Conformational Preferences and Antiproliferative Activity of Peptidomimetics Containing Methyl 1′-Aminoferrocene-1-carboxylate and Turn-Forming Homo- and Heterochiral Pro-Ala Motifs
Abstract
:1. Introduction
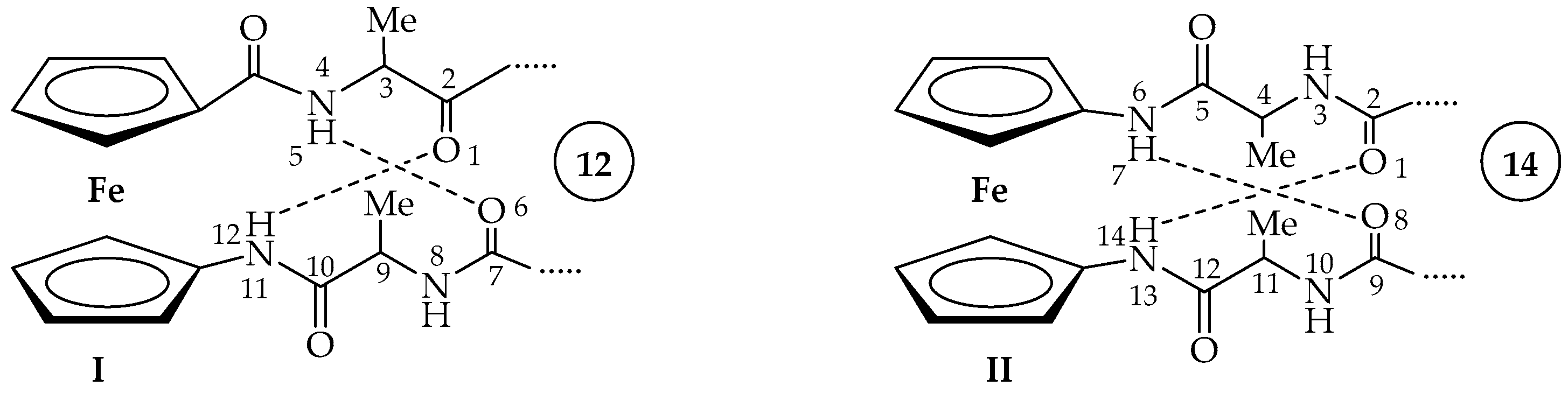
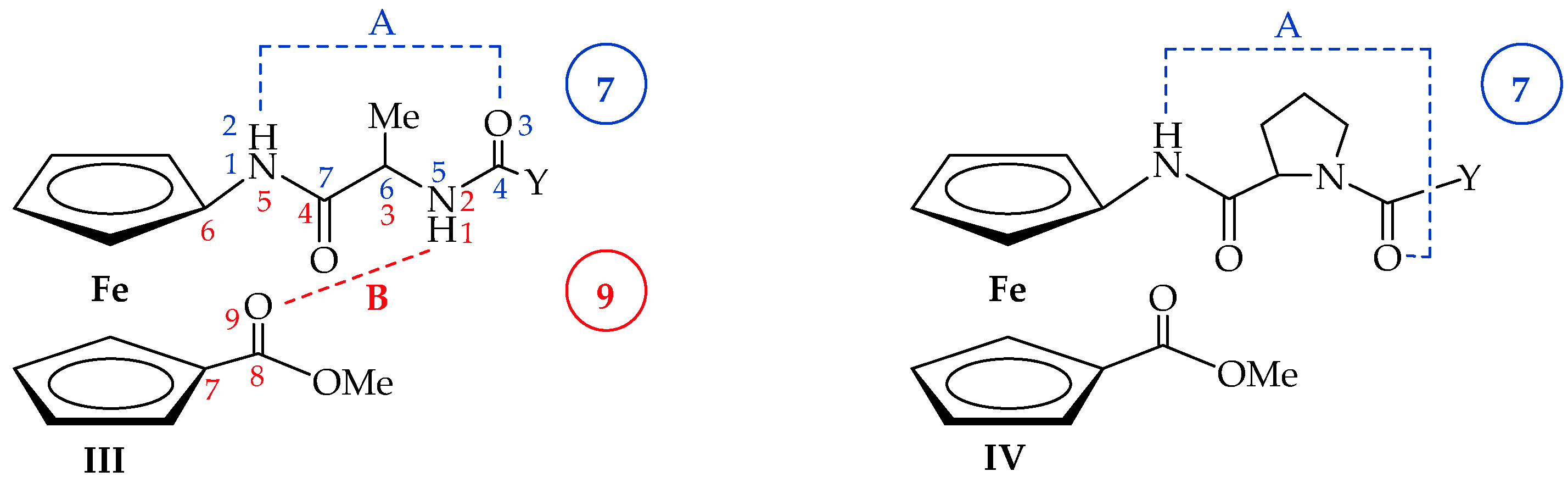
2. Results and Discussion
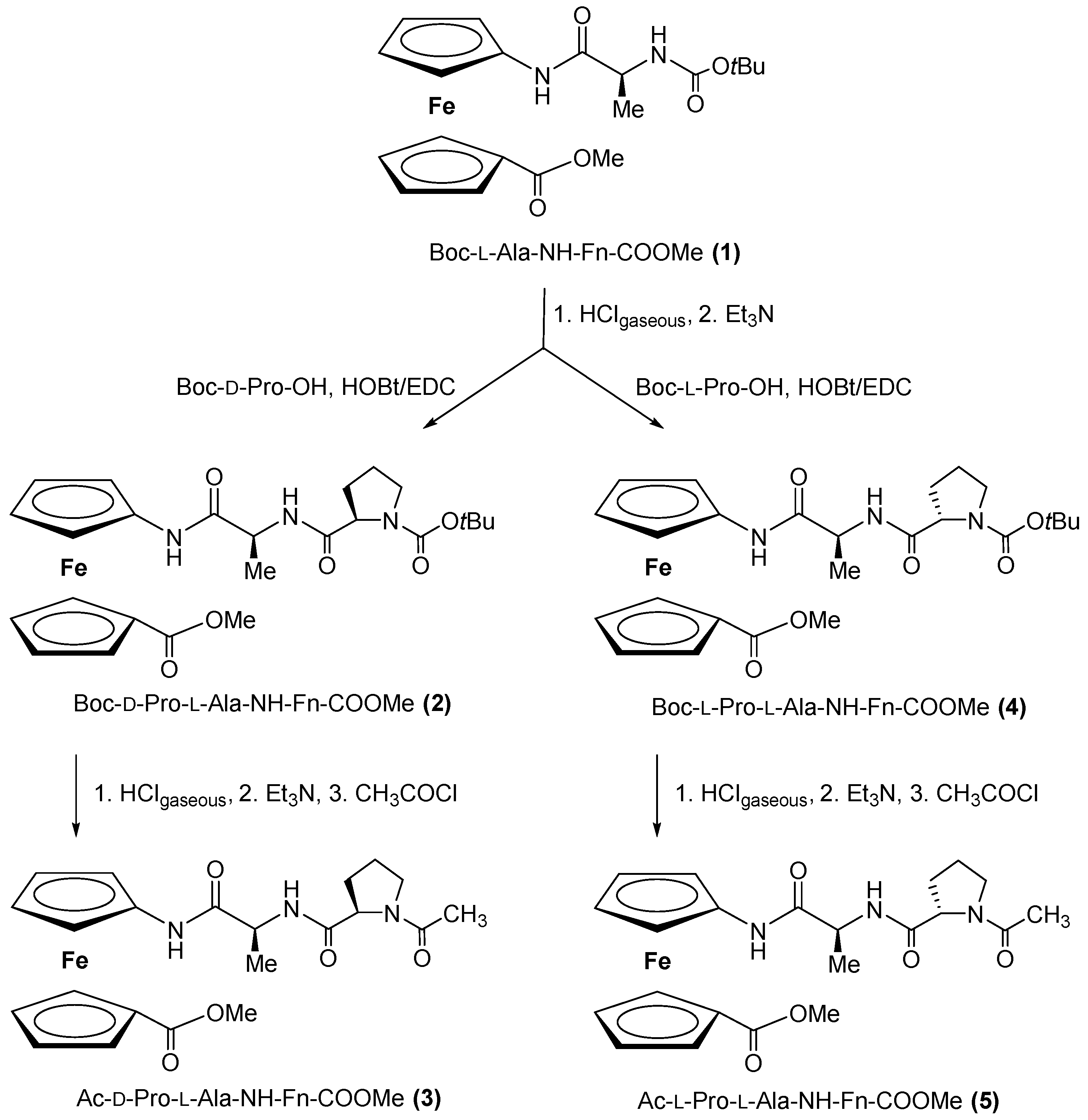
2.1. Synthesis of Peptides 2–5
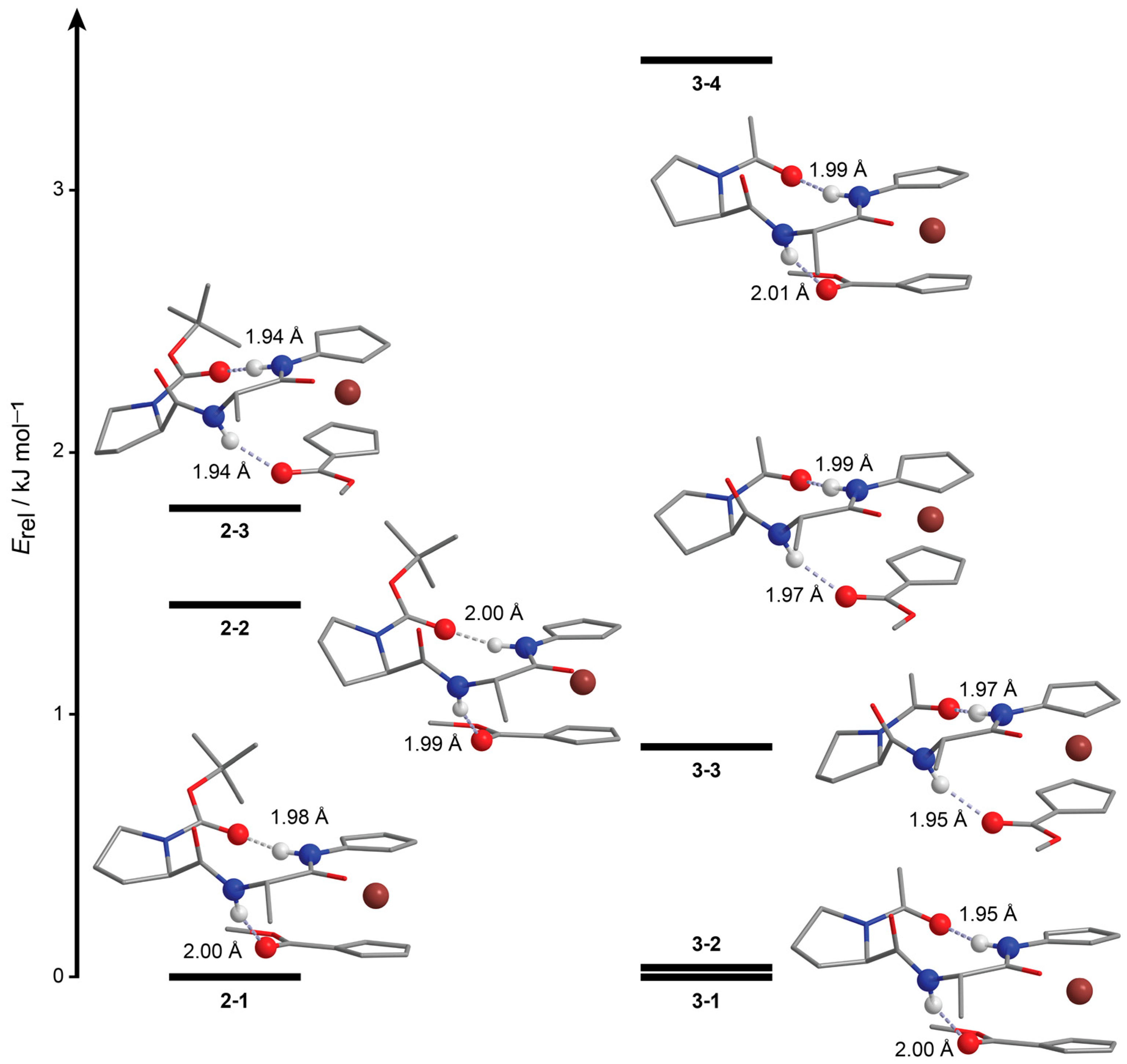
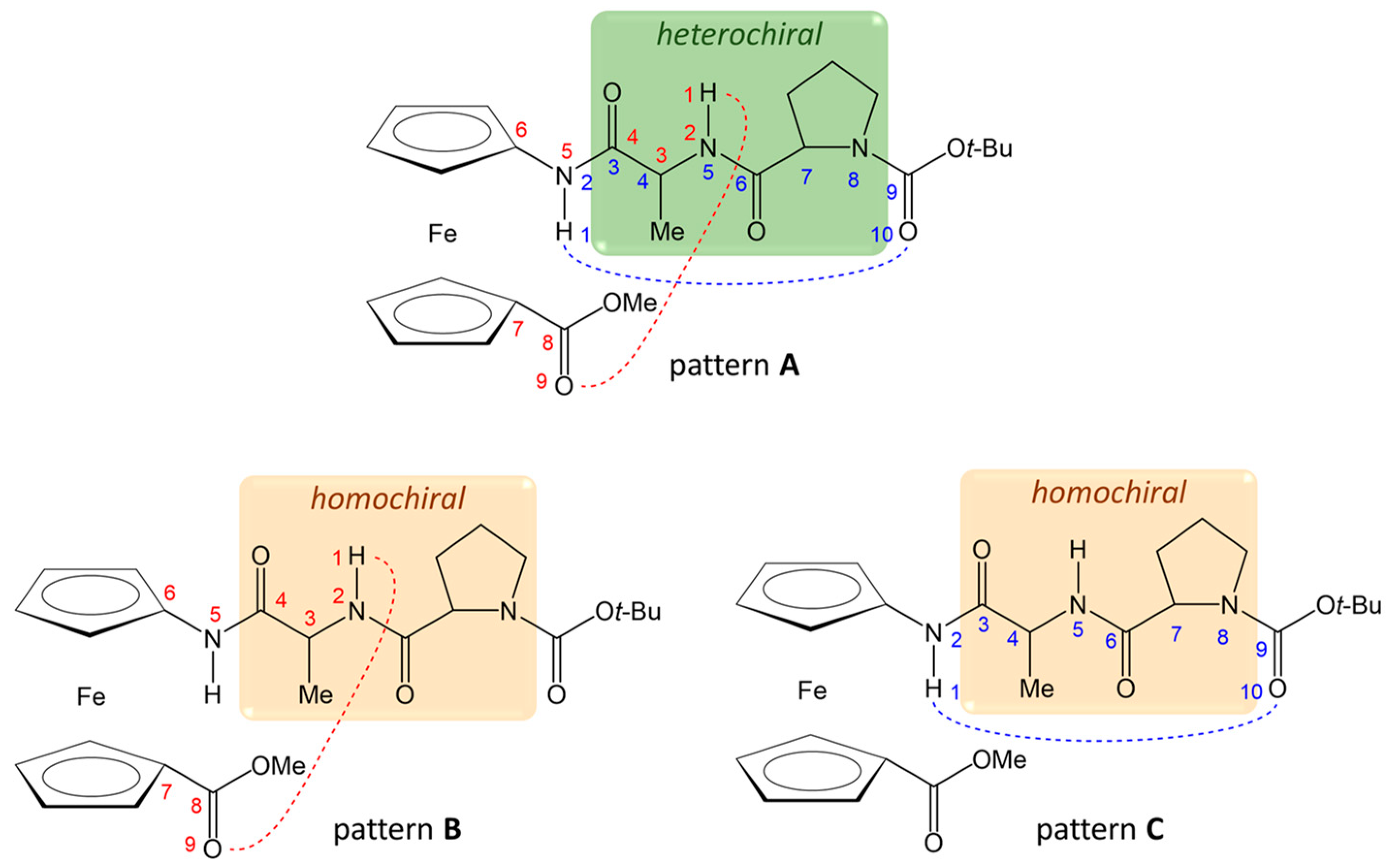
2.2. Computational Study
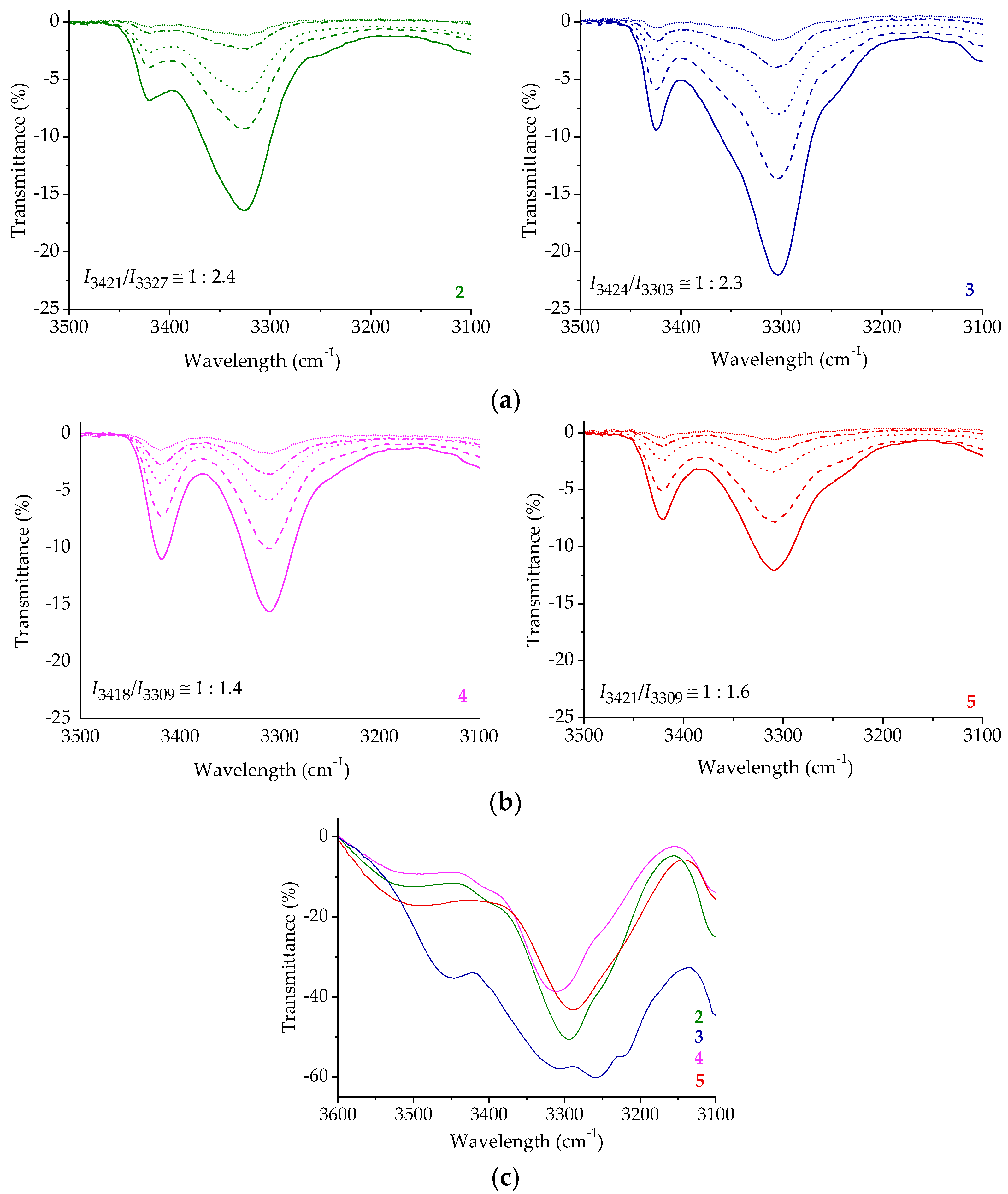
2.3. IR Spectroscopy
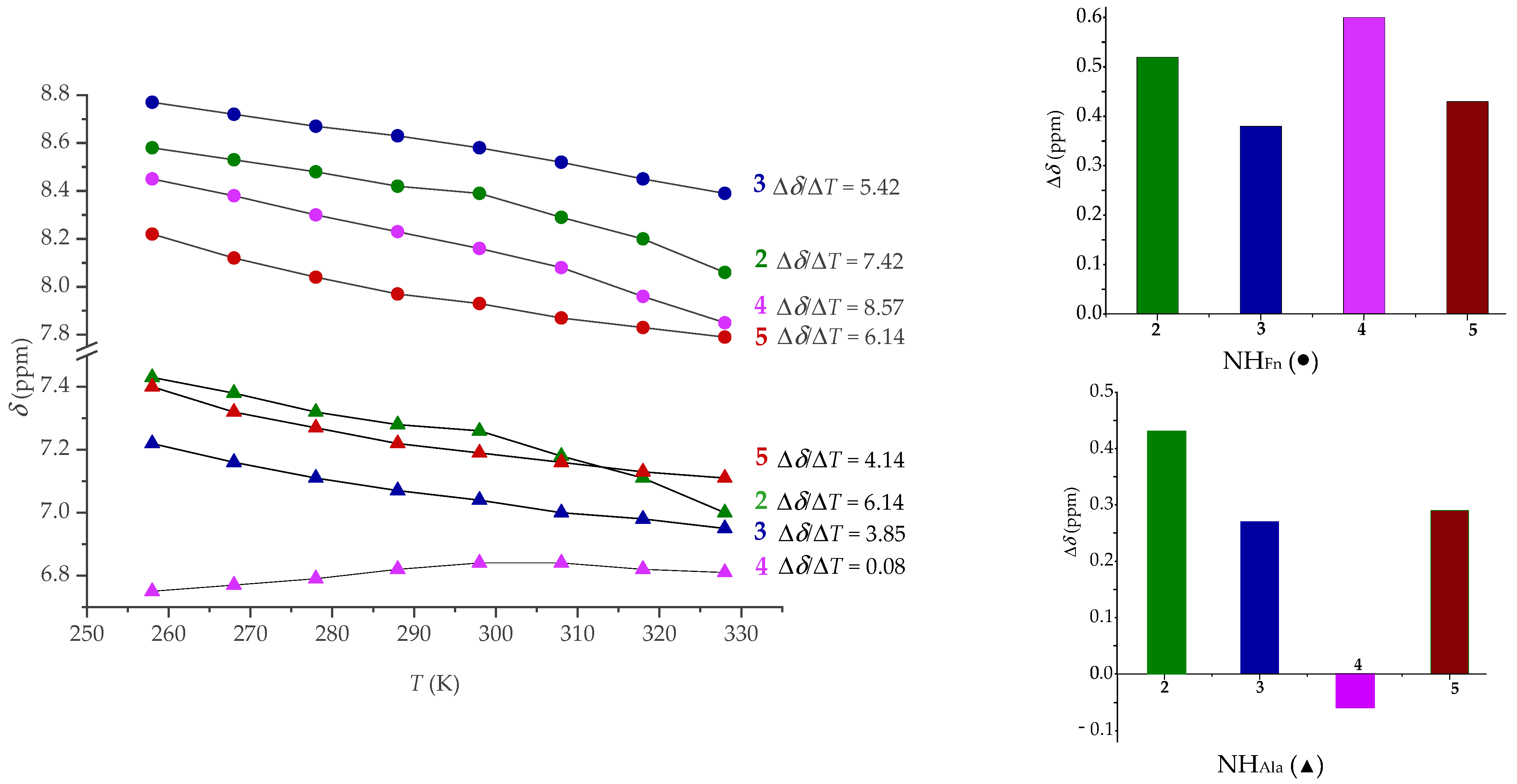
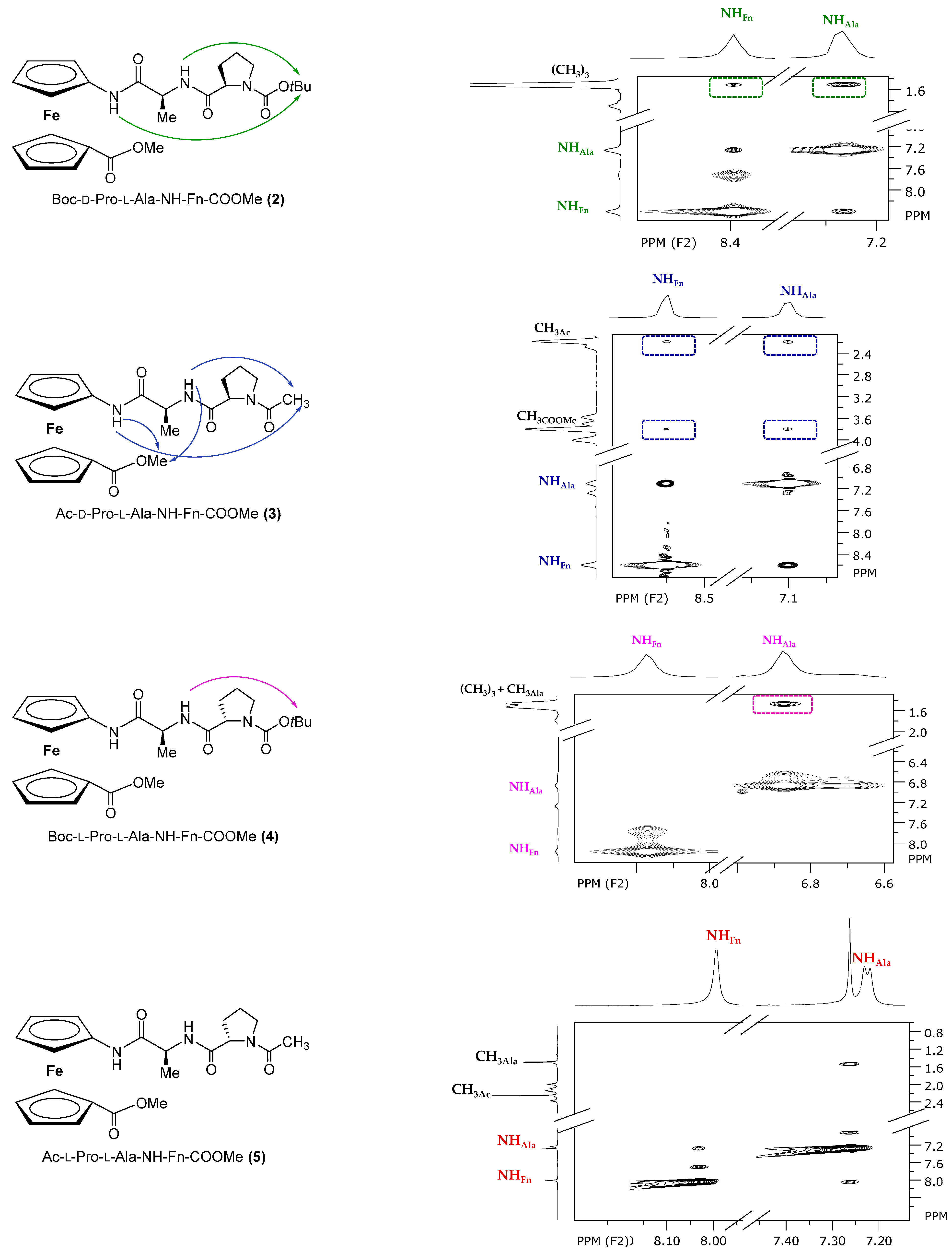
2.4. NMR Spectroscopy
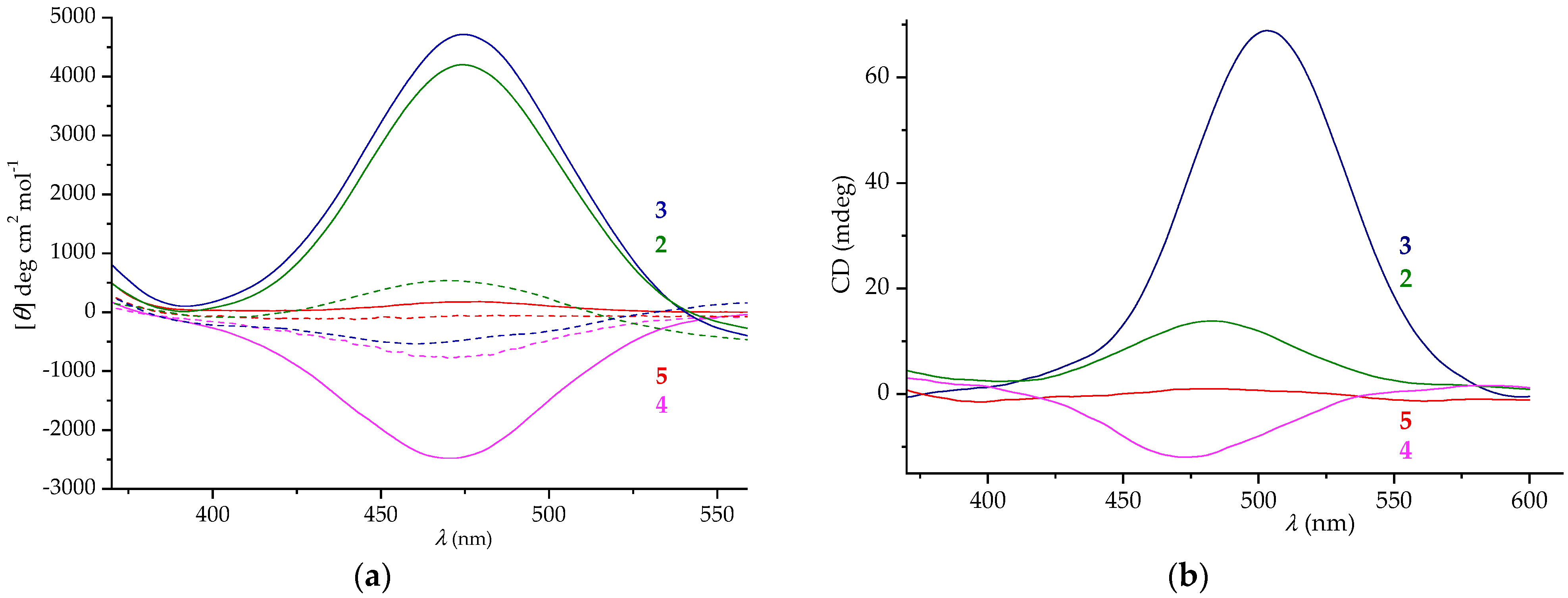
2.5. CD Spectroscopy
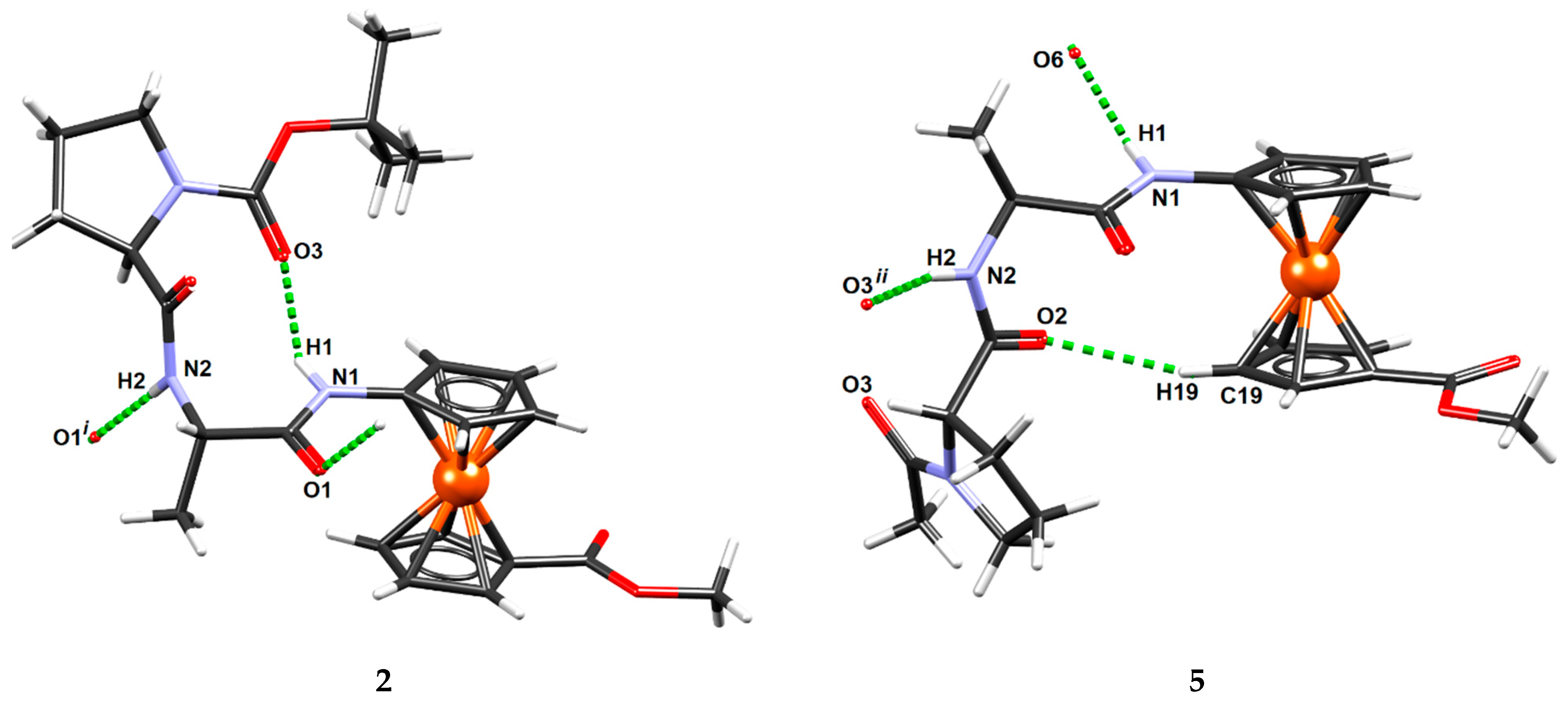
2.6. X-ray Crystal Structure Analysis
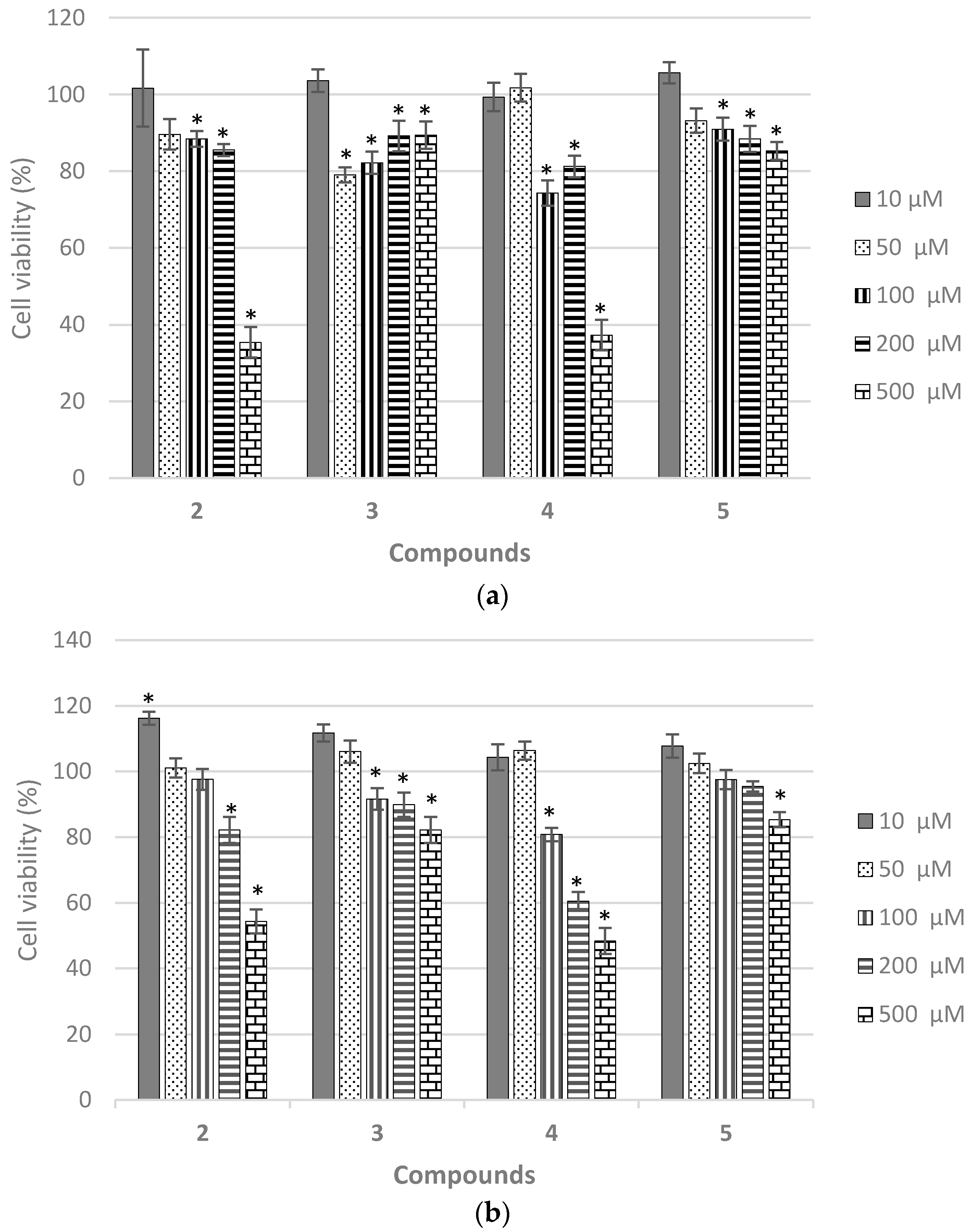
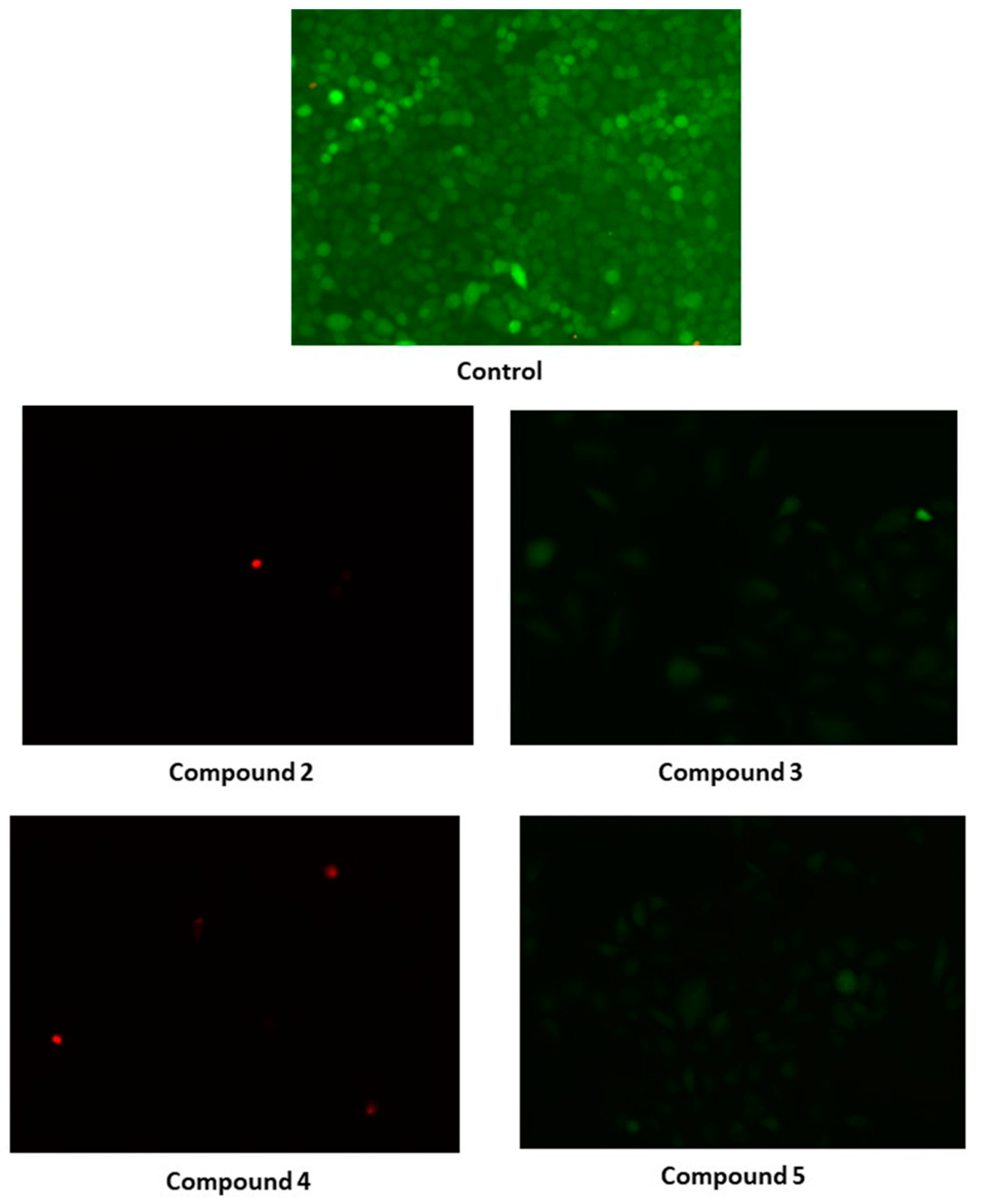
2.7. Biological Evaluation
3. Materials and Methods
3.1. General Procedure and Methods
3.1.1. Synthesis of Boc-d-Pro-l-Ala-NH-Fn-COOMe (2) and Boc-l-Pro-l-Ala-NH-Fn-COOMe (4)
3.1.2. Synthesis of Ac-d-Pro-l-Ala-NH-Fn-COOMe (3) and Ac-l-Pro-l-Ala-NH-Fn-COOMe (5)
3.1.3. Computational Details
3.1.4. Crystallographic Study
3.1.5. Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vagner, J.; Qu, H.; Hruby, V.J. Peptidomimetics, a synthetic tool of drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, Q.; He, J.; Jiang, Z.; Peng, C.; Tong, R.; Shi, J. Recent advances in the development of protein-protein interactions modulators: Mechanisms and clinical trials. Signal. Transduct. Target. Ther. 2020, 5, 213–235. [Google Scholar] [CrossRef]
- Hoang, H.N.; Hill, T.A.; Ruiz-Gómez, G.; Diness, F.; Mason, J.M.; Wu, C.; Abbenante, G.; Shepherd, N.E.; Fairlie, D.P. Twists or turns: Stabilising alpha vs. beta turns in tetrapeptides. Chem. Sci. 2019, 10, 10595–10600. [Google Scholar] [CrossRef] [Green Version]
- Laxio Arenas, J.; Kaffy, J.; Ongeri, S. Peptides and peptidomimetics as inhibitors of protein-protein interactions involving β-sheet secondary structures. Curr. Opin. Chem. Biol. 2019, 52, 157–167. [Google Scholar] [CrossRef]
