Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues
Abstract
1. Introduction
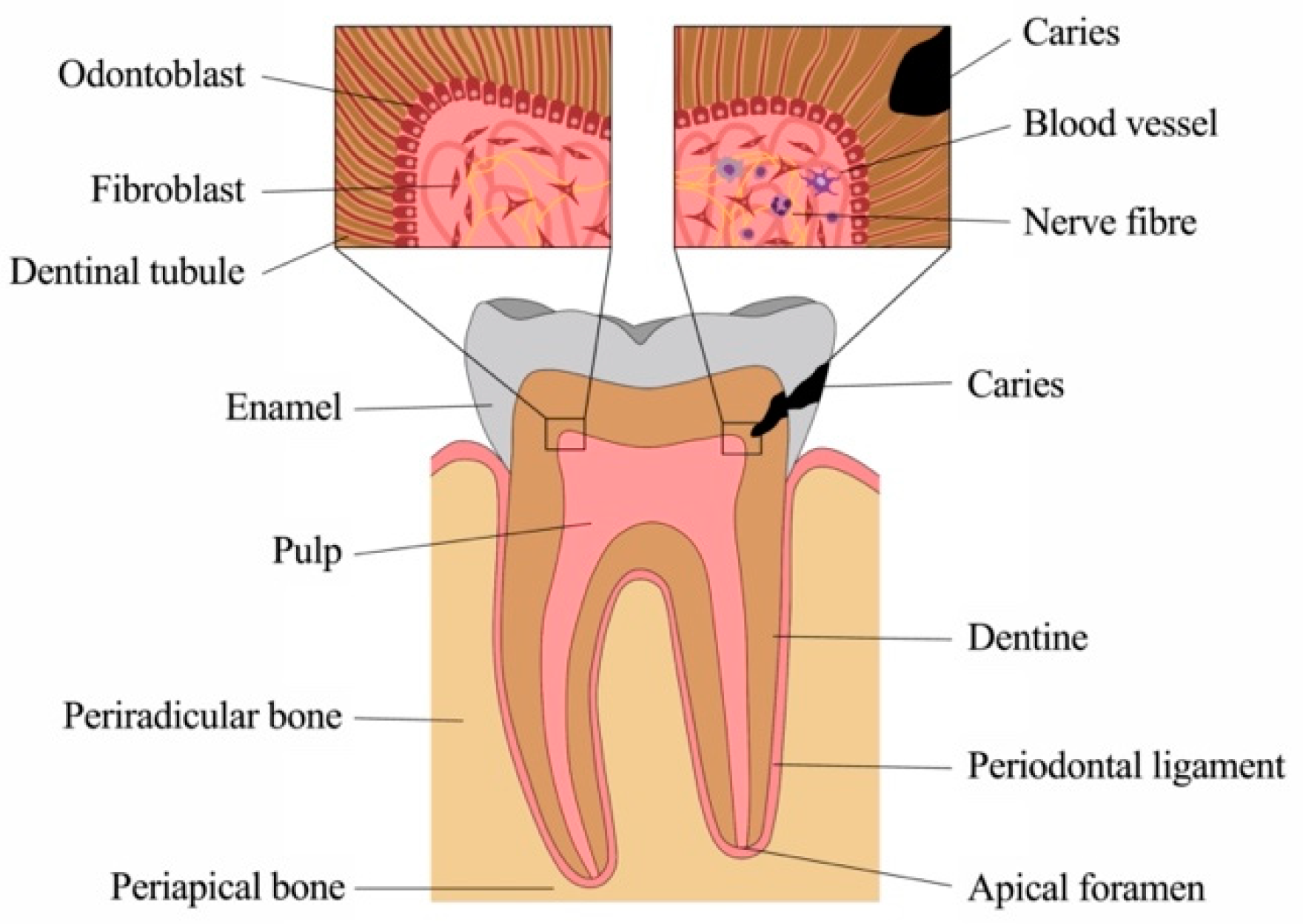
1.1. Anatomy and Physiology of Sound Dental Tissues
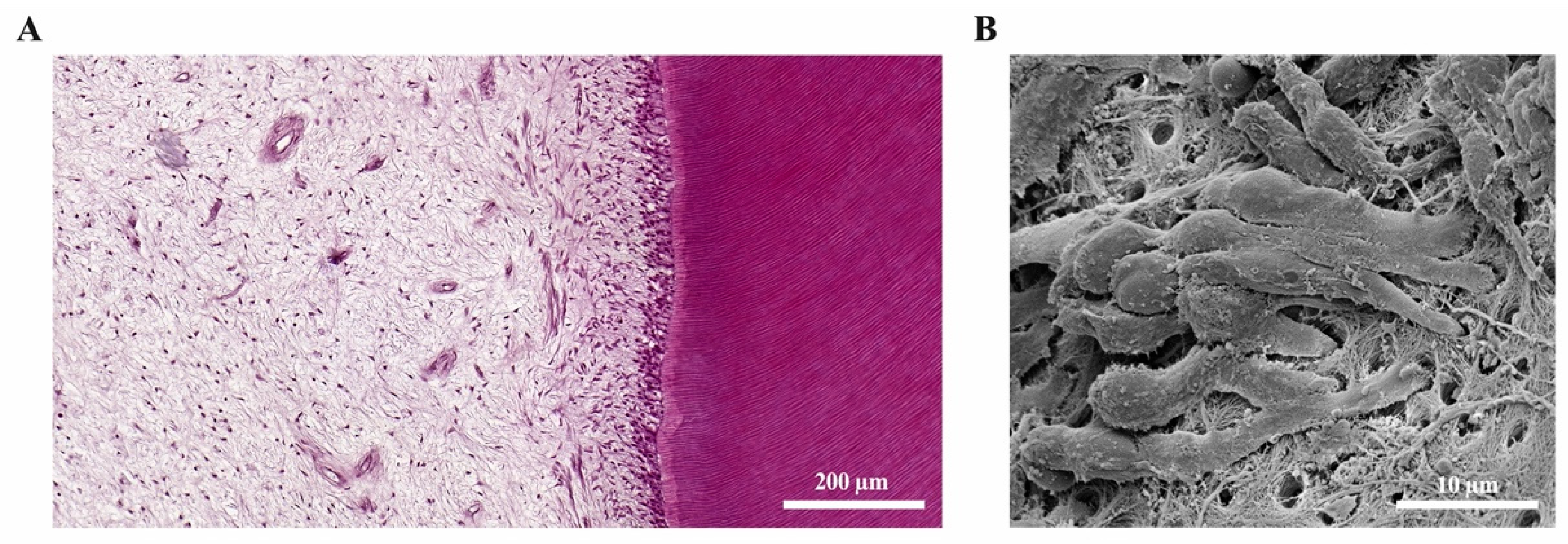
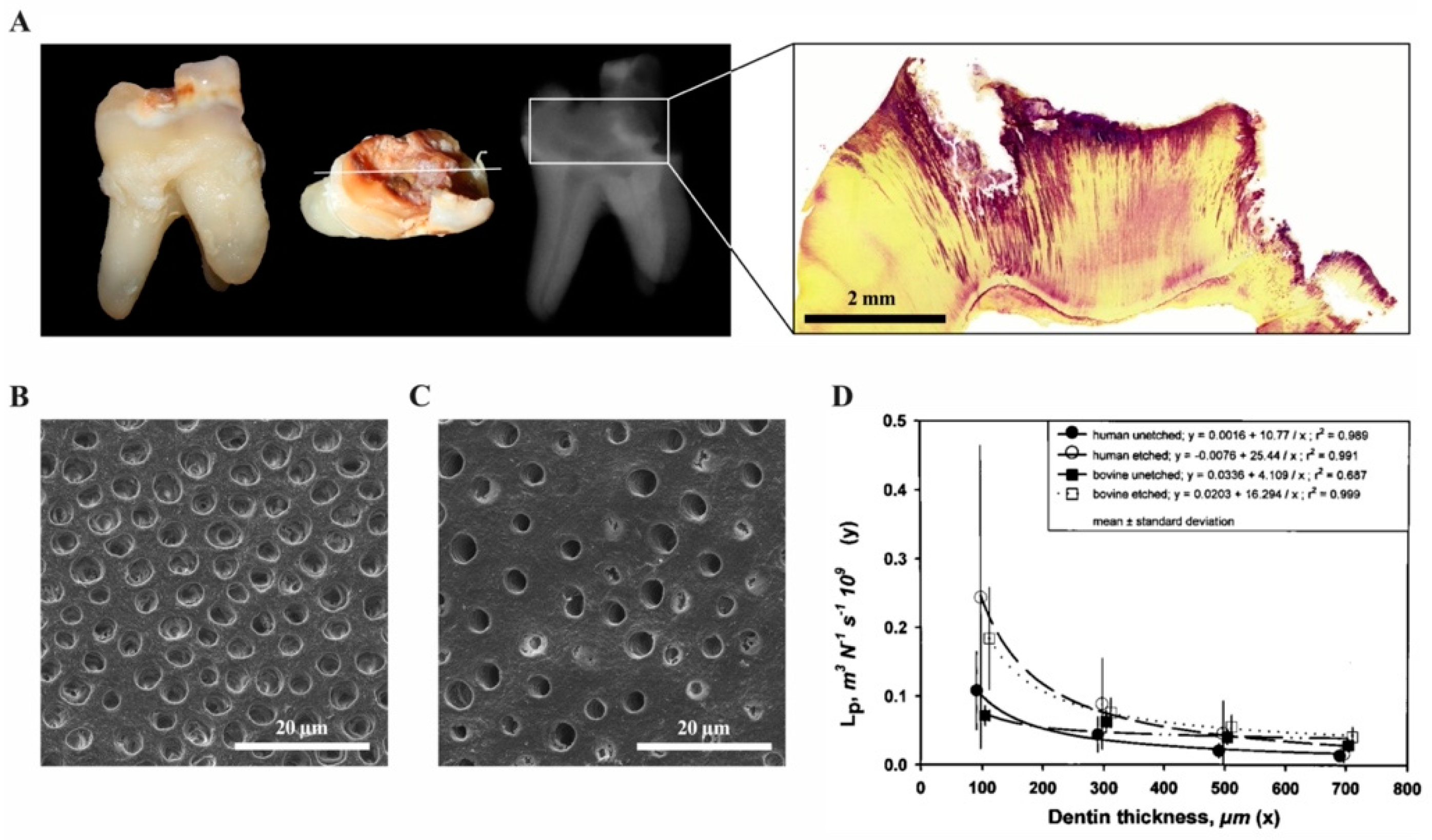
1.1.1. Physiology of the Dentine-Pulp Complex
1.1.2. Physiology of the Apical Periodontium and Periradicular Tissues
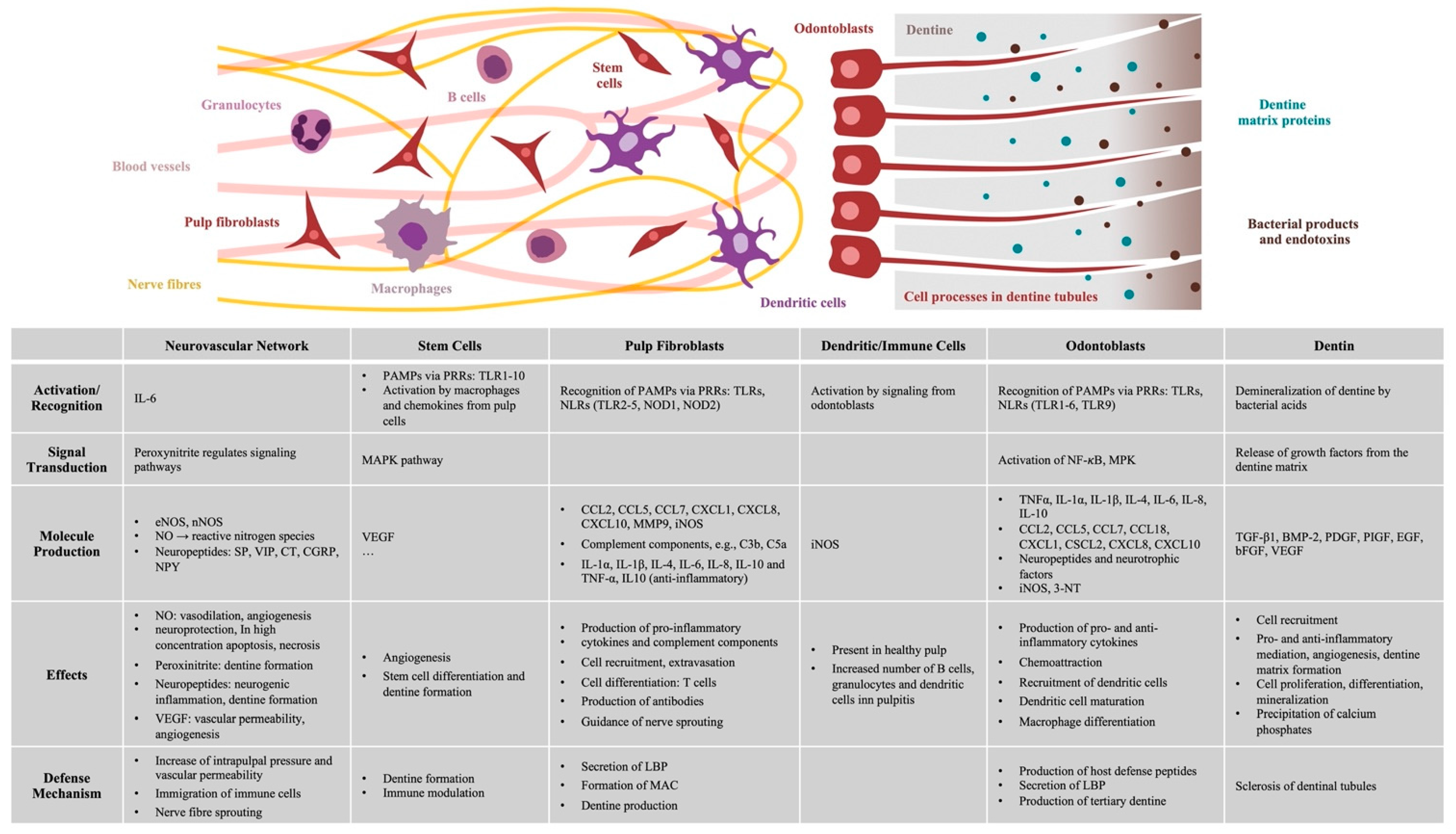
2. Inflammatory Response of the Dental Pulp
2.1. Recognition of Pathogens and Signal Transmission
2.2. The Role of Odontoblasts
2.3. Immunocompetence of Pulp Fibroblasts
2.4. Inflammatory Signaling Molecules and Accumulation of Immune Cells
2.5. Tertiary Dentine Formation
2.6. Vascular and Neuronal Networks
2.7. The Role of Signaling Molecules within the Dentine Matrix
3. Inflammatory Responses in the Periapical Bone
4. Resolution of Inflammatory Responses
4.1. Healing of the Dental Pulp
4.2. Healing of the Periradicular Bone
4.3. Stem cells in Repair and Regeneration
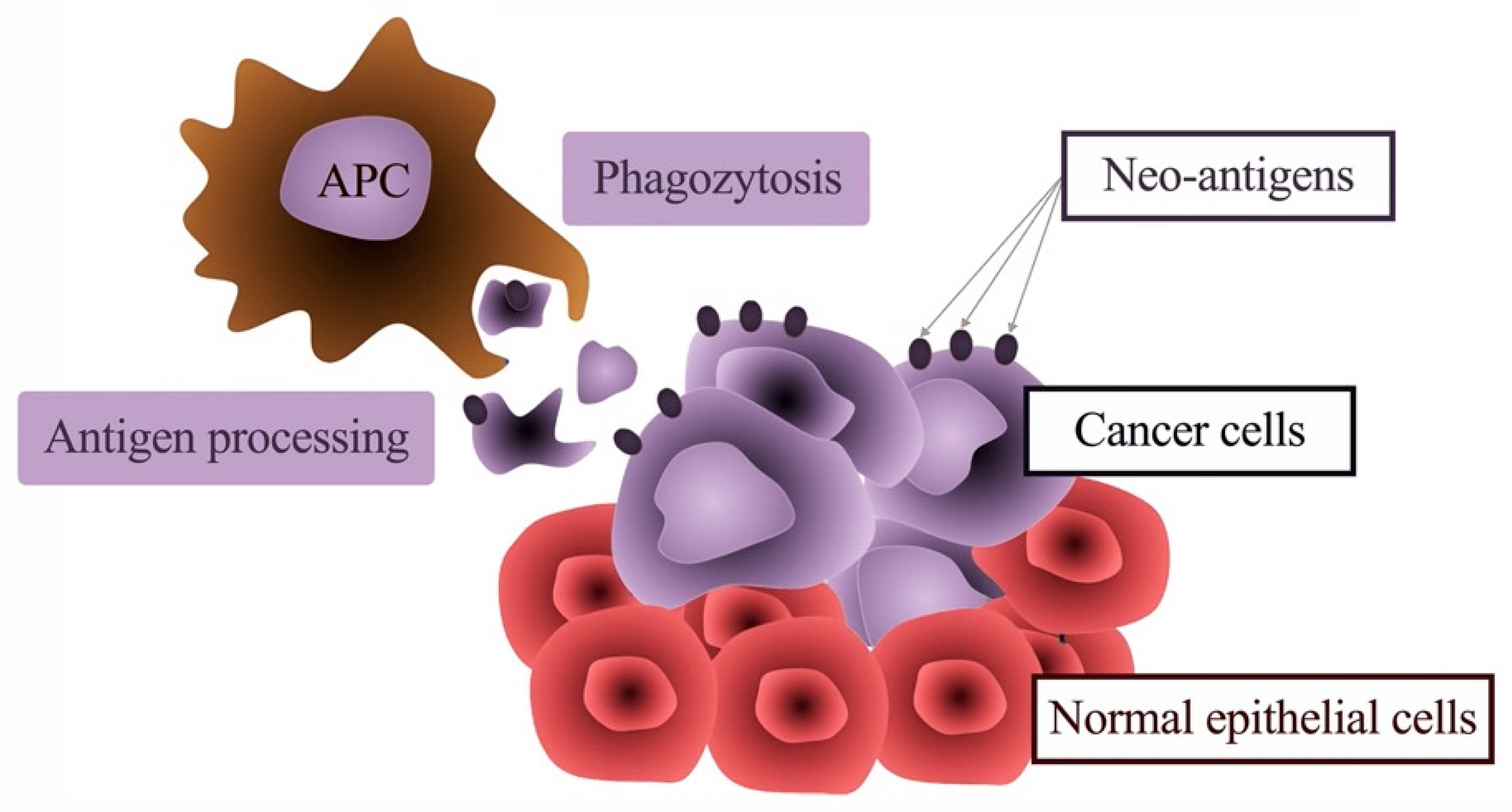
5. The Link between Inflammation and Malignant Transformation
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 3-NT | 3-nitrotyrosine |
| BCC | basal cell carcinoma |
| BCG | Bacille Calmette–Guerin |
| BD | beta-defensins |
| BDNF | brain-derived neurotrophic factor |
| bFGF | basic fibroblast growth factor |
| BH4 | tetrahydrobiopterin |
| BMP | bone morphogenetic protein |
| CCL | chemokine (C-C motif) ligand |
| CD | cluster of differentiation |
| CGRP | calcitonin gene-related peptide |
| CT | calcitonin |
| CXCL | chemokine (C-X-C motif) ligand |
| DC | dendritic cell |
| EGF | epidermal growth factor |
| ERM | epithelial cell rests of Malassez |
| HDP | host defense peptides |
| HERS | Hertwig’s epithelial root sheath |
| HLA | human leukocyte antigen |
| IFN | interferon |
| IL | interleukin |
| LBP | lipopolysaccharide-binding protein |
| LPS | lipopolysaccharide |
| LTA | lipoteichoic acid |
| MCSF | macrophage colony-stimulating factor |
| MAC | membrane attack complex |
| MHC | major histocompatibility complex |
| MPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor-κB |
| NGF | nerve growth factor |
| NK | natural killer |
| NLR | NOD-like receptors |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NPY | neuropeptide Y |
| OLP | oral leukoplakia |
| OONO- | peroxynitrite |
| OSCC | oral squamous cell carcinoma |
| PAMP | pathogen-associated molecular patterns |
| PDGF | platelet-derived growth factor |
| PIGF | placenta growth factor |
| PRR | pattern-recognition receptors |
| RNS | reactive nitrogen species |
| sGC | soluble guanylyl cyclase |
| SP | substance P |
| TGF | transforming growth factor |
| TLR | Toll-like receptors |
| TNF | tumor-necrosis-factor |
| Treg | regulatory T cell |
| VEGF | vascular endothelial growth factor |
| VIP | vasoactive intestinal polypeptide |
References
- Cordero, D.R.; Brugmann, S.; Chu, Y.; Bajpai, R.; Jame, M.; Helms, J.A. Cranial neural crest cells on the move: Their roles in craniofacial development. Am. J. Med. Genet. A 2011, 155A, 270–279. [Google Scholar] [CrossRef]
- Adameyko, I.; Fried, K. The nervous system orchestrates and integrates craniofacial development: A review. Front. Physiol. 2016, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.-G. Cranial neural crest: Migratory cell behavior and regulatory networks. Exp. Cell Res. 2014, 325, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.-L.; Liewehr, F.R. Innate immune responses of the dental pulp to caries. J. Endod. 2007, 33, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Staquet, M.-J.; Durand, S.H.; Colomb, E.; Romeas, A.; Vincent, C.; Bleicher, F.; Lebecque, S.; Farges, J.-C. Different roles of odontoblasts and fibroblasts in immunity. J. Dent. Res. 2008, 87, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Farges, J.-C.; Alliot-Licht, B.; Baudouin, C.; Msika, P.; Bleicher, F.; Carrouel, F. Odontoblast control of dental pulp inflammation triggered by cariogenic bacteria. Front. Physiol. 2013, 4, 326. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. 2011, 3, 711–735. [Google Scholar] [CrossRef]
- Chung, G.; Jung, S.J.; Oh, S.B. Cellular and molecular mechanisms of dental nociception. J. Dent. Res. 2013, 92, 948–955. [Google Scholar] [CrossRef]
- Jontell, M.; Gunraj, M.N.; Bergenholtz, G. Immunocompetent cells in the normal dental pulp. J. Dent. Res. 1987, 66, 1149–1153. [Google Scholar] [CrossRef]
- Goldberg, M.; Farges, J.-C.; Lacerda-Pinheiro, S.; Six, N.; Jegat, N.; Decup, F.; Septier, D.; Carrouel, F.; Durand, S.; Chaussain-Miller, C.; et al. Inflammatory and immunological aspects of dental pulp repair. Pharmacol. Res. 2008, 58, 137–147. [Google Scholar] [CrossRef]
- Gaudin, A.; Renard, E.; Hill, M.; Bouchet-Delbos, L.; Bienvenu-Louvet, G.; Farges, J.-C.; Cuturi, M.-C.; Alliot-Licht, B. Phenotypic analysis of immunocompetent cells in healthy human dental pulp. J. Endod. 2015, 41, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Krifka, S.; Widbiller, M.; Schröder, A.; Brochhausen, C.; Cataldi, A.; Hiller, K.-A.; Buchalla, W.; Schweikl, H. Distinguished properties of cells isolated from the dentin-pulp interface. Ann. Anat. 2020, 234, 151628. [Google Scholar] [CrossRef] [PubMed]
