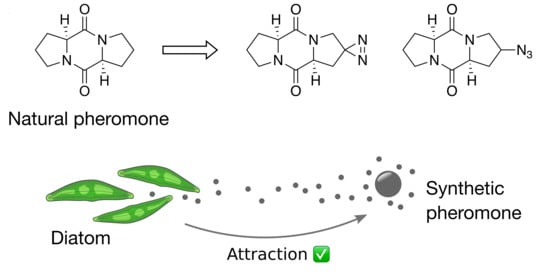
Altering the Sex Pheromone Cyclo(l-Pro-l-Pro) of the Diatom Seminavis robusta towards a Chemical Probe
Abstract
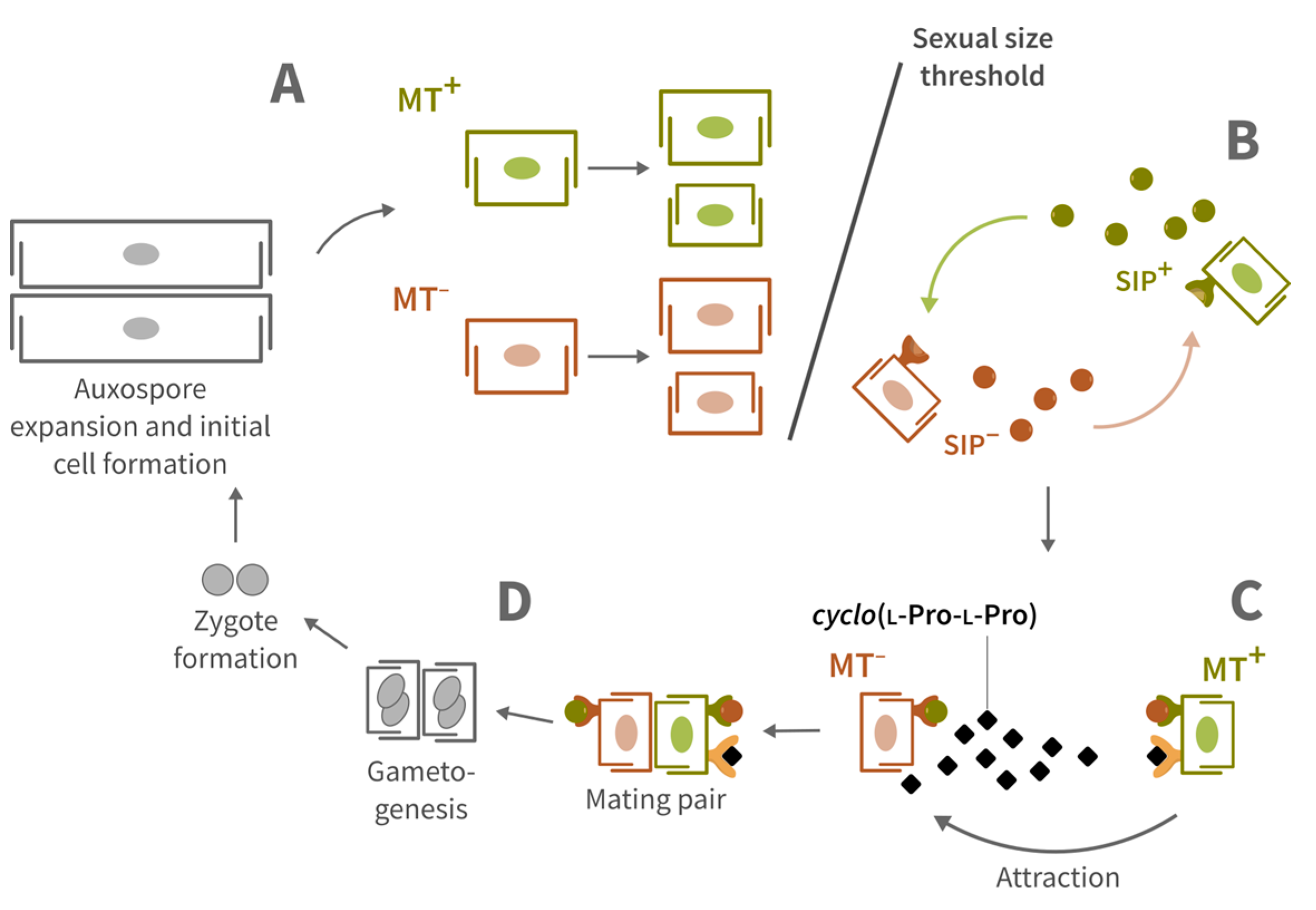
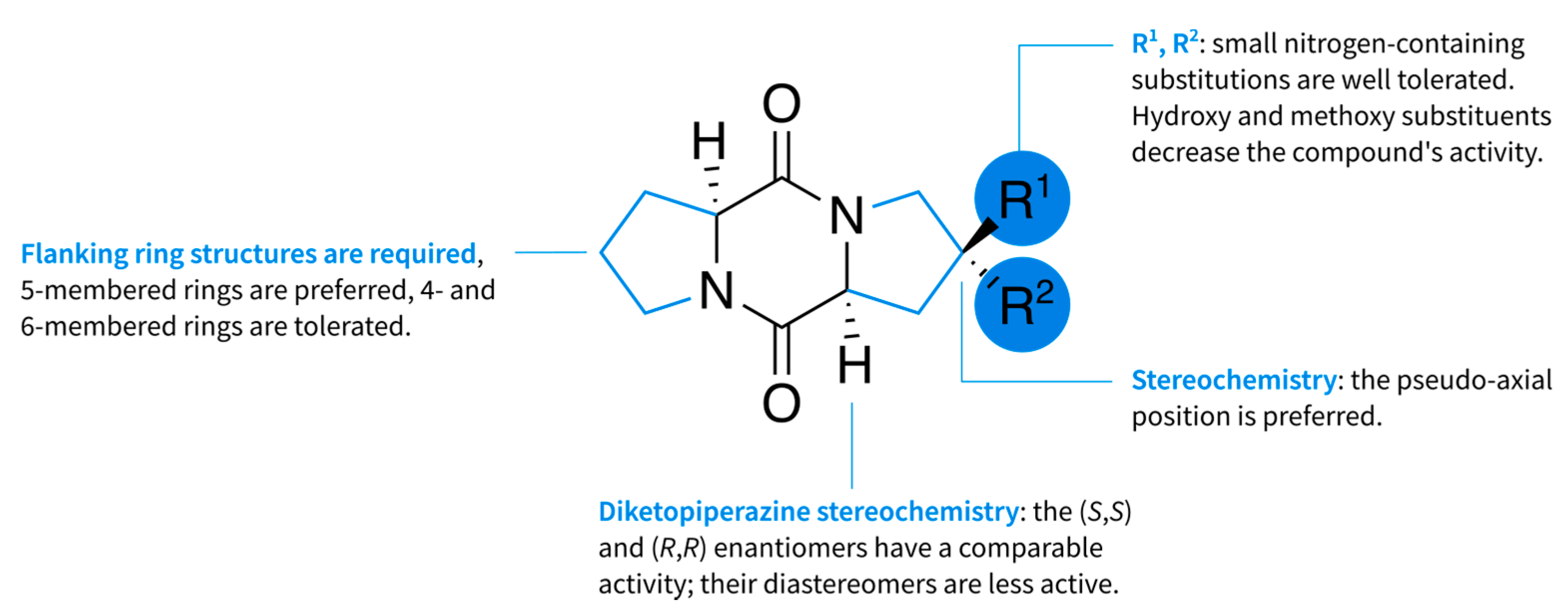
1. Introduction
2. Results
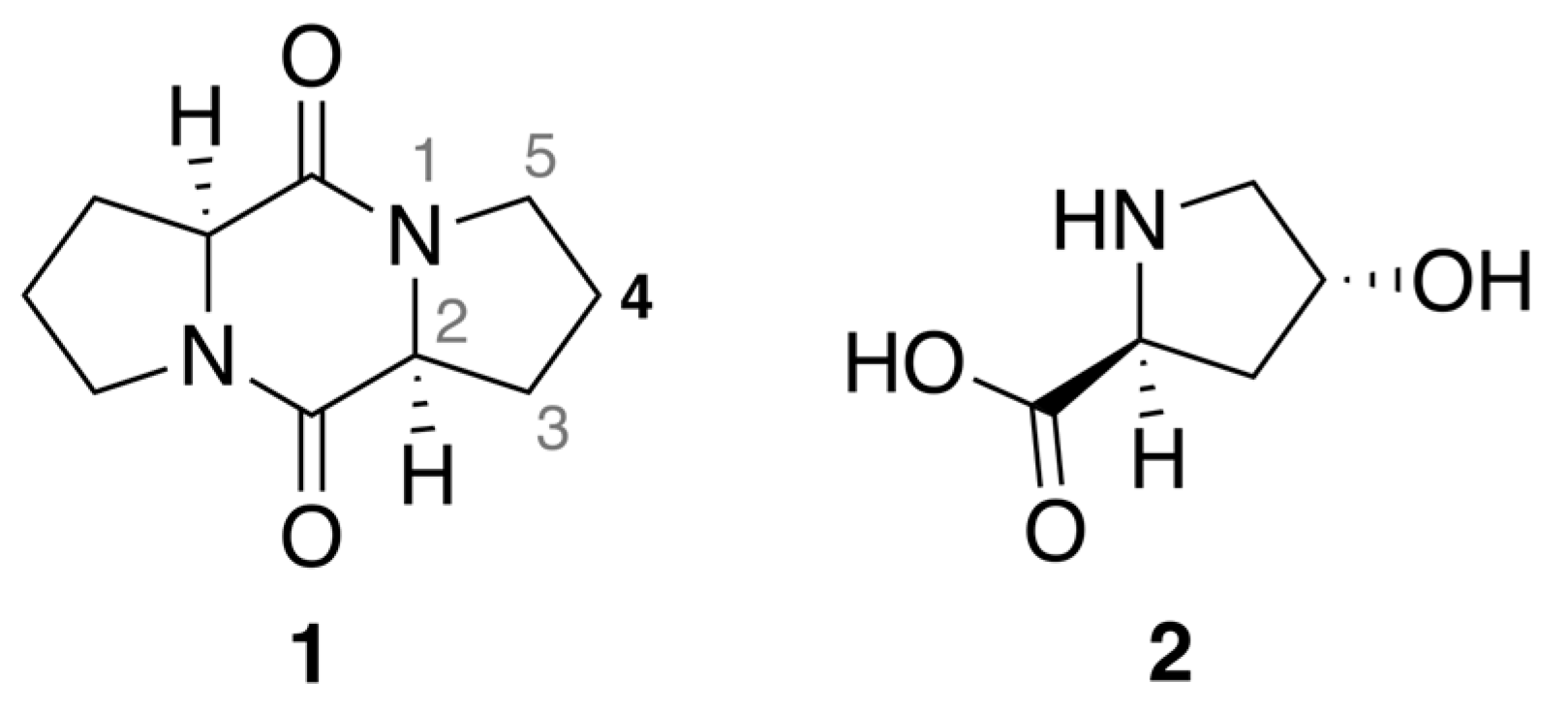
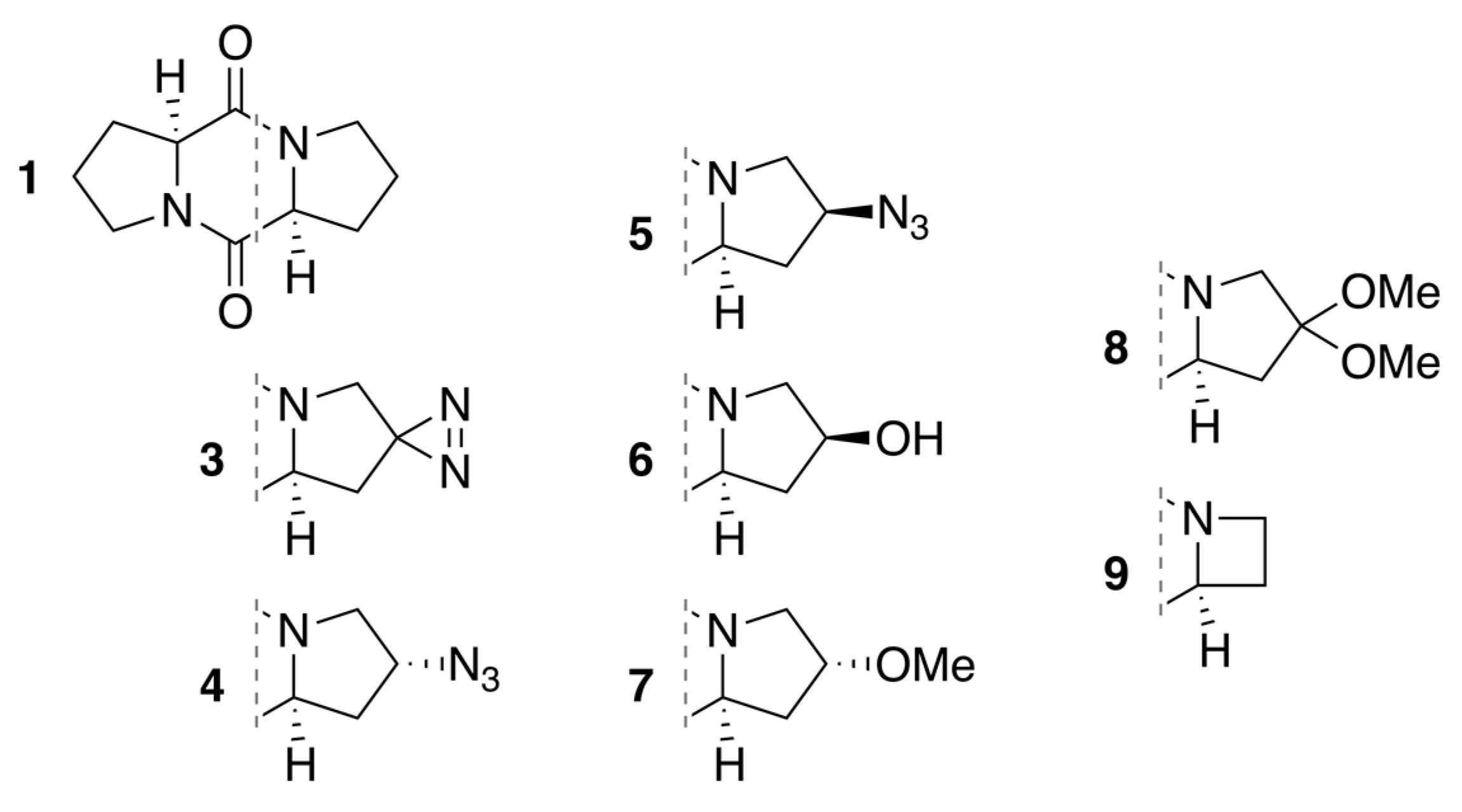
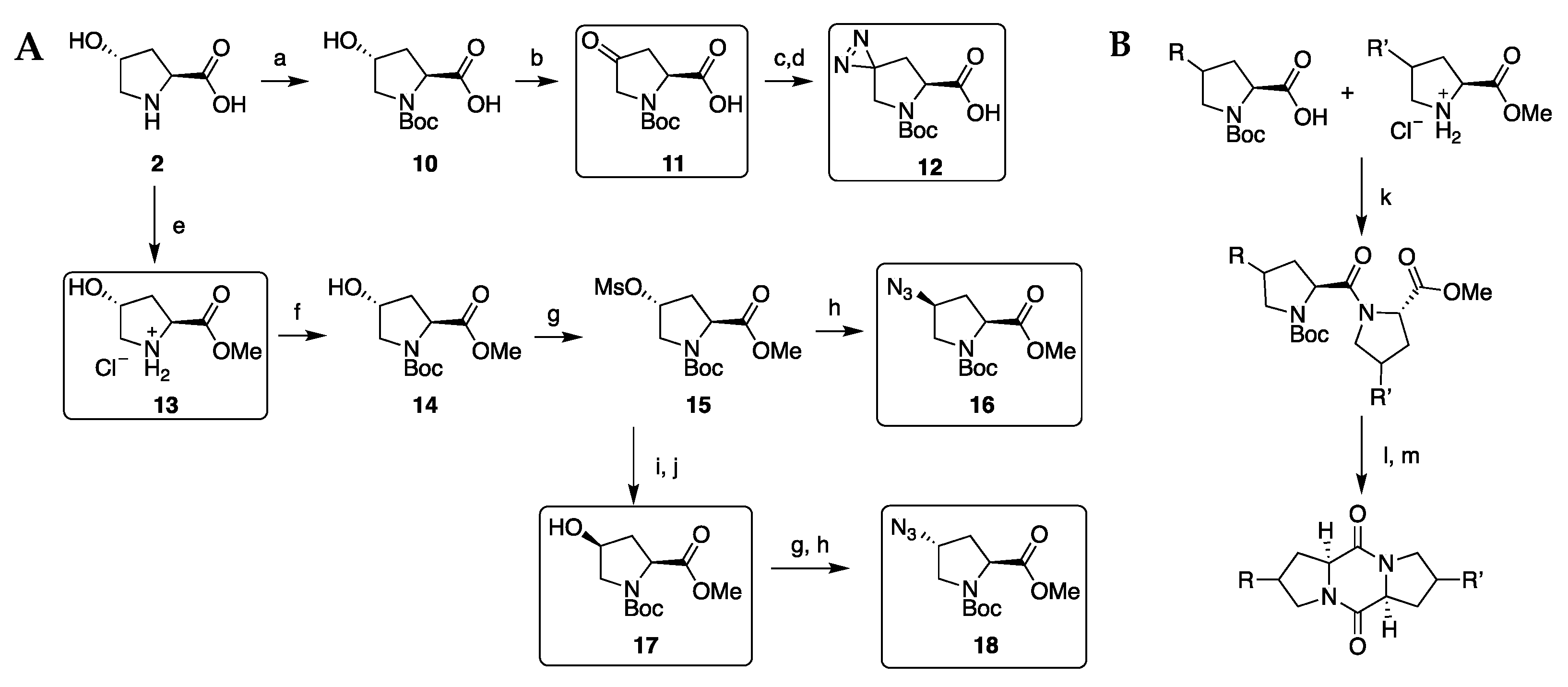
2.1. Diketopiperazine Synthesis
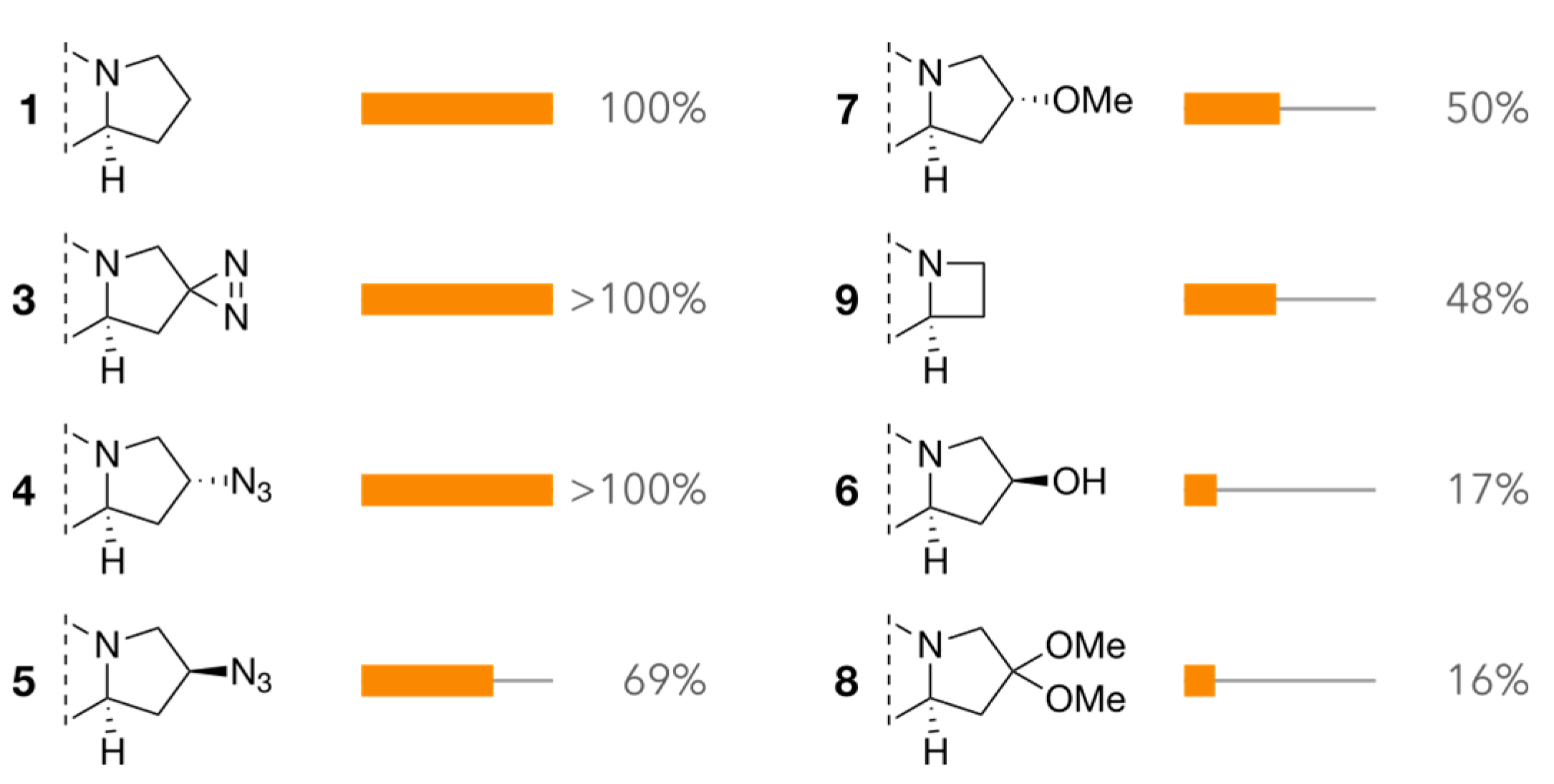
2.2. Diketopiperazine Phytotoxicity
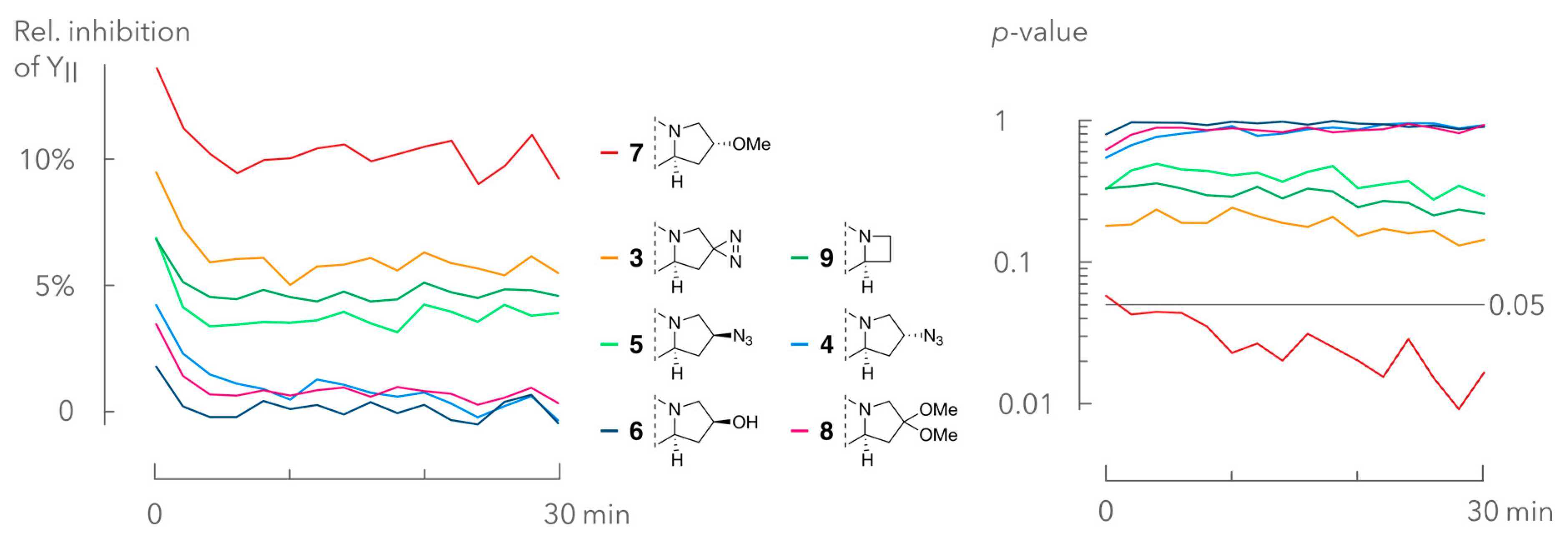
2.3. Interference Assay
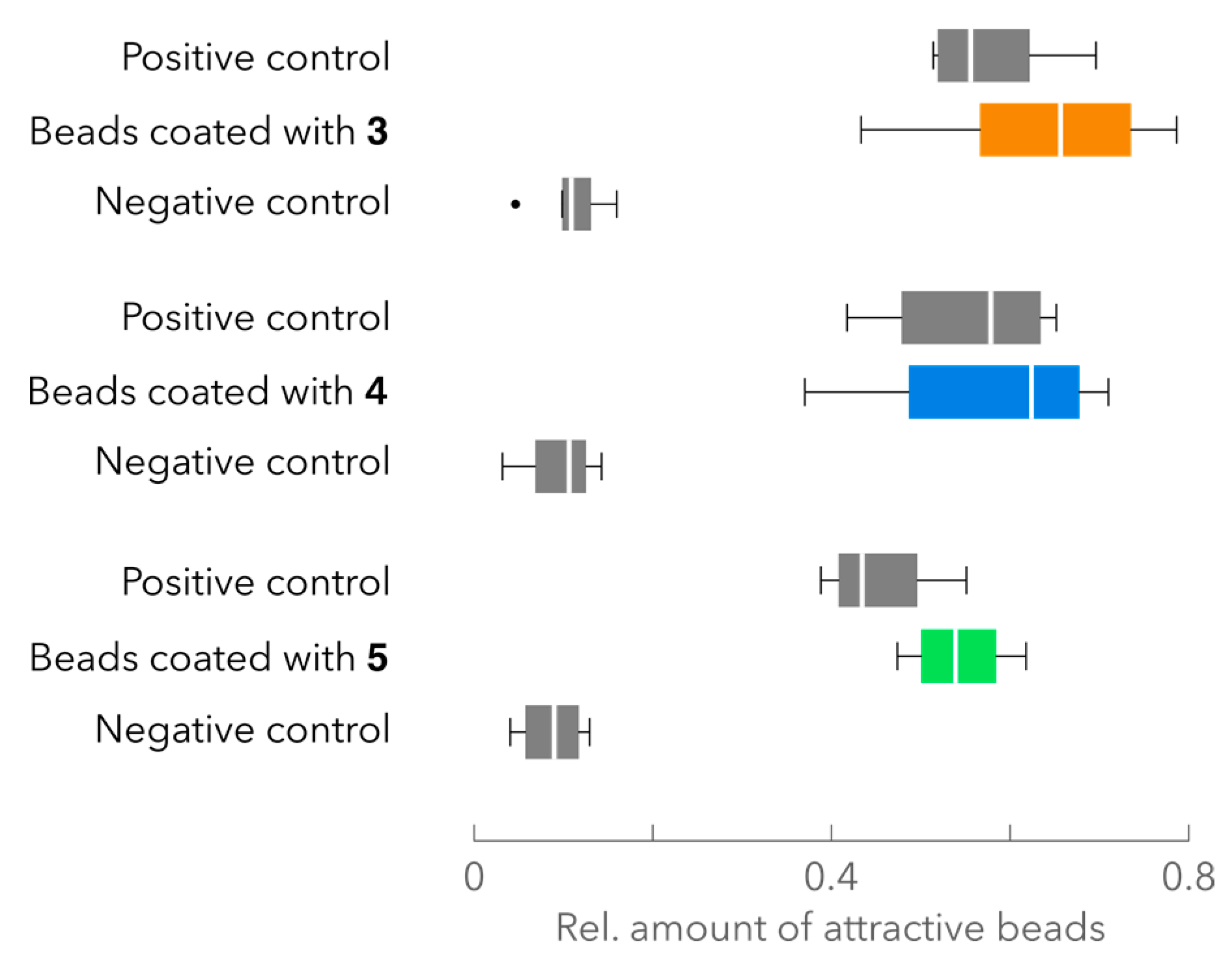
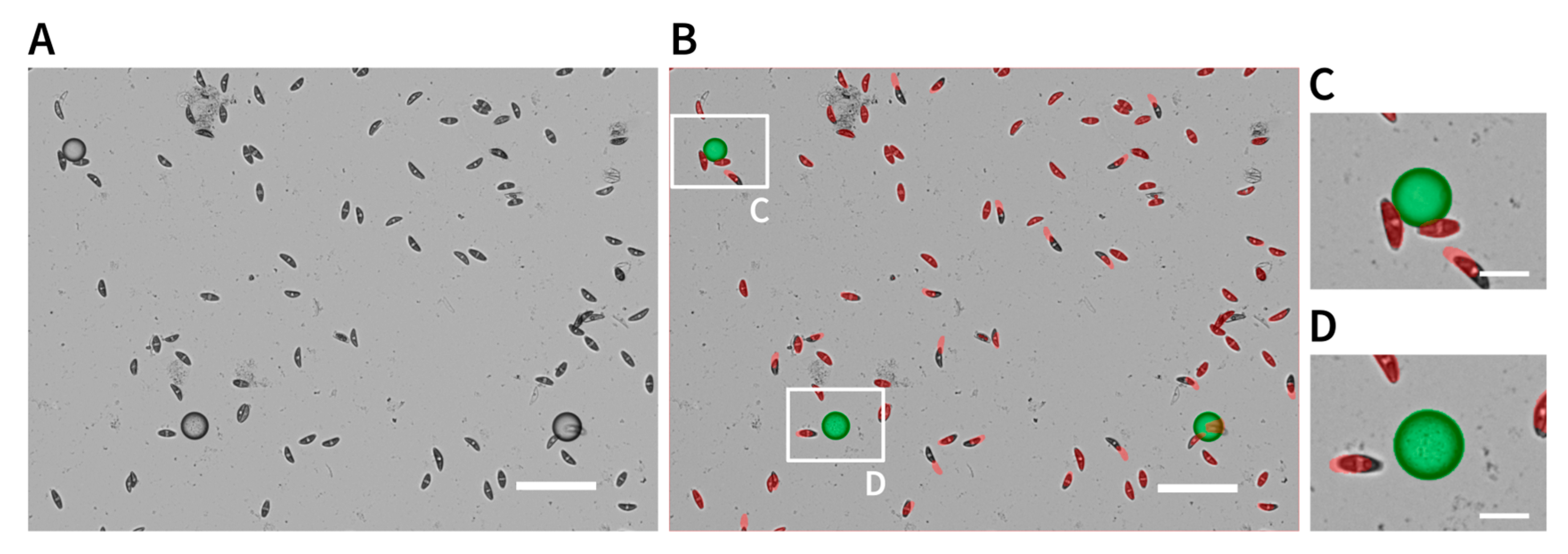
2.4. Attraction Assay
3. Discussion
4. Methods
4.1. Strain and Culture Conditions
4.2. Interference Assay
4.3. Attraction Assay
4.4. Microscopy and Image Processing
4.4.1. Automatic Image Capturing
4.4.2. Manual Image Capturing
4.5. Data Analysis
4.6. Phytotoxic Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CY5 | Cyanine 5 |
| GFP | Green Fluorescent Protein |
| GPCR | G protein-coupled receptor |
| MT | Mating type |
| PAL | Photoaffinity labeling |
| PAM | Pulse amplitude modulation |
| PSII | Photosystem II |
| SIP | Sex inducing pheromone |
| YII | Quantum yield of PSII |
References
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J.; Gordon, R. Beyond micromachining: The potential of diatoms. Trends Biotechnol. 1999, 17, 190–196. [Google Scholar] [CrossRef]
- Lopez, P.J.; Descles, J.; Allen, A.E.; Bowler, C.; Desclés, J.; Allen, A.E.; Bowler, C. Prospects in diatom research. Curr. Opin. Biotechnol. 2005, 16, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Daboussi, F.; Leduc, S.; Maréchal, A.; Dubois, G.; Guyot, V.; Perez-Michaut, C.; Amato, A.; Falciatore, A.; Juillerat, A.; Beurdeley, M.; et al. Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat. Commun. 2014, 5, 3831. [Google Scholar] [CrossRef] [PubMed]
