Protective Effect of Dinitrosyl Iron Complexes Bound with Hemoglobin on Oxidative Modification by Peroxynitrite
Abstract
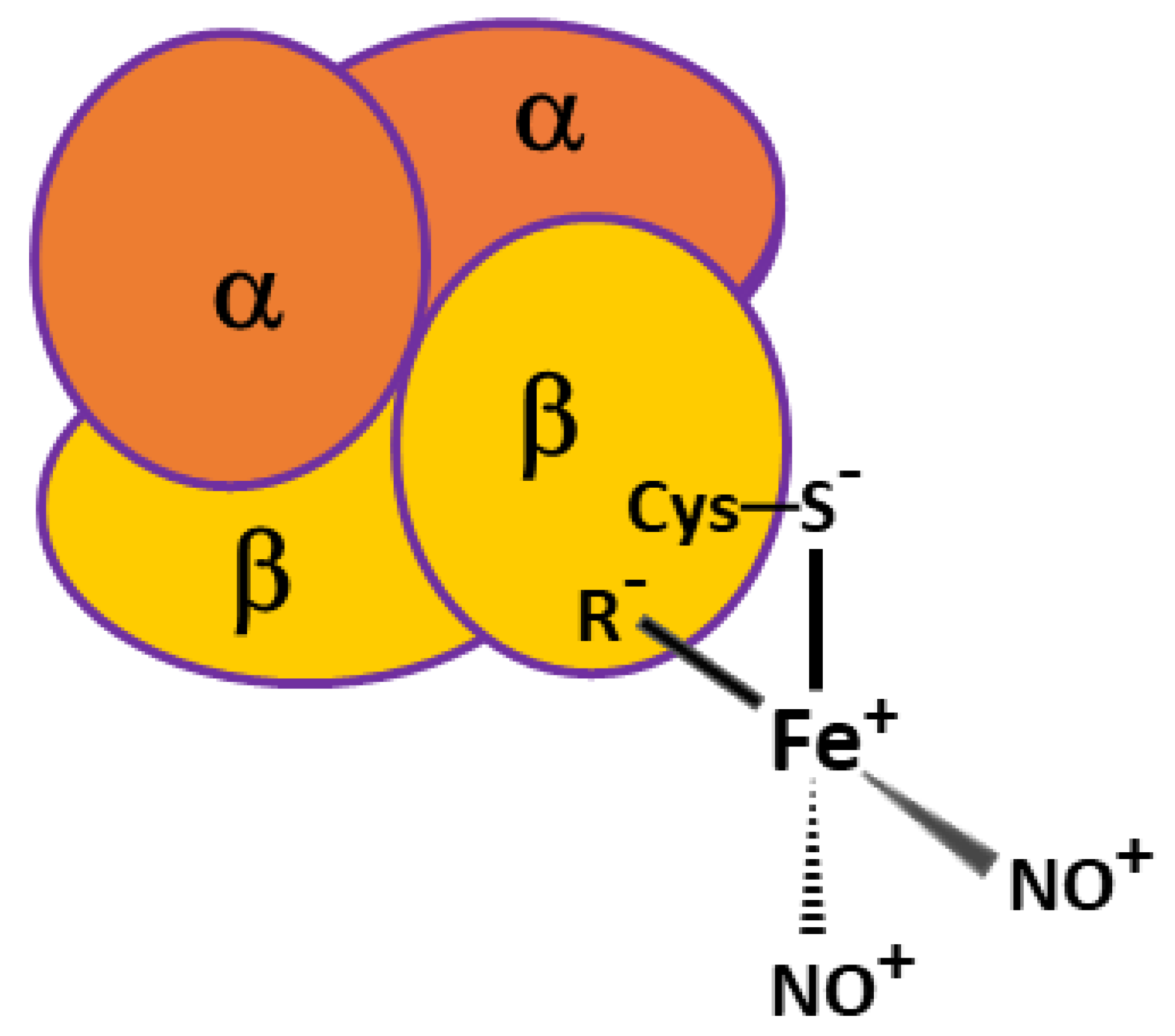
:1. Introduction
2. Results
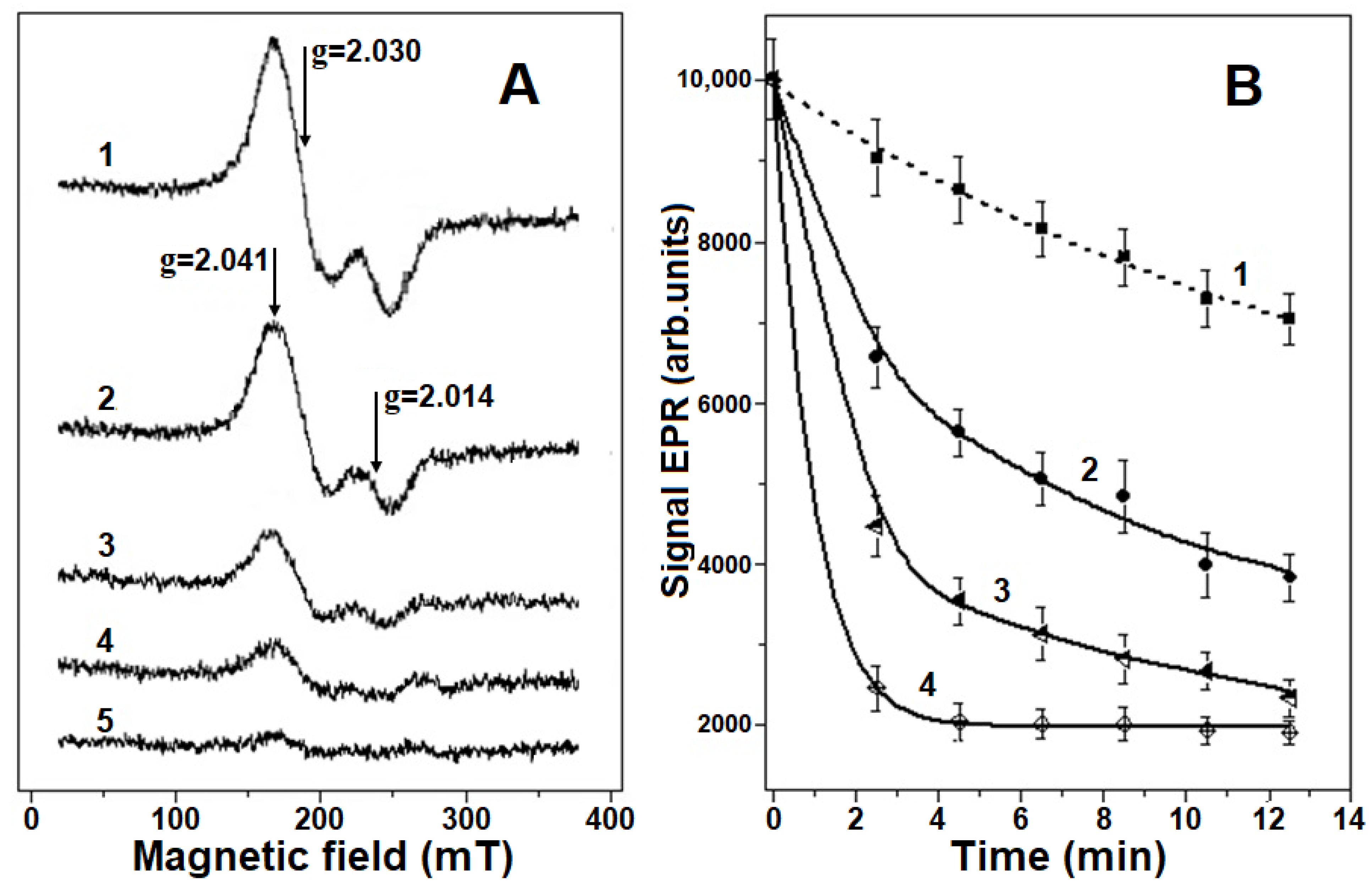
2.1. Destruction of Hb-DNICs with Peroxynitrite Action, Using EPR Spectroscopy
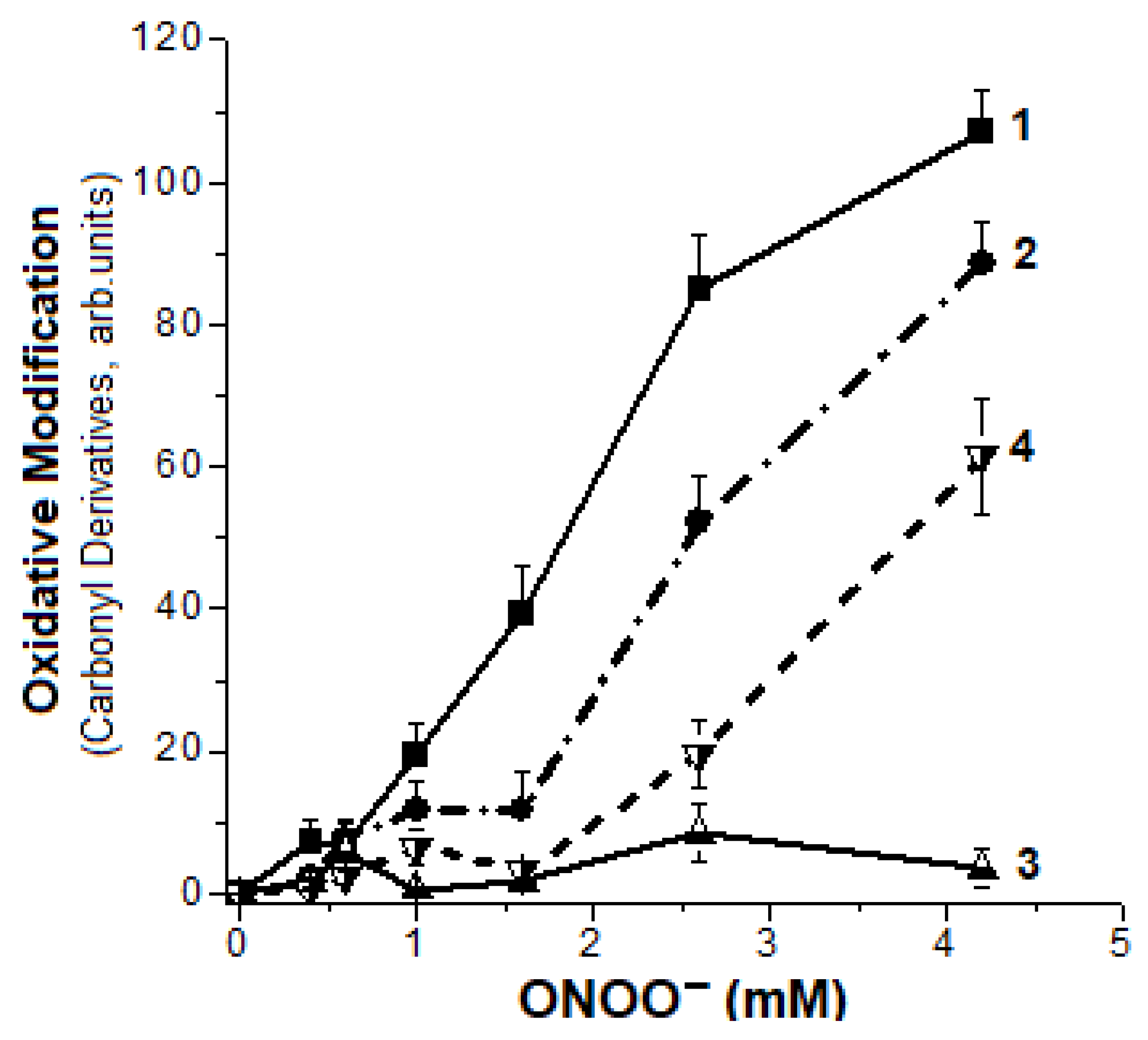
2.2. Formation of Hb Carbonyl Derivatives
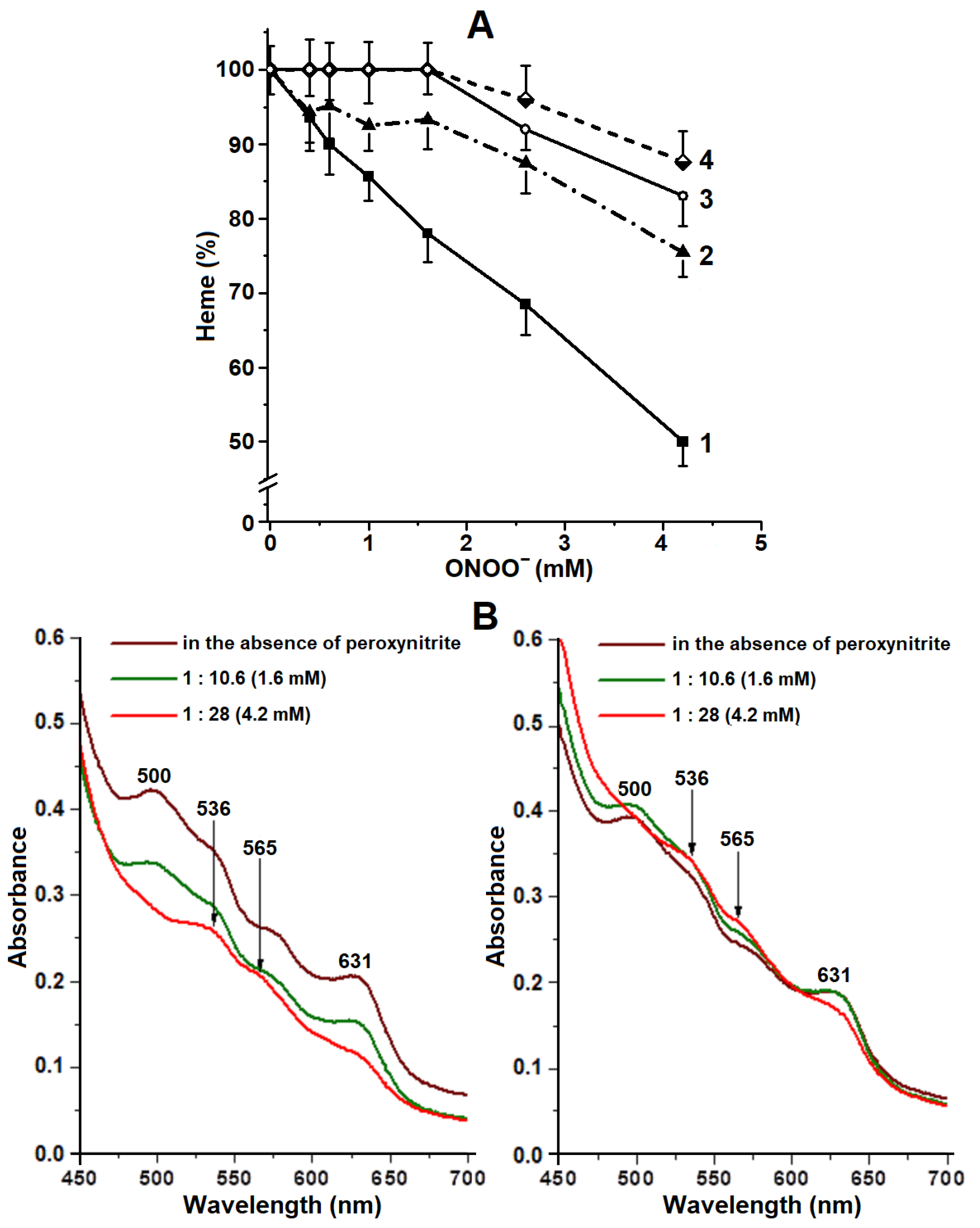
2.3. Degradation of Hb Heme Group
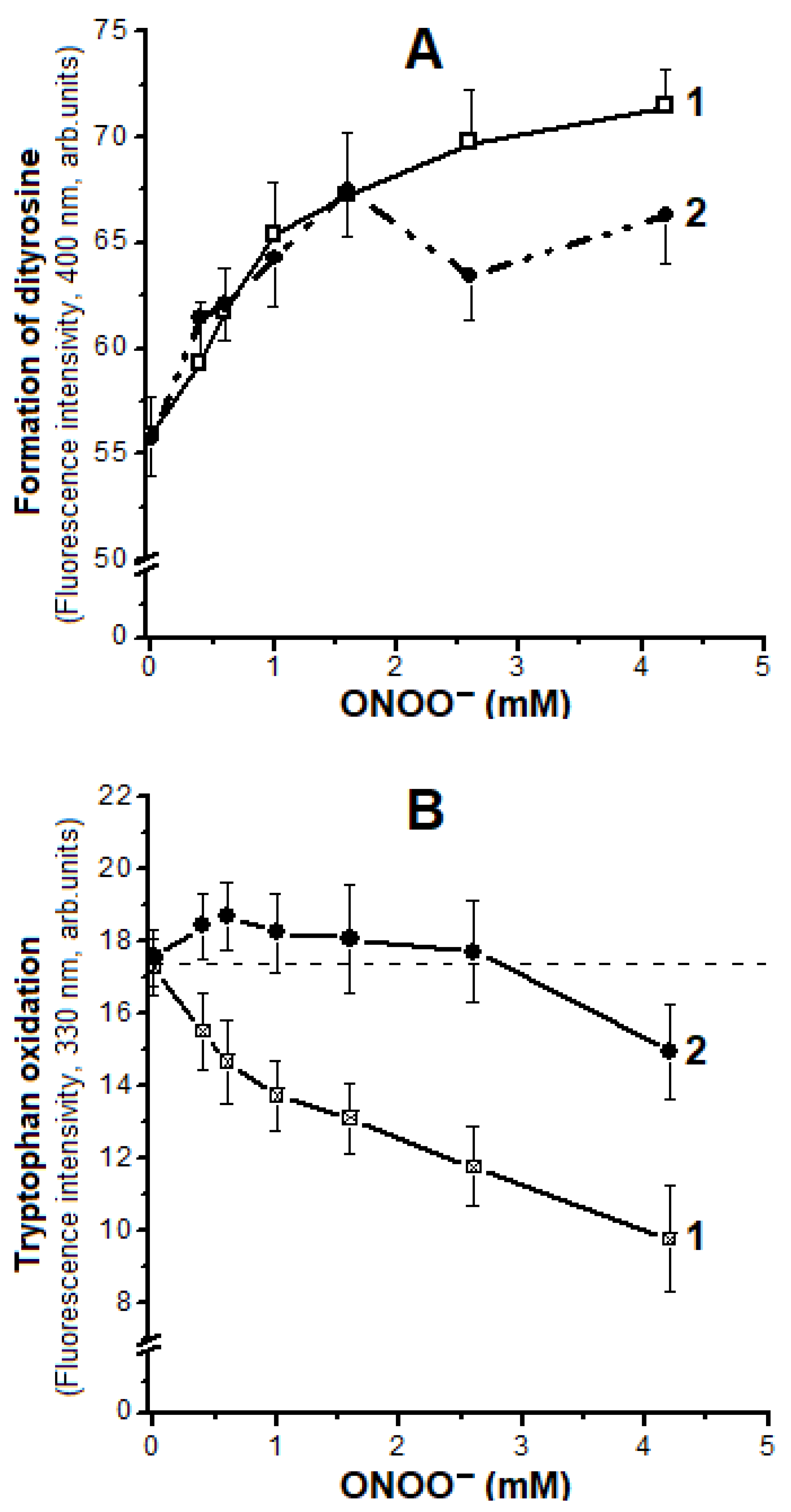
2.4. Oxidation of Tyrosine and Tryptophan Residues in Hb
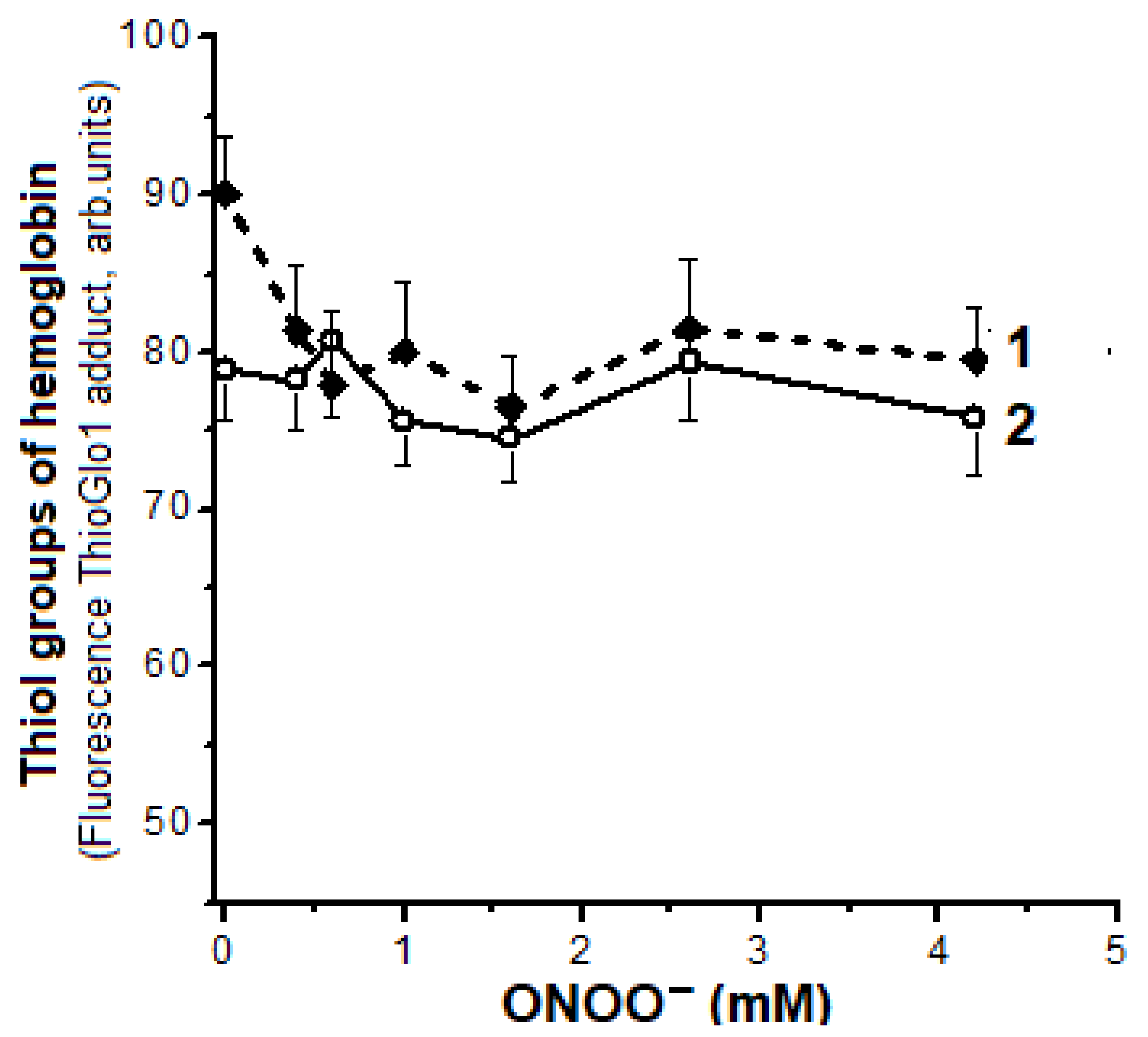
2.5. Oxidation of Hb Thiol Groups
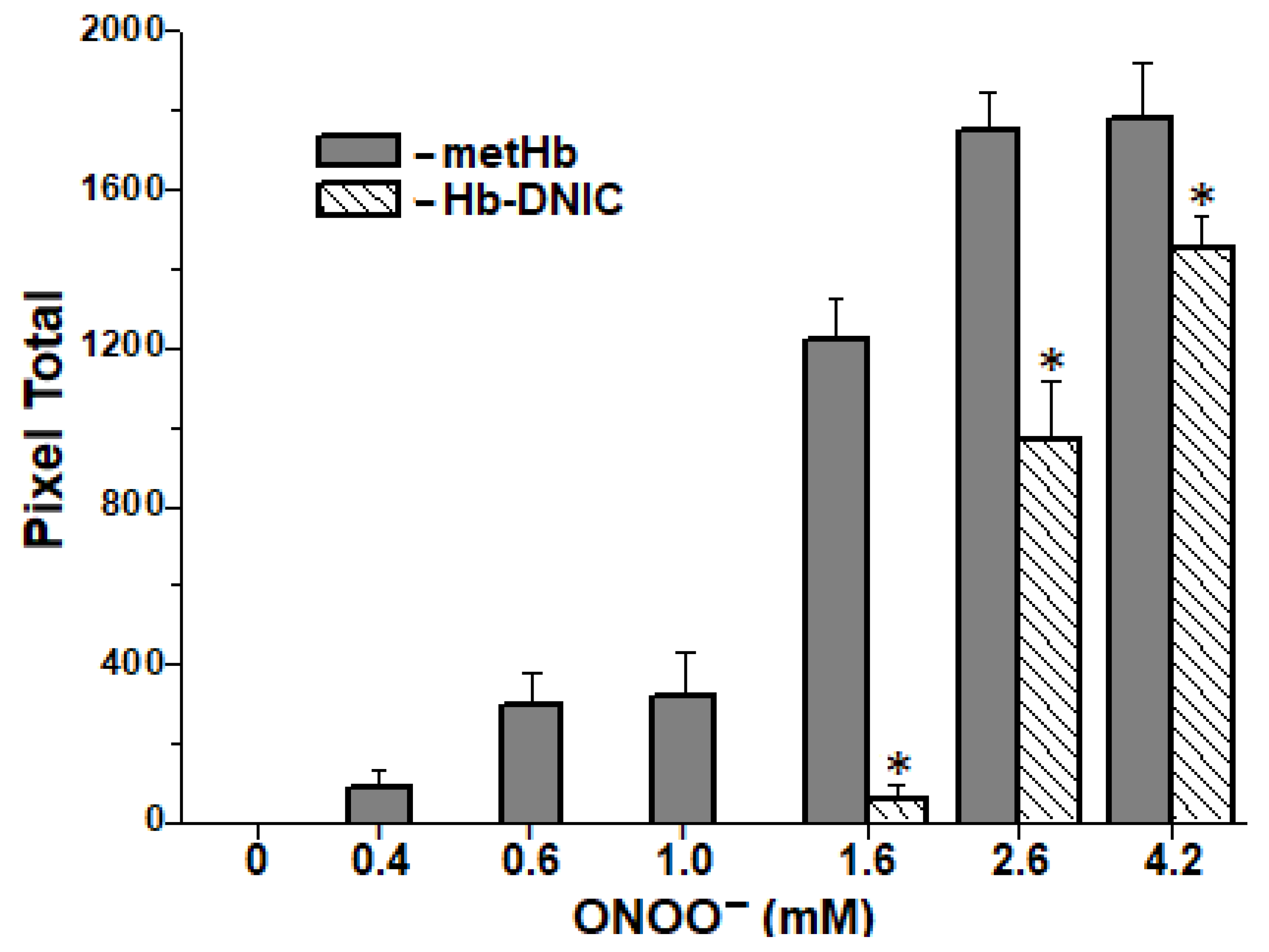
2.6. Formation of Interprotein Cross-Links
3. Discussion
↓
{(CysS−)(R−)} + FeIII + NO2−
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Registration of EPR Spectra
4.3. Determining of Protein Carbonyls
- S2 = (E363 + E370) × 3.5 + (E370 + E428) × 29 + (E430 + E434) × 2 + (E520 + E535) × 7.5.
- S1—aldehyde-dinitrophenylhydrazones,
- S2—neutral ketone-dinitrophenylhydrazones.
4.4. Measurements of Heme Groups
4.5. Measurement of Protein SH Groups
4.6. Identification of Tryptophan and Tyrosine Residues State
4.7. SDS-Electrophoresis in PAAG
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsai, M.-L.; Tsou, C.-C.; Liaw, W.-F. Dinitrosyl iron complexes (DNICs): From biomimetic synthesis and spectroscopic characterization toward unveiling the biological and catalytic roles of DNICs. Acc. Chem. Res. 2015, 48, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F. Dinitrosyl iron complexes with thiol-containing ligands as a “working form” of endogenous nitric oxide. Nitric Oxide 2016, 54, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-Y.; Chung, C.-W.; Santos, C.-W.; Villaflores, O.B.; Lu, T.-T. Fe in biosynthesis, translocations, and signal transduction of NO toward bioinorganic engineering of dinitrosyl iron complexes into NO delivery scaffolds for tissue engineering. Dalton Trans. 2019, 48, 9431–9453. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, H.; Brzóska, K.; Meczyńska-Wielgosz, S.; Rumianek, K.; Wójciuk, G.; Kruszewski, M. Dinitrosyl iron complexes—Structure and biological functions. Postępy Biochemii 2010, 56, 298–304. [Google Scholar] [PubMed]
- Vanin, A.F. What is the mechanism of nitric oxide conversion into nitrosonium ions ensuriong S-nitrosating processes in living organisms. Cell Biochem. Biophys. 2019, 77, 279–292. [Google Scholar] [CrossRef]