- García, C.C.; Sempere, F.V.; Diago, M.P.; Bowen, E.M. The post-endodontic periapical lesion: Histologic and etiopathogenic aspects. Med. Oral Patol. Oral Cir. Bucal 2007, 12, E585–E590. [Google Scholar] [PubMed]
- Fukada, S.Y.; Silva, T.A.; Garlet, G.P.; Rosa, A.L.; da Silva, J.S.; Cunha, F.Q. Factors involved in the T helper type 1 and type 2 cell commitment and osteoclast regulation in inflammatory apical diseases. Oral Microbiol. Immunol. 2009, 24, 25–31. [Google Scholar] [CrossRef]
- Xiong, J.; Gronthos, S.; Bartold, P.M. Role of the epithelial cell rests of Malassez in the development, maintenance and regeneration of periodontal ligament tissues. Periodontology 2000 2013, 63, 217–233. [Google Scholar] [CrossRef]
- Lin, L.M.; Huang, G.T.-J.; Rosenberg, P.A. Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J. Endod. 2007, 33, 908–916. [Google Scholar] [CrossRef]
- Love, R.M.; Jenkinson, H.F. Invasion of dentinal tubules by oral bacteria. Crit. Rev. Oral Biol. Med. 2002, 13, 171–183. [Google Scholar] [CrossRef]
- Schmalz, G.; Hiller, K.-A.; Nunez, L.J.; Stoll, J.; Weis, K. Permeability characteristics of bovine and human dentin under different pretreatment conditions. J. Endod. 2001, 27, 23–30. [Google Scholar] [CrossRef]
- Durand, S.H.; Flacher, V.; Roméas, A.; Carrouel, F.; Colomb, E.; Vincent, C.; Magloire, H.; Couble, M.-L.; Bleicher, F.; Staquet, M.-J.; et al. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J. Immunol. 2006, 176, 2880–2887. [Google Scholar] [CrossRef]
- Veerayutthwilai, O.; Byers, M.R.; Pham, T.-T.T.; Darveau, R.P.; Dale, B.A. Differential regulation of immune responses by odontoblasts. Oral Microbiol. Immunol. 2007, 22, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.-F.; Carrouel, F.; Colomb, E.; Durand, S.H.; Baudouin, C.; Msika, P.; Bleicher, F.; Vincent, C.; Staquet, M.-J.; Farges, J.-C. Toll-like receptor 2 activation by lipoteichoic acid induces differential production of pro-inflammatory cytokines in human odontoblasts, dental pulp fibroblasts and immature dendritic cells. Immunobiology 2010, 215, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Klingebiel, P.; Dörfer, C.E. Toll-like receptor expression profile of human dental pulp stem/progenitor cells. J. Endod. 2016, 42, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Staquet, M.-J.; Carrouel, F.; Keller, J.-F.; Baudouin, C.; Msika, P.; Bleicher, F.; Kufer, T.A.; Farges, J.-C. Pattern-recognition receptors in pulp defense. Adv. Dent. Res. 2011, 23, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Farges, J.-C.; Keller, J.-F.; Carrouel, F.; Durand, S.H.; Roméas, A.; Bleicher, F.; Lebecque, S.; Staquet, M.-J. Odontoblasts in the dental pulp immune response. J. Exp. Zool. Part B Mol. Dev. Evol. 2009, 312B, 425–436. [Google Scholar] [CrossRef]
- Farges, J.-C.; Romeas, A.; Melin, M.; Pin, J.-J.; Lebecque, S.; Lucchini, M.; Bleicher, F.; Magloire, H. TGF-beta1 induces accumulation of dendritic cells in the odontoblast layer. J. Dent. Res. 2003, 82, 652–656. [Google Scholar] [CrossRef]
- Farges, J.-C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental pulp defence and repair mechanisms in dental caries. Mediators Inflamm. 2015, 2015, 230251. [Google Scholar] [CrossRef]
- Renard, E.; Gaudin, A.; Bienvenu, G.; Amiaud, J.; Farges, J.-C.; Cuturi, M.C.; Moreau, A.; Alliot-Licht, B. Immune cells and molecular networks in experimentally induced pulpitis. J. Dent. Res. 2016, 95, 196–205. [Google Scholar] [CrossRef]
- Semple, F.; Dorin, J.R. β-Defensins: Multifunctional modulators of infection, inflammation and more? J. Innate Immun. 2012, 4, 337–348. [Google Scholar] [CrossRef]
- Pazgier, M.; Hoover, D.M.; Yang, D.; Lu, W.; Lubkowski, J. Human beta-defensins. Cell. Mol. Life Sci. 2006, 63, 1294–1313. [Google Scholar] [CrossRef]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.E.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E.W. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Kawsar, H.I.; Hirsch, S.A.; Zeng, C.; Jia, X.; Feng, Z.; Ghosh, S.K.; Zheng, Q.Y.; Zhou, A.; McIntyre, T.M.; et al. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS ONE 2010, 5, e10993. [Google Scholar] [CrossRef] [PubMed]
- Nijnik, A.; Pistolic, J.; Filewod, N.C.J.; Hancock, R.E.W. Signaling pathways mediating chemokine induction in keratinocytes by cathelicidin LL-37 and flagellin. J. Innate Immun. 2012, 4, 377–386. [Google Scholar] [CrossRef]
- Davidson, D.J.; Currie, A.J.; Reid, G.S.D.; Bowdish, D.M.E.; MacDonald, K.L.; Ma, R.C.; Hancock, R.E.W.; Speert, D.P. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004, 172, 1146–1156. [Google Scholar] [CrossRef]
- Pena, O.M.; Afacan, N.; Pistolic, J.; Chen, C.; Madera, L.; Falsafi, R.; Fjell, C.D.; Hancock, R.E.W. Synthetic cationic peptide IDR-1018 modulates human macrophage differentiation. PLoS ONE 2013, 8, e52449. [Google Scholar] [CrossRef]
