Role of Circulating Biomarkers in Platinum-Resistant Ovarian Cancer
Abstract
:1. Introduction
2. Methods
3. Circulating Biomarkers
3.1. Glycoprotein Biomarkers
3.1.1. CA125
3.1.2. HE4
3.1.3. Mesothelin
3.2. Liquid Biopsy
3.2.1. Circulating Tumor DNA
3.2.2. Circulating Tumor Cells
3.2.3. Extracellular Vesicles
3.3. Epigenetic and Genetic Biomarkers
3.3.1. Epigenetic Alteration Markers
MicroRNAs
DNA Methylation
Histone Modifications and Involved Enzymes
3.3.2. Genetic Alteration Markers
TP53 Mutations
Homologous Recombination and BRCA Genes
3.4. Angiogenic Biomarkers
3.5. Immune-Related Biomarkers
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Romero, I.; Bast, R.C., Jr. Minireview: Human Ovarian Cancer: Biology, current management, and paths to personalizing therapy. Endocrinology 2012, 153, 1593–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, W.E., 3rd; Maxwell, G.L.; Tian, C.; Sundorg, M.J.; Rose, G.S.; Rose, P.G.; Rubin, S.C.; Muggia, F.; McGuire, W.P. Gynecologic Oncology Group. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2008, 26, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.C.; Kitchener, H.; Bacon, M.; Marth, C.; Thigpen, T.; Trimble, E. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: Report from the fourth ovarian cancer consensus conference. Int. J. Gynecol. Cancer 2011, 21, 750–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomao, F.; D’Incalci, M.; Biagioli, E.; Peccatori, F.A.; Colombo, N. Restoring platinum sensitivity in recurrent ovarian cancer by extending the platinum-free interval: Myth or reality? Cancer 2017, 123, 3450–3459. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.K.; Pujdae-Lauraine, E.; Aoki, D.; Mirza, M.R.; Lorusso, D.; Oza, A.M.; du Bois, A.; Vergote, I.; Reuss, A.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recurrent disease. Ann. Oncol. 2017, 28, 727–732. [Google Scholar] [CrossRef]
- Davis, A.; Tinker, A.V.; Friedlander, M. “platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef]
- Markman, M.; Bookman, M.A. Second-Line Treatment of Ovarian Cancer. Oncologist 2000, 5, 26–35. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization & International Programme on Chemical Safety. Biomarkers in risk assessment: Validity and validation. 2001. Available online: https://apps.who.int/iris/handle/10665/42363 (accessed on 19 December 2021).
- National Cancer Institute. Tumor Markers in Common Use. 2019. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-markers-list (accessed on 19 December 2021).
- Strimbu, K.; Tavel, J. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Goossens, N.; Nakagawa, S.; Sun, X.; Hoshida, Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 2015, 4, 256–269. [Google Scholar] [CrossRef]
- Ruberg, S.J.; Shen, L. Personalized medicine: Four perspectives of tailored medicine. Stat. Biopharm. Res. 2015, 7, 214–229. [Google Scholar] [CrossRef]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C., Jr.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Han, L.Y.; Karavasilis, V.; va Hagen, T.; Nicum, S.; Thomas, K.; Harrison, M.; Papadopolos, P.; Blake, P.; Barton, D.P.J.; Gore, M.; et al. Doubling time of serum CA125 is an independent prognostic factor for survival in patients with ovarian cancer relapsing after first-line chemotherapy. Eur. J. Cancer 2010, 46, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Cosio, S.; Tana, R.; Genazzani, A.R. Serum and tissue biomarkers as predictive and prognostic variables in epithelial ovarian cancer. Crit. Rev. Oncol. Hematol. 2009, 69, 12–27. [Google Scholar] [CrossRef]
- Boivin, M.; Lane, D.; Piché, A.; Rancourt, C. CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol. Oncol. 2009, 115, 407–413. [Google Scholar] [CrossRef]
- Matte, I.; Garde-granger, P.; Bessette, P.; Piché, A. Serum CA125 and ascites leptin level ratio predicts baseline clinical resistance to first-line platinum-based treatment and poor prognosis in patients with high grade serous ovarian cancer. Am. J. Cancer Res. 2019, 9, 160–170. [Google Scholar]
- Lee, C.K.; Asher, R.; Friedlander, M.; Gebki, V.; Gonzales-Martyin, A.; Lortholay, A.; Lesoiin, A.; Kurzeder, C.; Largillier, R.; Hilpert, F.; et al. Development and validation of a prognostic nomogram for overall survival in patients with platinum-resistant ovarian cancer treated with chemotherapy. Eur. J. Cancer 2019, 117, 99–106. [Google Scholar] [CrossRef]
- Rustin, G.J.S.; van der Burg, M.E.L.; Griffin, C.L.; Guthrie, D.; Lamont, A.; Jayson, G.C.; Kristensen, G.; Mediola, C.; Coens, C.; Qian, W.; et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): A randomised trial. Lancet 2010, 376, 1155–1163. [Google Scholar] [CrossRef]
- James, N.E.; Chichester, C.; Ribeiro, J.R. Beyond the biomarker: Understanding the diverse roles of human epididymis protein 4 in the pathogenesis of epithelial ovarian cancer. Front. Oncol. 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, J.R.; Schorl, C.; Yano, N.; Romano, N.; Kim, K.K.; Singh, R.K.; Moore, R.G. HE4 promotes collateral resistance to cisplatin and paclitaxel in ovarian cancer cells. J. Ovarian Res. 2016, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.G.; Hill, E.K.; Horan, T.; Yano, N.; Kim, K.; MacLaughlan, S.; Lambert-Messerlian, G.; Tseng, Y.D.; Padlbury, J.F.; Miller, M.C.; et al. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci. Rep. 2014, 4, 3574. [Google Scholar] [CrossRef] [Green Version]
- Angioli, R.; Capriglione, S.; Aloisi, A.; Guzzo, F.; Luvero, D.; Miranda, A.; Damiani, P.; Montera, R.; Terranova, C.; Plotti, F. Can HE4 predict platinum response during first-line chemotherapy in ovarian cancer? Tumor Biol. 2014, 35, 7009–7015. [Google Scholar] [CrossRef]
- Lv, J.; Li, P. Mesothelin as a biomarker for targeted therapy. Biomark. Res. 2019, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of Ovarian Cancer Antigen CA125/MUC61 to Mesothelin Mediates Cell Adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Qian, M.; Ho, M. The Role of Mesothelin in Tumor Progression and Targeted Therapy. Anticancer Agents Med. Chem. 2013, 13, 276–280. [Google Scholar] [CrossRef]