- Padmaja, S.; Huie, R.E. The reaction of nitric oxide with organic peroxyl radicals. Biochem. Biophys. Res. Commun. 1993, 195, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Abalenikhina, Y.V.; Kosmachevskaya, O.V.; Topunov, A.F. Peroxynitrite: Toxic agent and signaling molecule. Appl. Biochem. Microbiol. 2020, 56, 611–625. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271 Pt 1, C1424–C1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuto, J.M.; Bartberger, M.; Dutton, A.S.; Paolocci, N. The physiological chemistry and biological activity of nitroxyl (HNO): The neglected, misunderstood, and enigmatic nitrogen oxide. Chem. Res. Toxicol. 2005, 18, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Prolo, C.; Álvarez, M.N.; Ríos, N.; Peluffo, G.; Radi, R.; Romero, N. Nitric oxide diffusion to red blood cells limits extracellular, but not intraphagosomal, peroxynitrite formation by macrophages. Free Radic. Biol. Med. 2015, 87, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shiva, S.; Kim-Shapiro, D.B.; Patel, R.P.; Ringwood, L.A.; Irby, C.E.; Huang, K.T.; Ho, C.; Hogg, N.; Schechter, A.N.; et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Investig. 2005, 115, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Balagopalakrishna, C.; Manoharan, P.T.; Abugo, O.O.; Rifkind, J.M. Production of superoxide from hemoglobin-bound oxygen under hypoxic conditions. Biochemistry 1996, 35, 6393–6398. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Gubkin, A.A.; Serezhenkov, V.A.; Lobysheva, I.I.; Kosmachevskaya, O.V.; Ruuge, E.K.; Lankin, V.Z.; Topunov, A.F.; Vanin, A.F. Interaction of reactive oxygen and nitrogen species with albumin—And hemoglobin bound dinitrosyl iron complexes. Nitric Oxide 2008, 18, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Kosmachevskaya, O.V.; Timoshin, A.A.; Vanin, A.F.; Topunov, A.F. Dinitrosyl iron complexes bound with haemoglobin as markers of oxidative stress. Methods Enzymol. 2008, 436, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Pietraforte, D.; Salzano, A.M.; Marino, G.; Minetti, M. Peroxynitrite-dependent modifications of tyrosine residues in hemoglobin. Formation of tyrosyl radical(s) and 3-nitrotyrosine. Amino Acids 2003, 25, 341–350. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Petrova, N.E.; Zabbarova, I.V.; Vanin, A.F.; Topunov, A.F.; Lankin, V.Z.; Ruuge, E.K. Interaction of oxoferrylmyoglobin and dinitrosyl iron complexes. Biochemistry 2004, 69, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Gorudko, I.V.; Kosmachevskaya, O.V.; Grigoryeva, D.V.; Panasenko, O.M.; Vanin, A.F.; Topunov, A.F.; Terekhova, M.S.; Sokolov, A.V.; Cherenkevich, S.N.; et al. Protective effect of dinitrosyl iron complexes with glutathione in red blood cell lysis induced by hypochlorous acid. Oxid. Med. Cell. Longev. 2019, 2019, e2798154. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Zhang, N.; Yang, J.; Ding, H. Nitric oxide-induced bacteriostasis ana modification of iron-sulphur proteins in Escherichia Coli. Mol. Microbiol. 2008, 70, 953–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Requena, J.R.; Levine, R.L.; Stadtman, E.R. Recent advances in the analysis of oxidized proteins. Amino Acids 2003, 25, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Campolo, N.; Bartesaghi, S.; Radi, R. Metal-catalyzed protein tyrosine nitration in biological systems. Redox Rep. 2014, 19, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M. Formation of fluorescent heme degradation products during the oxidation of hemoglobin by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1998, 247, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Exner, M.; Herold, S. Kinetic and mechanistic studies of the peroxynitrite-mediated oxidation of oxymyoglobin and oxyhemoglobin. Chem. Res. Toxicol. 2000, 13, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Alayash, A.I.; Ryan, B.A.; Cashon, R.E. Peroxynitrite mediated heme oxidation and protein modification of native and chemically modified hemoglobins. Arch. Biochem. Biophys. 1998, 349, 65–73. [Google Scholar] [CrossRef]
- De Marinis, E.; Casella, L.; Ciaccio, C.; Coletta, M.; Visca, P.; Ascenzi, P. Catalytic peroxidation of nitrogen monoxide and peroxynitrite by globins. IUBMB Life 2009, 61, 62–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herold, S.; Rehmann, F.-J.K. Kinetics of the reactions of nitrogen monoxide and nitrite with ferryl hemoglobin. Free Rad. Biol. Med. 2002, 34, 531–545. [Google Scholar] [CrossRef]
- Herold, S.; Shivashankar, K. Metmyoglobin and methemoglobin catalyze the isomerization of peroxynitrite to nitrate. Biochemistry 2003, 42, 14036–14046. [Google Scholar] [CrossRef]