- Dommisch, H.; Winter, J.; Açil, Y.; Dunsche, A.; Tiemann, M.; Jepsen, S. Human beta-defensin (hBD-1, -2) expression in dental pulp. Oral Microbiol. Immunol. 2005, 20, 163–166. [Google Scholar] [CrossRef]
- Dommisch, H.; Winter, J.; Willebrand, C.; Eberhard, J.; Jepsen, S. Immune regulatory functions of human beta-defensin-2 in odontoblast-like cells. Int. Endod. J. 2007, 40, 300–307. [Google Scholar] [CrossRef]
- Sass, V.; Schneider, T.; Wilmes, M.; Körner, C.; Tossi, A.; Novikova, N.; Shamova, O.; Sahl, H.-G. Human beta-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect. Immun. 2010, 78, 2793–2800. [Google Scholar] [CrossRef]
- Lee, J.-K.; Chang, S.W.; Perinpanayagam, H.; Lim, S.-M.; Park, Y.-J.; Han, S.H.; Baek, S.-H.; Zhu, Q.; Bae, K.-S.; Kum, K.-Y. Antibacterial efficacy of a human β-defensin-3 peptide on multispecies biofilms. J. Endod. 2013, 39, 1625–1629. [Google Scholar] [CrossRef]
- Okiji, T.; Jontell, M.; Belichenko, P.; Bergenholtz, G.; Dahlström, A. Perivascular dendritic cells of the human dental pulp. Acta Physiol. Scand. 1997, 159, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Okiji, T.; Kawashima, N.; Kosaka, T.; Matsumoto, A.; Kobayashi, C.; Suda, H. An immunohistochemical study of the distribution of immunocompetent cells, especially macrophages and Ia antigen-expressing cells of heterogeneous populations, in normal rat molar pulp. J. Dent. Res. 1992, 71, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Bergenholtz, G.; Nagaoka, S.; Jontell, M. Class II antigen expressing cells in experimentally induced pulpitis. Int. Endod. J. 1991, 24, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jontell, M.; Okiji, T.; Dahlgren, U.; Bergenholtz, G. Immune defense mechanisms of the dental pulp. Crit. Rev. Oral Biol. Med. 1998, 9, 179–200. [Google Scholar] [CrossRef]
- Telles, P.D.S.; Hanks, C.T.; Machado, M.A.A.M.; Nör, J.E. Lipoteichoic acid up-regulates VEGF expression in macrophages and pulp cells. J. Dent. Res. 2003, 82, 466–470. [Google Scholar] [CrossRef]
- Hahn, C.L.; Falkler, W.A.; Siegel, M.A. A study of T and B cells in pulpal pathosis. J. Endod. 1989, 15, 20–26. [Google Scholar] [CrossRef]
- Martin, F.E.; Nadkarni, M.A.; Jacques, N.A.; Hunter, N. Quantitative microbiological study of human carious dentine by culture and real-time PCR: Association of anaerobes with histopathological changes in chronic pulpitis. J. Clin. Microbiol. 2002, 40, 1698–1704. [Google Scholar] [CrossRef]
- Izumi, T.; Kobayashi, I.; Okamura, K.; Sakai, H. Immunohistochemical study on the immunocompetent cells of the pulp in human non-carious and carious teeth. Arch. Oral Biol 1995, 40, 609–614. [Google Scholar] [CrossRef]
- Hirao, K.; Yumoto, H.; Takahashi, K.; Mukai, K.; Nakanishi, T.; Matsuo, T. Roles of TLR2, TLR4, NOD2, and NOD1 in pulp fibroblasts. J. Dent. Res. 2009, 88, 762–767. [Google Scholar] [CrossRef]
- Chmilewsky, F.; Jeanneau, C.; Laurent, P.; About, I. Pulp fibroblasts synthesize functional complement proteins involved in initiating dentin-pulp regeneration. Am. J. Pathol. 2014, 184, 1991–2000. [Google Scholar]
- Jeanneau, C.; Rufas, P.; Rombouts, C.; Giraud, T.; Dejou, J.; About, I. Can pulp fibroblasts kill cariogenic bacteria? Role of complement activation. J. Dent. Res. 2015, 94, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; About, I.; Chung, S.H. Pulp fibroblasts control nerve regeneration through complement activation. J. Dent. Res. 2016, 95, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Byers, M.R.; Kvinnsland, I.; Bothwell, M. Analysis of low affinity nerve growth factor receptor during pulpal healing and regeneration of myelinated and unmyelinated axons in replanted teeth. J. Comp. Neurol. 1992, 326, 470–484. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, J.L.; Sloan, A.J.; Smith, A.J.; Landini, G.; Cooper, P.R. S100 and cytokine expression in caries. Infect. Immun. 2004, 72, 4102–4108. [Google Scholar] [PubMed]
- Farges, J.-C.; Carrouel, F.; Keller, J.-F.; Baudouin, C.; Msika, P.; Bleicher, F.; Staquet, M.-J. Cytokine production by human odontoblast-like cells upon Toll-like receptor-2 engagement. Immunobiology 2011, 216, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Fedele, S.; D’Aiuto, F.; Donos, N. Interleukin-6 in oral diseases: A review. Oral Dis. 2012, 18, 236–243. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Li, M.O.; Flavell, R.A. Contextual regulation of inflammation: A duet by transforming growth factor-beta and interleukin-10. Immunity 2008, 28, 468–476. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Kaji, R.; Kiyoshima-Shibata, J.; Nagaoka, M.; Nanno, M.; Shida, K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J. Immunol. 2010, 184, 3505–3513. [Google Scholar] [CrossRef]
