The Role of GSH in Intracellular Iron Trafficking
Abstract
1. Introduction
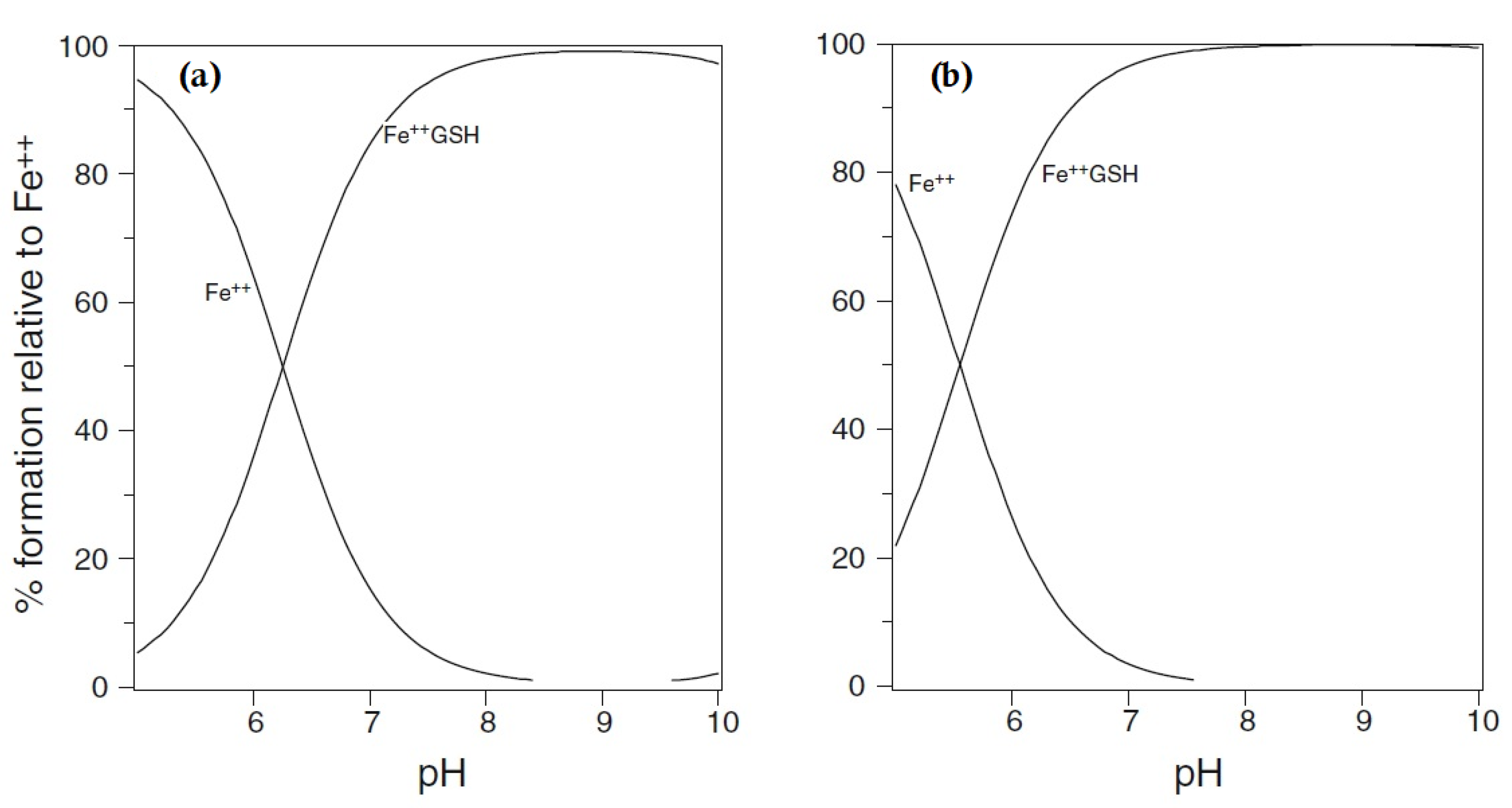
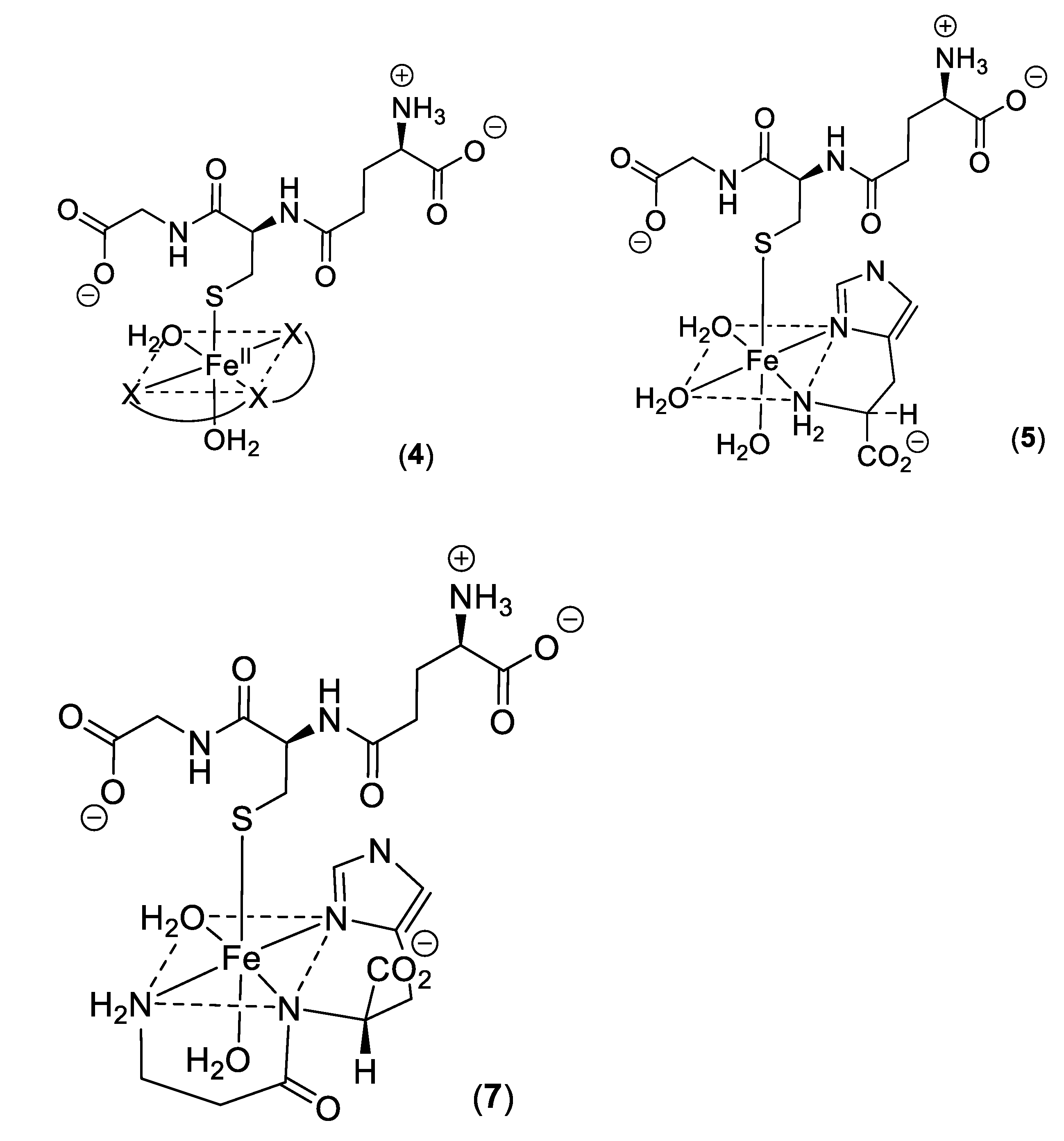
2. Cytosolic Thiol-Containing Iron(II) Ligands
3. Mitochondrial Labile Iron Pool and Iron Sulphur Cluster Biosynthesis
4. Intracellular Distribution of Iron
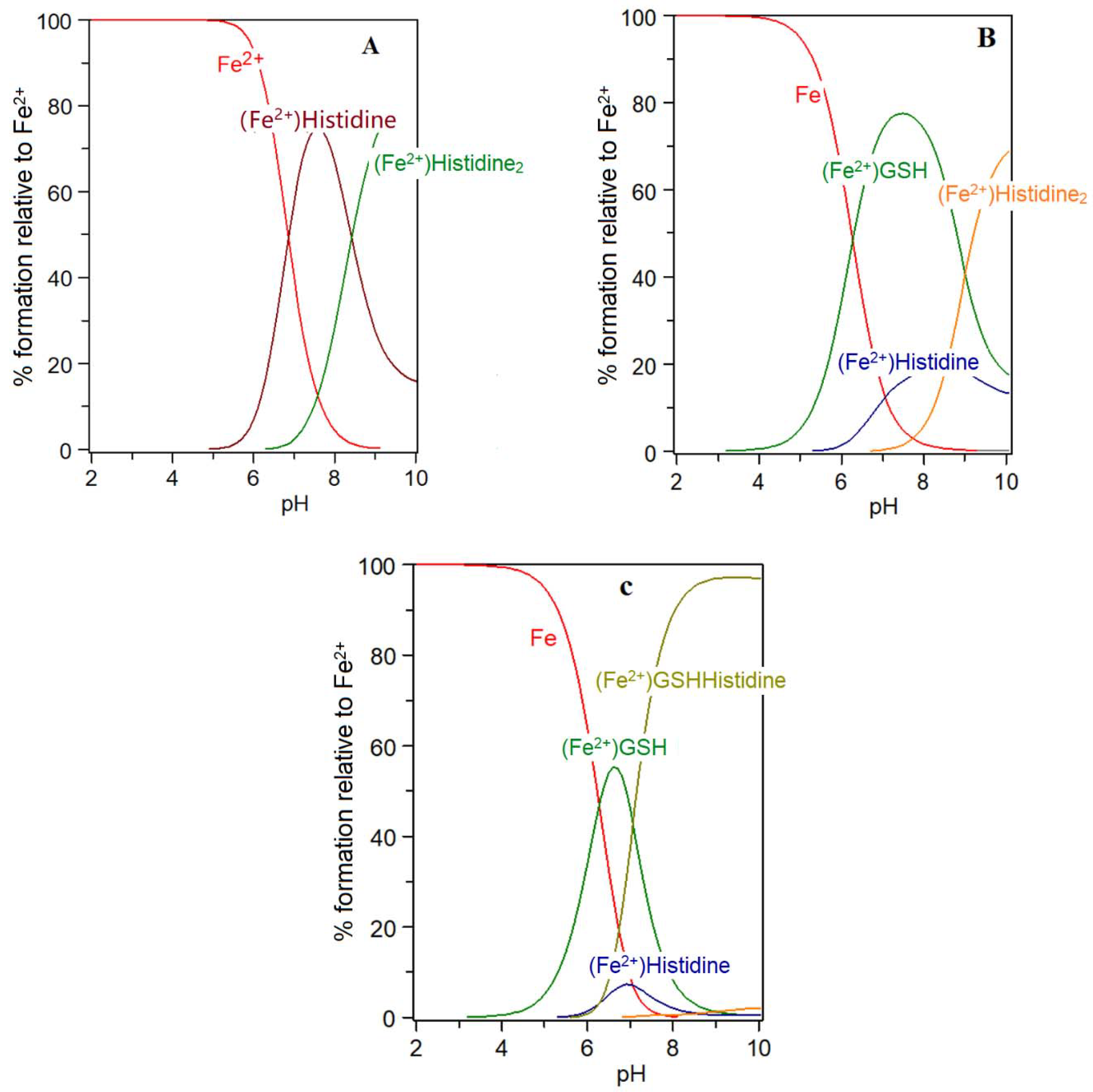
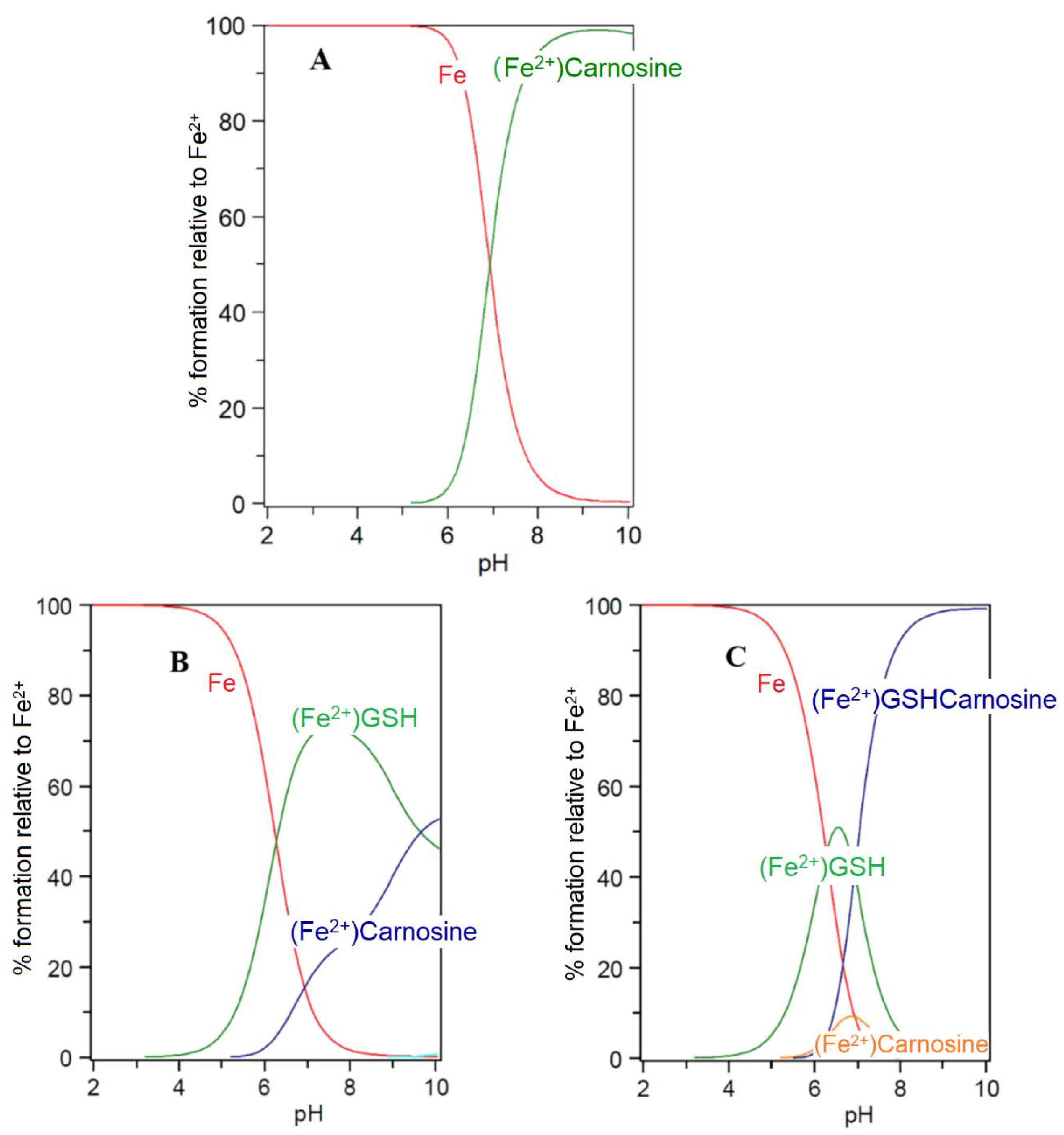
5. Carnosine and Histidine—Iron(II) Chelators of Physiological Significance
Conflicts of Interest
Abbreviations
| DMT | divalent metal transporter |
| FBXL5 | F-box and leucine-rich repeat protein 5 |
| HIF | hypoxia-inducing factor |
| IGFBP | insulin-like growth factor-binding protein |
| IRP | iron responsive protein |
| ISU | iron scaffold protein |
| PCBP | poly r C-binding protein |
References
- Lankford, C.D. Bacterial Assimilation of iron. CRC Rev. Microbiol. 1973, 2, 273–331. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1993. [Google Scholar]
- Crichton, R. Iron Metabolism, 3rd ed.; Wiley: Chichester, UK, 2009. [Google Scholar]
- Greenberg, G.R.; Wintrope, M.M. A labile iron pool. J. Biol. Chem. 1946, 165, 397–398. [Google Scholar] [CrossRef]
- Williams, R.J.P. Free manganese(II) and iron(II) cations can act as intracellular cell controls. FEBS Lett. 1982, 140, 3–10. [Google Scholar] [CrossRef]
- Loenarz, C.; Schofield, C.J. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat. Chem. Biol. 2008, 4, 152–156. [Google Scholar] [CrossRef]
- Ozer, A.; Bruick, R.K. Non-heme dioxygenases: Cellular sensors and regulators jelly rolled into one? Nat. Chem. Biol. 2007, 3, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Breuer, W.; Epsztejn, S.; Cabantchik, Z.I. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J. Biol. Chem. 1995, 270, 24209–24215. [Google Scholar] [CrossRef]
