Plasma miR-9-3p and miR-136-3p as Potential Novel Diagnostic Biomarkers for Experimental and Human Mild Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of TBI with Lateral Fluid-Percussion
2.3. Sampling of Plasma and Brain Tissue
2.4. Small RNA-Seq from Plasma
2.4.1. Library Preparation and Sequencing
2.4.2. Quantification of miRNAs and Differential Expression Analysis
2.4.3. Identification of Expression Pattern Differences with Machine Learning
2.5. Technical Validation of Small RNA-Seq Data
2.5.1. Selection of an Endogenous miRNA for Normalization of RT-qPCR
2.5.2. RT-qPCR of Selected miRNA Candidates
2.6. Analysis of Validated miRNAs in the Whole Cohort
2.6.1. Plasma Hemolysis Measurement
2.6.2. RT-qPCR of Validated miRNA Candidates
2.6.3. ddPCR of Validated miRNA Candidates
2.7. Analysis of the Validated miRNAs in Plasma from Human TBI Patients
2.7.1. ddPCR of Validated miRNA Candidates
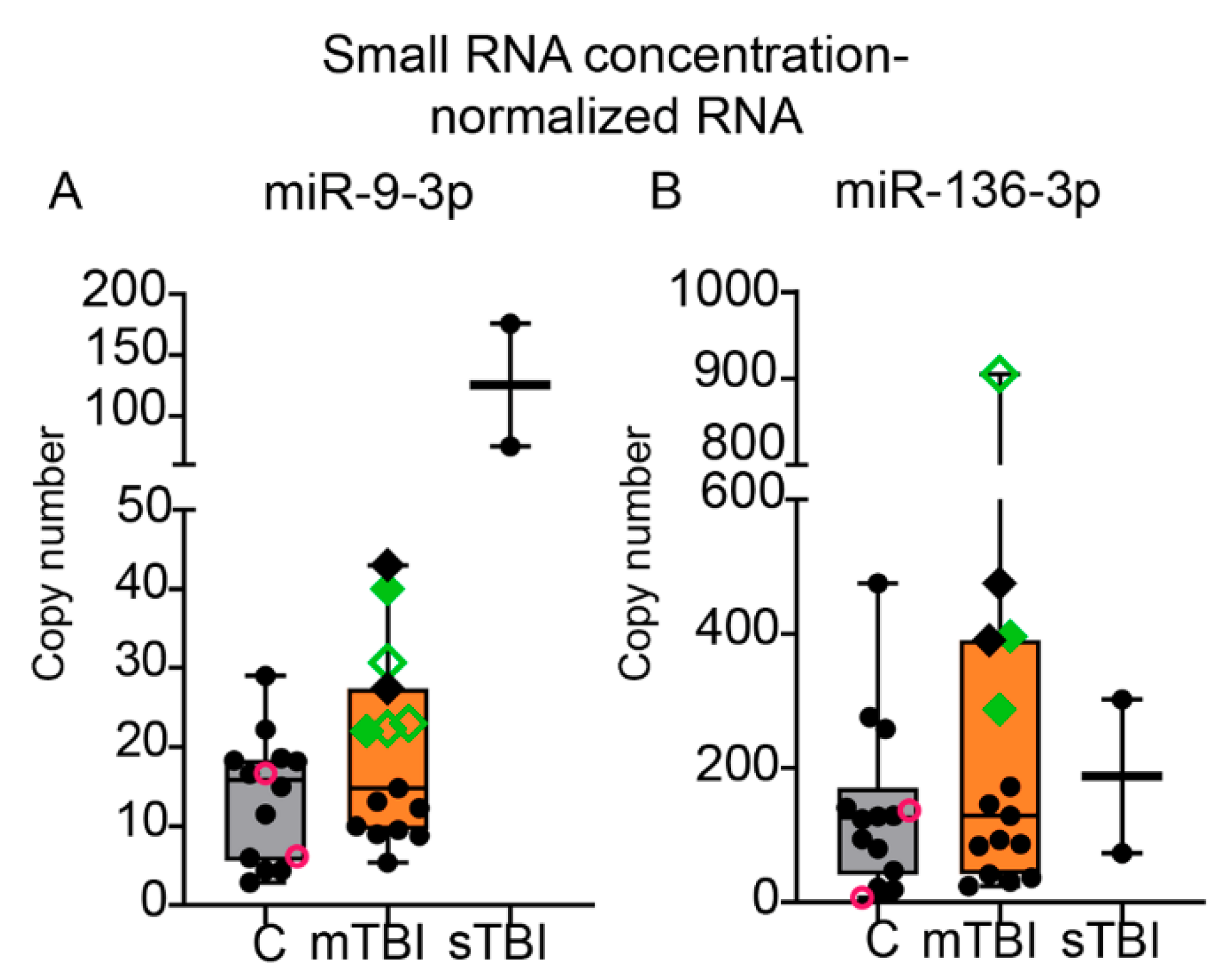
2.7.2. ddPCR from Small RNA Concentration-Normalized Samples
2.8. Statistical Analysis
3. Results
3.1. Impact Severity, Mortality, Duration of Postimpact Apnea, and Time to Righting
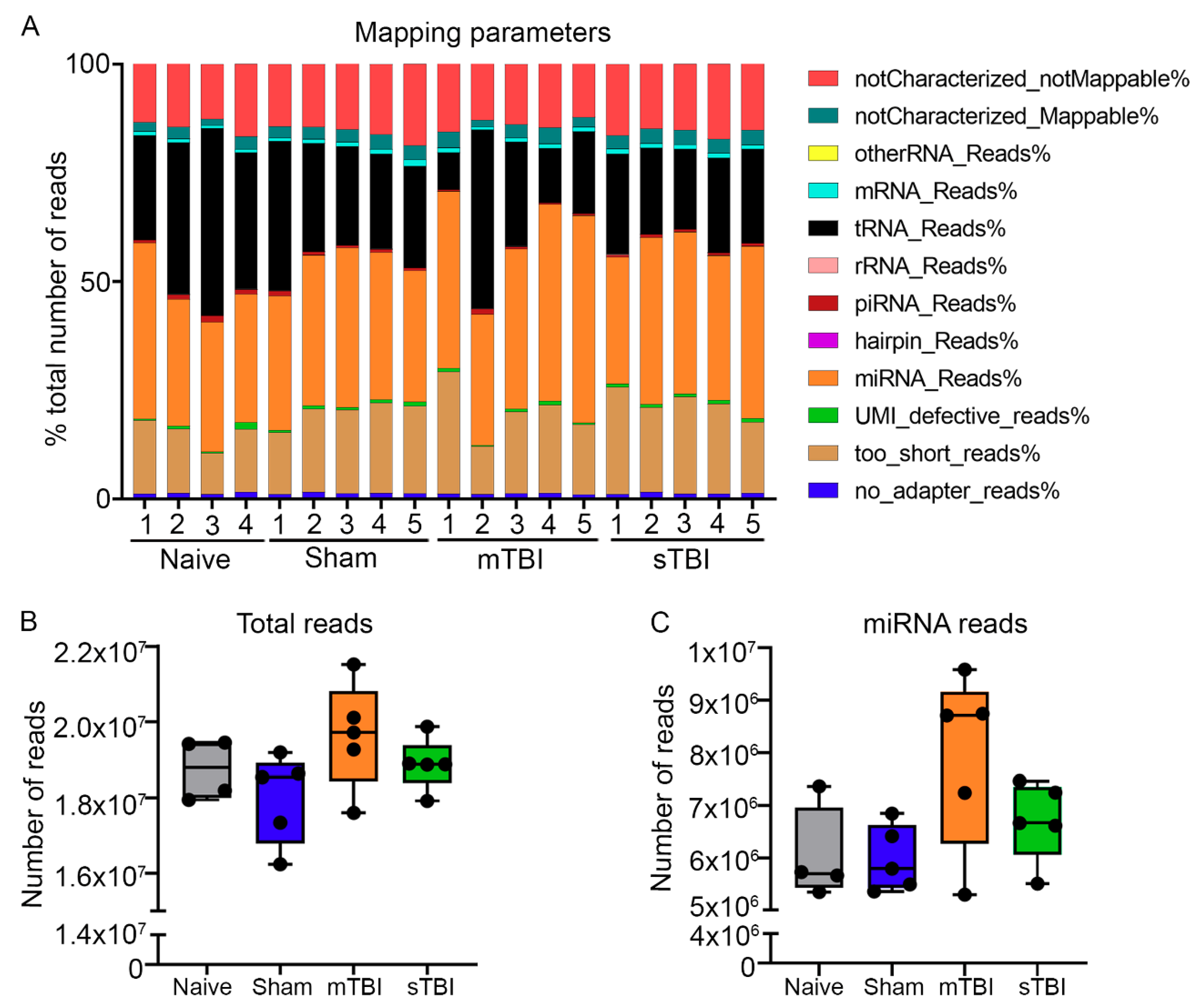
3.2. Quality Control Analysis from Tail-Vein Plasma and Extracted RNA Prior to Small RNA-Seq
3.2.1. Hemolysis Measurement with NanoDrop
3.2.2. Hemolysis Measurement with the ΔCq (miR-23a–miR-451) Method
3.2.3. Amplification of Endogenous miRNAs and Spike-In Controls
3.3. Primary miRNA Quantification
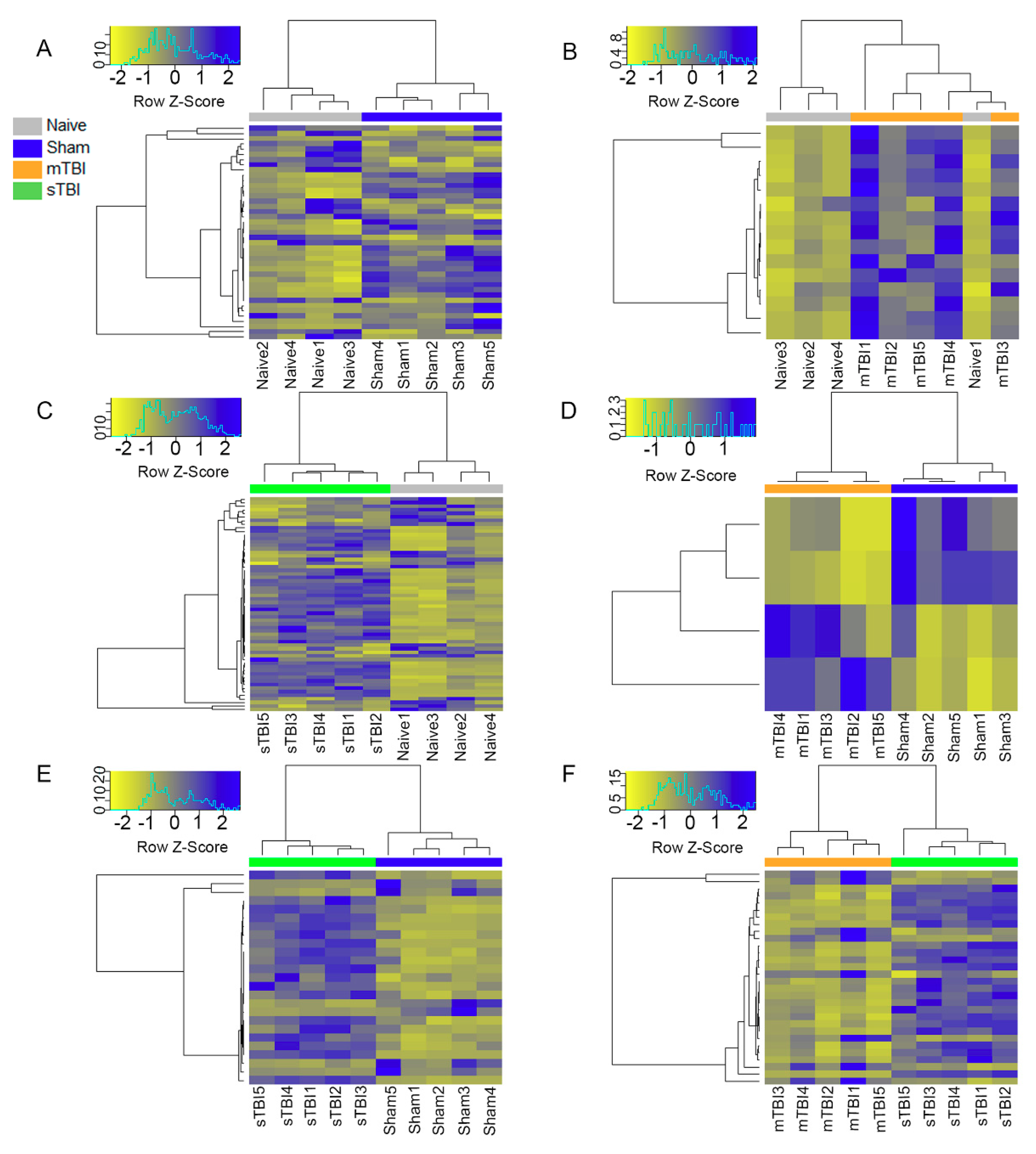
3.4. Differential Expression Analysis
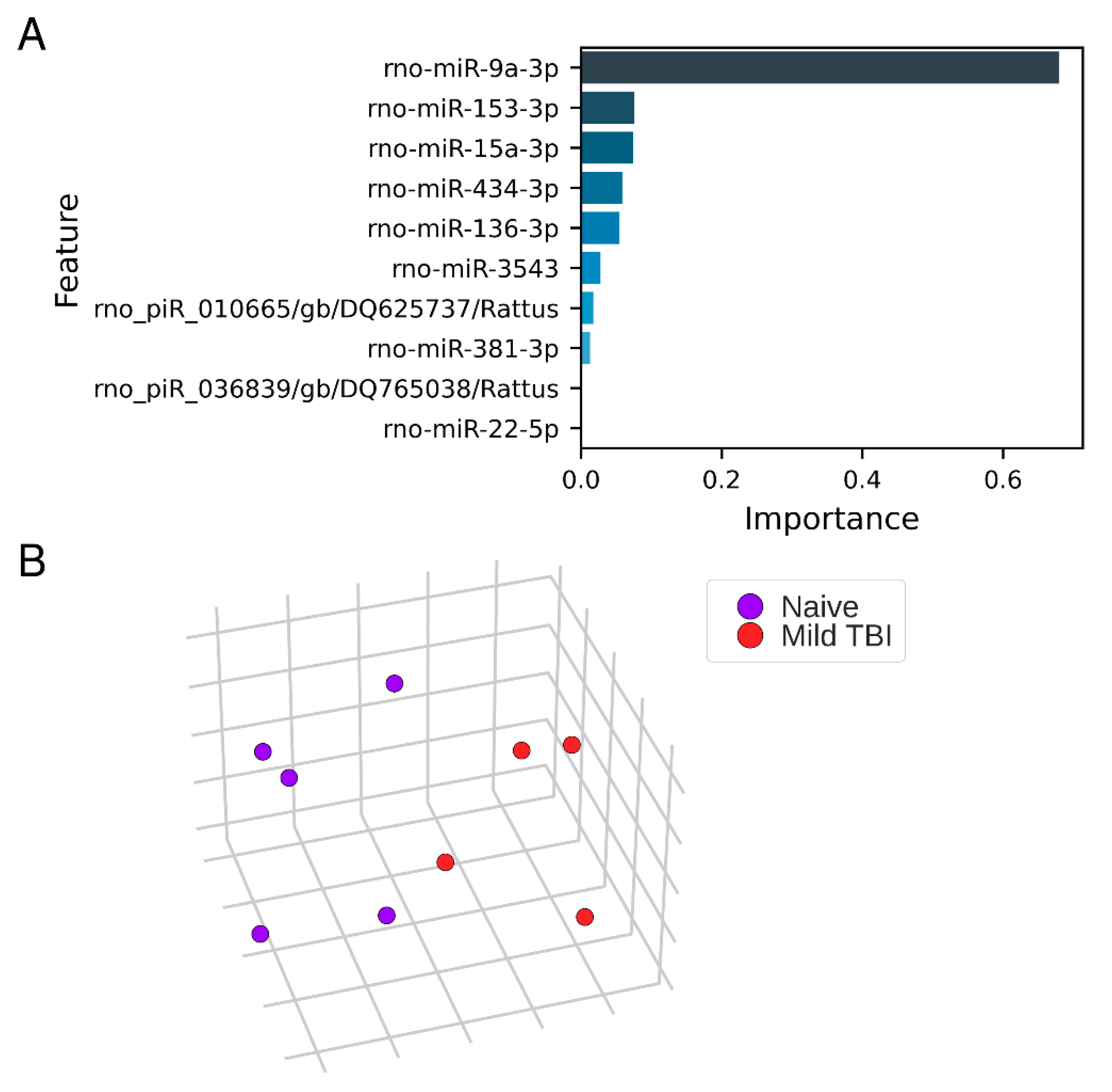
3.5. Expression Pattern Differences from Machine Learning
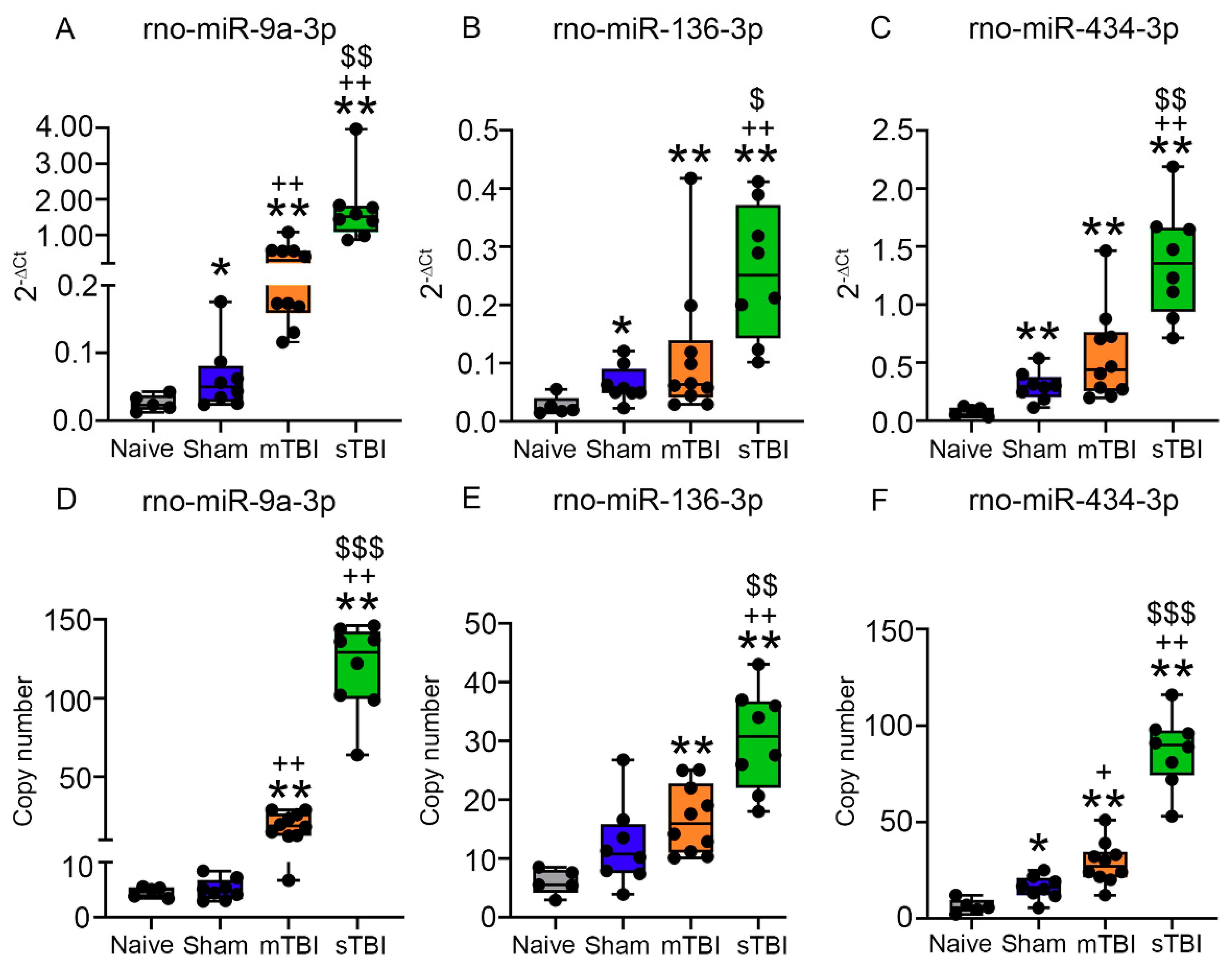
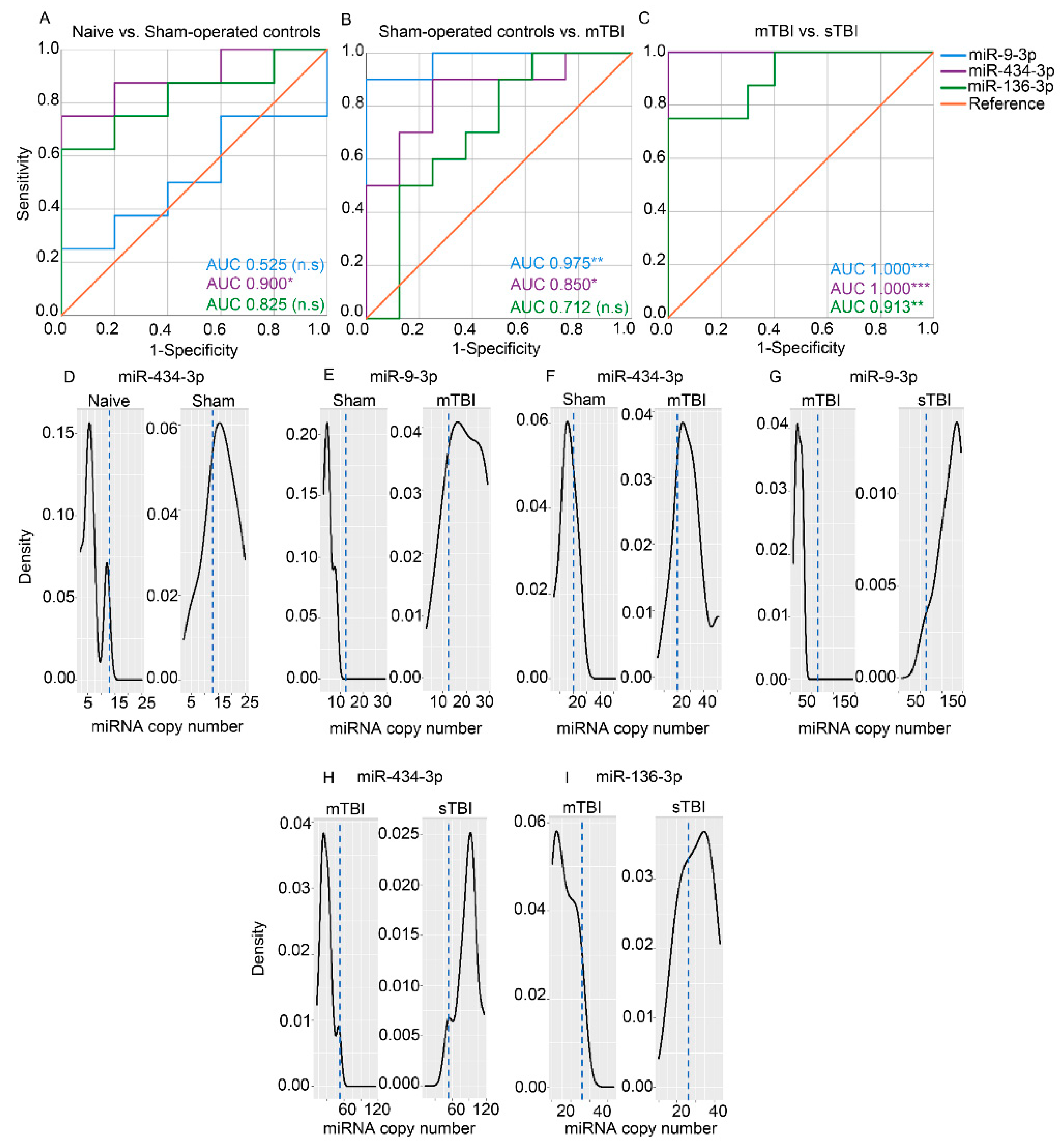
3.6. Technical Validation of Regulated rno-miR-9a-3p, rno-miR-153-3p, rno-miR-15a-3p, rno-miR-136-3p, and rno-miR-434-3p Levels in Samples Used for miR-Seq
3.6.1. miR-9a-3p
3.6.2. miR-136-3p
3.6.3. miR-434-3p
3.7. Validation of Regulated rno-miR-9a-3p, rno-miR-136-3p, and rno-miR-434-3p Plasma Levels in Whole Animal Cohort
3.7.1. Hemolysis
3.7.2. Plasma Levels of miR-9a-3p, miR-136-3p, and miR-434-3p Assessed with RT-qPCR
3.7.3. Absolute Copy Numbers of miR-9a-3p, miR-136-3p, and miR-434-3p in Plasma Assessed with ddPCR
3.8. ddPCR of hsa-miR-9-3p and hsa-miR-136-3p in Human TBI Plasma
3.8.1. Hemolysis
3.8.2. Effect of Sex, Age, and Injury-Sampling Interval on Plasma miR-9-3p and miR-136 Levels
3.8.3. Absolute Copy Numbers for miR-9-3p and miR-136-3p in Plasma Assessed with ddPCR
3.8.4. Association of miR-9-3p and miR-136 Levels with Plasma S100B Concentrations
4. Discussion
4.1. mTBI Led to an Acute Increase in Plasma miR-9a-3p, miR-136-3p, and miR-434-3p Levels
4.2. An Impact Severity -Dependent Increase was Observed in the Plasma miRNA Signature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position Statement: Definition of Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef]
- Anzai, Y.; Minoshima, S. Imaging of traumatic brain injury: Current and future. Imaging Med. 2011, 3, 153–165. [Google Scholar] [CrossRef]