- Magloire, H.; Romeas, A.; Melin, M.; Couble, M.L.; Bleicher, F.; Farges, J.-C. Molecular regulation of odontoblast activity under dentin injury. Adv. Dent. Res. 2001, 15, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J. Structure-function aspects in the nitric oxide synthases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Marletta, M.A. Nitric oxide synthase: Aspects concerning structure and catalysis. Cell 1994, 78, 927–930. [Google Scholar] [CrossRef]
- Griffith, O.W.; Stuehr, D.J. Nitric oxide synthases: Properties and catalytic mechanism. Annu. Rev. Physiol. 1995, 57, 707–736. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S.; Snyder, S.H. Nitric oxide, a novel neuronal messenger. Neuron 1992, 8, 3–11. [Google Scholar] [CrossRef]
- Garthwaite, J. Neuronal nitric oxide synthase and the serotonin transporter get harmonious. Proc. Natl. Acad. Sci. USA 2007, 104, 7739–7740. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010, 459, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Garthwaite, J. What is the real physiological NO concentration in vivo? Nitric Oxide 2009, 21, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Attwell, D. Assessing the physiological concentration and targets of nitric oxide in brain tissue. J. Physiol. 2008, 586, 3597–3615. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Förstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000, 190, 244–254. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M.G. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef] [PubMed]
- Gantner, B.N.; LaFond, K.M.; Bonini, M.G. Nitric oxide in cellular adaptation and disease. Redox Biol. 2020, 34, 101550. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, R.; Suzawa, T.; Miyamoto, Y.; Wang, X.; Takami, M.; Yamada, A.; Kamijo, R. Nitric oxide in pulp cell growth, differentiation, and mineralization. J. Dent. Res. 2007, 86, 163–168. [Google Scholar] [CrossRef]
- Lohinai, Z.; Balla, I.; Marczis, J.; Vass, Z.; Kovách, A.G. Evidence for the role of nitric oxide in the circulation of the dental pulp. J. Dent. Res. 1995, 74, 1501–1506. [Google Scholar] [CrossRef]
- Korkmaz, Y.; Baumann, M.A.; Steinritz, D.; Schröder, H.; Behrends, S.; Addicks, K.; Schneider, K.; Raab, W.H.-M.; Bloch, W. NO-cGMP signaling molecules in cells of the rat molar dentin-pulp complex. J. Dent. Res. 2005, 84, 618–623. [Google Scholar] [CrossRef]
- Felaco, M.; Di Maio, F.D.; De Fazio, P.; D’Arcangelo, C.; De Lutiis, M.A.; Varvara, G.; Grilli, A.; Barbacane, R.C.; Reale, M.; Conti, P. Localization of the e-NOS enzyme in endothelial cells and odontoblasts of healthy human dental pulp. Life Sci. 2000, 68, 297–306. [Google Scholar] [CrossRef]
- Asano, K.; Chee, C.B.; Gaston, B.; Lilly, C.M.; Gerard, C.; Drazen, J.M.; Stamler, J.S. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc. Natl. Acad. Sci. USA 1994, 91, 10089–10093. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.N.; Peroni, D.J.; Basclain, K.A.; Garlepp, M.J.; Thompson, P.J. Expression and activity of nitric oxide synthases in human airway epithelium. Am. J. Respir. Cell Mol. Biol. 1997, 16, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Reynolds, P.R.; Johns, R.A. Developmental expression of NOS isoforms in fetal rat lung: Implications for transitional circulation and pulmonary angiogenesis. Am. J. Physiol. 1996, 270, L88–L100. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W.; Fleischmann, B.K.; Lorke, D.E.; Andressen, C.; Hops, B.; Hescheler, J.; Addicks, K. Nitric oxide synthase expression and role during cardiomyogenesis. Cardiovasc. Res. 1999, 43, 675–684. [Google Scholar] [CrossRef]
- Di Nardo Di Maio, F.; Lohinai, Z.; D’Arcangelo, C.; De Fazio, P.E.; Speranza, L.; De Lutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M. Nitric oxide synthase in healthy and inflamed human dental pulp. J. Dent. Res. 2004, 83, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, Y.; Lang, H.; Beikler, T.; Cho, B.; Behrends, S.; Bloch, W.; Addicks, K.; Raab, W.H.-M. Irreversible inflammation is associated with decreased levels of the alpha1-, beta1-, and alpha2-subunits of sGC in human odontoblasts. J. Dent. Res. 2011, 90, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, L.; Darvann, T. A light microscopic study of odontoblastic and non-odontoblastic cells involved in tertiary dentinogenesis in well-defined cavitated carious lesions. Caries Res. 1999, 33, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Cassidy, N.; Perry, H.; Bègue-Kirn, C.; Ruch, J.V.; Lesot, H. Reactionary dentinogenesis. Int. J. Dev. Biol. 1995, 39, 273–280. [Google Scholar] [PubMed]
- Simon, S.R.J.; Berdal, A.; Cooper, P.R.; Lumley, P.J.; Tomson, P.L.; Smith, A.J. Dentin-pulp complex regeneration: From lab to clinic. Adv. Dent. Res. 2011, 23, 340–345. [Google Scholar] [CrossRef]
- Cooper, P.R.; Holder, M.J.; Smith, A.J. Inflammation and regeneration in the dentin-pulp complex: A double-edged sword. J. Endod. 2014, 40, S46–S51. [Google Scholar] [CrossRef]
- Widbiller, M.; Eidt, A.; Lindner, S.R.; Hiller, K.-A.; Schweikl, H.; Buchalla, W.; Galler, K.M. Dentine matrix proteins: Isolation and effects on human pulp cells. Int. Endod. J. 2018, 51 (Suppl. 4), e278–e290. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Signaling Molecules and Pulp Regeneration. J. Endod. 2017, 43, S7–S11. [Google Scholar] [CrossRef]