- Nair, R.V.; Baravkar, S.B.; Ingole, T.S.; Sanjayan, G.J. Synthetic turn mimetics and hairpin nucleators. Chem. Commun. 2014, 50, 13874–13884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barišić, L.; Dropučić, M.; Rapić, V.; Pritzkow, H.; Kirin, S.I.; Metzler-Nolte, N. The first oligopeptide derivative of 1′-aminoferrocene-1-carboxylic acid shows helical chirality with antiparallel strands. Chem. Commun. 2004, 17, 2004–2005. [Google Scholar] [CrossRef] [PubMed]
- Barišić, L.; Čakić, M.; Mahmoud, K.A.; Liu, Y.-N.; Kraatz, H.-B.; Pritzkow, H.; Kirin, S.I.; Metzler-Nolte, N.; Rapić, V. Helically Chiral Ferrocene Peptides Containing 1′-Aminoferrocene-1-Carboxylic Acid Subunits as Turn Inducers. Chem. Eur. J. 2006, 12, 4965–4980. [Google Scholar] [CrossRef]
- Barišić, L.; Rapić, V.; Metzler-Nolte, N. Incorporation of the Unnatural Organometallic Amino Acid 1′-Aminoferrocene-1-carboxylic Acid (Fca) into Oligopeptides by a Combination of Fmoc and Boc Solid-Phase Synthetic Methods. Eur. J. Inorg. Chem. 2006, 4019–4021. [Google Scholar] [CrossRef]
- Čakić Semenčić, M.; Siebler, D.; Heinze, K.; Rapić, V. Bis- and Trisamides Derived From 1′-Aminoferrocene-1-carboxylic Acid and α-Amino Acids: Synthesis and Conformational Analysis. Organometallics 2009, 28, 2028–2037. [Google Scholar] [CrossRef]
- Čakić Semenčić, M.; Heinze, K.; Förster, C.; Rapić, V. Bioconjugates of 1′-Aminoferrocene-1-carboxylic Acid with (S)-3-Amino-2-methylpropanoic Acid and L-Alanine. Eur. J. Inorg. Chem. 2010, 1089–1097. [Google Scholar] [CrossRef]
- Čakić Semenčić, M.; Barišić, L. Ferrocene Bioconjugates. Croat. Chem. Acta 2017, 90, 537–569. [Google Scholar] [CrossRef]
- Kovačević, M.; Kodrin, I.; Cetina, M.; Kmetič, I.; Murati, T.; Semenčić, M.Č.; Roca, S.; Barišić, L. The conjugates of ferrocene-1,1′-diamine and amino acids. A novel synthetic approach and conformational analysis. Dalton Trans. 2015, 44, 16405–16420. [Google Scholar] [CrossRef] [Green Version]