- Shin, S.S.; Bales, J.W.; Dixon, C.E.; Hwang, M. Structural imaging of mild traumatic brain injury may not be enough: Overview of functional and metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 2017, 11, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas, D.B.; Anderson, C.A.; Topkoff, J.; McAllister, T.W. Mild traumatic brain injury: A neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr. Dis. Treat. 2005, 1, 311–327. [Google Scholar]
- Agoston, D.V.; Shutes-David, A.; Peskind, E.R. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017, 31, 1195–1203. [Google Scholar] [CrossRef]
- Dadas, A.; Washington, J.; Diaz-Arrastia, R.; Janigro, D. Biomarkers in traumatic brain injury (TBI): A review. Neuropsychiatr. Dis. Treat. 2018, 14, 2989–3000. [Google Scholar] [CrossRef] [PubMed]
- Kawata, K.; Liu, C.Y.; Merkel, S.F.; Ramirez, S.H.; Tierney, R.T.; Langford, D. Blood biomarkers for brain injury: What are we measuring? Neurosci. Biobehav. Rev. 2016, 68, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.S.; Stein, S.C.; Swanson, R.; Guan, S.; Garcia, L.; Mehta, D.; Smith, D.H. Blood biomarkers for traumatic brain injury: A quantitative assessment of diagnostic and prognostic accuracy. Front. Neurol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Mondello, S.; Schmid, K.; Berger, R.P.; Kobeissy, F.; Italiano, D.; Jeromin, A.; Hayes, R.L.; Tortella, F.C.; Buki, A. The Challenge of Mild Traumatic Brain Injury: Role of Biochemical Markers in Diagnosis of Brain Damage. Med. Res. Rev. 2014, 34, 503–531. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Brolinson, P.G.; Büki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef]
- Calcagnile, O.; Anell, A.; Undén, J. The addition of S100B to guidelines for management of mild head injury is potentially cost saving. BMC Neurol. 2016, 16, 200. [Google Scholar] [CrossRef]
- Thelin, E.P.; Nelson, D.W.; Bellander, B.-M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir. 2017, 159, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; O Hansson, L.; Nilsson, O.; Dijlai-Merzoug, R.; Settergren, G. High serum S100B levels for trauma patients without head injuries. Neurosurgery 2001, 48, 1255–1260. [Google Scholar]
- Bloomfield, S.M.; McKinney, J.; Smith, L.; Brisman, J. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit. Care 2007, 6, 121–138. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Mazurek, M.; Litak, J.; Kamieniak, P.; Osuchowska, I.; Maciejewski, R.; Roliński, J.; Grajkowska, W.A.; Grochowski, C. Micro RNA Molecules as Modulators of Treatment Resistance, Immune Checkpoints Controllers and Sensitive Biomarkers in Glioblastoma Multiforme. Int. J. Mol. Sci. 2020, 21, 1507. [Google Scholar] [CrossRef]
- Redell, J.B.; Moore, A.N.; Ward, N.H., III; Hergenroeder, G.W.; Dash, P.K. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma 2010, 27, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Bhomia, M.; Balakathiresan, N.S.; Wang, K.K.; Papa, L.; Maheshwari, R.K. A Panel of Serum MiRNA Biomarkers for the Diagnosis of Severe to Mild Traumatic Brain Injury in Humans. Sci. Rep. 2016, 6, 28148. [Google Scholar] [CrossRef]