- Ten Cate, J.M. In situ models, physico-chemical aspects. Adv. Dent. Res. 1994, 8, 125–133. [Google Scholar] [CrossRef]
- Widbiller, M.; Schmalz, G. Endodontic regeneration: Hard shell, soft core. Odontology 2020, 2015, 1–10. [Google Scholar]
- Caviedes-Bucheli, J.; Muñoz, H.R.; Azuero-Holguín, M.M.; Ulate, E. Neuropeptides in dental pulp: The silent protagonists. J. Endod. 2008, 34, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Rodd, H.D.; Boissonade, F.M. Vascular status in human primary and permanent teeth in health and disease. Eur. J. Oral Sci. 2005, 113, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, C.L.; Byers, M.R. Inflammation of rat molar pulp and periodontium causes increased calcitonin gene-related peptide and axonal sprouting. Anat. Rec. 1988, 222, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Byers, M.R.; Taylor, P.E. Effect of sensory denervation on the response of rat molar pulp to exposure injury. J. Dent. Res. 1993, 72, 613–618. [Google Scholar] [CrossRef]
- Hanoun, M.; Maryanovich, M.; Arnal-Estapé, A.; Frenette, P.S. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 2015, 86, 360–373. [Google Scholar] [CrossRef]
- Maita, E.; Simpson, M.D.; Tao, L.; Pashley, D.H. Fluid and protein flux across the pulpodentine complex of the dog in vivo. Arch. Oral Biol. 1991, 36, 103–110. [Google Scholar] [CrossRef]
- Matthews, B.; Vongsavan, N. Interactions between neural and hydrodynamic mechanisms in dentine and pulp. Arch. Oral Biol. 1994, 39, 87S–95S. [Google Scholar] [CrossRef]
- Nagaoka, S.; Miyazaki, Y.; Liu, H.J.; Iwamoto, Y.; Kitano, M.; Kawagoe, M. Bacterial invasion into dentinal tubules of human vital and nonvital teeth. J. Endod. 1995, 21, 70–73. [Google Scholar] [CrossRef]
- Mastrangelo, F.; Sberna, M.T.; Tettamanti, L.; Cantatore, G.; Tagliabue, A.; Gherlone, E. Vascular endothelial growth factor and nitric oxide synthase expression in human tooth germ development. J. Biol. Regul. Homeost. Agents 2016, 30, 421–432. [Google Scholar]
- Canzobre, M.C.; Ríos, H. Nicotinamide adenine dinucleotide phosphate/neuronal nitric oxide synthase-positive neurons in the trigeminal subnucleus caudalis involved in tooth pulp nociception. J. Neurosci. Res. 2011, 89, 1478–1488. [Google Scholar] [CrossRef]
- Towler, P.K.; Bennett, G.S.; Moore, P.K.; Brain, S.D. Neurogenic oedema and vasodilatation: Effect of a selective neuronal NO inhibitor. Neuroreport 1998, 9, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Williamson, D.J.; Kaube, H.; Goadsby, P.J. Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 2002, 137, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Joo, K.M.; Chung, Y.H.; Lee, Y.J.; Kim, J.; Lee, B.H.; Shin, D.H.; Lee, K.H.; Cha, C.I. Vasoactive intestinal peptide (VIP) and VIP mRNA decrease in the cerebral cortex of nNOS knock-out(-/-) mice. Brain Res. 2003, 978, 233–240. [Google Scholar] [CrossRef]
- Yao, G.; Man, Y.-H.; Li, A.-R.; Guo, Y.; Dai, Y.; Wang, P.; Zhou, Y.-F. NO up-regulates migraine-related CGRP via activation of an Akt/GSK-3β/NF-κB signaling cascade in trigeminal ganglion neurons. Aging 2020, 12, 6370–6384. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Daiber, A.; Ullrich, V.; Mülsch, A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- McNeill, E.; Channon, K.M. The role of tetrahydrobiopterin in inflammation and cardiovascular disease. Thromb. Haemost. 2012, 108, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Daiber, A. Role of endothelial and macrophage tetrahydrobiopterin in development and progression of atherosclerosis: BH4 puzzle solved? Cardiovasc. Res. 2018, 114, 1310–1312. [Google Scholar] [CrossRef]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- Finkelman, R.D.; Mohan, S.; Jennings, J.C.; Taylor, A.K.; Jepsen, S.; Baylink, D.J. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J. Bone Miner. Res. 1990, 5, 717–723. [Google Scholar]
- Roberts-Clark, D.J.; Smith, A.J. Angiogenic growth factors in human dentine matrix. Arch. Oral Biol. 2000, 45, 1013–1016. [Google Scholar] [CrossRef]
- Widbiller, M.; Schweikl, H.; Bruckmann, A.; Rosendahl, A.; Hochmuth, E.; Lindner, S.R.; Buchalla, W.; Galler, K.M. Shotgun Proteomics of Human Dentin with Different Prefractionation Methods. Sci. Rep. 2019, 9, 4457. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, J.L.; Regatieri, C.V.; Jarrouge, T.R.; Cavalheiro, R.P.; Sampaio, L.O.; Nader, H.B. Heparan sulfate proteoglycans: Structure, protein interactions and cell signaling. An. Acad. Bras. Cienc. 2009, 81, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.M.; Sugars, R.V.; Wendel, M.; Smith, A.J.; Waddington, R.J.; Cooper, P.R.; Sloan, A.J. TGF-beta/extracellular matrix interactions in dentin matrix: A role in regulating sequestration and protection of bioactivity. Calcif. Tissue Int. 2009, 85, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Patel, Y.; Murray, J.; Patel, K.V.; Sumathipala, R.; Sobel, M.; Wijelath, E.S. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005, 6, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, R.; Ruehl, M.; Tiling, N.; Ackermann, R.; Schmid, M.; Riecken, E.O.; Schuppan, D. Collagens serve as an extracellular store of bioactive interleukin 2. J. Biol. Chem. 2000, 275, 38170–38175. [Google Scholar] [CrossRef]