- Kroth, P.G.; Bones, A.M.; Daboussi, F.; Ferrante, M.I.; Jaubert, M.; Kolot, M.; Nymark, M.; Río Bártulos, C.; Ritter, A.; Russo, M.T.; et al. Genome editing in diatoms: Achievements and goals. Plant Cell Rep. 2018, 37, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Chepurnov, V.A.; Mann, D.G.; von Dassow, P.; Vanormelingen, P.; Gillard, J.; Inzé, D.; Sabbe, K.; Vyverman, W. In search of new tractable diatoms for experimental biology. BioEssays 2008, 30, 692–702. [Google Scholar] [CrossRef]
- Osuna-Cruz, C.M.; Bilcke, G.; Vancaester, E.; De Decker, S.; Bones, A.M.; Winge, P.; Poulsen, N.; Bulankova, P.; Verhelst, B.; Audoor, S.; et al. The Seminavis robusta genome provides insights into the evolutionary adaptations of benthic diatoms. Nat. Commun. 2020, 11, 3320. [Google Scholar] [CrossRef]
- Chepurnov, V.A.; Mann, D.G.; Vyverman, W.; Sabbe, K.; Danielidis, D.B. Sexual Reproduction, Mating System, and Protoplast Dynamics of Seminavis (BAcillariophyceae). J. Phycol. 2002, 38, 1004–1019. [Google Scholar] [CrossRef]
- Gillard, J.; Frenkel, J.; Devos, V.; Sabbe, K.; Paul, C.; Rempt, M.; Inzé, D.; Pohnert, G.; Vuylsteke, M.; Vyverman, W. Metabolomics Enables the Structure Elucidation of a Diatom Sex Pheromone. Angew. Chem. Int. Ed. 2013, 52, 854–857. [Google Scholar] [CrossRef]
- Bilcke, G.; Van den Berge, K.; De Decker, S.; Bonneure, E.; Poulsen, N.; Bulankova, P.; Osuna-Cruz, C.M.; Dickenson, J.; Sabbe, K.; Pohnert, G.; et al. Mating type specific transcriptomic response to sex inducing pheromone in the pennate diatom Seminavis robusta. ISME J. 2020, 570. [Google Scholar] [CrossRef]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Huang, R.-M.; Yi, X.-X.; Zhou, Y.; Su, X.; Peng, Y.; Gao, C.-H. An Update on 2,5-Diketopiperazines from Marine Organisms. Mar. Drugs 2014, 12, 6213–6235. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, X.; Xu, T.; Yang, X.; Liu, Y. Diketopiperazines from marine organisms. Chem. Biodivers. 2010, 7, 2809–2829. [Google Scholar] [CrossRef] [PubMed]
- Lembke, C.; Stettin, D.; Speck, F.; Ueberschaar, N.; De Decker, S.; Vyverman, W.; Pohnert, G. Attraction Pheromone of The Benthic Diatom Seminavis robusta: Studies on Structure-Activity Relationships. J. Chem. Ecol. 2018, 44, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Thornton, E.R.; Westheimer, F.H. The photolysis of diazoacetylchymotrypsin. J. Biol. Chem. 1962, 237, 3006–3008. [Google Scholar] [CrossRef]