- Petrat, F.; de Groot, H.; Sustmann, R.; Raven, U. The chelatable iron pool in living cells: A methodically defined quantity. Biol. Chem. 2002, 383, 489–502. [Google Scholar] [CrossRef]
- Helm, L.; Merbach, A.E. Inorganic and bioin-organic solvent exchange mechanisms. Chem. Rev. 2005, 105, 1922–1959. [Google Scholar] [CrossRef]
- Miller, J.P.G.; Perkins, D.J. Model experiments for the study of iron transfer from transferrin to ferritin. Eur. J. Biochem. 1969, 10, 146–151. [Google Scholar] [CrossRef]
- Weaver, J.; Pollack, S. Low-Mr iron isolated from guinea pig reticulocytes as AMP-Fe and ATP-Fe complexes. Biochem. J. 1989, 261, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Veiga, N.; Torres, J.; Mansell, D.; Freeman, S.; Domínguez, S.; Barker, C.J.; Díaz, A.; Kremer, C. “Chelatable iron pool”: Inositol 1,2,3-trisphosphate fulfils the conditions required to be a safe cellular iron ligand. J. Biol. Inorg. Chem. 2009, 14, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, L.R.; Hart, D.O.; Goetz, D.H.; Green, M.R.A. Mammalian siderophore synthesised by an enzyme with a bacterial homolog involved in enterobactin production. Cell 2010, 141, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Morley, C.G.D.; Bezkorovainy, A. Identification of the iron chelate in hepatocyte cytosol. IRCS Med. Sci. 1983, 11, 1106–1107. [Google Scholar]
- Timberlake, C.F. Iron-malate and iron-citrate complexes. J. Chem. Soc. 1964, 5078–5085. [Google Scholar] [CrossRef]
- Harris, D.C.; Aisen, P. Facilitation of Fe(II) autoxidation by Fe(III) complexing agents. Biochim. Biophys. Acta 1973, 329, 156–158. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X.L. Glutathione: A key component of the cytoplasmic labile iron pool. Biometals 2011, 24, 1179–1187. [Google Scholar] [CrossRef]