- Kovačević, M.; Kodrin, I.; Roca, S.; Molčanov, K.; Shen, Y.; Adhikari, B.; Kraatz, H.B.; Barišić, L. Helically Chiral Peptides That Contain Ferrocene-1,1′-diamine Scaffolds as a Turn Inducer. Chem. Eur. J. 2017, 23, 10372–10395. [Google Scholar] [CrossRef]
- Moriuchi, T.; Ohmura, S.D.; Moriuchi-Kawakami, T. Chirality Induction in Bioorganometallic Conjugates. Inorganics 2018, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Barišić, L.; Kovačević, M.; Mamić, M.; Kodrin, I.; Mihalić, Z.; Rapić, V. Synthesis and Conformational Analysis of Methyl N-Alanyl-1-aminoferrocene-1-carboxylate. Eur. J. Inorg. Chem. 2012, 11, 1810–1822. [Google Scholar] [CrossRef]
- Kovačević, M.; Molčanov, K.; Radošević, K.; Srček, G.V.; Roca, S.; Čače, A.; Barišić, L. Conjugates of 1′-aminoferrocene-1-carboxylic acid and proline: Synthesis, conformational analysis and biological evaluation. Molecules 2014, 21, 12852–12880. [Google Scholar] [CrossRef] [Green Version]
- Kovač, V.; Čakić Semenčić, M.; Kodrin, I.; Roca, S.; Rapić, V. Ferrocene-dipeptide conjugates derived from aminoferrocene and 1-acetyl-1′-aminoferrocene: Synthesis and conformational studies. Tetrahedron 2013, 69, 10497–10506. [Google Scholar] [CrossRef]
- Nuskol, M.; Studen, B.; Meden, A.; Kodrin, I.; Čakić Semenčić, M. Tight turn in dipeptide bridged ferrocenes: Synthesis, X-ray structural, theoretical and spectroscopic studies. Polyhedron 2019, 161, 137–144. [Google Scholar] [CrossRef]
- Nuskol, M.; Šutalo, P.; Đaković, M.; Kovačević, M.; Kodrin, I.; Čakić, S.M. Testing the Potential of the Ferrocene Chromophore as a Circular Dichroism Probe for the Assignment of the Screw-Sense Preference of Tripeptides. Organometallics 2021, 40, 1351–1362. [Google Scholar] [CrossRef]
- Ganguly, H.K.; Basu, G. Conformational landscape of substituted prolines. Biophys. Rev. 2020, 12, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Byun, B.J.; Song, I.K.; Chung, Y.J.; Ryu, K.H.; Kang, Y.K. Conformational preferences of X-Pro sequences: Ala-Pro and Aib-Pro motifs. J. Phys. Chem. B. 2010, 114, 14077–14086. [Google Scholar] [CrossRef]
