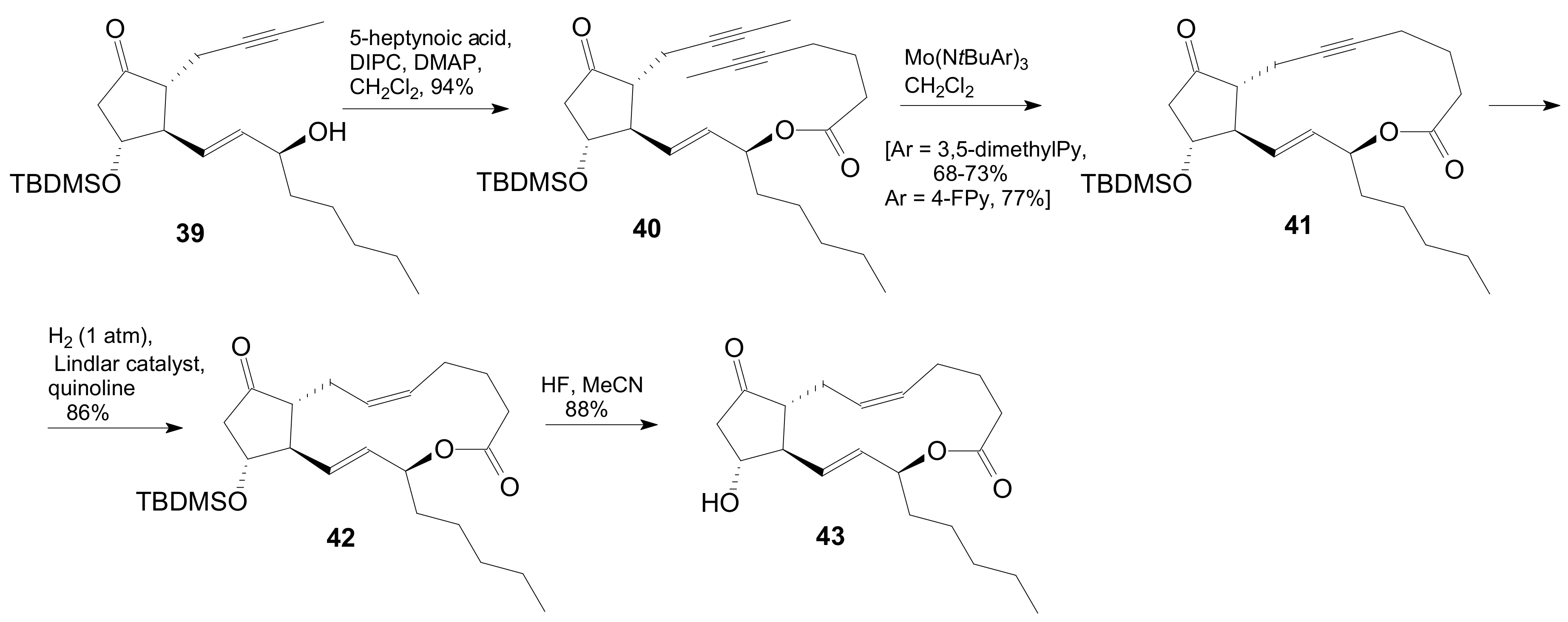









| Entry | Compound | R | Lactonization Method | Yield (%) | mp |
|---|---|---|---|---|---|
| 1 | 28a, 29a, 30a | 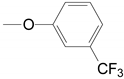 | A THF | 75.0, 3 Steps | 113–118 °C |
| 2 | 28a, 29a, 30a |  | B Benzoyl chloride | 80.3, 3 steps | |
| 3 | 28a, 29a, 30a |  11,15-triethylsilyl | B Benzoyl chloride | 61.6 | |
| 4 | 28a, 29a, 30a |  | B 2,4,6-trichlorobenzoyl chloride | 82.4, 3 steps | |
| 5 | 28a, 29a, 30a |  | C | 72.0, 3 steps | |
| 6 | 28b 29b 30b | 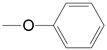 15-difluoro | A Xylene | 48.0, 3 Steps 90.0 | |
| 7 | 28c, 29c, 30c | 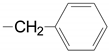 | B | 58.6, 3 steps 56.5 | |
| 8 | 28d, 29d, 30d |  13,14-H2 | A Xylene | 68.0, 3 steps 78.6 | 114–118 °C |
| 9 | 28e, 29e, 11-THF 29e, 30e | C6H13 | AXylene | 75.0, 3 Steps 75.0% | 57–60 °C |
| Entry | Compound | R* | Method | Yield (%) |
|---|---|---|---|---|
| 1 | 34a | X = H; 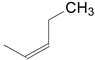 | A | 7134 |
| 2 | ent-34a | X = H;  | A | 7434 |
| 3 | 34b | X = H;  | A | 6234 |
| 4 | 34c | X = H;  | A | 5034 |
| 5 | 34d | X = H; 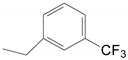 | A | 6134 |
| 6 | 34e | X = H; 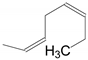 | A | 6234 |
| 7 | 34f | X = Cl;  | B | 7334 |
| 8 | 34g + 34h | 8β-Me-34a + 8α-Me-34a | C | 59 (38 + 21)34 |
| 9 | 34i | X = H, R = C4H9 | D | 7235 |
| 10 | 34j | X = Cl, R = (CH2)3CH3 | D | 6935 |
| 11 | 34k | X = Cl, R = (CH2)3CF3 | D | 7335 |
| 12 | 34l | X = I, R = (CH2)3CF3 | D | 5635 |
| Entry | Compd | R * | Yield (%) | Entry | Compd | R | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 52a, 53a, epi-53a 54a 55a 56a 57a | 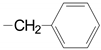 | 99.4 50.8; 93.3 34.4 87.0 85.0 61.0 63.7 | 3 | 52c, 53c, epi-53c 54c 55c 56c 57c |  13,14-H2 | 63.5 62.1 25.3 84.2, 90 85.0 72.6 49.0, 83 |