- Whitfield, N.L.; Kreimier, E.L.; Verdial, F.C.; Skovgaard, N.; Olson, K.R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signalling. Am. J. Physiol. 2008, 294, R1930–R1937. [Google Scholar]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulphide concentrations are orders of magnitude lower than presently accepted levels. Am. J. Physiol. 2008, 295, R1479–R1485. [Google Scholar]
- Luther, G.W.; Rickard, D.T.; Theberge, S.; Olroyd, A. Determination of metal sulphide stability constants of Mn2+, Fe2+, Co2+, Ni2+, Ci2+, and Zn2+ by voltammetric methods. Environ. Sci. Technol. 1996, 30, 671–679. [Google Scholar] [CrossRef]
- Albert, A. Quantitative studies of the avidity of naturally occurring substances for trace metals. 2. Amino-acids having three ionizing groups. J. Chem. Soc. 1952, 50, 690–697. [Google Scholar] [CrossRef]
- Kondo, T.; Dale, G.L.; Beutler, E. Thiol transport from human red blood cells. Methods Enzymol. 1995, 252, 72–82. [Google Scholar] [PubMed]
- Soboll, S.; Gründel, S.; Harris, J.; Kolb-Bachofen, V.; Ketterer, B.; Sies, H. The content of glutathione and glutathione S-transferases and the glutathione peroxidase activity in rat liver nuclei determined by a non-aqueous technique of cell fractionation. Biochem. J. 1995, 311, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.Y.; Silver, J.; Wilson, M.T. Studies of the reactions of ferric iron with glutathione and some related thiols. Inorg. Chim. Acta 1983, 78, 1–11. [Google Scholar] [CrossRef]
- Fuhr, J.; Rabenstein, D. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX Binding of cadmium, zinc, lead, and mercury by glutathione. J. Am. Chem. Soc. 1973, 95, 6944–6948. [Google Scholar] [CrossRef]
- Ba, L.A.; Doering, M.; Burkholz, T.; Jacob, C. Metal trafficking: From maintaining the metal homeostasis to future drug design. Metallomics 2009, 1, 292–311. [Google Scholar] [CrossRef]
- Martin, H.B.; Edsall, J.T. The association of divalent cations with glutathione. J. Am. Chem. Soc. 1959, 81, 4044–4047. [Google Scholar] [CrossRef]