- Kotzyba-Hibert, F.; Kapfer, I.; Goeldner, M. Recent Trends in Photoaffinity Labeling. Angew. Chem. Int. Ed. 1995, 34, 1296–1312. [Google Scholar] [CrossRef]
- Ge, S.-S.; Chen, B.; Wu, Y.-Y.; Long, Q.-S.; Zhao, Y.-L.; Wang, P.-Y.; Yang, S. Current advances of carbene-mediated photoaffinity labeling in medicinal chemistry. RSC Adv. 2018, 8, 29428–29454. [Google Scholar] [CrossRef]
- Dubinsky, L.; Krom, B.P.; Meijler, M.M. Diazirine based photoaffinity labeling. Bioorg. Med. Chem. 2012, 20, 554–570. [Google Scholar] [CrossRef]
- Soethoudt, M.; Stolze, S.C.; Westphal, M.V.; van Stralen, L.; Martella, A.; van Rooden, E.J.; Guba, W.; Varga, Z.V.; Deng, H.; van Kasteren, S.I.; et al. Selective Photoaffinity Probe That Enables Assessment of Cannabinoid CB 2 Receptor Expression and Ligand Engagement in Human Cells. J. Am. Chem. Soc. 2018, 140, 6067–6075. [Google Scholar] [CrossRef]
- Müskens, F.M.; Ward, R.J.; Herkt, D.; van de Langemheen, H.; Tobin, A.B.; Liskamp, R.M.J.; Milligan, G. Design, synthesis, and evaluation of a diazirine photoaffinity probe for ligand-based receptor capture targeting G protein–coupled receptors. Mol. Pharmacol. 2019, 95, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, R.; Sakai, K.; Otani, Y.; Wadatsu, T.; Sakata, Y.; Nishikawa, Y.; Tanaka, M.; Yamashita, Y.; Hayashi, M.; Kondo, K.; et al. Novel Tetrafunctional Probes Identify Target Receptors and Binding Sites of Small-Molecule Drugs from Living Systems. ACS Chem. Biol. 2020, 15, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, H.; Momose, T.; Handa, C.; Ozawa, T. Photoaffinity Labeling of the Human A 2A Adenosine Receptor and Cross-link Position Analysis by Mass Spectrometry. ACS Med. Chem. Lett. 2017, 8, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Demko, Z.P.; Sharpless, K.B. A click chemistry approach to tetrazoles by Huisgen 1,3-dipolar cycloaddition: Synthesis of 5-acyltetrazoles from azides and acyl cyanides. Angew. Chem. Int. Ed. 2002, 41, 2113–2116. [Google Scholar] [CrossRef]