- Cheng, W.F.; Huang, C.-Y.; Chang, M.-C.; Hu, Y.-H.; Chiang, Y.-C.; Chen, Y.-L.; Chen, C.-A. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br. J. Cancer 2009, 100, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Badgwell, D.; Lu, Z.; Cole, L.; Frische, H.; Atkinson, E.N.; Somers, E.; Allard, J.; Moore, R.G.; Lu, K.H.; Bast, R.C., Jr. Urinary mesothelin provides greater sensitivity for early stage ovarian cancer than serum mesothelin, urinary hCG free beta subunit and urinary hCG beta core fragment. Gynecol. Oncol. 2007, 106, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Scholler, N.; Fu, N.; Ye, Z.; Goodman, G.E.; Hellstrom, K.E.; Hellstrom, I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc. Natl. Acad. Sci. USA 1999, 96, 11531–11536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Zhang, L.; Chen, Y.; Qing, C. Circulating cell-free DNA and circulating tumor cells, the ‘liquid biopsies’ in ovarian cancer. J. Ovarian Res. 2017, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Esposito, A.; Criscitiello, C.; Locatelli, M.; Milano, M.; Curigliano, G. Liquid biopsies for solid tumors: Understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol. Ther. 2016, 157, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Li, M.; Dressman, D.; He, Y.; Shen, D.; Szabo, S.; Diaz, L.A., Jr.; Goodman, S.N.; Davis, K.A.; Juhl, H.; et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 16368–16373. [Google Scholar] [CrossRef] [Green Version]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.; Kaper, F.; Dawson, S.-J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl. Med. 2012, 4, 136ra68. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef] [Green Version]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the possible origin and mechanism of circulating DNA: Apoptosis and active DNA release. Clin. Chim. Acta 2001, 313, 139–142. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Gormally, E.; Caboux, E.; Vineis, P.; Hainaut, P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: Practical aspects and biological significance. Mutat. Res. 2007, 635, 105–117. [Google Scholar] [CrossRef]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar]
- Stroun, M.; Anker, P.; Lyautey, J.; Lederrey, C.; Maurice, P.A. Isolation and characterization of DNA from the plasma of cancer patients. Eur. J. Cancer Clin. Oncol. 1987, 23, 707–712. [Google Scholar] [CrossRef]
- Marzese, D.M.; Hirose, H.; Hoon, D.S.B. Diagnostic and prognostic value of circulating tumor-related DNA in cancer patients. Expert Rev. Mol. Diagn. 2013, 13, 827–844. [Google Scholar] [CrossRef]
- Kuhlmann, J.D.; Schwarzenbach, H.; Wimberger, P.; Poetsch, M.; Kimmig, R.; Kasimir-Bauer, S. LOH at 6q and 10q in fractionated circulating DNA of ovarian cancer patients is predictive for tumor cell spread and overall survival. BMC Cancer 2012, 12, 325. [Google Scholar] [CrossRef] [Green Version]
- Cohen, P.A.; Flowers, N.; Tong, S.; Hannan, N.; Pertile, M.D.; Hui, L. Abnormal plasma DNA profiles in early ovarian cancer using a non-invasive prenatal testing platform: Implications for cancer screening. BMC Med. 2016, 14, 126. [Google Scholar] [CrossRef] [Green Version]
- Vanderstichele, A.; Busschaert, P.; Smeets, D.; Landolfo, C.; Van Nieuwenhuysen, E.; Leunen, K.; Neven, P.; Amant, F.; Mahner, S.; Braicu, E.I.; et al. Chromosomal instability in cell-free DNA as a highly specific biomarker for detection of ovarian cancer in women with adnexal masses. Clin. Cancer Res. 2017, 23, 2223–2231. [Google Scholar] [CrossRef] [Green Version]
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat. Commun. 2018, 9, 5068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mari, R.; Mamessier, E.; Lambaudie, E.; Provansal, M.; Birnbaum, D.; Bertucci, F.; Sabatier, R. Liquid biopsies for ovarian carcinoma: How blood tests may improve the clinical management of a deadly disease. Cancers 2019, 11, 774. [Google Scholar] [CrossRef] [Green Version]
- Steffensen, K.D.; Madsen, C.V.; Andersen, R.F.; Waldstrøm, M.; Adimi, P.; Jakobsen, A. Prognostic importance of cell-free DNA in chemotherapy resistant ovarian cancer treated with bevacizumab. Eur. J. Cancer 2014, 50, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Comino-Mendez, I.; de Bruijn, I.; Tian, L.; Meisel, J.L.; Garcia-Murillas, I.; Fribbens, C.; Cutts, R.; Martelotto, L.G.; Ng, C.K.Y.; et al. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin. Cancer Res. 2017, 23, 6708–6720. [Google Scholar] [CrossRef] [Green Version]
- Diehl, F.; Schmidt, L.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Sedlackova, T.; Repiska, G.; Celec, P.; Szemes, T.; Minarik, G. Fragmentation of DNA affects the accuracy of the DNA quantitation by the commonly used methods. Biol. Proced. Online 2013, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Ignatiadis, M.; Lee, M.; Jeffrey, S.S. Circulating tumor cells and circulating tumor DNA: Challenges and opportunities on the path to clinical utility. Clin. Cancer Res. 2015, 21, 4786–4800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehm, T.; Sagalowsky, A.; Clifford, E.; Beitsch, P.; Saboorian, H.; Euhus, D.; Meng, S.; Morrison, L.; Tucker, T.; Lane, N.; et al. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin. Cancer Res. 2002, 8, 2073–2084. [Google Scholar] [PubMed]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.J.; Uhr, J.; Terstappen, L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6879–6904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, C.J.; Moreno, J.G.; Pienta, K.J.; Gross, S.; Repollet, M.; O’hara, S.M.; Russel, T.; Terstappen, L.W.M.M. Apoptosis of circulating tumor cells in prostate cancer patients. Cytometry A 2004, 62, 46–53. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Koo, K.H.; Sung, J.Y.; Yun, U.-J.; Kim, H. Anoikis resistance: An essential prerequisite for tumor metastasis. Int. J. Cell Biol. 2012, 2012, 306879. [Google Scholar] [CrossRef] [Green Version]
- Van Berckelaer, C.; Brouwers, A.J.; Peeters, D.J.E.; Tjalma, W.; Trinh, X.B.; van Dam, P.A. Current and future role of circulating tumor cells in patients with epithelial ovarian cancer. Eur. J. Surg. Oncol. 2016, 42, 1772–1779. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Oskarsson, T.; Acharyya, S.; Nguyen, D.X.; Zhang, X.H.-F.; Norton, L.; Massague, J. Tumor Self-Seeding by Circulating Cancer Cells. Cell 2009, 139, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Qiao, X.; Shi, H.; Han, X.; Liu, W.; Tian, X.; Zeng, X. Circulating tumor-associated neutrophils (cTAN) contribute to circulating tumor cell survival by suppressing peripheral leukocyte activation. Tumor Biol. 2016, 37, 5397–5404. [Google Scholar] [CrossRef]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R.F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L.E.; et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int. J. Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.A.; Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. 2013, 91, 411–429. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Kolostova, K.; Pinkas, M.; Jalabova, A.; Pospisilova, E.; Svobodova, P.; Spicka, J.; Cegan, M.; Matkowski, R.; Bobek, V. Molecular characterization of circulating tumor cells in ovarian cancer. Am. J. Cancer Res. 2016, 6, 973–980. [Google Scholar] [PubMed]
- Chebouti, I.; Kuhlmann, J.D.; Buderath, P.; Weber, S.; Wimberger, P.; Bokeloh, Y.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. ERCC1-expressing circulating tumor cells as a potential diagnostic tool for monitoring response to platinum-based chemotherapy and for predicting post-therapeutic outcome of ovarian cancer. Oncotarget 2017, 8, 24303–24313. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular analysis of circulating tumour cells—Biology and biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144. [Google Scholar] [CrossRef]