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Zumbrennen, K.B.; Paw, B.H.; Ward, D.M.; Kaplan, J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell Biol. 2009, 29, 1007–1016. [Google Scholar] [CrossRef]
- Atkinson, A.; Winge, D.R. Metal Acquisition and Availability in the Mitochondria. Chem. Rev. 2009, 109, 4708–4721. [Google Scholar] [CrossRef]
- Chen, Z.; Lash, L.H. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J. Pharmacol. Exp. Ther. 1998, 285, 608–618. [Google Scholar]
- Lash, L.H. Mitochondrial glutathione transport: Physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006, 163, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Bowman, E.J.; Ikuma, H.; Stein, H.J. Citric Acid cycle activity in mitochondria isolated from mung bean hypocotyls. Plant Physiol. 1976, 58, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, R.A.; Hiltunen, J.K.; Hassinen, I.E. Mitochondrial membrane potential, transmembrane difference in the NAD+ redox potential and the equilibrium of the glutamate-aspartate translocase in the isolated perfused rat heart. Biochim. Biophys. Acta 1982, 681, 286–291. [Google Scholar] [CrossRef]
- Go, Y.M.; Jones, D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Campanella, A.; Rovelli, E.; Santambrogio, P.; Cozzi, A.; Taroni, F.; Levi, S. Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: Hypothesis for a protective role in Friedreich ataxia. Hum. Mol. Genet. 2009, 18, 1–11. [Google Scholar] [CrossRef]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 2008, 1780, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Wells, W.W.; Yang, Y.; Deits, T.L.; Gran, Z.R. Thioltransferases. Adv. Enzymol. Relat. Areas Mol. Biol. 1993, 66, 149–201. [Google Scholar]