- MacKinnon, A.L.; Taunton, J. Target Identification by Diazirine Photo-Cross-Linking and Click Chemistry. In Current Protocols in Chemical Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 55–73. ISBN 9780470559277. [Google Scholar]
- Murale, D.P.; Hong, S.C.; Haque, M.M.; Lee, J.-S. Photo-affinity labeling (PAL) in chemical proteomics: A handy tool to investigate protein-protein interactions (PPIs). Proteome Sci. 2016, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, A.; Hayes, W. Development and application of diazirines in biological and synthetic macromolecular systems. Soft Matter 2005, 1, 178–205. [Google Scholar] [CrossRef]
- Romero-Diaz, C.; Campos, S.M.; Herrmann, M.A.; Lewis, K.N.; Williams, D.R.; Soini, H.A.; Novotny, M.V.; Hews, D.K.; Martins, E.P. Structural Identification, Synthesis and Biological Activity of Two Volatile Cyclic Dipeptides in a Terrestrial Vertebrate. Sci. Rep. 2020, 10, 4303. [Google Scholar] [CrossRef]
- Baer, B.; Maile, R.; Schmid-Hempel, P.; Morgan, E.D.; Jones, G.R. Chemistry of a mating plug in bumblebees. J. Chem. Ecol. 2000, 26, 1869–1875. [Google Scholar] [CrossRef]
- De Kesel, J.; Gómez-Rodríguez, R.; Bonneure, E.; Mangelinckx, S.; Kyndt, T. The Use of PTI-Marker Genes to Identify Novel Compounds that Establish Induced Resistance in Rice. Int. J. Mol. Sci. 2020, 21, 317. [Google Scholar] [CrossRef]
- Keller, O.; Keller, W.E.; van Look, G.; Wersin, G. tert-Butoxycarbonylation of amino acids and their derivatives: N-tert-butoxycarbonyl-l-phenylalanine. Org. Synth. 1985, 63, 160–170. [Google Scholar] [CrossRef]
- Van der Meijden, B.; Robinson, J.A. Synthesis and application of photoproline—A photoactivatable derivative of proline. Arkivoc 2011, 4, 130–136. [Google Scholar] [CrossRef]
- Marusawa, H.; Setoi, H.; Sawada, A.; Kuroda, A.; Seki, J.; Motoyama, Y.; Tanaka, H. Synthesis and Biological Activity of 1-Phenylsulfonyl-4-Phenylsulfonylaminopyrrolidine Derivatives as Thromboxane A2 Receptor Antagonists. Bioorg. Med. Chem. 2002, 10, 1399–1415. [Google Scholar] [CrossRef]
