Effect of Mineral-Balanced Deep-Sea Water on Kidney Function and Renal Oxidative Stress Markers in Rats Fed a High-Salt Diet
Abstract
1. Introduction
2. Results
2.1. Clinical Symptoms
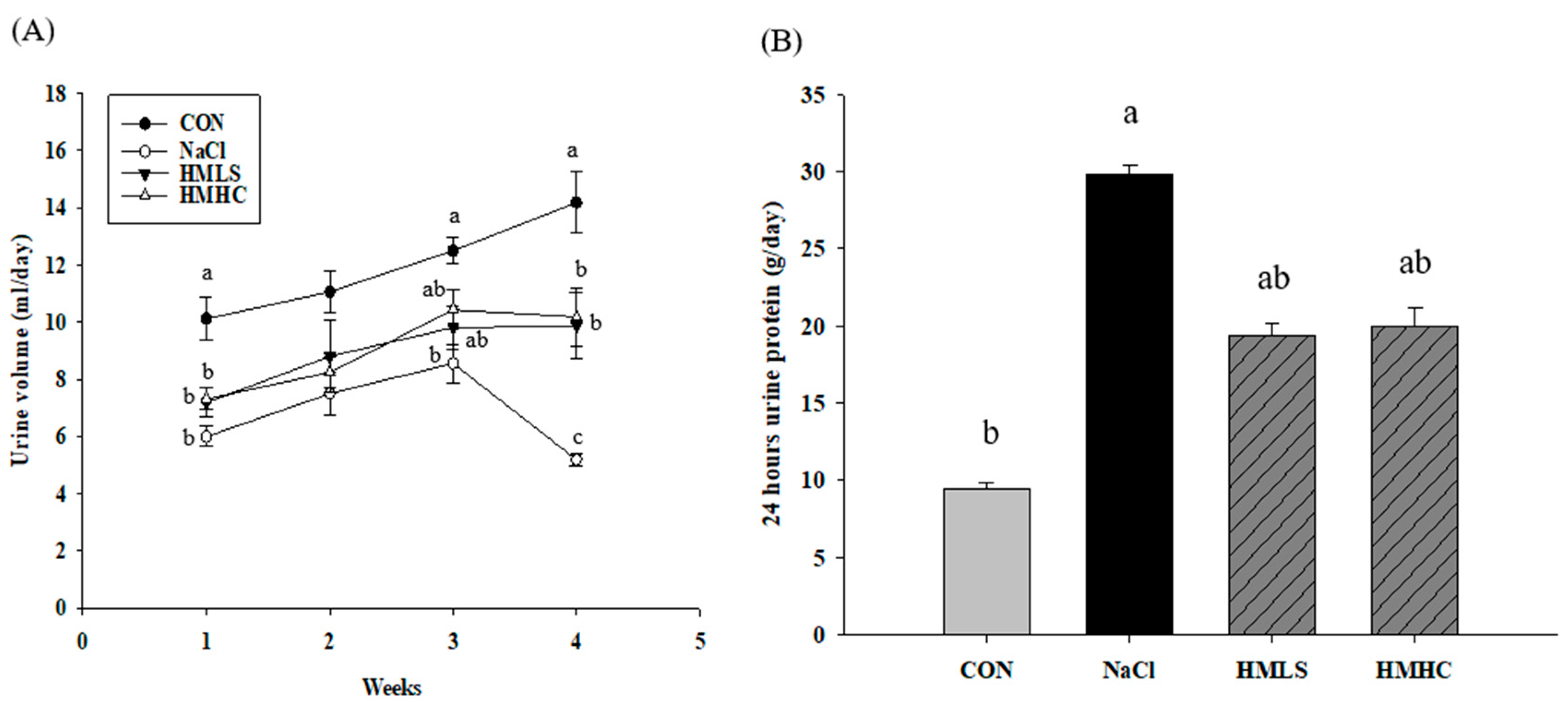
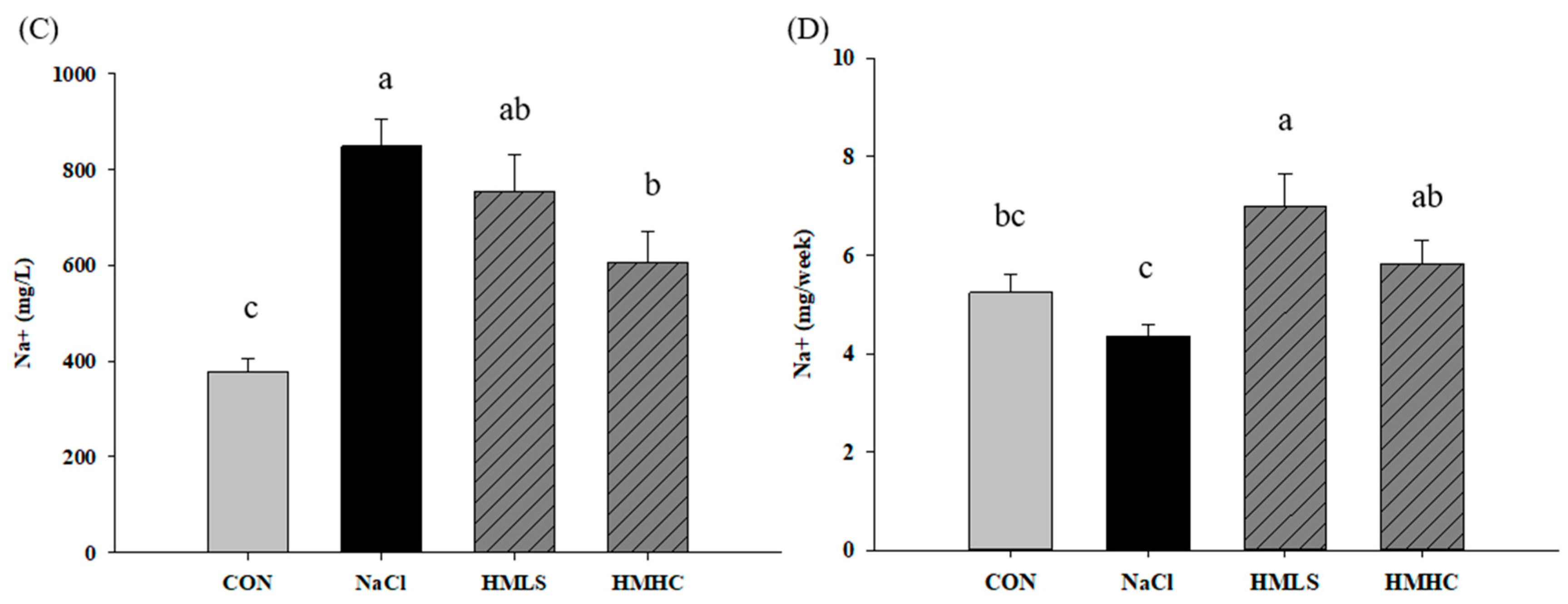
2.2. Urine Volume, Sodium Excretion, and Urine Protein
2.3. Kidney Injury Markers
2.4. Relative mRNA Expression of Genes Related to Kidney Function
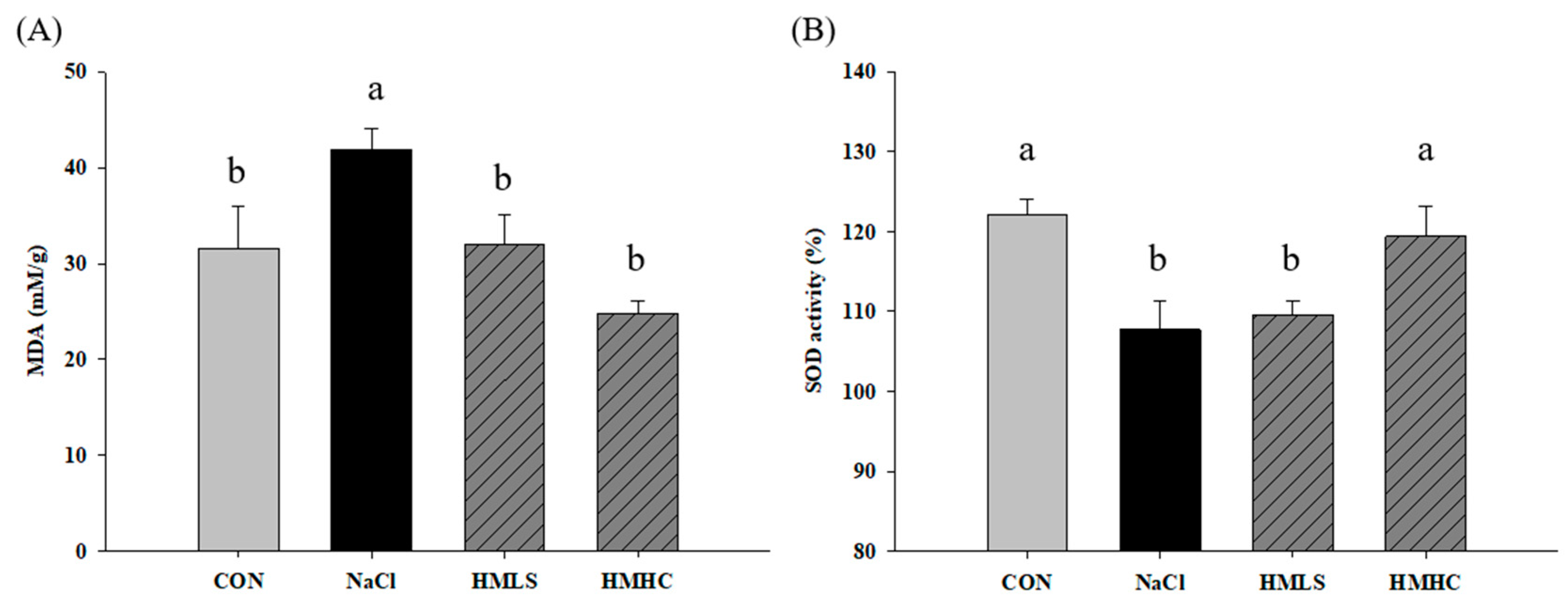
2.5. Oxidative Stress and Antioxidant Activity
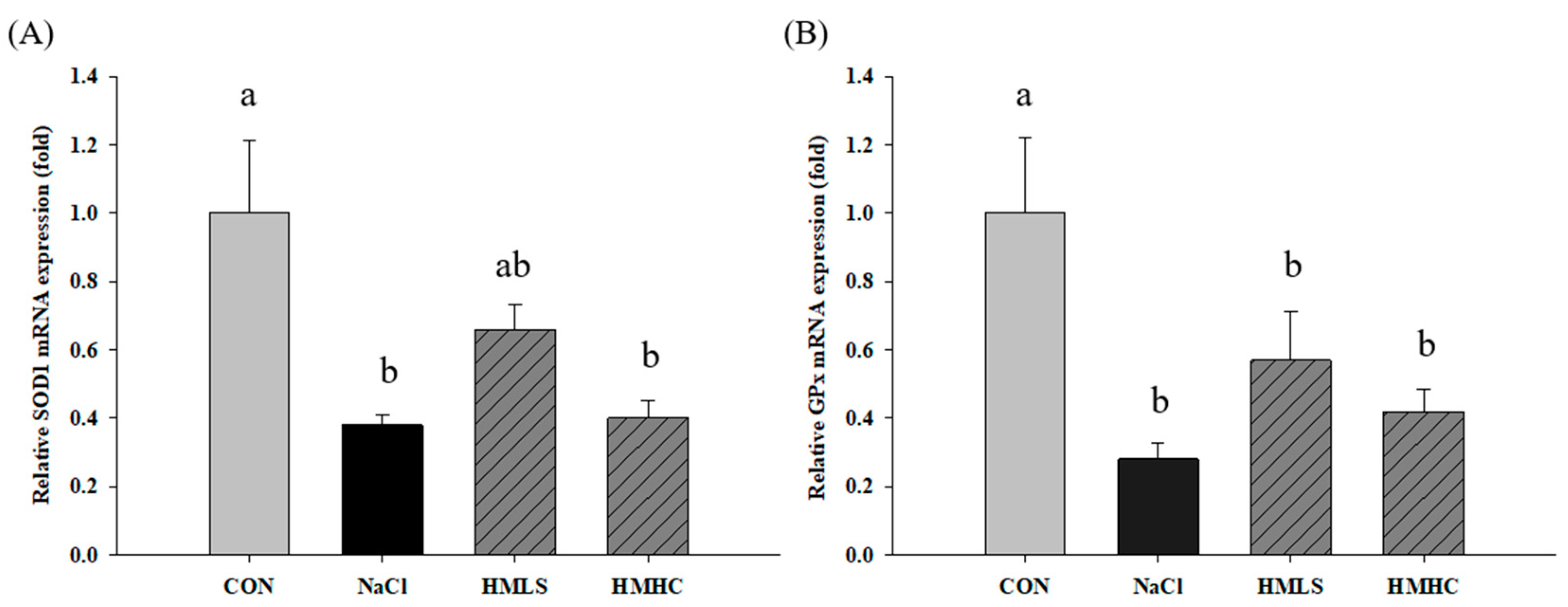
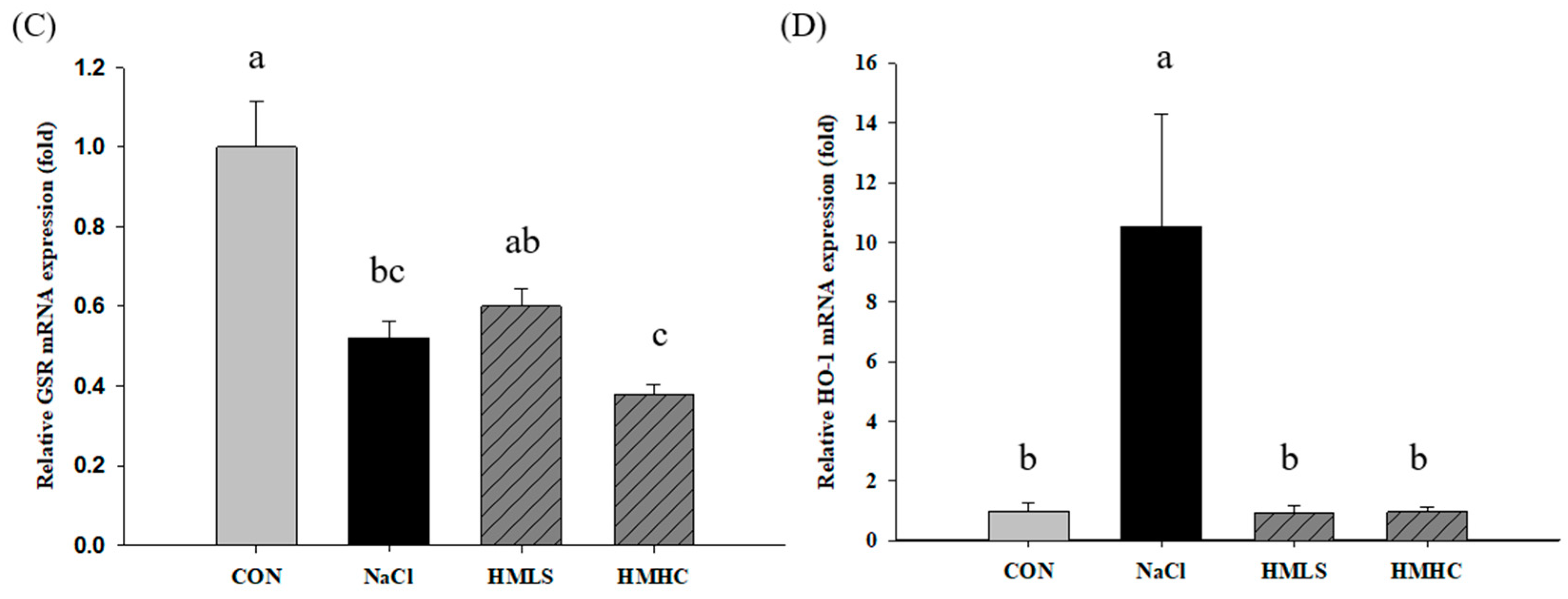
2.6. Relative mRNA Expression of Antioxidant Genes
2.7. Total RNA-Sequencing and Differentially Expressed Gene Analysis
2.8. GO and KEGG Enrichment Analyses of DEG
3. Discussion
4. Materials and Methods
4.1. Preparation of Mineral-Balanced Deep Sea Water (DSW)
4.2. Animals
4.3. Measurement of Urine Protein