- Mesecke, N.; Mittler, S.; Eckers, E.; Herrmann, J.M.; Depante, M. Two Novel Monothiol Glutaredoxins from Saccharomyces cerevisiae Provide Further Insight into Iron-Sulfur Cluster Binding, Oligomerization, and Enzymatic Activity of Glutaredoxins. Biochemistry 2008, 47, 1452–1463. [Google Scholar] [CrossRef]
- Rouhier, N.; Couturier, J.; Johnson, M.K.; Jacquot, J.P. Glutaredoxins: Roles in iron homeostasis. Trends Biochem. Sci. 2009, 35, 43–52. [Google Scholar] [CrossRef]
- Li, H.; Mapolelo, D.T.; Dingra, N.N.; Naik, S.G.; Lees, N.S.; Hoffman, B.M.; Riggs-Gelasco, P.J.; Huynh, B.H.; Johnson, M.K.; Outten, C.E. The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation. Biochemistry 2009, 48, 9569–9581. [Google Scholar] [CrossRef]
- Qi, W.; Cowan, J.A. Mechanism of glutaredoxin—ISU [2Fe–2S] cluster exchange. Chem. Commun. 2011, 47, 4989–4991. [Google Scholar] [CrossRef] [PubMed]
- Ehrensberger, K.M.; Bird, A.J. Hammering out details: Regulating metal levels in eukaryotes. Trends Biochem. Sci. 2011, 36, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Lill, R.; Hoffmann, B.; Molik, S.; Pierik, A.J.; Rietzschel, N.; Stchling, O.; Uzarska, M.A.; Webert, H.; Wilbrecht, C.; Muhlenhoff, U. The role of mitochondria in cellular iron–sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta 2012, 1823, 1491–1508. [Google Scholar] [CrossRef] [PubMed]
- Lill, R. Function and biogenesis of iron-sulphur proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef]
- Sipos, K.; Lange, H.; Fekete, Z.; Uilmann, P.; Lill, R.; Kispal, G. Maturation of cytosolic iron-sulfur proteins requires glutathione. J. Biol. Chem. 2002, 277, 26944–26949. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pallesen, L.J.; Spang, R.J.; Walden, W.E. Cytosolic Iron-Sulfur Cluster Assembly (CIA) System: Factors, Mechanism, and Relevance to Cellular Iron Regulation. J. Biol. Chem. 2010, 285, 26745–26751. [Google Scholar] [CrossRef]