- Kuhlmann, J.D.; Wimberger, P.; Bankfalvi, A.; Keller, T.; Scholer, S.; Aktas, B.; Buderath, P.; Hauch, S.; Otterbach, F.; Kimmig, R.; et al. ERCC1-positive circulating tumor cells in the blood of ovarian cancer patients as a predictive biomarker for platinum resistance. Clin. Chem. 2014, 60, 1282–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obermayr, E.; Castillo-Tong, D.C.; Pils, D.; Speiser, P.; Braicu, I.; Van Gorp, T.; Mahner, S.; Sehouli, J.; Vergote, I.; Zellinger, R. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance—A study of the OVCAD consortium. Gynecol. Oncol. 2013, 128, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Poveda, A.; Kaye, S.B.; McCormack, R.; Wang, S.; Parekh, T.; Ricci, D.; Lebedinsky, C.A.; Tercero, J.C.; Zintl, P.; Monk, B.J. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol. Oncol. 2011, 122, 567–572. [Google Scholar] [CrossRef]
- Lee, M.; Kim, E.J.; Cho, Y.; Kim, S.; Chung, H.H.; Park, N.H.; Song, Y.-S. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol. Oncol. 2017, 145, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Stott, S.; Toner, M.; Maheswaran, S.; Haber, D.A. Circulating tumor cells: Approaches to isolation and characterization. J. Cell Biol. 2011, 192, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.J. Circulating tumor cells: Will they be clinically useful? J. Natl. Cancer Inst. 2010, 102, 146–148. [Google Scholar] [CrossRef] [Green Version]
- Alix-Panabières, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Brouwer, A.; De Laere, B.; Peeters, D.; Peeters, M.; Salgado, R.; Dirix, L.; Van Laere, S. Evaluation and consequences of heterogeneity in the circulating tumor cell compartment. Oncotarget 2016, 7, 48625–48643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnoletto, C.; Corrà, F.; Minotti, L.; Baldassari, F.; Crudele, F.; Cook, W.J.J.; Di Leva, G.; d’Adamo, A.P.; Gasparini, P.; Volinia, S. Heterogeneity in Circulating Tumor Cells: The Relevance of the Stem-Cell Subset. Cancers 2019, 11, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.B.; Lim, C.T.; Lim, W.-T. Single-Cell Analysis of Circulating Tumor Cells: Why Heterogeneity Matters. Cancers 2019, 11, 1595. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.K.S.; Yue, P.Y.K.; Huang, R.-L.; Lai, H.-C.; Cheung, A.N.Y.; Tse, K.Y.; Ngan, H.Y.S.; Wong, A.S.T. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat. Commun. 2018, 9, 2270. [Google Scholar] [CrossRef]
- Chang, L.; Ni, J.; Zhu, Y.; Pang, B.; Graham, P.; Zhang, H.; Li, Y. Liquid biopsy in ovarian cancer: Recent advances in circulating extracellular vesicle detection for early diagnosis and monitoring progression. Theranostics 2019, 9, 4130–4140. [Google Scholar] [CrossRef]
- Soung, Y.H.; Ford, S.; Zhang, V.; Chung, J. Exosomes in cancer diagnostics. Cancers 2017, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- De Wever, O.; Derycke, L.; Hendrix, A.; De Meerleer, G.; Godeau, F.; Depypere, H.; Bracke, M. Soluble cadherins as cancer biomarkers. Clin. Exp. Metastasis 2007, 24, 685–697. [Google Scholar] [CrossRef]
- Peng, P.; Yan, Y.; Keng, S. Exosomes in the ascites of ovarian cancer patients: Origin and effects on anti-tumor immunity. Oncol. Rep. 2011, 25, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Szajnik, M.; Derbis, M.; Lach, M.; Patalas, P.; Michalak, M.; Drzewiecka, H.; Szpurek, D.; Nowakowski, A.J.; Spaczynski, M.; Baranowski, W.; et al. Exosomes in Plasma of Patients with Ovarian Carcinoma: Potential Biomarkers of Tumor Progression and Response to Therapy. Gynecol. Obstet. 2013. [Google Scholar] [CrossRef] [Green Version]
- Ayers, L.; Pink, R.; Carter, D.R.F.; Nieuwland, R. Clinical requirements for extracellular vesicle assays. J. Extracell. Vesicles 2019, 8, 1593755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeshima, H.; Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis. Oncol. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Li, Z.; Liu, J. MiRNAs in primary cutaneous lymphomas. Cell Prolif. 2015, 48, 271–277. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Shen, J.; Law, P.T.Y.; Chan, M.T.V.; Wu, W.K.K. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget 2015, 6, 13914–13921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, N.; Chen, Q.; Situ, H.; Xie, T.; Zhang, J.; Peng, C.; Lin, Y.; Chen, J. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014, 5, 7013–7026. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Liao, X.; Lu, N.; Liu, W.; Wong, C.-W. Chromatin-modifying drugs induce miRNA-153 expression to suppress Irs-2 in glioblastoma cell lines. Int. J. Cancer 2011, 129, 2527–2531. [Google Scholar] [CrossRef] [PubMed]
- Ujifuku, K.; Mitsutake, N.; Takakura, S.; Matsuse, M.; Saenko, V.; Suzuki, K.; Hayashi, K.; Matsuo, T.; Kamada, K.; Nagata, I.; et al. MiR-195, miR-455-3p and miR-10a* are implicated in acquired temozolomide resistance in glioblastoma multiforme cells. Cancer Lett. 2010, 296, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef]
- Zhou, J.; Gong, G.; Tan, H.; Dai, F.; Zhu, X.; Chen, Y.; Wang, J.; Liu, Y.; Chen, P.; Wu, X.; et al. Urinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinoma. Oncol. Rep. 2015, 33, 2915–2923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaksman, O.; Tropé, C.; Davidson, B.; Reich, R. Exosome-derived miRNAs and ovarian carcinoma progression. Carcinogenesis 2014, 35, 2113–2120. [Google Scholar] [CrossRef]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Cir. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Remaley, A.T. Lipid-based carriers of microRNAs and intercellular communication. Curr. Opin. Lipidol. 2012, 23, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Zernecke, A.; Bidagekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Debecke, B.; Hristov, M.; Koppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef]
- Shen, W.; Song, M.; Liu, J.; Qiu, G.; Li, T.; Hu, Y.; Liu, H. MiR-26a promotes ovarian cancer proliferation and tumorigenesis. PLoS ONE 2014, 9, e86871. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, L.; Cayan, F.; Gorur, A.; Skbayir, S.; Yaroglu, H.Y.; Unal, N.D.; Tamer, L. Circulating microRNA expression profiles in ovarian cancer. J. Obstet. Gynaecol. 2014, 34, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.; Zou, Y.; Wu, L.; Pei, M.; Jiang, Y. miR-145 promotes miR-133b expression through c-myc and DNMT3A-mediated methylation in ovarian cancer cells. J. Cell. Physiol. 2020, 235, 4291–4301. [Google Scholar] [CrossRef] [PubMed]