- Martin, V.; Legrand, B.; Vezenkov, L.L.; Berthet, M.; Subra, G.; Calmès, M.; Bantignies, J.-L.; Martinez, J.; Amblard, M. Turning Peptide Sequences into Ribbon Foldamers by a Straightforward Multicyclization Reaction. Angew. Chem. Int. Ed. 2015, 54, 13966–13970. [Google Scholar] [CrossRef]
- Metrano, A.J.; Abascal, N.C.; Mercado, B.Q.; Paulson, E.K.; Hurtley, A.E.; Miller, S.J. Diversity of Secondary Structure in Catalytic Peptides with β-Turn-Biased Sequences. J. Am. Chem. Soc. 2017, 139, 492–516. [Google Scholar] [CrossRef]
- Fiorina, V.J.; Dubois, R.J.; Brynes, S. Ferrocenyl polyamines as agents for the chemoimmunotherapy of cancer. J. Med. Chem. 1978, 21, 393–395. [Google Scholar] [CrossRef]
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 6–29. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 66. [Google Scholar] [CrossRef]
- Wang, R.; Chen, H.; Yan, W.; Zheng, M.; Zhang, T.; Zhang, Y. Ferrocene-containing hybrids as potential anticancer agents: Current developments, mechanisms of action and structure-activity relationships. Eur. J. Med. Chem. 2020, 190, 112109–112129. [Google Scholar] [CrossRef] [PubMed]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Defining the hydrogen bond: An account (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1619–1636. [Google Scholar] [CrossRef]
- Fornaro, T.; Burini, D.; Biczysko, M.; Barone, V. Hydrogen-bonding effects on infrared spectra from anharmonic computations: Uracil-water complexes and uracil dimers. J. Phys. Chem. A 2015, 119, 4224–4236. [Google Scholar] [CrossRef] [PubMed]
- Ananthanarayanan, V.S.; Cameron, T.S. Proline-containing β-turns. Int. J. Pept. Protein Res. 1988, 31, 399–411. [Google Scholar] [CrossRef]
- Vincenzi, M.; Mercurio, F.A.; Leone, M. NMR Spectroscopy in the Conformational Analysis of Peptides: An Overview. Curr. Med. Chem. 2021, 28, 2729–2782. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Pardi, A.; Wuethrich, K. Hydrogen bond length and proton NMR chemical shifts in proteins. J. Am. Chem. Soc. 1983, 105, 5948–5949. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Sun, X.-Y.; Tang, Q.; Song, J.-J.; Li, X.-Q.; Gong, B.; Liu, R.; Lu, Z.-L. An unnatural tripeptide structure containing intramolecular double H-bond mimics a turn-hairpin conformation. Org. Biomol. Chem. 2021, 19, 4359–4363. [Google Scholar] [CrossRef]