4.4. Analysis of Trace Minerals in Urine
4.5. Measurement of Kidney Injury Markers in Urine
4.6. Total RNA Extraction and Quantitative Reverse Transcription (qRT-PCR)
4.7. Measurement of Malondialdehyde (MDA)
4.8. Measurement of Superoxide Dismutase (SOD) Activity
4.9. Statistical Analysis
4.10. RNA-Sequencing
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarke, L.S. Temporal trends in dietary sodium intake among adults aged ≥ 19 years—United States, 2003–2016. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1478–1482. [Google Scholar] [CrossRef]
- He, F.J.; Burnier, M.; MacGregor, G.A. Nutrition in cardiovascular disease: Salt in hypertension and heart failure. Eur. Heart J. 2011, 32, 3073–3080. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Kuwabara, M.; Andres-Hernando, A.; Li, N.; Cicerchi, C.; Jensen, T.; Orlicky, D.J.; Roncal-Jimenez, C.A.; Ishimoto, T.; Nakagawa, T. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 3138–3143. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5, S23–S30. [Google Scholar] [CrossRef]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Fellner, R.C.; Cook, A.K.; O’Connor, P.M.; Zhang, S.; Pollock, D.M.; Inscho, E.W. High-salt diet blunts renal autoregulation by a reactive oxygen species-dependent mechanism. Am. J. Physiol.-Ren. Physiol. 2014, 307, F33–F40. [Google Scholar] [CrossRef]
- Hosohata, K. Biomarkers for chronic kidney disease associated with high salt intake. Int. J. Mol. Sci. 2017, 18, 2080. [Google Scholar] [CrossRef]
- Huang, P.; Shen, Z.; Liu, J.; Huang, Y.; Chen, S.; Yu, W.; Wang, S.; Ren, Y.; Li, X.; Tang, C. Hydrogen sulfide inhibits high-salt diet-induced renal oxidative stress and kidney injury in Dahl rats. Oxidative Med. Cell. Longev. 2016, 2016, 2807490. [Google Scholar] [CrossRef]