- Yang, T.; Song, J.; Bu, X.; Wang, C.; Wu, J.; Cai, J.; Wan, S.; Fan, C.; Zhang, C.; Wang, J. Elevated serum miR-93, miR-191, and miR-499 are noninvasive bi-omarkers for the presence and progression of traumatic brain injury. J. Neurochem. 2016, 137, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Ragusa, M.; Davies, D.; Su, Z.; Hazeldine, J.; Lazzarino, G.; Hill, L.J.; Crombie, N.; Foster, M.; Purrello, M.; et al. MicroRNAs as novel biomarkers for the diagnosis and prognosis of mild and severe traumatic brain injury. J. Neurotrauma 2017, 34, 1948–1956. [Google Scholar] [CrossRef]
- Qin, X.; Li, L.; Lv, Q.; Shu, Q.; Zhang, Y.L.; Wang, Y. Expression profile of plasma microRNAs and their roles in diagnosis of mild to severe traumatic brain injury. PLoS ONE 2018, 13, e0204051. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bu, X.; Li, Z.; Wu, J.; Wang, C.; Li, D.; Song, J.; Wang, J. Screening the expression of several miRNAs from TaqMan Low Density Array in traumatic brain injury: miR-219a-5p regulates neuronal apoptosis by modulating CCNA2 and CACUL1. J. Neurochem. 2019, 150, 202–217. [Google Scholar] [CrossRef]
- Papa, L.; Slobounov, S.M.; Breiter, H.C.; Walter, A.; Bream, T.; Seidenberg, P.; Bailes, J.E.; Bravo, S.; Johnson, B.; Kaufman, D.; et al. Elevations in microRNA biomarkers in serum are associated with measures of concussion, neurocognitive function, and Subconcussive trauma over a single national col-legiate athletic association division I season in collegiate football players. J. Neurotrauma 2019, 36, 1343–1351. [Google Scholar] [CrossRef]
- LaRocca, D.; Barns, S.; Hicks, S.D.; Brindle, A.; Williams, J.; Uhlig, R.; Johnson, P.; Neville, C.; Middleton, F.A. Comparison of serum and saliva miRNAs for identifi-cation and characterization of mTBI in adult mixed martial arts fighters. PLoS ONE 2019, 14, e0207785. [Google Scholar] [CrossRef]
- Mitra, B.; Rau, T.F.; Surendran, N.; Brennan, J.H.; Thaveenthiran, P.; Sorich, E.; Fitzgerald, M.C.; Rosenfeld, J.V.; Patel, S.A. Plasma micro-RNA biomarkers for diagnosis and prognosis after traumatic brain injury: A pilot study. J. Clin. Neurosci. 2017, 38, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hergenroeder, G.W.; Redell, J.B.; Moore, A.N.; Dash, P.K. Biomarkers in the Clinical Diagnosis and Management of Traumatic Brain Injury. Mol. Diagn. Ther. 2008, 12, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.I.; Stabenfeldt, S.E. Current trends in biomarker discovery and analysis tools for traumatic brain injury. J. Biol. Eng. 2019, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Balakathiresan, N.S.; Bhomia, M.; Chandran, R.; Chavko, M.; McCarron, R.M.; Maheshwari, R.K. MicroRNA Let-7i Is a Promising Serum Biomarker for Blast-Induced Traumatic Brain Injury. J. Neurotrauma 2012, 29, 1379–1387. [Google Scholar] [CrossRef]
- Vuokila, N.; Das Gupta, S.; Huusko, R.; Tohka, J.; Puhakka, N.; Pitkänen, A. Elevated Acute Plasma miR-124-3p Level Relates to Evolution of Larger Cortical Lesion Area after Traumatic Brain Injury. Neuroscience 2020, 433, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chandran, R.; Barry, E.S.; Bhomia, M.; Hutchison, M.A.; Balakathiresan, N.S.; Grunberg, N.E.; Maheshwari, R.K. Identification of Serum MicroRNA Signatures for Diagnosis of Mild Traumatic Brain Injury in a Closed Head Injury Model. PLoS ONE 2014, 9, e112019. [Google Scholar] [CrossRef]