- Llinás, M.M.; Klein, M.P. Solution conformation of the ferrichromes. VI. Charge relay at the peptide bond. Proton magnetic resonance study of solvation effects on the amide electron density distribution. J. Am. Chem. Soc. 1975, 97, 4731–4737. [Google Scholar] [CrossRef]
- Stevens, E.S.; Sugawara, N.; Bonora, G.M.; Toniolo, C. Conformational analysis of linear peptides. 3. Temperature dependence of NH chemical shifts in chloroform. J. Am. Chem. Soc. 1980, 102, 7048–7050. [Google Scholar] [CrossRef]
- Kessler, H. Conformation and Biological Activity of cyclic Peptides. Angew. Chem. Int. Ed. Engl. 1982, 21, 512–523. [Google Scholar] [CrossRef]
- Iqbal, M.; Balaram, P. Aggregation of apolar peptides in organic solvents. Concentration dependence of 1H-nmr parameters for peptide NH groups in 310 helical decapeptide fragment of suzukacillin. Biopolymers 1982, 21, 1427–1433. [Google Scholar] [CrossRef]
- Vijayakumar, E.K.S.; Balaram, P. Stereochemistry of a-aminoisobutyric acid peptides in solution. Helical conformations of protected decapeptides with repeating Aib-L-Ala and Aib-L-Val sequences. Biopolymers 1983, 22, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.H.; Neidigh, J.W.; Harris, S.M.; Lee, G.M.; Liu, Z.; Tong, H. Extracting Information from the Temperature Gradients of Polypeptide NH Chemical Shifts. 1. The Importance of Conformational Averaging. J. Am. Chem. Soc. 1997, 119, 8547–8561. [Google Scholar] [CrossRef]
- Baxter, N.J.; Williamson, M.P. Temperature dependence of 1H chemical shifts in proteins. J. Biomol. NMR 1997, 9, 359–369. [Google Scholar] [CrossRef]