- Chalker, J.M.; Gunnoo, S.B.; Boutureira, O.; Gerstberger, S.C.; Fernández-González, M.; Bernardes, G.J.L.; Griffin, L.; Hailu, H.; Schofield, C.J.; Davis, B.G. Methods for converting cysteine to dehydroalanine on peptides and proteins. Chem. Sci. 2011, 2, 1666. [Google Scholar] [CrossRef]
- Campbell, J.; Lin, Q.; Geske, G.D.; Blackwell, H.E. New and Unexpected Insights into the Modulation of LuxR-Type Quorum Sensing by Cyclic Dipeptides. ACS Chem. Biol. 2009, 4, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Chiba, J.; Takayama, G.; Takashi, T.; Yokoyama, M.; Nakayama, A.; Baldwin, J.J.; McDonald, E.; Moriarty, K.J.; Sarko, C.R.; Saionz, K.W.; et al. Synthesis, biological evaluation, and pharmacokinetic study of prolyl-1-piperazinylacetic acid and prolyl-4-piperidinylacetic acid derivatives as VLA-4 antagonists. Bioorg. Med. Chem. 2006, 14, 2725–2746. [Google Scholar] [CrossRef]
- Vičar, J.; Smolíková, J.; Bláha, K. Amino acids and peptides. CXV. 2,5-Piperazinediones with an anneled azetidine ring; Synthesis and infrared spectra. Collect. Czechoslov. Chem. Commun. 1973, 38, 1957–1970. [Google Scholar] [CrossRef]
- Ji, X.; Xia, C.; Wang, J.; Su, M.; Zhang, L.; Dong, T.; Li, Z.; Wan, X.; Li, J.; Li, J.; et al. Design, synthesis and biological evaluation of 4-fluoropyrrolidine-2-carbonitrile and octahydrocyclopenta[b]pyrrole-2-carbonitrile derivatives as dipeptidyl peptidase IV inhibitors. Eur. J. Med. Chem. 2014, 86, 242–256. [Google Scholar] [CrossRef]
- Abraham, D.J.; Mokotoff, M.; Sheh, L.; Simmons, J.E. Design, synthesis, and testing of antisickling agents. 2. Proline derivatives designed for the donor site. J. Med. Chem. 1983, 26, 549–554. [Google Scholar] [CrossRef]
- Chen, A.; Gomez, R.; Oballa, R.; Powell, D.; Roppe, J.; Seiders, T.; Sheng, T. Aliphatic prolinamide derivatives. WO2017222917A1, 28 December 2017. [Google Scholar]