- Resnick, K.E.; Alder, H.; Hagan, J.P.; Richardson, D.L.; Croce, C.M.; Cohn, D.E. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol. Oncol. 2009, 112, 55–59. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, H.; Cong, H.; Wang, X.; Ni, H.; Shen, X.; Ju, S. Diagnostic Model of Serum miR-193a-5p, HE4 and CA125 Improves the Diagnostic Efficacy of Epithelium Ovarian Cancer. Pathol. Oncol. Res. 2018, 24, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Staicu, C.E.; Predescu, D.-V.; Rusu, C.M.; Radu, B.M.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.-C. Role of microRNAs as Clinical Cancer Biomarkers for Ovarian Cancer: A Short Overview. Cells 2020, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Kjersem, J.B.; Ikdahl, T.; Lingjaerde, O.C.; Guren, T.; Tveit, K.M.; Kure, E.H. Plasma microRNAs predicting clinical outcome in metastatic colorectal cancer patients receiving first-line oxaliplatin-based treatment. Mol. Oncol. 2014, 8, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-N.; Chen, G.-S.; Hong, S.-J. Circulating MicroRNAs in gynecological malignancies: From detection to prediction. Exp. Hematol. Oncol. 2014, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Zhang, L.; Zhao, Y.; Yang, D.; Song, F.; Wen, Y.; Hao, Q.; Hu, Z.; Zhang, W.; Chen, K. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS ONE 2013, 8, e77853. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.A.; Skaar, T.C.; Liu, Y.; Nephew, K.P.; Matei, D. Carboplatin with decitabine therapy, in recurrent platinum resistant ovarian cancer, alters circulating miRNAs concentrations: A pilot study. PLoS ONE 2015, 10, e0141279. [Google Scholar] [CrossRef] [Green Version]
- Vigneron, N.; Vernon, M.; Meryet-Figuiere, M.; Lambert, B.; Briand, M.; Louis, M.-H.; Krieger, S.; Joly, F.; Lheureus, S.; Blanc-Fournier, C.; et al. Predictive relevance of circulating miR-622 in patients with newly diagnosed and recurrent high-grade serous ovarian carcinoma. Clin. Chem. 2020, 66, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Talens, R.P.; Boomsma, D.I.; Tobi, E.W.; Kremer, D.; Jukema, J.W.; Willemsen, G.; Putter, H.; Slagboom, P.E.; Heijmans, B.T. Variation, patterns, and temporal stability of DNA methylation: Considerations for epigenetic epidemiology. FASEB J. 2010, 24, 3135–3144. [Google Scholar] [CrossRef]
- Teschendorff, A.E.; Menon, U.; Gentry-Maharaj, A.; Ramus, S.J.; Gayther, S.A.; Apostolidou, S.; Jones, A.; Lechner, M.; Beck, S.; Jacobs, I.J.; et al. An epigenetic signature in peripheral blood predicts active ovarian cancer. PLoS ONE 2009, 4, e8274. [Google Scholar] [CrossRef] [Green Version]
- De Caceres, I.I.; Battagli, C.; Esteller, M.; Herman, J.G.; Dulaimi, E.; Edelson, M.I.; Bergman, C.; Ehya, H.; Eisenberg, B.L.; Cairns, P. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004, 64, 6476–6481. [Google Scholar] [CrossRef] [Green Version]
- Losi, L.; Fonda, S.; Saponaro, S.; Chelbi, S.T.; Lancellotti, C.; Gozzi, G.; Alberti, L.; Fabbiani, L.; Botticelli, L.; Benhatter, J. Distinct DNA methylation profiles in ovarian tumors: Opportunities for novel biomarkers. Int. J. Mol. Sci. 2018, 19, 1559. [Google Scholar] [CrossRef] [Green Version]
- Cacan, E. Epigenetic regulation of RGS2 (Regulator of G-protein signaling 2) in chemoresistant ovarian cancer cells. J. Chemother. 2017, 29, 173–178. [Google Scholar] [CrossRef]
- Gifford, G.; Paul, J.; Vasey, P.A.; Kaye, S.B.; Brown, R. The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin. Cancer Res. 2004, 10, 4420–4426. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.-P.; Chen, L.-Y.; Huang, R.-L.; Su, P.-H.; Chan, M.W.Y.; Chang, C.-C.; Yu, M.-H.; Wang, P.-H.; Yen, M.-S.; Nephew, K.P.; et al. Hypomethylation signature of tumor-initiating cells predicts poor prognosis of ovarian cancer patients. Hum. Mol. Genet. 2014, 23, 1894–1906. [Google Scholar] [CrossRef] [Green Version]
- Tomar, T.; Alkema, N.; Schreuder, L.; Meersma, G.J.; de Meyer, T.; van Criekinge, W.; Klip, H.G.; Fiegl, H.; van Nieuwenhuysen, E.; Vergote, I.; et al. Methylome analysis of extreme chemoresponsive patients identifies novel markers of platinum sensitivity in high-grade serous ovarian cancer. BMC Med. 2017, 15, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacan, E. Epigenetic-mediated immune suppression of positive co-stimulatory molecules in chemoresistant ovarian cancer cells. Cell Biol. Int. 2017, 41, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Bonito, N.A.; Borley, J.; Wilhelm-Benartzi, C.S.; Ghaem-Maghami, S.; Brown, R. Epigenetic regulation of the homeobox gene MSX1 associates with platinum-resistant disease in high-grade serous epithelial ovarian cancer. Clin. Cancer Res. 2016, 22, 3097–3104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leon, M.; Cardenas, H.; Vieth, E.; Emerson, R.; Segar, M.; Liu, Y.; Nephew, K.; Matei, D. Transmembrane protein 88 (TMEM88) promoter hypomethylation is associated with platinum resistance in ovarian cancer. Gynecol. Oncol. 2016, 142, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balch, C.; Huang, T.H.-M.; Brown, R.; Nephew, K.P. The epigenetics of ovarian cancer drug resistance and resensitization. Am. J. Obstet.Gynecol. 2004, 191, 1552–1572. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.A.; Strathdee, G.; Sludden, J.; Kaye, S.B.; Brown, R. Reversal of drug resistance in human tumor xenografts by 2′-deoxy-5-azacytidine-induced demethylation of the hMLH1 gene promoter. Cancer Res. 2000, 60, 6039–6044. [Google Scholar]
- Li, Y.; Hu, W.; Shen, D.-Y.; Kavanagh, J.J.; Fu, S. Azacitidine enhances sensitivity of platinum-resistant ovarian cancer cells to carboplatin through induction of apoptosis. Am. J. Obstet. Gynecol. 2009, 200, 177.e1–177.e9. [Google Scholar] [CrossRef]
- Gomyo, Y.; Sasaki, J.-I.; Branch, C.; Roth, J.A.; Mukhopadhyay, T. 5-Aza-2′-deoxycytidine upregulates caspase-9 expression cooperating with p53-induced apoptosis in human lung cancer cells. Oncogene 2004, 23, 6779–6787. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Balch, C.; Schilder, J.; Breen, T.; Zhang, S.; Shen, C.; Li, L.; Kulesavage, C.; Snyder, A.J.; Nephew, K.P.; et al. A phase 1 and pharmacodynamic study of decitabine in combination with carboplatin in patients with recurrent, platinum-resistant, epithelial ovarian cancer. Cancer 2010, 116, 4043–4053. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Zuo, Q.; Pilrose, J.; Wang, Y.; Shen, C.; Li, M.; Wulfridge, P.; Matei, D.; Nephew, K.P. Decitabine reactivated pathways in platinum resistant ovarian cancer. Oncotarget 2014, 5, 3579–3589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matei, D.; Shen, C.; Fang, F.; Schilder, J.; Li, M.; Zeng, A.A.; Pilrose, J.M.; Kulesavage, C.; Balch, C.; Berry, W.; et al. A phase II study of decitabine and carboplatin in recurrent platinum (Pt)-resistant ovarian cancer (OC). J. Clin. Oncol. 2011, 29, 5011. [Google Scholar] [CrossRef]
- Glasspool, R.M.; Brown, R.; Gore, M.E.; Rustin, G.J.S.; McBeish, I.A.; Wilson, R.H.; Peldge, S.; Paul, J.; Mackean, M.; Hall, G.D.; et al. A randomised, phase II trial of the DNA-hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in combination with carboplatin vs. carboplatin alone in patients with recurrent, partially platinum-sensitive ovarian cancer. Br. J. Cancer 2014, 110, 1923–1929. [Google Scholar] [CrossRef]