- Chandran, R.; Sharma, A.; Bhomia, M.; Balakathiresan, N.S.; Knollmann-Ritschel, B.E.; Maheshwari, R.K. Differential expression of microRNAs in the brains of mice subjected to increasing grade of mild traumatic brain injury. Brain Inj. 2016, 31, 106–119. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, T.K.; Vink, R.; Noble, L.J.; Yamakami, I.; Fernyak, S.; Soares, H.; Faden, A. Traumatic brain injury in the rat: Characterization of a lateral fluid-percussion model. Neuroscience 1989, 28, 233–244. [Google Scholar] [CrossRef]
- Kharatishvili, I.; Nissinen, J.; McIntosh, T.; Pitkänen, A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 2006, 140, 685–697. [Google Scholar] [CrossRef]
- Andrade, P.; Banuelos-Cabrera, I.; Lapinlampi, N.; Paananen, T.; Ciszek, R.; Ndode-Ekane, X.E. Acute non-convulsive sta-tus epilepticus after experimental traumatic brain injury in rats. J. Neurotrauma 2019, 36, 1890–1907. [Google Scholar] [CrossRef] [PubMed]
- Ndode-Ekane, X.E.; Santana-Gomez, C.; Casillas-Espinosa, P.M.; Ali, I.; Brady, R.D.; Smith, G.; Andrade, P.; Immonen, R.; Puhakka, N.; Hudson, M.R.; et al. Harmonization of lateral fluid-percussion injury model production and post-injury monitoring in a preclinical multicenter biomarker discovery study on post-traumatic epileptogenesis. Epilepsy Res. 2019, 151, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, E.A.; Puhakka, N.; Mills, J.D.; Srivastava, P.K.; Johnson, M.R.; Roncon, P.; Das Gupta, S.; Karttunen, J.; Simonato, M.; Łukasiuk, K.; et al. Standardization procedure for plasma biomarker analysis in rat models of epileptogenesis: Focus on circulating microRNAs. Epilepsia 2017, 58, 2013–2024. [Google Scholar] [CrossRef]
- Ekamnaksh, A.; Puhakka, N.; Ali, I.; Smith, G.; Aniceto, R.; McCullough, J.; Das Gupta, S.; Ndode-Ekane, X.E.; Brady, R.; Casillas-Espinosa, P.; et al. Harmonization of pipeline for preclinical multicenter plasma protein and miRNA biomarker discovery in a rat model of post-traumatic epileptogenesis. Epilepsy Res. 2019, 149, 92–101. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 2013, 10, 1003. [Google Scholar] [CrossRef]
- Gupta, S.D.; Ndode-Ekane, X.E.; Puhakka, N.; Pitkänen, A. Droplet digital polymerase chain reaction-based quantification of circulating microRnAs using small RnA concentration normalization. Sci. Rep. 2020, 10, 9012. [Google Scholar] [CrossRef]
- Thiele, C. Cutpointr: Determine and Evaluate Optimal Cutpoints in Binary Classification Tasks; R Packaging Version; R Foundation: Vienna, Austria, 2019; p. 1. [Google Scholar]
- Lapinlampi, N.; Andrade, P.; Paananen, T.; Hämäläinen, E.; Ekolle Ndode-Ekane, X.; Puhakka, N.; Pitkänen, A. Postinjury weight rather than cognitive or behavioral impairment predicts development of posttraumatic epilepsy after lateral fluid-percussion injury in rats. Epilepsia 2020, 61, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Blondal, T.; Nielsen, S.J.; Baker, A.; Andreasen, D.; Mouritzen, P.; Teilum, M.W.; Dahlsveen, I.K. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef]