| 2 | 52b 53b, epi-53b 54b 55b 56b 57b | 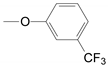 | 70.7 51.7 39.6 75.0 83.0 38.5 46.0 | 4 | 52d 53d, epi-53d 54d 55d 56d 57d |  | 55.5 46.1 43.2 85.0 35.0 77.5 |
| Step | Compound | R, R1, R2, R4 | Reaction Conditions | Yield (%) | [α]D (c = 1% in THF) |
|---|---|---|---|---|---|
| 2 | 117a | R = C6H5 | A | (quant) | +24.16 |
| 117b | R = p-O2NC6H5 | A | 94.5 | +21.15° | |
| ±117c | R = CH3 | A | 68.6 | ||
| 2a | 115a | R1 = CH3 | B | quant | +45.5 |
| 115b | R1 = CH2CH3 | B1 | quant | +45.58 | |
| 3 | 207a | R = C6H5R1 = CH3, R2 = TBDMS | C | quant | +30.49 |
| 207b | R = C6H5R1 = CH3, R2 = THP | E | quant | ||
| 207c | R = p-O2NC6H5R1 = CH3, R2 = THP | E | quant | +18.1 | |
| 3a | 205a | R1 = CH3, R4 = TBDMS | C | >89.6 | +22.98 |
| 205b and 205c | R1 = CH3, R4 = TrR1 = CH3, R4 = Tr +5-OTr | D | 85 6.5 | 14.63 +21.67 | |
| 205c | R1 = CH3, R4 = Tr +5-OTr | D1 | 83.3 | ||
| 205d | R1 = CH3, R4 = TBDMS + 5-OTHP | E | 82.1 | +27.8 | |
| 205e | R1 = CH3, R4 = TBDMS + 5-OTr | D | ~quant | +21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tănase, C.; Pintilie, L.; Tănase, R.E. Lactones in the Synthesis of Prostaglandins and Prostaglandin Analogs. Int. J. Mol. Sci. 2021, 22, 1572. https://doi.org/10.3390/ijms22041572
Tănase C, Pintilie L, Tănase RE. Lactones in the Synthesis of Prostaglandins and Prostaglandin Analogs. International Journal of Molecular Sciences. 2021; 22(4):1572. https://doi.org/10.3390/ijms22041572
Chicago/Turabian StyleTănase, Constantin, Lucia Pintilie, and Raluca Elena Tănase. 2021. "Lactones in the Synthesis of Prostaglandins and Prostaglandin Analogs" International Journal of Molecular Sciences 22, no. 4: 1572. https://doi.org/10.3390/ijms22041572
APA StyleTănase, C., Pintilie, L., & Tănase, R. E. (2021). Lactones in the Synthesis of Prostaglandins and Prostaglandin Analogs. International Journal of Molecular Sciences, 22(4), 1572. https://doi.org/10.3390/ijms22041572