- Wood, J.M.; Furkert, D.P.; Brimble, M.A. Total Synthesis and Stereochemical Revision of the 2-Formylpyrrole Alkaloid Hemerocallisamine I. J. Nat. Prod. 2017, 80, 1926–1929. [Google Scholar] [CrossRef]
- Anteunis, M.J.O.; Callens, R.; Asher, V.; Sleeckx, J. Ring Conformational Aspects of Proline and Hydroxyproline. High Conformational Purity of Pro in DKP’s and Hydantoïns. Bull. Sociétés Chim. Belg. 1978, 87, 41–60. [Google Scholar] [CrossRef]
- Sleeckx, J.J.M.; Anteunis, M.J.O. Revisited Conformational Aspects of Pro and Hyp Pyrrolidine Rings In Bicyclic Peptide Systems. Bull. Sociétés Chim. Belg. 1985, 94, 187–198. [Google Scholar] [CrossRef]
- Young, P.E.; Madison, V.; Blout, E.R. Cyclic peptides. VI. Europium-assisted nuclear magnetic resonance study of the solution conformations of cyclo(l-Pro-l-Pro) and cyclo(l-Pro-d-Pro). J. Am. Chem. Soc. 1973, 95, 6142–6144. [Google Scholar] [CrossRef] [PubMed]
- Haasnoot, C.A.G.; de Leeuw, F.A.A.M.; Altona, C. The relationship between proton-proton NMR coupling constants and substituent electronegativities–I. Tetrahedron 1980, 36, 2783–2792. [Google Scholar] [CrossRef]
- Subirós-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An Efficient Additive for Peptide Synthesis to Replace the Benzotriazole-Based HOBt and HOAt with a Lower Risk of Explosion. Chem. A Eur. J. 2009, 15, 9394–9403. [Google Scholar] [CrossRef] [PubMed]
- Jad, Y.E.; Khattab, S.N.; de la Torre, B.G.; Govender, T.; Kruger, H.G.; El-Faham, A.; Albericio, F. EDC·HCl and Potassium Salts of Oxyma and Oxyma-B as Superior Coupling Cocktails for Peptide Synthesis. Eur. J. Org. Chem. 2015, 2015, 3116–3120. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Pan, J.; Yang, H. Synergistic algicidal effect and mechanism of two diketopiperazines produced by Chryseobacterium sp. strain GLY-1106 on the harmful bloom-forming Microcystis aeruginosa. Sci. Rep. 2015, 5, 14720. [Google Scholar] [CrossRef]
- Tan, S.; Hu, X.; Yin, P.; Zhao, L. Photosynthetic inhibition and oxidative stress to the toxic Phaeocystis globosa caused by a diketopiperazine isolated from products of algicidal bacterium metabolism. J. Microbiol. 2016, 54, 364–375. [Google Scholar] [CrossRef]