- Fu, S.; Hu, W.; Iyer, R.; Kavanagh, J.J.; Coleman, R.; Levenback, C.F.; Sood, S.K.; Wolf, J.K.; Gershenson, D.M.; Markman, M.; et al. Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer 2011, 117, 1661–1669. [Google Scholar] [CrossRef]
- Oza, A.M.; Matulonis, U.A.; Secord, A.A.; Nemunaitis, J.; Roman, L.D.; Blagden, S.P.; Banerjee, S.; McGuire, W.P.; Ghamande, S.; Birrer, M.J.; et al. A Randomized Phase II Trial of Epigenetic Priming with Guadecitabine and Carboplatin in Platinum-resistant, Recurrent Ovarian Cancer. Clin. Cancer Res. 2020, 26, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, K.; Nalejska, E.; Kubiak, M.; Wojtysiak, J.; Żołna, Ł.; Kowalewski, J.; Lewandowska, M.A. Prognostic and Predictive Epigenetic Biomarkers in Oncology. Mol. Diagn. Ther. 2019, 23, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yörüker, E.E.; Holdenrieder, S.; Gezer, U. Potential of circulating nucleosome-associated histone modifications in cancer. Transl. Cancer Res. 2018, 72, S185–S191. [Google Scholar] [CrossRef]
- McAnena, P.; Brown, J.A.L.; Kerin, M.J. Circulating nucleosomes and nucleosome modifications as biomarkers in cancer. Cancers 2017, 9, 5. [Google Scholar] [CrossRef]
- Stoetzer, O.J.; Fersching, D.M.I.; Salat, C.; Steinkohl, O.; Gabka, C.J.; Hamann, U.; Braun, M.; Feller, A.-M.; Heinemann, V.; Siegele, B.; et al. Prediction of response to neoadjuvant chemotherapy in breast cancer patients by circulating apoptotic biomarkers nucleosomes, DNAse, cytokeratin-18 fragments and surviving. Cancer Lett. 2013, 336, 140–148. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gezer, U.; Holdenrieder, S. Post-translational histone modifications in circulating nucleosomes as new biomarkers in colorectal cancer. In Vivo 2014, 28, 287–292. [Google Scholar]
- Gezer, U.; Yörüker, E.E.; Keskin, M.; Kulle, C.B.; Dharuman, Y.; Holdenrieder, S. Histone methylation marks on circulating nucleosomes as novel blood-based biomarker in colorectal cancer. Int. J. Mol. Sci. 2015, 16, 29654–29662. [Google Scholar] [CrossRef]
- Thålin, C.; Lundstrom, S.; Seignez, C.; Daleskog, M.; Lundstrom, A.; Henriksson, P.; Helleday, T.; Phillipson, M.; Wallen, H.; Demers, M. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Hwang, J.-W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Su, L.; Chen, W.Y. The emerging and diverse roles of sirtuins in cancer: A clinical perspective. Onco. Targets. Ther. 2013, 6, 1399–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mvunta, D.H.; Miyamoto, T.; Asaka, R.; Yamada, Y.; Ando, H.; Higuchi, S.; Ida, K.; Kashima, H.; Shiozawa, T. SIRT1 Regulates the Chemoresistance and Invasiveness of Ovarian Carcinoma Cells. Transl. Oncol. 2017, 10, 621–631. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W. Emerging Roles of SIRT1 in Cancer Drug Resistance. Genes Cancer 2013, 4, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modesitt, S.C.; Sill, M.; Hoffman, J.S.; Bender, D.P. Gynecologic Oncology Group. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 109, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-T.; Lai, H.-C.; Lee, H.-Y.; Lin, W.-H.; Chang, C.-C.; Chu, T.-Y.; Lin, Y.-W.; Lee, K.-D.; Yu, M.-H. Valproic acid resensitizes cisplatin-resistant ovarian cancer cells. Cancer Sci. 2008, 99, 1218–1226. [Google Scholar] [CrossRef]
- Shen, L.; Cui, J.; Pang, Y.-X.; Ma, Y.-H.; Liu, P.-S. 3-deazaneplanocin A is a promising therapeutic agent for ovarian cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 2915–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falchook, G.S.; Fu, S.; Naing, A.; Hong, D.S.; Hu, W.; Moulder, S.; Wheler, J.L.; Sood, A.K.; Bustinza-Linares, E.; Parkhurst, K.L.; et al. Methylation and histone deacetylase inhibition in combination with platinum treatment in patients with advanced malignancies. Investig. New Drugs 2013, 31, 1192–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 tumor suppressor gene: Important milestones at the various steps of tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.A.; Etemadmoghadam, D.E.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A.; et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 2010, 221, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köbel, M.; Piskorz, A.M.; Lee, S.; Lui, S.; LePage, C.; Marass, F.; Rosenfeld, N.; Mes Masson, A.-M.; Brenton, J.D. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J. Pathol. Clin. Res. 2016, 2, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hainaut, P.; Hollstein, M. p53 and Human Cancer: The First Ten Thousand Mutations. Adv. Cancer Res. 2000, 77, 81–137. [Google Scholar] [CrossRef]
- Bullock, A.N.; Fersht, A.R. Rescuing the function of mutant p53. Nat. Rev. Cancer 2001, 1, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Brosh, R.; Rotter, V. When mutants gain new powers: News from the mutant p53 field. Nat. Rev. Cancer 2009, 9, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Oren, M.; Rotter, V. Mutant p53 gain-of-function in cancer. Cold Spring Harb. Perspect. Bio. 2010, 2, a001107. [Google Scholar] [CrossRef] [PubMed]
- Lubin, R.; Schlichtholz, B.; Teillaud, J.L.; Garay, E.; Bussel, A.; Wild, C.P. p53 Antibodies in Patients with Various Types of Cancer: Assay, Identification, and Characterization. Clin. Cancer Res. 1995, 1, 1463–1469. [Google Scholar]
- Soussi, T. p53 Antibodies in the sera of patients with various types of cancer: A review. Cancer Res. 2000, 60, 1777–1788. [Google Scholar] [PubMed]
- Qiu, T.; Yang, Q.; Li, X.-R.; Yang, H.; Qiu, L.-L.; Wang, L. Detection of serum anti-p53 antibodies from patients with ovarian cancer in China: Correlation to clinical parameters. Cancer Investig. 2007, 25, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Garziera, M.; Montico, M.; Bidoli, E.; Scalone, S.; Sorio, R.; Giorda, G.; Lucia, E.; Toffoli, G. Prognostic role of serum antibody immunity to p53 oncogenic protein in ovarian cancer: A systematic review and a meta-analysis. PLoS ONE 2015, 10, e0140351. [Google Scholar] [CrossRef]
- Gadducci, A.; Ferdeghini, M.; Buttitta, F.; Cosio, S.; Fanucchi, A.; Annicchiarico, C.; Gagetti, O.; Bevilacqua, G.; Genazzani, A.R. Assessment of the prognostic relevance of serum anti-p53 antibodies in epithelial ovarian cancer. Gynecol. Oncol. 1999, 72, 76–81. [Google Scholar] [CrossRef]
- Gadducci, A.; Ferdeghini, M.; Buttitta, F.; Fanucchi, A.; Annicchiarico, C.A.; Prontera, C.; Bevilacqua, G.; Genazzani, A.R. Preoperative serum antibodies against the p53 protein in patients with ovarian and endometrial cancer. Anticancer Res. 1996, 16, 3519–3523. [Google Scholar]
- Vitale, S.R.; Groenendijk, F.H.; van Marion, R.; Beaufort, C.M.; Helmijr, J.C.; Dubbink, H.J.; Dinjens, W.N.M.; Ewing-Graham, P.C.; Smolders, R.; van Doorn, H.C.; et al. TP53 mutations in serum circulating cell-free tumor DNA as longitudinal biomarker for high-grade serous ovarian cancer. Biomolecules 2020, 10, 415. [Google Scholar] [CrossRef] [Green Version]