- Paralkar, V.M.; Vukicevic, S.; Reddi, A.H. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: Implications for development. Dev. Biol. 1991, 143, 303–308. [Google Scholar] [CrossRef]
- Dung, S.Z.; Gregory, R.L.; Li, Y.; Stookey, G.K. Effect of lactic acid and proteolytic enzymes on the release of organic matrix components from human root dentin. Caries Res. 1995, 29, 483–489. [Google Scholar] [CrossRef]
- Widbiller, M.; Austah, O.; Lindner, S.R.; Sun, J.; Diogenes, A.R. Neurotrophic proteins in dentin and their effect on trigeminal sensory neurons. J. Endod. 2019, 45, 729–735. [Google Scholar] [CrossRef]
- Smith, A.J.; Scheven, B.A.; Takahashi, Y.; Ferracane, J.L.; Shelton, R.M.; Cooper, P.R. Dentine as a bioactive extracellular matrix. Arch. Oral Biol. 2012, 57, 109–121. [Google Scholar] [CrossRef]
- Cassidy, N.; Fahey, M.; Prime, S.S.; Smith, A.J. Comparative analysis of transforming growth factor-beta isoforms 1-3 in human and rabbit dentine matrices. Arch. Oral Biol. 1997, 42, 219–223. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Smith, A.J.; Cooper, P.R.; Nör, J.E.; Smith, G. Angiogenic activity of dentin matrix components. J. Endod. 2011, 37, 26–30. [Google Scholar] [CrossRef]
- Smith, A.J.; Murray, P.E.; Sloan, A.J.; Matthews, J.B.; Zhao, S. Trans-dentinal stimulation of tertiary dentinogenesis. Adv. Dent. Res. 2001, 15, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Eidt, A.; Wölflick, M.; Lindner, S.R.; Schweikl, H.; Hiller, K.-A.; Buchalla, W.; Galler, K.M. Interactive effects of LPS and dentine matrix proteins on human dental pulp stem cells. Int. Endod. J. 2018, 51, 877–888. [Google Scholar] [CrossRef]
- He, W.-X.; Wang, Z.; Luo, Z.; Yu, Q.; Jiang, Y.; Zhang, Y.; Zhou, Z.; Smith, A.J.; Cooper, P.R. LPS promote the odontoblastic differentiation of human dental pulp stem cells via MAPK signaling pathway. J. Cell Physiol. 2015, 230, 554–561. [Google Scholar] [CrossRef]
- Teixeira-Salum, T.B.; Rodrigues, D.B.R.; Gervásio, A.M.; Souza, C.J.A.; Rodrigues, V.; Loyola, A.M. Distinct Th1, Th2 and Treg cytokines balance in chronic periapical granulomas and radicular cysts. J. Oral Pathol. Med. 2010, 39, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, P.; Teles, R.; D’Souza, R. Periapical inflammatory responses and their modulation. Crit. Rev. Oral Biol. Med. 1998, 9, 498–521. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Schlittenbauer, T.; Moebius, P.; Büttner-Herold, M.; Ries, J.; Preidl, R.; Geppert, C.-I.; Neukam, F.W.; Wehrhan, F. Macrophage polarization differs between apical granulomas, radicular cysts, and dentigerous cysts. Clin. Oral Investig. 2018, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Ries, J.; Büttner-Herold, M.; Geppert, C.-I.; Kesting, M.; Wehrhan, F. Differences in inflammation and bone resorption between apical granulomas, radicular cysts, and dentigerous cysts. J. Endod. 2019, 45, 1200–1208. [Google Scholar]
- Silva, L.A.B.D.; Sá, M.A.R.; Melo, R.A.; Pereira, J.D.S.; Silveira, É.J.D.D.; Miguel, M.C.D.C. Analysis of CD57+ natural killer cells and CD8+ T lymphocytes in periapical granulomas and radicular cysts. Braz. Oral Res. 2017, 31, e106. [Google Scholar] [CrossRef]
- Rodini, C.O.; Lara, V.S. Study of the expression of CD68+ macrophages and CD8+ T cells in human granulomas and periapical cysts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; McLachlan, J.L.; Simon, S.; Graham, L.W.; Smith, A.J. Mediators of inflammation and regeneration. Adv. Dent. Res. 2011, 23, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, L.; Demant, S.; Dabelsteen, S. Depth and activity of carious lesions as indicators for the regenerative potential of dental pulp after intervention. J. Endod. 2014, 40, S76–S81. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Fishbein, M.C.; Lew, H.; Maroko, P.R.; Braunwald, E. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation 1978, 57, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Shimono, M. Repair dentinogenesis following transplantation into normal and germ-free animals. Proc. Finn. Dent. Soc. 1992, 88 (Suppl. 1), 183–194. [Google Scholar]
- El Karim, I.A.; Linden, G.J.; Irwin, C.R.; Lundy, F.T. Neuropeptides regulate expression of angiogenic growth factors in human dental pulp fibroblasts. J. Endod. 2009, 35, 829–833. [Google Scholar] [CrossRef]
- Kline, L.W.; Yu, D.C. Effects of calcitonin, calcitonin gene-related peptide, human recombinant bone morphogenetic protein-2, and parathyroid hormone-related protein on endodontically treated ferret canines. J. Endod. 2009, 35, 866–869. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Luukko, K. Neurotrophins in odontogenesis. Int. J. Dev. Biol. 1995, 39, 195–202. [Google Scholar]