- Mühlenhoff, U.; Molik, S.; Godoy, J.R.; Uzarska, M.A.; Richter, N.; Seubert, A.; Zhang, Y.; Stubbe, J.; Pierrel, F.; Herrers, E.; et al. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 2010, 12, 373–385. [Google Scholar] [CrossRef]
- Ye, H.; Rouault, T.A. Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry 2010, 49, 4945–4956. [Google Scholar] [CrossRef]
- Qi, W.; Li, J.; Chain, C.Y.; Pasquevich, G.A.; Pasquevich, A.F.; Cowan, J.A. Glutathione-complexed iron-sulfur clusters. Reaction intermediates and evidence for a template effect promoting assembly and stability. Chem. Commun. 2013, 49, 6313–6315. [Google Scholar] [CrossRef][Green Version]
- Kühn, L. Iron regulatory proteins and their role in controlling iron metabolism. Metallomics 2015, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Ryu, M. Special delivery: Distributing iron in the cytosol of mammalian cells. Front. Pharmacol. 2014, 5, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Frey, A.G.; Palenchar, D.J.; Achar, S.; Bullough, Z.; Vashisht, A.; Wohlschlegel, J.A.; Philpott, C.C. A PCBP-BolA2 chaperone complex delivers iron for cytosolic [2Fe-2S] cluster assembly. Nat. Chem. Biol. 2019, 15, 872–881. [Google Scholar] [CrossRef]
- Williams, R.J.P.; Fraústo da Silva, J.J.R. The distribution of elements in cells. Coord. Chem. Rev. 2000, 200–202, 247–348. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Iron speciation in the cytosol: An overview. Dalton Trans. 2013, 42, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Patel, S.J.; Protchenko, O. Management versus miscues in the cytosolic labile iron pool: The varied functions of iron chaperones. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118830. [Google Scholar] [CrossRef] [PubMed]