- Parkinson, C.A.; Gale, D.; Piskorz, A.M.; Biggs, H.; Hodgkin, C.; Addley, H.; Freeman, S.; Moyle, P.; Sala, E.; Sayal, K.; et al. Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study. PLoS Med. 2016, 13, e1002198. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.N.; Kachnic, L.A. Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene 2003, 22, 5784–5791. [Google Scholar] [CrossRef] [Green Version]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous recombination deficiency: Exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Khan, S.; Sun, Y.; Hess, K.; Shmulevich, I.; Sood, A.K.; Zhang, W. Association of BRCA1 and BRCA2 Mutations with Survival, Chemotherapy Sensitivity, and Gene Mutator Phenotype in Patients With Ovarian Cancer. JAMA 2011, 306, 1557–1565. [Google Scholar] [CrossRef] [Green Version]
- Vencken, P.M.L.H.; Kriege, M.; Hoogwerf, D.; Beugelink, S.; van der burg, M.E.L.; Hooning, M.J.; Berns, E.M.; Jager, A.; Collee, M.; Burger, C.W.; et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann. Oncol. 2011, 22, 1346–1352. [Google Scholar] [CrossRef]
- Harter, P.; Johnson, T.; Berton-Rigaud, D.; Park, S.-Y.; Friedlander, M.; Del Capo, J.M.; Shimada, M.; Forget, F.; Mirza, M.R.; Colombo, N.; et al. BRCA1/2 mutations associated with progression-free survival in ovarian cancer patients in the AGO-OVAR 16 study. Gynecol. Oncol. 2016, 140, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Lee, M.; Kim, H.S.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.-S. Effect of BRCA mutational status on survival outcome in advanced-stage high-grade serous ovarian cancer. J. Ovarian Res. 2019, 12, 40. [Google Scholar] [CrossRef]
- Rudaitis, V.; Zvirblis, T.; Kanopiene, D.; Janulynaite, D.; Griskevicius, L.; Janavicius, R. BRCA1/2 mutation status is an independent factor of improved survival for advanced (stage III-IV) ovarian cancer. Int. J. Gynecol. Cancer 2014, 24, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Damia, G.; Broggini, M. Platinum resistance in ovarian cancer: Role of DNA repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damia, G.; Imperatori, L.; Stefanini, M.; D’Incalci, M. Sensitivity of CHO mutant cell lines with specific defects in nucleotide excision repair to different anti-cancer agents. Int. J. Cancer 1996, 66, 779–783. [Google Scholar] [CrossRef]
- Damia, G.; D’Incalci, M. Targeting DNA repair as a promising approach in cancer therapy. Eur. J. Cancer 2007, 43, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Traganos, F.; Wlodkowic, D. Impaired DNA damage response—An Achilles’ heel sensitizing cancer to chemotherapy and radiotherapy. Eur. J. Pharmacol. 2009, 625, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Deans, A.J.; West, S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 2011, 11, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.-H.; Li, J.; Chen, P.; Jiang, H.-G.; Wu, M.; Chen, Y.-C. RNA interferences targeting the Fanconi anemia/BRCA pathway upstream genes reverse cisplatin resistance in drug-resistant lung cancer cells. J. Biomed. Sci. 2015, 22, 77. [Google Scholar] [CrossRef]
- Alsop, K.; Fereday, S.; Meldrum, C.; DeFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian ovarian cancer study group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomao, F.; Bardhi, E.; Di Pinto, A.; Sassu, C.M.; Biagioli, E.; Petrella, M.C.; Palaia, I.; Muzii, L.; Colombo, N.; Panici, P.B. Parp inhibitors as maintenance treatment in platinum sensitive recurrent ovarian cancer: An updated meta-analysis of randomized clinical trials according to BRCA mutational status. Cancer Treat. Rev. 2019, 80, 101909. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; Christensen, R.D.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Perol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Domchek, S.M.; Aghajanian, C.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016, 140, 199–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, S.L.; Brough, R.; Lord, C.J.; Natrajan, R.; Vatcheva, R.; Levine, D.A.; Boyd, J.; Reis-Filho, J.S.; Ashworth, A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature 2008, 451, 1111–1115. [Google Scholar] [CrossRef]
- Sakai, W.; Swisher, E.M.; Karlan, B.Y.; Agarwal, M.K.; Higgins, J.; Friedman, C.; Villegas, E.; Jacquemont, C.; Farrugia, D.J.; Couch, F.J.; et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 2008, 451, 1116–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, K.K.; Swisher, E.M.; Taniguchi, T. Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci. 2011, 102, 663–669. [Google Scholar] [CrossRef] [Green Version]
- De Mattos-Arruda, L.; Weigelt, B.; Cortes, J.; Won, H.H.; Ng, C.K.Y.; Nuciforo, P.; Bidard, F.-C.; Aura, C.; Saura, C.; Peg, V.; et al. Capturing intra-tumor genetic heterogeneity by de novo mutation profiling of circulating cell-free tumor DNA: A proof-of-principle. Ann. Oncol. 2014, 25, 1729–1735. [Google Scholar] [CrossRef]
- Murtaza, M.; Dawson, S.-J.; Tsui, D.W.Y.; Gale, D.; Forshew, T.; Piskorz, A.M.; Parkinson, C.; Chin, S.-F.; Kingsbury, Z.; Wong, A.S.; et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013, 497, 108–112. [Google Scholar] [CrossRef]
- Garcia-Murillas, I.; Schiavon, G.; Weigelt, B.; Ng, C.; Hrebien, S.; Cutts, R.J.; Cheang, M.; Osin, P.; Nerurkar, A.; Kozarewa, I.; et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl. Med. 2015, 7, 302ra133. [Google Scholar] [CrossRef]
- Quigley, D.; Akumkal, J.J.; Wyatt, A.W.; Kothari, V.; Foye, A.; Lioyd, P.; Aggarwal, R.; Kim, W.; Lu, E.; Schwartzman, J.; et al. Analysis of circulating cell-free DnA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 2017, 7, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Christie, E.L.; Fereday, S.; Doig, K.; Pattnaik, S.; Dawson, S.-J.; Bowtell, D.D.L. Reversion of BRCA1/2 germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian cancer. J. Clin. Oncol. 2017, 35, 1274–1280. [Google Scholar] [CrossRef]
- Lin, K.K.; Harrell, M.I.; Oza, A.M.; Oaknin, A.; Ray-Coquard, I.; Tinker, A.V.; Helman, E.; Radke, M.R.; Say, C.; Vo, L.-T.; et al. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2019, 9, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Senger, D.R.; Van de water, L.; Brown, L.F.; Nagy, J.A.; Yeo, K.T.; Berse, B.; Jackman, R.W.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993, 12, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Terai, Y.; Kumagai, K.; Ueki, K.; Yamaguchi, H.; Akise, D.; Uei, M. Vascular endothelial growth factor C gene expression is closely related to invasion phenotype in gynecological tumor cells. Gynecol. Oncol. 2001, 82, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor. Eur. J. Cancer 1996, 32, 2413–2422. [Google Scholar] [CrossRef]
- Salven, P.; Mäenpää, H.; Orpana, A.; Alitalo, K.; Joensuu, H. Serum vascular endothelial growth factor is often elevated in disseminated cancer. Clin. Cancer Res. 1997, 3, 647–651. [Google Scholar] [PubMed]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Oxygen sensing and metabolic homeostasis. Mol. Cell Endocrinol. 2014, 397, 51–58. [Google Scholar] [CrossRef]