- Amano, O.; Bringas, P.; Takahashi, I.; Takahashi, K.; Yamane, A.; Chai, Y.; Nuckolls, G.H.; Shum, L.; Slavkin, H.C. Nerve growth factor (NGF) supports tooth morphogenesis in mouse first branchial arch explants. Dev. Dyn. 1999, 216, 299–310. [Google Scholar] [CrossRef]
- Arany, S.; Koyota, S.; Sugiyama, T. Nerve growth factor promotes differentiation of odontoblast-like cells. J. Cell Biochem. 2009, 106, 539–545. [Google Scholar] [CrossRef]
- Childs, D.R.; Murthy, A.S. Overview of wound healing and management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.F. Diabetes mellitus as a modulating factor of endodontic infections. J. Dent. Educ. 2003, 67, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Liu, R.; Oates, T.W. Diabetes-enhanced inflammation and apoptosis: Impact on periodontal pathosis. Periodontology 2000 2007, 45, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Thomas, B.; Ramesh, A. Comparison of neutrophil functions in diabetic and healthy subjects with chronic generalized periodontitis. J. Indian Soc. Periodontol. 2008, 12, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Tard, C.; Rouxel, O.; Lehuen, A. Regulatory role of natural killer T cells in diabetes. Biomed. J. 2015, 38, 484–495. [Google Scholar] [CrossRef]
- Cintra, L.T.A.; Samuel, R.O.; Azuma, M.M.; Ribeiro, C.P.; Narciso, L.G.; de Lima, V.M.F.; Sumida, D.H.; Coclete, G.A.; Dezan Junior, E.; Gomes-Filho, J.E. Apical periodontitis and periodontal disease increase serum IL-17 levels in normoglycemic and diabetic rats. Clin. Oral Investig. 2014, 18, 2123–2128. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef]
- Dimitrova-Nakov, S.; Baudry, A.; Harichane, Y.; Kellermann, O.; Goldberg, M. Pulp stem cells: Implication in reparative dentin formation. J. Endod. 2014, 40, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Neves, V.C.M.; Yianni, V.; Sharpe, P.T. Macrophage modulation of dental pulp stem cell activity during tertiary dentinogenesis. Sci. Rep. 2020, 10, 20216–20219. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Cleaton-Jones, P.E.; Austin, J.C.; Andreasen, J.O. Pulp reactions to exposure after experimental crown fractures or grinding in adult monkeys. J. Endod. 1982, 8, 391–397. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hobson, J.; Gummadidala, P.; Silverstrim, B.; Grier, D.; Bunn, J.; James, T.; Rincon, M. Acute inflammation induced by the biopsy of mouse mammary tumors promotes the development of metastasis. Breast Cancer Res. Treat. 2013, 139, 391–401. [Google Scholar] [CrossRef]
- Mukthinuthalapati, P.K.; Gotur, R.; Ghabril, M. Incidence, risk factors and outcomes of de novo malignancies post liver transplantation. World J. Hepatol. 2016, 8, 533–544. [Google Scholar] [CrossRef]
- Džambová, M.; Sečníková, Z.; Jiráková, A.; Jůzlová, K.; Viklický, O.; Hošková, L.; Göpfertovà, D.; Hercogová, J. Malignant melanoma in organ transplant recipients: Incidence, outcomes, and management strategies: A review of literature. Dermatol. Ther. 2016, 29, 64–68. [Google Scholar] [CrossRef]
- Francis, A.; Johnson, D.W.; Craig, J.C.; Wong, G. Incidence and predictors of cancer following kidney transplantation in childhood. Am. J. Transplant. 2017, 17, 2650–2658. [Google Scholar] [CrossRef]
- Scanlon, C.S.; Van Tubergen, E.A.; Inglehart, R.C.; D’Silva, N.J. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J. Dent. Res. 2013, 92, 114–121. [Google Scholar] [CrossRef]
- Tsantoulis, P.K.; Kastrinakis, N.G.; Tourvas, A.D.; Laskaris, G.; Gorgoulis, V.G. Advances in the biology of oral cancer. Oral Oncol. 2007, 43, 523–534. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Kitahara, H.; Kobayashi, Y.; Kato, K.; Bou-Gharios, G.; Nakamura, H.; Kawashiri, S. Regulation of PD-L1 expression in a high-grade invasive human oral squamous cell carcinoma microenvironment. Int. J. Oncol. 2017, 50, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Büttner-Herold, M.; Hyckel, P.; Moebius, P.; Distel, L.; Ries, J.; Amann, K.; Neukam, F.W.; Wehrhan, F. Small oral squamous cell carcinomas with nodal lymphogenic metastasis show increased infiltration of M2 polarized macrophages--an immunohistochemical analysis. J. Cranio-Maxillofac. Surg. 2014, 42, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Katou, F.; Ohtani, H.; Nakayama, T.; Yoshie, O.; Hashimoto, K. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med. Oral Pathol Oral Radiol. Endod. 2010, 109, 744–752. [Google Scholar] [CrossRef]
- Shah, W.; Yan, X.; Jing, L.; Zhou, Y.; Chen, H.; Wang, Y. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol. Immunol. 2011, 8, 59–66. [Google Scholar] [CrossRef]
- Ino, Y.; Yamazaki-Itoh, R.; Shimada, K.; Iwasaki, M.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 2013, 108, 914–923. [Google Scholar] [CrossRef]
- Kurahara, H.; Shinchi, H.; Mataki, Y.; Maemura, K.; Noma, H.; Kubo, F.; Sakoda, M.; Ueno, S.; Natsugoe, S.; Takao, S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J. Surg. Res. 2011, 167, e211–e219. [Google Scholar] [CrossRef]