- Miska, E.A.; Alvarez-Saavedra, E.; Townsend, M.; Yoshii, A.; Šestan, N.; Rakic, P. Microarray analysis of microRNA expres-sion in the developing mammalian brain. Genome Biol. 2004, 5, R68. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression profiling of mammalian mi-croRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentia-tion. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific MicroRNAs from Mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell. Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef]
- Sim, S.E.; Lim, C.S.; Kim, J.I.; Seo, D.; Chun, H.; Yu, N.K.; Lee, J.; Kang, S.J.; Ko, H.G.; Choi, J.H.; et al. The brain-enriched microRNA miR-9-3p regulates synaptic plas-ticity and memory. J. Neurosci. 2016, 36, 8641–8652. [Google Scholar] [CrossRef]
- Ferretti, E.; De Smaele, E.; Po, A.; Di Marcotullio, L.; Tosi, E.; Espinola, M.S.; Di Rocco, C.; Riccardi, R.; Giangaspero, F.; Farcomeni, A.; et al. MicroRNA profiling in human medulloblasto-ma. Int. J. Cancer 2009, 124, 568–577. [Google Scholar] [CrossRef]
- Senyuk, V.; Zhang, Y.; Liu, Y.; Ming, M.; Premanand, K.; Zhou, L.; Chen, P.; Chen, J.; Rowley, J.D.; Nucifora, G.; et al. Critical role of miR-9 in myelopoiesis and EVI1-induced leukemogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 5594–5599. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Abraham, E. MicroRNAs in immune response and macrophage polarization. Arter. Thromb. Vasc. Biol. 2013, 33, 170–177. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Z.-L.; Zhao, W.-T.; Fan, Q.-R.; Wang, S.-C.; Li, J.; Zhang, Y.-Q.; Shi, J.-W.; Lin, X.-L.; Yang, S.; et al. miR-9 modulates the expression of interferon-regulated genes and MHC class I molecules in human nasopharyngeal carcinoma cells. Biochem. Biophys. Res. Commun. 2013, 431, 610–616. [Google Scholar] [CrossRef]
- Nowek, K.; Wiemer, E.A.C.; Jongen-Lavrencic, M. The versatile nature of miR-9/9* in human cancer. Oncotarget 2018, 9, 20838. [Google Scholar] [CrossRef]
- Wang, P.; Ma, H.; Zhang, Y.; Zeng, R.; Yu, J.; Liu, R.; Jin, X.; Zhao, Y. Plasma Exosome-derived MicroRNAs as Novel Biomarkers of Traumatic Brain Injury in Rats. Int. J. Med Sci. 2020, 17, 437–448. [Google Scholar] [CrossRef]
- Sørensen, S.S.; Nygaard, A.-B.; Carlsen, A.L.; Heegaard, N.H.H.; Bak, M.; Christensen, T. Elevation of brain-enriched miRNAs in cerebrospinal fluid of patients with acute ischemic stroke. Biomark. Res. 2017, 5, 24. [Google Scholar] [CrossRef]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Han, R.; Liu, H.; Sun, D.; Liu, X. Downregulation of serum brain specific microRNA is associated with in-flammation and infarct volume in acute ischemic stroke. J. Clin. Neurosci. 2015, 22, 291–295. [Google Scholar] [CrossRef]
- Jovičić, A.; Roshan, R.; Moisoi, N.; Pradervand, S.; Moser, R.; Pillai, B.; Luthi-Carter, R. Comprehensive Expression Analyses of Neural Cell-Type-Specific miRNAs Identify New Determinants of the Specification and Maintenance of Neuronal Phenotypes. J. Neurosci. 2013, 33, 5127–5137. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.S.; Saba, R.; Campbell, K.; Majer, A.; Medina, S.; Burton, L.; Booth, T.F.; Chong, P.; Westmacott, G.R.; Dutta, S.M.; et al. MicroRNA abundance is altered in synaptoneurosomes during prion disease. Mol. Cell. Neurosci. 2016, 71, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. MicroRNA-136-3p inhibits glioma tumorigenesis in vitro and in vivo by targeting KLF7. World J. Surg. Oncol. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Chen, W.; Yang, Y.; Chen, B.; Lu, P.; Zhan, L.; Yu, Q.; Cao, K.; Li, Q. MiR-136 targets E2F1 to reverse cisplatin chemosensitivity in glioma cells. J. Neuro Oncol. 2014, 120, 43–53. [Google Scholar] [CrossRef]