- Bandiera, E.; Franceschini, R.; Specchia, C.; Bignotti, E.; Trevisiol, C.; Gion, M.; Pecorelli, S.; Santin, A.D.; Ravaggi, A. Prognostic Significance of Vascular Endothelial Growth Factor Serum Determination in Women with Ovarian Cancer. ISRN Obstet. Gynecol. 2012, 2012, 245756. [Google Scholar] [CrossRef] [Green Version]
- Abendstein, B.; Daxenbichler, G.; Windbichler, G.; Zeimet, A.G.; Geurts, A.; Sweep, F.; Marth, C. Predictive value of uPA, PAI-1, HER-2 and VEGF in the serum of ovarian cancer patients. Anticancer Res. 2000, 20, 569–572. [Google Scholar] [PubMed]
- Steffensen, K.D.; Waldstrøm, M.; Brandslund, I.; Jakobsen, A. The relationship of VEGF polymorphisms with serum VEGF levels and progression-free survival in patients with epithelial ovarian cancer. Gynecol. Oncol. 2010, 117, 109–116. [Google Scholar] [CrossRef]
- Soyama, H.; Miyamoto, M.; Matsuura, H.; Iwahashi, H.; Kakimoto, S.; Ishibashi, H.; Sakamoto, T.; Hada, T.; Suminokura, J.; Takano, M. Rapid decrease in serum VEGF-A levels may be a worse prognostic biomarker for patients with platinum-resistant recurrent ovarian cancer treated with bevacizumab and gemcitabine. Cancer Chemother. Pharmacol. 2020, 85, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Smerdel, M.P.; Steffensen, K.D.; Waldstrøm, M.; Brandslund, I.; Jakobsen, A. The predictive value of serum VEGF in multiresistant ovarian cancer patients treated with bevacizumab. Gynecol. Oncol. 2010, 118, 167–171. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, S.; Liu, Y.; Zhai, L.; Sun, X. Prognostic value of systemic inflammatory markers in ovarian Cancer: A PRISMA-compliant meta-analysis and systematic review. BMC Cancer 2018, 18, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Yan, Q.; Li, S.; Li, B.; Feng, Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark. 2016, 17, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choi, H.-Y.; Lee, M.; Suh, D.H.; Kim, K.; No, J.H.; Chung, H.H.; Kim, Y.B.; Song, Y.S. Systemic inflammatory response markers and CA-125 levels in ovarian clear cell carcinoma: A two center cohort study. Cancer Res. Treat. 2016, 48, 250–258. [Google Scholar] [CrossRef]
- Marchetti, C.; Romito, A.; Musella, A.; Santo, G.; Palais, I.; Perniola, G.; Di Donato, V.; Muzii, L.; Panici, P.B. Combined Plasma Fibrinogen and Neutrophil Lymphocyte Ratio in Ovarian Cancer Prognosis May Play a Role? Int. J. Gynecol. Cancer 2018, 28, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Palaia, I.; Tomao, F.; Sassu, C.M.; Musacchio, L.; Panici, P.B. Immunotherapy for ovarian cancer: Recent advances and combination therapeutic approaches. Onco. Targets. Ther. 2020, 13, 6109–6129. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- Bansal, P.; Osman, D.; Gan, G.N.; Simon, G.R.; Boumber, Y. Recent advances in immunotherapy in metastatic NSCLC. Front. Oncol. 2016, 6, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santangelo, G.; Caruso, G.; Palaia, I.; Tomao, F.; Perniola, G.; Di Violante, D.; Fischetti, M.; Muzii, L.; Benedetti, P. The emerging role of precision medicine in the treatment of ovarian cancer. Expert Rev. Precis. Med. Drug Dev. 2020, 5, 283–297. [Google Scholar] [CrossRef]
- Di Donato, V.; Caruso, G.; Bogani, G.; Giannini, A.; D’Oria, O.; Perniola, G.; Palaia, I.; Plotti, F.; Angioli, R.; Muzii, L.; et al. Preoperative frailty assessment in patients undergoing gynecologic oncology surgery: A systematic review. Gynecol Oncol. 2021, 161, 11–19. [Google Scholar] [CrossRef]
- Caruso, G.; Musacchio, L.; Santangelo, G.; Palaia, I.; Tomao, F.; Di Donato, V.; Perniola, G.; Salutari, V.; Panici, P.B. Ovarian Cancer Metastasis to the Breast: A Case Report and Review of the Literature. Case Rep. Oncol. 2020, 13, 1317–1324. [Google Scholar] [CrossRef]
- Palaia, I.; Caruso, G.; Di Donato, V.; Perniola, G.; Ferrazza, G.; Panzini, E.; Scudo, M.; Di Pinto, A.; Muzii, L.; Panici, P.B. Peri-operative blood management of Jehovah’s Witnesses undergoing cytoreductive surgery for advanced ovarian cancer. Blood Transfus. 2021. [Google Scholar] [CrossRef]
- Di Donato, V.; Di Pinto, A.; Giannini, A.; Caruso, G.; D’Oria, O.; Tomao, F.; Fischetti, M.; Perniola, G.; Palaia, I.; Muzii, L.; et al. Modified fragility index and surgical complexity score are able to predict postoperative morbidity and mortality after cytoreductive surgery for advanced ovarian cancer. Gynecol Oncol. 2021, 161, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.; Clayton, A.; Mason, M.D.; Jasani, B.; Adams, M.; Tabi, Z. Recovery of CD8+ T-cell function during systemic chemotherapy in advanced ovarian cancer. Cancer Res. 2005, 65, 7000–7006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Feng, Q.-M.; Wang, Y.; Shi, J.; Ge, H.-L.; Di, W. The immunologic aspects in advanced ovarian cancer patients treated with paclitaxel and carboplatin chemotherapy. Cancer Immunol. Immunother. 2010, 59, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Montfort, A.; Pearce, O.; Maniati, E.; Vincent, B.; Bixby, L.; Böhm, S.; Dowe, T.; Wilkes, E.H.; Chakravarty, P.; Thompson, R.; et al. A strong B cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin. Cancer Res. 2017, 23, 250–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671–97682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.L.; Selenica, P.; Zhou, Q.; Iasonos, A.; Callahan, M.; Feit, N.Z.; Boland, J.; Vazquez-Garcia, I.; Mandelker, D.; Zehir, A.; et al. BRCA Mutations, Homologous DNA Repair Deficiency, Tumor Mutational Burden, and Response to Immune Checkpoint Inhibition in Recurrent Ovarian Cancer. JCO Precis. Oncol. 2020, 4, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mahoney, K.M.; Giobbie-Hurder, A.; Zhao, F.; Lee, S.; Liao, X.; Rodig, S.; Li, J.; Wu, X.; Butterfield, L.H.; et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol. Res. 2017, 5, 480–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, A.; Fossati, M.; Buzzonetti, A.; Scambia, G.; Fattorossi, A. A robust immune system conditions the response to abagovomab (anti-idiotypic monoclonal antibody mimicking the CA125 protein) vaccination in ovarian cancer patients. Immunol. Lett. 2017, 191, 35–39. [Google Scholar] [CrossRef]
- Battaglia, A.; Buzzonetti, A.; Fossati, M.; Scambia, G.; Fattorossi, A.; Madiyalakan, M.R.; Mahnke, Y.D.; Nicodemus, C. Translational immune correlates of indirect antibody immunization in a randomized phase II study using scheduled combination therapy with carboplatin/paclitaxel plus oregovomab in ovarian cancer patients. Cancer Immunol. Immunother. 2020, 69, 383–397. [Google Scholar] [CrossRef] [PubMed]
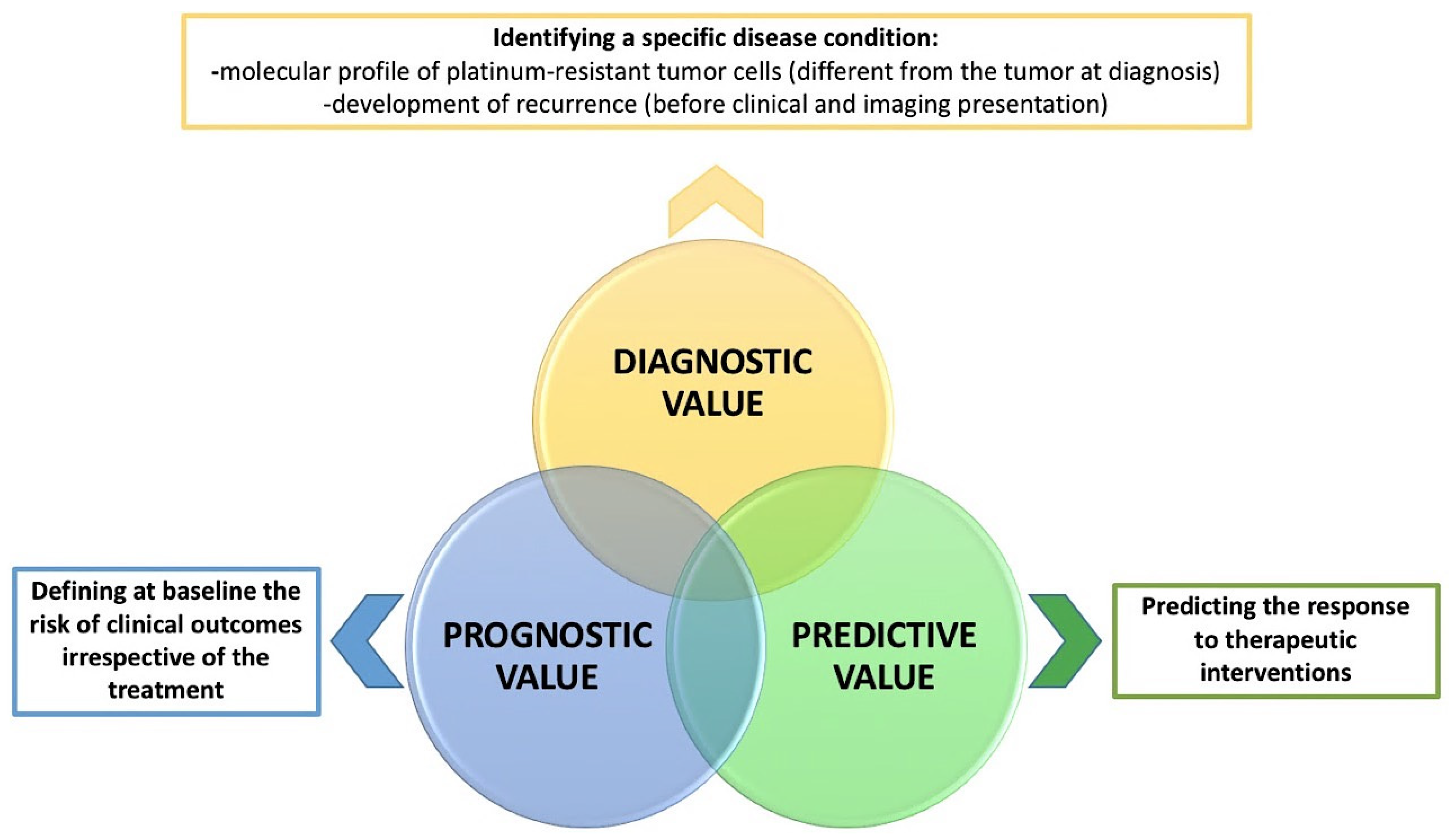
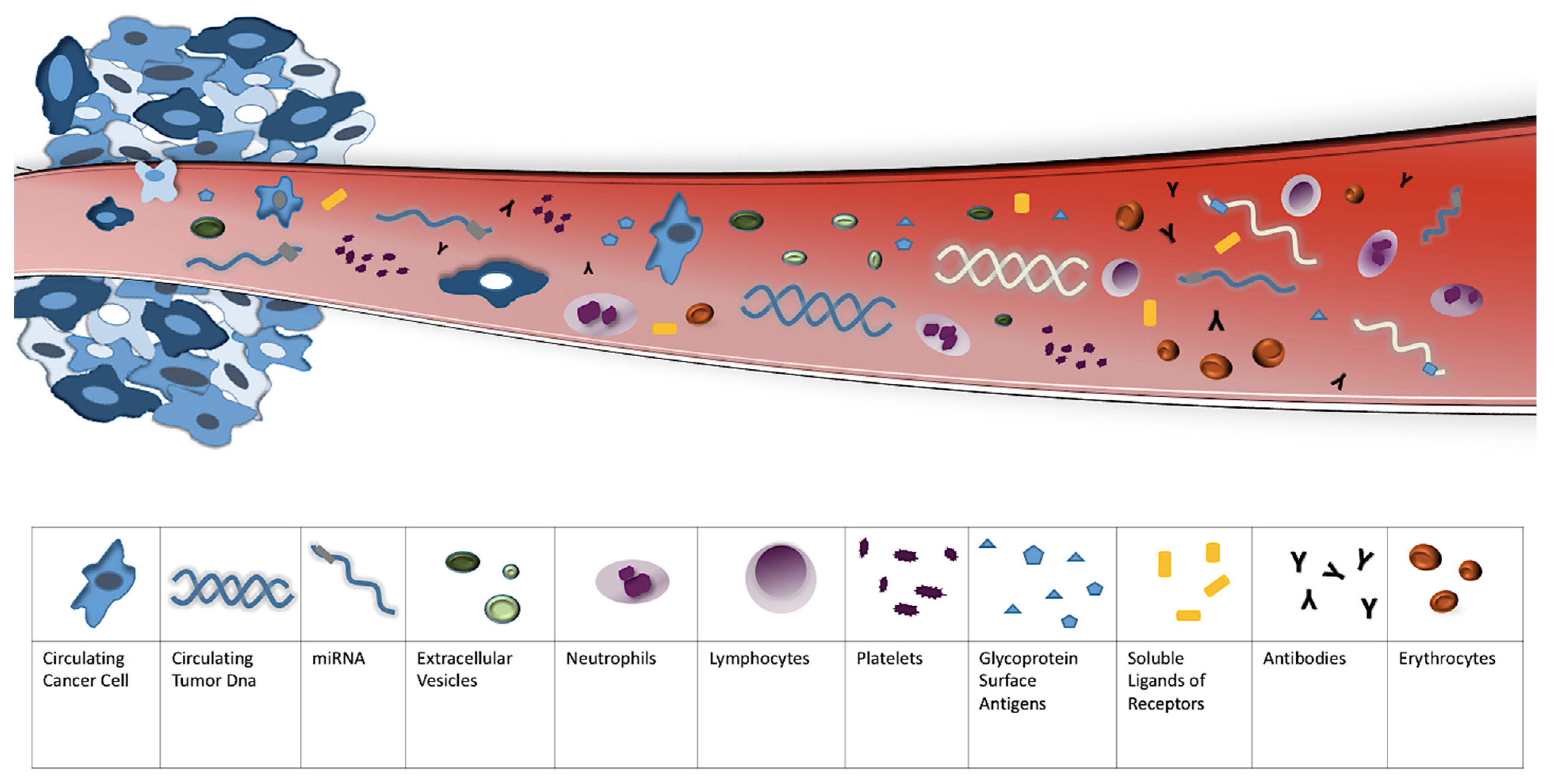
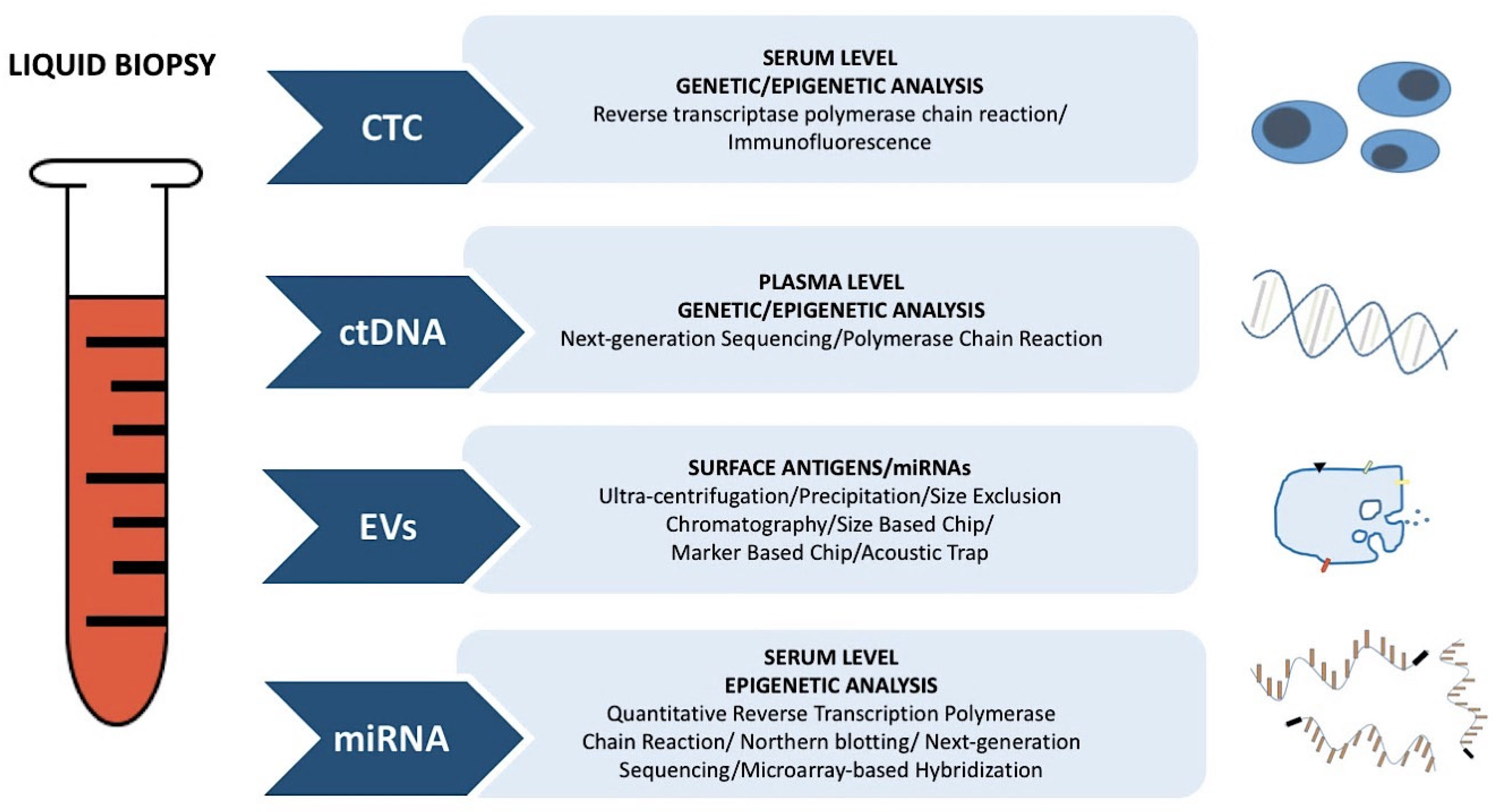

| Circulating Biomarkers and Liquid Biopsy | Tumor Biopsy |
|---|---|
| Material derived from cancer detectable in bloodstream, urine, or peritoneal fluid | Material obtained from a sampling of tissue lesion |
| Non-invasive procedure | High invasive procedure |