- Consalvey, M.; Perkins, R.G.; Paterson, D.M.; Underwood, G.J.C. PAM FLUORESCENCE: A BEGINNERS GUIDE FOR BENTHIC DIATOMISTS. Diatom Res. 2005, 20, 1–22. [Google Scholar] [CrossRef]
- Magnusson, M.; Heimann, K.; Quayle, P.; Negri, A.P. Additive toxicity of herbicide mixtures and comparative sensitivity of tropical benthic microalgae. Mar. Pollut. Bull. 2010, 60, 1978–1987. [Google Scholar] [CrossRef]
- Sjollema, S.B.; MartínezGarcía, G.; van der Geest, H.G.; Kraak, M.H.S.; Booij, P.; Vethaak, A.D.; Admiraal, W. Hazard and risk of herbicides for marine microalgae. Environ. Pollut. 2014, 187, 106–111. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Dahms, H.-U.; Lee, J.-S.; Kim, H.C.; Lee, W.C.; Shin, K.-H. Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol. Environ. Saf. 2014, 104, 51–71. [Google Scholar] [CrossRef]
- Podola, B.; Melkonian, M. A long-term operating algal biosensor for the rapid detection of volatile toxic compounds. J. Appl. Phycol. 2003, 15, 415–424. [Google Scholar] [CrossRef]
- Grzesiuk, M.; Wacker, A.; Spijkerman, E. Photosynthetic sensitivity of phytoplankton to commonly used pharmaceuticals and its dependence on cellular phosphorus status. Ecotoxicology 2016, 25, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Stock, F.; Bilcke, G.; De Decker, S.; Osuna-Cruz, C.M.; Van den Berge, K.; Vancaester, E.; De Veylder, L.; Vandepoele, K.; Mangelinckx, S.; Vyverman, W. Distinctive Growth and Transcriptional Changes of the Diatom Seminavis robusta in Response to Quorum Sensing Related Compounds. Front. Microbiol. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Quayle, P.; Schmidt, S.; Escher, B.I.; Mueller, J.F. Methodology and evaluation of a highly sensitive algae toxicity test based on multiwell chlorophyll fluorescence imaging. Biosens. Bioelectron. 2007, 22, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Stock, F.; Syrpas, M.; Graff van Creveld, S.; Backx, S.; Blommaert, L.; Dow, L.; Stock, W.; Ruysbergh, E.; Lepetit, B.; Bailleul, B.; et al. N-acyl homoserine lactone derived tetramic acids impair photosynthesis in Phaeodactylum tricornutum. ACS Chem. Biol. 2019, 14, 198–203. [Google Scholar] [CrossRef]