- Vera-Aviles, M.; Vantana, E.; Kardinasari, E.; Koh, N.L.; Latude-Dada, G.O. Protective role of histidine supplementation against oxidative stress damage in the management of anemia of chronic kidney disease. Pharmaceuticals 2018, 11, 111. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Xing, L.; Chee, M.E.; Zhang, H.; Zhang, W.; Mine, Y. Carnosine—A natural bioactive dipeptide: Bioaccessibility, bioavailability and health benefits. J. Food Bioact. 2019, 5, 8–17. [Google Scholar] [CrossRef]
- Baran, E.J. Metal complexes of carnosine. Biochemistry 2000, 65, 789–797. [Google Scholar]
- Boakye, A.A.; Zhang, D.; Guo, L.; Zheng, Y.; Hoetker, D.; Zhao, J.; Posa, D.K.; Ng, C.K.; Zheng, H.; Kumar, A.; et al. Carnosine supplementation enhances post ischemic hind limb revascularization. Front. Physiol. 2019, 10, 751. [Google Scholar] [CrossRef]
- Brown, C.E.; Antholine, W.E. Chelation Chemistry of Carnosine. Evidence that Mixed. Complexes May Occur In Vivo. J. Chem. Phys. 1979, 83, 3314–3319. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Carnosine and its possible roles in nutrition and health. Adv. Food Nut. Res. 2009, 57, 87–154. [Google Scholar]
- Tamba, M.; Torreggiani, A. A pulse radiolysis study of carnosine in aqueous solution. Int. J. Radiat. Biol. 1999, 74, 333–340. [Google Scholar]
- Lee, Y.T.; Hsu, C.C.; Lin, M.H.; Liu, K.S.; Yin, M.C. Histidine and carnosine delay diabetic deterioration in mice and protect human low-density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Soliman, K.; Mohamed, A.; Metwally, N. Attenuation of some metabolic deteriorations induced by diabetes mellitus using carnosine. J. Appl. Sci. 2007, 7, 2252–2260. [Google Scholar]
- Forsberg, E.A.; Botusan, I.R.; Wang, J.; Peters, V.; Ansurudeen, I.; Brismar, K.; Catrina, S.B. Carnosine decreases IGFBP1 production in db/db mice through suppression of HIF-1. J. Endocrinol. 2015, 225, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Klessens, C.Q.F.; Baelde, H.J.; Singler, B.; Varaar, A.M.; Zutinic, A.; Drozak, J.; Zschocke, J.; Schmitt, C.P.; de Heer, E. Intrinsic carnosine metabolism in the human kidney. Amino Acids 2015, 47, 2541–2550. [Google Scholar] [CrossRef]