| Real-time follow up | Impracticable for real-time follow up |
| Quick and easily repeatable procedure for obtaining the samples | Difficult to repeat and depend on the correctness of the procedure |
| No surgical complication or pain | Risk of surgical complication and pain |
| Lack of well-defined practice rules and standardizing protocols | Clinically validated and standard for histologic diagnosis |
| Less cost (with some exceptions) | High cost |
| Assessment of tumor heterogeneity in different phases of the disease | Failure to reflect tumor heterogeneity |
| Low concentrations and easily degradable material | Higher concentration and fixed material |
| Less specificity | Higher specificity |
| Specialized laboratory | Histology laboratory |
| Type of Circulating Biomarker | |
|---|---|
| Glycoprotein Biomarkers | CA 125 |
| HE4 | |
| Mesothelin | |
| Liquid Biopsy | ctDNA |
| CTCs | |
| EVs | |
| Epigenetic and Genetic Markers | miRNA |
| DNA methylation | |
| Histone modification | |
| TP53 mutation | |
| HRD-BRCA1/2 mutation | |
| Immune-Related Biomarkers | NLR |
| PLR | |
| Circulating T-cell | |
| Circulating B-cell | |
| sPD-1/sPD-L1 | |
| MDSC4 | |
| NMLR | |
| Angiogenic Markers | sVEGF |
| Author, Year | Material and Methods | Results | Conclusions |
|---|---|---|---|
| Kuhlmann JD. 2014 [66] |
| Platinum resistance ERCC1+ CTCs vs. ERCC1−CTC OR, 8.5 (1.7–43.6), p = 0.01 | The presence of CTCs expressing ERCC1 is an independent predictor of platinum resistance |
| Obermayr E. 2013 [67] |
| Frequency of CTCs with overexpression of PPIC gene in PtR vs. platinum sensible patients at follow up: 35.7% vs. 10.1%, p = 0.024 | CTCs with overexpression of PPIC gene correlate with platinum resistance |
| Poveda A. 2011 [68] |
| -PFS ≥2 CTCs vs. <2 CTCs: 3.2 months vs. 6.6 months; p = 0.0024. -OS ≥ 2 CTCs vs. <2 CTCs: 12.4 months vs. 20.6 months; p = 0.0017. -Multivariate analysis: PFS HR 1.58 (0.99–2.53) p = 0.058 -Multivariate analysis: OS HR 1.54 (0.93–2.54) p = 0.096 | Levels of CTCs seem to correlate with platinum resistance and worse survival, but data are inconsistent |
| Lee M. 2017 [69] |
| -OS pts with CTCs cluster vs. pts without CTCs cluster: 21 vs. 74 months, p = 0.008. -Multivariate analysis OS: HR 1.3 (0.94–17.149) p = 0.94 −65.2% of patients with CTCs cluster showed platinum resistance (p = 0.001). | Levels of CTCs seem to correlate with platinum resistance and worse survival, but data are inconsistent |
| Author, Year | Material and Methods | Results | Conclusions |
|---|---|---|---|
| Benson EA. 2015 [106] |
|
| miRNA analysis predicts the response to chemotherapy and prognosis. |
| Vigneron N. 2020 [107] |
| OS miRNA > 0.34 zmol/mL vs. <0.34 zmol/mL: 7.9 months vs. 20.6 months, HR 3.15, p = 0.006 | miRNA analysis predicts prognosis |
| Author, Year | Material and Methods | Results | Conclusions |
|---|---|---|---|
| Losi L. 2018 [111] |
| % of hypermethylated promoter genes:
| OC is characterized by a slight increase of hypermethylation |
| De Caceres II. 2004 [110] |
| % of hypermethylated BRCA 1 and/or RASSF1A: 68% (regardless FIGO stage) vs. 0% in control group. | Promoter hypermethylation is a common and relatively early event in ovarian tumorigenesis |
| Cacan E. 2016 [112] |
| The expression of positive co-stimulatory molecules of T cell, OX-40L and 4-1BBL, is suppressed due to DNA hypermethylation and histone deacetylation in chemo-resistant cells compared to parental chemo-sensitive OC cells. | Hypermethylation correlates with chemo-resistance in OC |
| Gifford G. 2004 [113] |
|
| The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients |
| Teschendorff AE. 2009 [109] |
|
| Hypomethylation is correlated with OC |
| Liao P. 2014 [114] |
| In case of hypomethylation of ATG4A and HIST1H2BN in OTICs:
| In OTICs, hypomethylation of ATG4A and HIST1H2BN is associated with poor prognosis |
| Author, Year | Material and Methods | Results | Conclusions |
|---|---|---|---|
| Zhu Y. 2018 [198] |
| PLR > cut off - OS: metaHR 2.53 (2.16–2.96) - PFS: metaHR 2.48, (2.10–2.96) NLR > cut off - OS: metaHR 2.21 (1.95–2.52) - PFS: metaHR 1.36 (1.17–1.57) | Higher value of PLR and NLR are associated with worse ovarian cancer survival |
| Miao Y. 2016 [199] |
| Predictive values for platinum resistance: - PLR > 207: SN 60.42%, SP 85.48%, p < 0.001 - NLR > 3.02: SN 75%, SP 81.45%, p < 0.001 | Assessment of NLR and PLR has potential clinical value in predicting platinum resistance in patients with EOC |
| Kim HS. 2016 [200] | 109 pts with CCOC (18.3% PtR) | PLR ≥ 205.4 predicted non-CR (accuracy, 71.6%) Predictive values for platinum resistance: - NLR ≥ 2.8: SN 68.4%, SP 65.1%, p < 0.01 - PLR ≥ 178.3: SN 68.4, SP 55.4%, p = 0.02 | NLR and PLR value correlate with platinum resistance in patients with CCOC |
| Diagnostic Value | Prognostic Value | Predictive Value | Currently Used in Clinical Practice | Limits | |
|---|---|---|---|---|---|
| Glicoprotein markers |  |  |  | Low specificity | |
| ctDNA |  |  | High fragmentation, low stability, and low quantity in bloodstream | ||
| CTCs |  |  | Controversial data, scarcity in the bloodstream. Short half-life after blood draw | ||
| EVs |  |  | Need of clinically validated test | ||
| Micro RNAs |  |  |  | High cost and scarce availability of the test | |
| DNA methilation |  |  | Less sensitive test | ||
| Histone modification |  | Need of further investigation about treatment efficacy | |||
| TP53 (Ab and ctDNA) |  |  | Scarce data from PtR OC | ||
| BRCA (somatic and germinal) |  |  |  | Reversion mutation | |
| Immune related biomarkers |  |  |  | Low specificity, not universally established cut off, scarce data from PtR OC | |
| Angiogenic markers |  |  | Scarce and controversial data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassu, C.M.; Palaia, I.; Boccia, S.M.; Caruso, G.; Perniola, G.; Tomao, F.; Di Donato, V.; Musella, A.; Muzii, L. Role of Circulating Biomarkers in Platinum-Resistant Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 13650. https://doi.org/10.3390/ijms222413650
Sassu CM, Palaia I, Boccia SM, Caruso G, Perniola G, Tomao F, Di Donato V, Musella A, Muzii L. Role of Circulating Biomarkers in Platinum-Resistant Ovarian Cancer. International Journal of Molecular Sciences. 2021; 22(24):13650. https://doi.org/10.3390/ijms222413650
Chicago/Turabian StyleSassu, Carolina Maria, Innocenza Palaia, Serena Maria Boccia, Giuseppe Caruso, Giorgia Perniola, Federica Tomao, Violante Di Donato, Angela Musella, and Ludovico Muzii. 2021. "Role of Circulating Biomarkers in Platinum-Resistant Ovarian Cancer" International Journal of Molecular Sciences 22, no. 24: 13650. https://doi.org/10.3390/ijms222413650
APA StyleSassu, C. M., Palaia, I., Boccia, S. M., Caruso, G., Perniola, G., Tomao, F., Di Donato, V., Musella, A., & Muzii, L. (2021). Role of Circulating Biomarkers in Platinum-Resistant Ovarian Cancer. International Journal of Molecular Sciences, 22(24), 13650. https://doi.org/10.3390/ijms222413650