- Cierpicki, T.; Otlewski, J. Amide proton temperature coefficients as hydrogen bond indicators in proteins. J. Biomol. NMR 2001, 21, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Cierpicki, T.; Zhukov, I.; Byrd, R.A.; Otlewski, J. Hydrogen bonds in human ubiquitin reflected in temperature coefficients of amide protons. J. Magn. Reson. 2002, 157, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Young, P.E.; Sullivan, C.E.; Torchia, D.A. Detection of cis and trans X-Pro peptide bonds in proteins by 13C NMR: Application to collagen. Proc. Natl. Acad. Sci. USA 1984, 81, 4800–4803. [Google Scholar] [CrossRef] [Green Version]
- O’Neal, K.D.; Chari, M.V.; Mcdonald, C.H.; Cook, R.G.; Yu-Lee, L.Y.; Morrisett, J.D.; Shearer, W.T. Multiple cis-trans conformers of the prolactin receptor proline-rich motif (PRM) peptide detected by reverse-phase HPLC, CD and NMR spectroscopy. Biochem. J. 1996, 315, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Bandara, H.M.; Friss, T.R.; Enriquez, M.M.; Isley, W.; Incarvito, C.; Frank, H.A.; Gascon, J.; Burdette, S.C. Proof for the concerted inversion mechanism in the trans->cis isomerization of azobenzene using hydrogen bonding to induce isomer locking. J. Org. Chem. 2010, 75, 4817–4827. [Google Scholar] [CrossRef]
- Siebler, C.; Maryasin, B.; Kümin, M.; Erdmann, R.S.; Rigling, C.; Grünenfelder, C.; Ochsenfeld, C.; Wennemers, H. Importance of Dipole Moments and Ambient Polarity for the Conformation of Xaa-Pro Moieties–A Combined Experimental and Theoretical Study. Chem. Sci. 2015, 6, 6725–6730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollastrini, M.; Lipparini, F.; Pasquinelli, L.; Balzano, F.; Barretta, G.U.; Pescitelli, G.; Angelici, G. A Proline Mimetic for the Design of New Stable Secondary Structures: Solvent-Dependent Amide Bond Isomerization of (S)-Indoline-2-carboxylic Acid Derivatives. J. Org. Chem. 2021, 86, 7946–7954. [Google Scholar] [CrossRef]
- Deetz, M.J.; Fahey, J.E.; Smith, B.D. NMR studies of hydrogen bonding interactions with secondary amide and urea groups. J. Phys. Org. Chem. 2001, 14, 463–467. [Google Scholar] [CrossRef]
- Pignataro, M.F.; Herrera, M.G.; Dodero, V.I. Evaluation of Peptide/Protein Self-Assembly and Aggregation by Spectroscopic Methods. Molecules 2020, 25, 4854. [Google Scholar] [CrossRef]