- Johnson, W.M.; Wilson-Delfosse, A.L.; Mieyal, J.J. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 2012, 4, 1399–1440. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, T.; Bai, R.; Xie, Y. Hydroxypyridinone-based iron chelators with broad-ranging biological activities. J. Med. Chem. 2020, 63, 14470–14501. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Hobart, L.J.; Seibel, I.; Yeargans, G.S.; Seidler, N.W. Anti-Crosslinking Properties of Carnosine: Significance of histidine. Life Sci. 2004, 75, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Rama Rao, K.V.; Reddy, P.V.B.; Tong, X.; Norenberg, M.D. Brain edema in acute liver failure: Inhibition by L-histidine. Am. J. Pathol. 2010, 176, 1400–1408. [Google Scholar] [CrossRef] [PubMed]





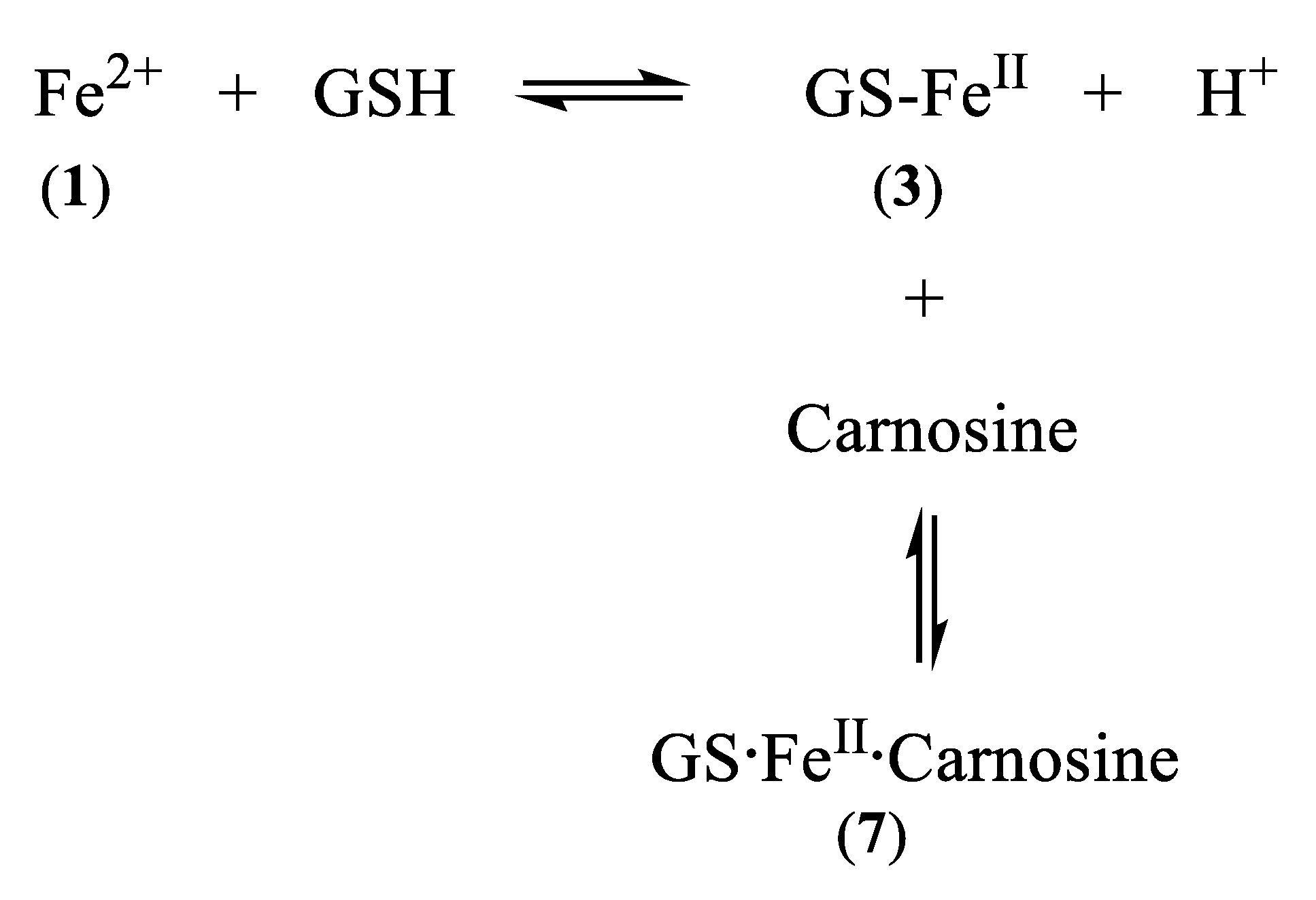
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hider, R.; Aviles, M.V.; Chen, Y.-L.; Latunde-Dada, G.O. The Role of GSH in Intracellular Iron Trafficking. Int. J. Mol. Sci. 2021, 22, 1278. https://doi.org/10.3390/ijms22031278
Hider R, Aviles MV, Chen Y-L, Latunde-Dada GO. The Role of GSH in Intracellular Iron Trafficking. International Journal of Molecular Sciences. 2021; 22(3):1278. https://doi.org/10.3390/ijms22031278
Chicago/Turabian StyleHider, Robert, Mayra Vera Aviles, Yu-Lin Chen, and Gladys Oluyemisi Latunde-Dada. 2021. "The Role of GSH in Intracellular Iron Trafficking" International Journal of Molecular Sciences 22, no. 3: 1278. https://doi.org/10.3390/ijms22031278
APA StyleHider, R., Aviles, M. V., Chen, Y.-L., & Latunde-Dada, G. O. (2021). The Role of GSH in Intracellular Iron Trafficking. International Journal of Molecular Sciences, 22(3), 1278. https://doi.org/10.3390/ijms22031278