- Zhong, Y.; Yu, C.; Qin, W. LncRNA SNHG14 promotes inflammatory response induced by cerebral ischemia/reperfusion injury through regulating miR-136-5p /ROCK1. Cancer Gene Ther. 2019, 26, 234–247. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Zhu, D.; Liu, P.; Yin, J.; Liu, D.; Zheng, M.; Gao, J.; Zhang, C.; Gao, Y. miR-136-3p targets PTEN to regulate vascularization and bone formation and ameliorates alcohol-induced osteopenia. FASEB J. 2020, 34, 5348–5362. [Google Scholar] [CrossRef]
- Kitahara, Y.; Nakamura, K.; Kogure, K.; Minegishi, T. Role of microRNA-136-3p on the Expression of Luteinizing Hormone-Human Chorionic Gonadotropin Receptor mRNA in Rat Ovaries1. Biol. Reprod. 2013, 89, 114. [Google Scholar] [CrossRef]
- Lin, Y.; Dan, H.; Lu, J. Overexpression of microRNA-136-3p Alleviates Myocardial Injury in Coronary Artery Disease via the Rho A/ROCK Signaling Pathway. Kidney Blood Press. Res. 2020, 45, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Ravanidis, S.; Bougea, A.; Papagiannakis, N.; Koros, C.; Simitsi, A.M.; Pachi, I.; Breza, M.; Stefanis, L.; Doxakis, E. Validation of Differentially Expressed Brain-Enriched microRNAs in the Plasma of Parkinson’s Disease Patients. Ann. Clin. Transl. Neurol. 2020, 7, 1594–1607. [Google Scholar] [CrossRef]
- Ravanidis, S.; Bougea, A.; Papagiannakis, N.; Maniati, M.; Koros, C.; Simitsi, A.; Bozi, M.; Pachi, I.; Stamelou, M.; Paraskevas, G.P.; et al. Circulating Brain-Enriched MicroRNAs for Detection and Discrimination of Idiopathic and Genetic Parkinson’s Disease. Mov. Disord. 2019, 35, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Liu, H.; Zhang, L.; Lv, W.; Hu, X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef] [PubMed]
- Burgos, K.; Malenica, I.; Metpally, R.; Courtright, A.; Rakela, B.; Beach, T.; Shill, H.; Adler, C.; Sabbagh, M.; Villa, S.; et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PLoS ONE 2014, 9, e94839. [Google Scholar]
- Raheja, R.; Regev, K.; Healy, B.C.; Mazzola, M.A.; Beynon, V.; Von Glehn, F.; Paul, A.; Diaz-Cruz, C.; Gholipour, T.; Glanz, B.I.; et al. Correlating serum micrornas and clinical parameters in amyotrophic lateral sclerosis. Muscle Nerve 2018, 58, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, A.; Williams, M.H.; Miranda, R.C.; Sohrabji, F. Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clin. Sci. 2014, 127, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Manakov, S.A.; Morton, A.; Enright, A.J.; Grant, S.G.N. A Neuronal Transcriptome Response Involving Stress Pathways is Buffered by Neuronal microRNAs. Front. Behav. Neurosci. 2012, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Q.-Y.; Edson, J.; Zhang, Z.H.; Li, X.; Wei, W.; Bredy, T.; Reutens, D.C. Genome-wide microRNA profiling in brain and blood samples in a mouse model of epileptogenesis. Epilepsy Res. 2020, 166, 106400. [Google Scholar] [CrossRef]