- Rogers, D.M.; Jasim, S.B.; Dyer, N.T.; Auvray, F.; Réfrégiers, M.; Hirst, J.D. Electronic Circular Dichroism Spectroscopy of Proteins. Chemistry 2019, 5, 2751–2774. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Stap, J.; Rodermond, H.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Li, L.; Thomas, R.M.M.; Suzuki, H.; de Brabander, J.K.; Wang, X.; Harran, P.G. A small molecule Smac mimic potentiates TRAIL-and TNFalpha-mediated cell death. Science 2004, 305, 1471–1474. [Google Scholar] [CrossRef] [Green Version]
- Mabonga, L.; Kappo, A.P. Peptidomimetics: A Synthetic Tool for Inhibiting Protein–Protein Interactions in Cancer. Int. J. Pept. Res. Ther. 2020, 26, 225–241. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger, E. Maestro, Version 9.7; Schrödinger: New York, NY, USA, 2014. [Google Scholar]
- Schrödinger, E. MacroModel, version 10.3. Schrödinger: New York, NY, USA, 2014. [Google Scholar]
- Mohamadi, F.; Richards, N.G.J.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel—An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll, Version 19.02.13; TK Gristmill Software: Overland Park, KS, USA, 2017. [Google Scholar]
- Koch, U.; Popelier, P.L.A.J. Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Popelier, P.L.A.J. Characterization of a dihydrogen bond on the basis of the electron density. J. Phys. Chem. A 1998, 102, 1873–1878. [Google Scholar] [CrossRef]
- Rigaku, P.R.O. CrysAlis, Version: 1.171.39.46; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2018. [Google Scholar]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. CheckCIF validation ALERTS: What they mean and how to respond. Acta Crystallogr. E76 2020, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Kraljić, K.; Brkan, V.; Škevin, D.; Gaurina Srček, V.; Radošević, K. Canolol Dimer, a Biologically Active Phenolic Compound of Edible Rapeseed Oil. Lipids 2019, 54, 189–200. [Google Scholar] [CrossRef]
- Radošević, K.; Rudjer, N.; Slivac, I.; Mihajlović, M.; Dumić, J.; Kniewald, Z.; Gaurina Srček, V. Cytotoxic and Apoptotic Effects of 17α-Ethynylestradiol and Diethylstilbestrol on CHO−K1 Cells. Food Technol. Biotechnol. 2011, 49, 447–452. [Google Scholar]