- Romero, N.; Radi, R.; Linares, E.; Augusto, O.; Detweiler, C.D.; Mason, R.P.; Denicola, A. Reaction of human hemoglobin with peroxynitrite. J. Biol. Chem. 2003, 278, 44049–44057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbunov, N.V.; Osipov, A.N.; Day, B.W.; Zayas-Rivera, B.; Kagan, V.E.; Elsayed, N.M. Reduction of ferrylmyoglobin and ferrylhemoglobin by nitric oxide: A protective mechanism against ferryl hemoprotein-induced oxidations. Biochemistry 1995, 34, 6689–6699. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, N.V.; Yalowich, J.C.; Gaddam, A.; Thampatty, P.; Ritov, V.B.; Kisin, E.R.; Elsayed, N.M.; Kagan, V.E. Nitric oxide prevents oxidative damage produced by tert-butyl hydroperoxide in erythroleukemia cells via nitrosylation of heme and non-heme iron. Electron paramagnetic resonance evidence. J. Biol. Chem. 1997, 272, 12328–12341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagan, V.E.; Kozlov, A.V.; Tyurina, Y.Y.; Shvedova, A.A.; Yalowich, J.C. Antioxidant mechanisms of nitric oxide against iron-catalyzed oxidative stress in cells. Antioxid. Redox Signal. 2001, 3, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Tyurina, Y.Y.; Gorbunov, N.V.; Tyurin, V.A.; Castranova, V.; Kommineni, C.; Ojimba, J.; Gandley, R.; McLaughlin, M.K.; Kagan, V.E. Tert-butyl hydroperoxide/hemoglobin-induced oxidative stress and damage to vascular smooth muscle cells: Different effects of nitric oxide and nitrosothiols. Biochem. Pharmacol. 1999, 57, 989–1001. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Gubkin, A.A.; Gubkina, S.A.; Gudkov, L.L.; Lakomkin, V.L.; Topunov, A.F.; Vanin, A.F.; Ruuge, E.K. Interaction between albumin-bound dinitrosyl iron complexes and reactive oxygen species. Biophysics 2007, 52, 336–339. [Google Scholar] [CrossRef]
- Tomoda, A.; Sugimoto, K.; Suhara, M.; Takeshita, M.; Yoneyama, Y. Haemichrome formation from haemoglobin subunits by hydrogen peroxide. Biochem. J. 1978, 171, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Vergara, A.; Vitagliano, L.; Verde, C.; di Prisco, G.; Mazzarella, L. Spectroscopic and crystallographic characterization of bis-histidyl adducts in tetrameric hemoglobins. Methods Enzymol. 2008, 436, 425–444. [Google Scholar] [PubMed]
- Reeder, B.J.; Grey, M.; Silaghi-Dumitrescu, R.-L.; Svistunenko, D.A.; Bülow, L.; Cooper, C.E.; Wilson, M.T. Tyrosine residues as redox cofactors in human hemoglobin. J. Biol. Chem. 2008, 283, 30780–30787. [Google Scholar] [CrossRef] [Green Version]