- Shang, F.-F.; Xia, Q.-J.; Liu, W.; Xia, L.; Qian, B.-J.; You, L.; He, M.; Yang, J.; Wang, T.-H. miR-434-3p and DNA hypomethylation co-regulate eIF5A1 to increase AChRs and to improve plasticity in SCT rat skeletal muscle. Sci. Rep. 2016, 6, 22884. [Google Scholar] [CrossRef]
- Pardo, P.S.; Hajira, A.; Boriek, A.M.; Mohamed, J.S. MicroRNA-434-3p regulates age-related apoptosis through eIF5A1 in the skeletal muscle. Aging 2017, 9, 1012–1029. [Google Scholar] [CrossRef]
- Zhen, S.H.; Chen, Y.A.; Li, Z.L.; Yuan, T.E.; Wen, L.I.; Wang, J.S.; Ye, C.S.; Chang, G.Q.; Huang, X.L.; Li, X.X.; et al. Differentially expressed microRNAs at different stages of atherosclerosis in ApoE-deficient mice. Chin. Med. J. 2013, 126, 515–520. [Google Scholar]
- Jung, H.J.; Lee, K.-P.; Milholland, B.; Shin, Y.J.; Kang, J.S.; Kwon, K.-S.; Suh, Y. Comprehensive miRNA Profiling of Skeletal Muscle and Serum in Induced and Normal Mouse Muscle Atrophy During Aging. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2017, 72, 1483–1491. [Google Scholar] [CrossRef]
- Siracusa, J.; Koulmann, N.; Bourdon, S.; Goriot, M.-E.; Banzet, S. Circulating miRNAs as Biomarkers of Acute Muscle Damage in Rats. Am. J. Pathol. 2016, 186, 1313–1327. [Google Scholar] [CrossRef]
- Rau, C.-S.; Wu, S.-C.; Yang, J.C.-S.; Lu, T.-H.; Wu, Y.-C.; Chen, Y.-C.; Tzeng, S.-L.; Wu, C.-J.; Hsieh, C.-H. Profiling the circulating miRNAs in mice exposed to gram-positive and gram-negative bacteria by Illumina small RNA deep sequencing. J. Biomed. Sci. 2015, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Mainieri, A.; Haig, D. Retrotransposon gag-like 1 (RTL1) and the molecular evolution of self-targeting imprinted microRNAs. Biol. Direct 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Hylin, M.J.; Orsi, S.A.; Zhao, J.; Bockhorst, K.; Perez, A.; Moore, A.N.; Dash, P.K. Behavioral and Histopathological Alterations Resulting from Mild Fluid Percussion Injury. J. Neurotrauma 2013, 30, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Eakin, K.; Miller, J.P. Mild Traumatic Brain Injury Is Associated with Impaired Hippocampal Spatiotemporal Representation in the Absence of Histological Changes. J. Neurotrauma 2012, 29, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.R.; MacFabe, D.F.; Foley, K.A.; Taylor, R.; Cain, D.P. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long–Evans rat: Support for an animal model of concussion. Behav. Brain. Res. 2011, 224, 326–335. [Google Scholar] [CrossRef]
- Cole, J.T.; Yarnell, A.; Kean, W.S.; Gold, E.; Lewis, B.; Ren, M.; McMullen, D.C.; Jacobowitz, D.M.; Pollard, H.B.; O’Neill, J.T.; et al. Craniotomy: True Sham for Traumatic Brain Injury, or a Sham of a Sham? J. Neurotrauma 2011, 28, 359–369. [Google Scholar] [CrossRef]
- Mondello, S.; Shear, D.A.; Bramlett, H.M.; Dixon, C.E.; Schmid, K.E.; Dietrich, W.D.; Wang, K.K.; Hayes, R.L.; Glushakova, O.; Catania, M.; et al. Insight into pre-clinical models of traumatic brain injury using circulating brain damage biomarkers: Operation brain trauma therapy. J. Neurotrauma 2016, 33, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Lagraoui, M.; Latoche, J.R.; Cartwright, N.G.; Sukumar, G.; Dalgard, C.L.; Schaefer, B.C. Controlled cortical impact and craniotomy induce strikingly similar profiles of inflammatory gene expression, but with distinct kinetics. Front. Neurol. 2012, 3, 155. [Google Scholar] [CrossRef]