- Benedetti, E.; Goodman, M.; Marsh, R.; Rapoport, H.; Musich, J. Cyclo-L-prolyl-L-prolyl, C10H14N2O2. Cryst. Struct. Commun. 1975, 4, 641–645. [Google Scholar]
- Siemion, I.Z.; Wieland, T.; Pook, K.-H. Influence of the Distance of the Proline Carbonyl from the β and γ Carbon on the 13C Chemical Shifts. Angew. Chem. Int. Ed. 1975, 14, 702–703. [Google Scholar] [CrossRef]
- Siemion, I.Z. Conformational investigations on cyclic dipeptides containing a proline residue by means of 13C n.m.r. spectroscopy. Org. Magn. Reson. 1976, 8, 432–435. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing (3.5); R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonneure, E.; De Baets, A.; De Decker, S.; Van den Berge, K.; Clement, L.; Vyverman, W.; Mangelinckx, S. Altering the Sex Pheromone Cyclo(l-Pro-l-Pro) of the Diatom Seminavis robusta towards a Chemical Probe. Int. J. Mol. Sci. 2021, 22, 1037. https://doi.org/10.3390/ijms22031037
Bonneure E, De Baets A, De Decker S, Van den Berge K, Clement L, Vyverman W, Mangelinckx S. Altering the Sex Pheromone Cyclo(l-Pro-l-Pro) of the Diatom Seminavis robusta towards a Chemical Probe. International Journal of Molecular Sciences. 2021; 22(3):1037. https://doi.org/10.3390/ijms22031037
Chicago/Turabian StyleBonneure, Eli, Amber De Baets, Sam De Decker, Koen Van den Berge, Lieven Clement, Wim Vyverman, and Sven Mangelinckx. 2021. "Altering the Sex Pheromone Cyclo(l-Pro-l-Pro) of the Diatom Seminavis robusta towards a Chemical Probe" International Journal of Molecular Sciences 22, no. 3: 1037. https://doi.org/10.3390/ijms22031037
APA StyleBonneure, E., De Baets, A., De Decker, S., Van den Berge, K., Clement, L., Vyverman, W., & Mangelinckx, S. (2021). Altering the Sex Pheromone Cyclo(l-Pro-l-Pro) of the Diatom Seminavis robusta towards a Chemical Probe. International Journal of Molecular Sciences, 22(3), 1037. https://doi.org/10.3390/ijms22031037