- Radi, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of its functional effects. Acc. Chem. Res. 2012, 46, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Vlasova, I.I. Peroxidase activity of human hemoproteins: Keeping the fire under control. Molecules 2018, 23, 2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Deterding, L.J.; Jiang, J.; Bonini, M.G.; Tomer, K.B.; Ramirez, D.C.; Mason, R.P. Electron transfer between a tyrosyl radical and a cysteine residue in hemoproteins: Spin trapping analysis. J. Am. Chem. Soc. 2007, 129, 13493–13501. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Delsignore, M.E. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J. Biol. Chem. 1987, 262, 9908–9913. [Google Scholar] [CrossRef]
- Gunther, M.R.; Sturgeon, B.E.; Mason, R.P. Nitric oxide trapping of the tyrosyl radical-chemistry and biochemistry. Toxicology 2002, 177, 1–9. [Google Scholar] [CrossRef]
- Schopfer, M.P.; Mondal, B.; Lee, D.-H.; Sarjeant, A.A.N.; Karlin, K.D. Heme/O2/•NO Nitric oxide dioxygenase (NOD) reactivity: Phenolic nitration via a putative heme-peroxynitrite intermediate. J. Am. Chem. Soc. 2009, 131, 11304–11305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietraforte, D.; Minetti, M. One-electron oxidation pathway of peroxynitrite decomposition in human blood plasma: Evidence for the formation of protein tryptophan-centred radicals. Biochem. J. 1997, 321, 734–750. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, B.; Rubbo, H.; Kirk, M.; Barnes, S.; Freeman, B.A.; Radi, R. Peroxynitrite-dependent tryptophan nitration. Chem. Res. Toxicol. 1996, 9, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Vitturi, D.A.; Sun, C.-W.; Harper, V.M.; Thrash-Williams, B.; Cantu-Medellin, N.; Chacko, B.K.; Peng, N.; Dai, Y.; Wyss, J.M.; Townes, T.; et al. Antioxidant functions for the hemoglobin β93 cysteine residue in erythrocytes and in the vascular compartment in vivo . Free Radic. Biol. Med. 2013, 55, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, Y.; Joseph, J.; Kalyanaraman, B. Intramolecular electron transfer between tyrosyl radical and cysteine residue inhibits tyrosine nitration and induces thiyl radical formation in model peptides treated with myeloperoxidase, H2O2, and NO2−: EPR spin-trapping studies. J. Biol. Chem. 2005, 280, 40684–40698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Buehler, P.W.; Boykins, R.A.; Venable, R.M.; Alayash, A.I. Structural