- Shimizu, S.; Hiratsuka, H.; Koike, K.; Tsuchihashi, K.; Sonoda, T.; Ogi, K.; Miyakawa, A.; Kobayashi, J.; Kaneko, T.; Igarashi, T.; et al. Tumor-infiltrating CD8+ T-cell density is an independent prognostic marker for oral squamous cell carcinoma. Cancer Med. 2019, 8, 80–93. [Google Scholar] [CrossRef]
- Weber, M.; Iliopoulos, C.; Moebius, P.; Büttner-Herold, M.; Amann, K.; Ries, J.; Preidl, R.; Neukam, F.W.; Wehrhan, F. Prognostic significance of macrophage polarization in early stage oral squamous cell carcinomas. Oral Oncol. 2016, 52, 75–84. [Google Scholar] [CrossRef]
- Scheifele, C.; Reichart, P.A. Oral leukoplakia in manifest squamous epithelial carcinoma. A clinical prospective study of 101 patients. Mund Kiefer Gesichtschir. 1998, 2, 326–330. [Google Scholar] [CrossRef]
- Sudbø, J.; Reith, A. Which putatively pre-malignant oral lesions become oral cancers? Clinical relevance of early targeting of high-risk individuals. J. Oral Pathol. Med. 2003, 32, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Reibel, J. Prognosis of oral pre-malignant lesions: Significance of clinical, histopathological, and molecular biological characteristics. Crit. Rev. Oral Biol. Med. 2003, 14, 47–62. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Reibel, J.; Bouquot, J.; Dabelsteen, E. Oral epithelial dysplasia classification systems: Predictive value, utility, weaknesses and scope for improvement. J. Oral Pathol. Med. 2008, 37, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Fleskens, S.; Slootweg, P. Grading systems in head and neck dysplasia: Their prognostic value, weaknesses and utility. Head Neck Oncol. 2009, 1, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gnjatic, S.; Nagata, Y.; Jager, E.; Stockert, E.; Shankara, S.; Roberts, B.L.; Mazzara, G.P.; Lee, S.Y.; Dunbar, P.R.; Dupont, B.; et al. Strategy for monitoring T cell responses to NY-ESO-1 in patients with any HLA class I allele. Proc. Natl. Acad. Sci. USA 2000, 97, 10917–10922. [Google Scholar] [CrossRef]
- Marchetti, A.; Di Lorito, A.; Buttitta, F. Why anti-PD1/PDL1 therapy is so effective? Another piece in the puzzle. J. Thorac. Dis. 2017, 9, 4863–4866. [Google Scholar] [CrossRef]
- Grigore, A.; Albulescu, A.; Albulescu, R. Current methods for tumor-associated macrophages investigation. J. Immunoass. Immunochem. 2018, 39, 119–135. [Google Scholar] [CrossRef]
- Lacerda Mariano, L.; Ingersoll, M.A. Bladder resident macrophages: Mucosal sentinels. Cell Immunol. 2018, 330, 136–141. [Google Scholar] [CrossRef]
- Saluja, M.; Gilling, P. Intravesical bacillus Calmette-Guérin instillation in non-muscle-invasive bladder cancer: A review. Int. J. Urol. 2018, 25, 18–24. [Google Scholar] [CrossRef]
- Bahner, J.D.; Bordeaux, J.S. Non-melanoma skin cancers: Photodynamic therapy, cryotherapy, 5-fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin. Dermatol. 2013, 31, 792–798. [Google Scholar] [CrossRef]
- Müller, E.; Christopoulos, P.F.; Halder, S.; Lunde, A.; Beraki, K.; Speth, M.; Øynebråten, I.; Corthay, A. Toll-like receptor ligands and interferon-γ synergize for induction of antitumor M1 macrophages. Front. Immunol. 2017, 8, 1383. [Google Scholar] [CrossRef]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef]
- Wolf, K.-D.; Bootz, F.; Beck, J.; Bikowski, K.; Böhme, P.; Budach, W.; Burkhardt, A.; Danker, H.; Eberhardt, W.; Engers, K.; et al. Mundhöhlenkarzinom. In Leitlinienprogramm Onkologie; AWMW, Deutschen Krebsgesellschaft e.V. und Deutschen Krebshilfe e.V.: Berlin, Germany, 2012; pp. 1–119. [Google Scholar]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal stem cells: Key players in cancer progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef]
- Stevens, A.; Zuliani, T.; Olejnik, C.; LeRoy, H.; Obriot, H.; Kerr-Conte, J.; Formstecher, P.; Bailliez, Y.; Polakowska, R.R. Human dental pulp stem cells differentiate into neural crest-derived melanocytes and have label-retaining and sphere-forming abilities. Stem Cells Dev. 2008, 17, 1175–1184. [Google Scholar] [CrossRef]
- Neuhaus, K.W. Teeth: Malignant neoplasms in the dental pulp? Lancet Oncol. 2007, 8, 75–78. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480. https://doi.org/10.3390/ijms22031480
Galler KM, Weber M, Korkmaz Y, Widbiller M, Feuerer M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. International Journal of Molecular Sciences. 2021; 22(3):1480. https://doi.org/10.3390/ijms22031480
Chicago/Turabian StyleGaller, Kerstin M., Manuel Weber, Yüksel Korkmaz, Matthias Widbiller, and Markus Feuerer. 2021. "Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues" International Journal of Molecular Sciences 22, no. 3: 1480. https://doi.org/10.3390/ijms22031480
APA StyleGaller, K. M., Weber, M., Korkmaz, Y., Widbiller, M., & Feuerer, M. (2021). Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. International Journal of Molecular Sciences, 22(3), 1480. https://doi.org/10.3390/ijms22031480