- Schober, M.E.; Block, B.; Requena, D.F.; Hale, M.A.; Lane, R.H. Developmental traumatic brain injury decreased brain derived neurotrophic factor expression late after injury. Metab. Brain Dis. 2012, 27, 167–173. [Google Scholar] [CrossRef]
- Sashindranath, M.; Daglas, M.; Medcalf, R. Evaluation of gait impairment in mice subjected to craniotomy and traumatic brain injury. Behav. Brain Res. 2015, 286, 33–38. [Google Scholar] [CrossRef]
- Wu, J.C.-C.; Chen, K.-Y.; Yo, Y.-W.; Huang, S.-W.; Shih, H.-M.; Chiu, W.-T.; Chiang, Y.-H.; Shiau, C.-Y. Different sham procedures for rats in traumatic brain injury experiments induce corresponding increases in levels of trauma markers. J. Surg. Res. 2013, 179, 138–144. [Google Scholar] [CrossRef]
- Mayeux, J.P.; Teng, S.X.; Katz, P.S.; Gilpin, N.W.; Molina, P.E. Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats. Behav. Brain Res. 2015, 279, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across hu-man tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef] [PubMed]


| Clinical Characteristics | Controls (n = 14) | mTBI (n = 15) | sTBI (n = 2) |
|---|---|---|---|
| Age (mean ± SD) | 63 ± 6 | 68 ± 12 | 63 ± 3 |
| Female (n (%)) | 4 (29) | 5 (33) | 0 (0) |
| GCS (mean ± SD) | - | 14.8 ± 0.6 | 3.5 ± 0.7 |
| Post-traumatic amnesia (n (%)) | - | 12 (80) | - |
| Hours from injury to plasma sampling (mean ± SD) | - | 11 ± 12 | - |
| Plasma S100B (µg/L) (mean ± SD) | - | 0.2 ± 0.1 | - |
| Plasma S100B > 0.1 µg/L (n (%)) | - | 11 (73) | - |
| Alcohol consumed (n (%)) | - | 3 (20) | 1 (50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das Gupta, S.; Ciszek, R.; Heiskanen, M.; Lapinlampi, N.; Kukkonen, J.; Leinonen, V.; Puhakka, N.; Pitkänen, A. Plasma miR-9-3p and miR-136-3p as Potential Novel Diagnostic Biomarkers for Experimental and Human Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 1563. https://doi.org/10.3390/ijms22041563
Das Gupta S, Ciszek R, Heiskanen M, Lapinlampi N, Kukkonen J, Leinonen V, Puhakka N, Pitkänen A. Plasma miR-9-3p and miR-136-3p as Potential Novel Diagnostic Biomarkers for Experimental and Human Mild Traumatic Brain Injury. International Journal of Molecular Sciences. 2021; 22(4):1563. https://doi.org/10.3390/ijms22041563
Chicago/Turabian StyleDas Gupta, Shalini, Robert Ciszek, Mette Heiskanen, Niina Lapinlampi, Janne Kukkonen, Ville Leinonen, Noora Puhakka, and Asla Pitkänen. 2021. "Plasma miR-9-3p and miR-136-3p as Potential Novel Diagnostic Biomarkers for Experimental and Human Mild Traumatic Brain Injury" International Journal of Molecular Sciences 22, no. 4: 1563. https://doi.org/10.3390/ijms22041563
APA StyleDas Gupta, S., Ciszek, R., Heiskanen, M., Lapinlampi, N., Kukkonen, J., Leinonen, V., Puhakka, N., & Pitkänen, A. (2021). Plasma miR-9-3p and miR-136-3p as Potential Novel Diagnostic Biomarkers for Experimental and Human Mild Traumatic Brain Injury. International Journal of Molecular Sciences, 22(4), 1563. https://doi.org/10.3390/ijms22041563