Basis of Peroxide-mediated Changes in Human Hemoglobin. J. Biol. Chem. 2007, 282, 4894–4907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobysheva, I.I.; Serezhenkov, V.A.; Vanin, A.F. Interaction of peroxynitrite and hydrogen peroxide with dinitrosyl iron complexes containing thiol ligands in vitro. Biochemistry 1999, 64, 153–158. [Google Scholar]
- Alayash, A.I. βCysteine 93 in human hemoglobin: A gateway to oxidative stability in health and disease. Lab. Investig. 2021, 101, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.-C.; Liaw, W.-F. Transformation of the {Fe(NO)2}9 dinitrosyl iron complexes (DNICs) into S-nitrosothiols (RSNOs) triggered by acid-base pairs. Chemistry 2011, 17, 13358–13366. [Google Scholar] [CrossRef]
- Vanin, A.F.; van Faassen, E. DNICs: Physico-Chemical Properties and Their Observation in Cells and Tissues. In Radicals for Life: The Various Forms of Nitric Oxide; Van Faassen, E., Vanin, A.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 17–74. [Google Scholar]
- Tran, N.G.; Kalyvas, H.; Skodje, K.M.; Hayashi, T.; Moënne-Loccoz, P.; Callan, P.E.; Shearer, J.; Kirschenbaum, L.J.; Kim, E. Phenol nitration induced by an {Fe(NO)2}10 dinitrosyl iron complex. J. Am. Chem. Soc. 2011, 133, 1184–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Shang, P. The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Metallomics 2018, 10, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.C.; Bosworth, C.A.; Hennon, S.W.; Mahtani, H.A.; Bergonia, H.A.; Lancaster, J.R. Nitric oxide induced conversion of cellular chelatable iron into macromolecular-bound paramagnetic diinitrosyliron complexes. J. Biol. Chem. 2008, 283, 28926–28933. [Google Scholar] [CrossRef] [Green Version]
- Damasceno, F.C.; Condeles, A.L.; Lopes, A.K.B.; Facci, R.R.; Linares, E.; Truzzi, D.R.; Augusto, O.; Toledo, J.C., Jr. The labile iron pool attenuates peroxynitrite-dependent damage and can no longer be considered solely a pro-oxidative cellular iron source. J. Biol. Chem. 2018, 293, 8530–8542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese-Krott, M.M.; Kelm, M. Endothelial nitric oxide synthase in red blood cells: Key to a new erythrocrine function? Redox Biology 2014, 2, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damasceno, F.C.; Toledo, J.C.; Condeles, A.L.; Lopes, A.K.B.; Facci, R.R.; Linares, E. Can cellular labile iron pool be considered solely a pro-oxidant species in cells? FASEB J. 2019, 33 (Suppl. S1), 351.4. [Google Scholar] [CrossRef]