| Compound | HeLa Cells | MCF-7 Cells |
|---|---|---|
| 2 | 436.1959 µM | n.d. 1 |
| 3 | n.d. 1 | n.d. 1 |
| 4 | 370.3969 µM | 270.6925 µM |
| 5 | n.d. 1 | n.d. 1 |
| PE (%) | Concentration | SF (2) | SF (3) | SF (4) | SF (5) |
|---|---|---|---|---|---|
| 31.75 | 100 μM | 0.1575 | 1.0394 | 0 | 0.7874 |
| 500 μM | 0 | 0 | 0 | 0.0315 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, M.; Čakić Semenčić, M.; Radošević, K.; Molčanov, K.; Roca, S.; Šimunović, L.; Kodrin, I.; Barišić, L. Conformational Preferences and Antiproliferative Activity of Peptidomimetics Containing Methyl 1′-Aminoferrocene-1-carboxylate and Turn-Forming Homo- and Heterochiral Pro-Ala Motifs. Int. J. Mol. Sci. 2021, 22, 13532. https://doi.org/10.3390/ijms222413532
Kovačević M, Čakić Semenčić M, Radošević K, Molčanov K, Roca S, Šimunović L, Kodrin I, Barišić L. Conformational Preferences and Antiproliferative Activity of Peptidomimetics Containing Methyl 1′-Aminoferrocene-1-carboxylate and Turn-Forming Homo- and Heterochiral Pro-Ala Motifs. International Journal of Molecular Sciences. 2021; 22(24):13532. https://doi.org/10.3390/ijms222413532
Chicago/Turabian StyleKovačević, Monika, Mojca Čakić Semenčić, Kristina Radošević, Krešimir Molčanov, Sunčica Roca, Lucija Šimunović, Ivan Kodrin, and Lidija Barišić. 2021. "Conformational Preferences and Antiproliferative Activity of Peptidomimetics Containing Methyl 1′-Aminoferrocene-1-carboxylate and Turn-Forming Homo- and Heterochiral Pro-Ala Motifs" International Journal of Molecular Sciences 22, no. 24: 13532. https://doi.org/10.3390/ijms222413532
APA StyleKovačević, M., Čakić Semenčić, M., Radošević, K., Molčanov, K., Roca, S., Šimunović, L., Kodrin, I., & Barišić, L. (2021). Conformational Preferences and Antiproliferative Activity of Peptidomimetics Containing Methyl 1′-Aminoferrocene-1-carboxylate and Turn-Forming Homo- and Heterochiral Pro-Ala Motifs. International Journal of Molecular Sciences, 22(24), 13532. https://doi.org/10.3390/ijms222413532