- Carballal, S.; Bartesaghi, S.; Radi, R. Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim. Biophys. Acta 2014, 1840, 768–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amici, A.; Levine, R.L.; Tsai, L.; Stadtman, E.R. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J. Biol. Chem. 1989, 264, 3341–3346. [Google Scholar] [CrossRef]
- Sampson, J.B.; Rosen, H.; Beckman, J.S. Peroxynitrite dependent tyrosine nitration catalyzed by superoxide dismutase, myeloperoxidase and horseradish peroxidase. Methods Enzymol. 1996, 269, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Daiber, A.; Shoun, H.; Peterson, J.A.; Ullrich, V. Rapid reactions of peroxynitrite with heme-thiolate proteins as the basis for protection of prostacyclin synthase from inactivation by nitration. Arch. Biochem. Biophys. 2000, 376, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Yamakura, F.; Taka, H.; Fujimura, T.; Murayama, K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J. Biol. Chem. 1998, 273, 14085–14089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shumaev, K.B.; Kosmachevskaya, O.V.; Grachev, D.I.; Timoshin, A.A.; Topunov, A.F.; Lankin, V.Z.; Ruuge, E.K. A possible mechanism of the antioxidant action of dinitrosyl iron complexes. Biochem. Suppl. Ser. B Biomed. Chem. 2021, 15, 313–319. [Google Scholar] [CrossRef]
- Kovacs, I.; Lindermayr, C. Nitric oxide-based protein modification: Formation and site-specificity of protein S-nitrosylation. Front. Plant Sci. 2013, 4, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An emerging paradigm of redox signaling. Antioxidants 2019, 8, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation: Determinants of specificity and enzymatic regulation of S-nitrosothiol-based signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.A.; Neuman, N.I.; Muñoz, M.; Álvarez, L.A.; Bikiel, D.E.; Brondino, C.D.; Ivanović-Burmazović, I.; Miljkovic, J.L.; Filipovic, M.R.; Martí, M.A.; et al. Nitric oxide is reduced to HNO by proton-coupled nucleophilic attack by ascorbate, tyrosine, and other alcohols. A new route to HNO in biological media? J. Am. Chem. Soc. 2015, 137, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Borodulin, R.R.; Kubrina, L.N.; Mikoyan, V.D.; Poltorakov, A.P.; Shvydkiy, O.V.; Burbaev, D.S.; Serezhenkov, V.A.; Yakhontova, E.R.; Vanin, A.F. Dinitrosyl iron complexes with glutathione as NO and NO+ donors. Nitric Oxide 2013, 29, 4–16. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Shumaev, K.B.; Novikova, N.N.; Topunov, A.F. Effect of iron—Nitric oxide complexes on the reactivity of hemoglobin cysteines. Appl. Biochem. Microbiol. 2020, 56, 512–520. [Google Scholar] [CrossRef]
- Beckman, J.S. Synthesis of peroxynitrite by a reaction of nitrite with acidified hydrogen peroxide in the presence of a quencher. Methods Enzymol. 1994, 233, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Schmorak, J.; Lewin, M. The determination of small concentrations of carbonyl groups in starch by the cyanohydrin method. Anal. Chem. 1961, 33, 1403–1405. [Google Scholar] [CrossRef]
- Riggs, A. Preparation of blood hemoglobins of vertebrates. Methods Enzymol. 1981, 76, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Hoff, S.; Larsen, F.H.; Andersen, M.L.; Lund, M.N. Quantification of protein thiols using ThioGlo1 fluorescent derivatives and HPLC separation. Analyst 2013, 138, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmachevskaya, O.V.; Nasybullina, E.I.; Shumaev, K.B.; Novikova, N.N.; Topunov, A.F. Protective Effect of Dinitrosyl Iron Complexes Bound with Hemoglobin on Oxidative Modification by Peroxynitrite. Int. J. Mol. Sci. 2021, 22, 13649. https://doi.org/10.3390/ijms222413649
Kosmachevskaya OV, Nasybullina EI, Shumaev KB, Novikova NN, Topunov AF. Protective Effect of Dinitrosyl Iron Complexes Bound with Hemoglobin on Oxidative Modification by Peroxynitrite. International Journal of Molecular Sciences. 2021; 22(24):13649. https://doi.org/10.3390/ijms222413649
Chicago/Turabian StyleKosmachevskaya, Olga V., Elvira I. Nasybullina, Konstantin B. Shumaev, Natalia N. Novikova, and Alexey F. Topunov. 2021. "Protective Effect of Dinitrosyl Iron Complexes Bound with Hemoglobin on Oxidative Modification by Peroxynitrite" International Journal of Molecular Sciences 22, no. 24: 13649. https://doi.org/10.3390/ijms222413649
APA StyleKosmachevskaya, O. V., Nasybullina, E. I., Shumaev, K. B., Novikova, N. N., & Topunov, A. F. (2021). Protective Effect of Dinitrosyl Iron Complexes Bound with Hemoglobin on Oxidative Modification by Peroxynitrite. International Journal of Molecular Sciences, 22(24), 13649. https://doi.org/10.3390/ijms222413649