- Wolf, M. Mineral (Mal) adaptation to kidney disease—Young investigator award address: American society of Nephrology kidney week 2014. Clin. J. Am. Soc. Nephrol. 2015, 10, 1875–1885. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Sun, N.; Liang, M.; Tian, Z. Mitochondrial dysfunction and altered renal metabolism in Dahl salt-sensitive rats. Kidney Blood Press. Res. 2017, 42, 587–597. [Google Scholar] [CrossRef]
- Trolliet, M.R.; Rudd, M.A.; Loscalzo, J. Oxidative stress and renal dysfunction in salt-sensitive hypertension. Kidney Blood Press. Res. 2001, 24, 116–123. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.R.; Wilkinson, N.; Madison, R.; Felix, T. Vitamins and minerals functioning as antioxidants with supplementation considerations. In Florida Ruminant Nutrition Symposium; Best Western Gateway Grand: Gainesville, FL, USA, 2007; pp. 30–31. [Google Scholar]
- Lukaski, H.C. Vitamin and mineral status: Effects on physical performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Hans, C.P.; Chaudhary, D.P.; Bansal, D.D. Effect of magnesium supplementation on oxidative stress in alloxanic diabetic rats. Magnes. Res. 2003, 16, 13–19. [Google Scholar]
- Nani, M.; Zura, S.; Majid, F.; Jaafar, A.; Mahdzir, A.; Musa, M. Potential health benefits of deep sea water: A review. Evid.-Based Complementary Altern. Med. 2016, 2016, 6520475. [Google Scholar]
- Jo, S.M.; Nam, J.; Park, G.; Kim, B.G.; Jeong, G.-H.; Hurh, B.S.; Kim, J.Y. Effects of calcium and magnesium-balanced deep sea water on antioxidation in kidney cells. J. Appl. Biol. Chem. 2021, 64, 165–170. [Google Scholar] [CrossRef]
- Ba Aqeel, S.H.; Sanchez, A.; Batlle, D. Angiotensinogen as a biomarker of acute kidney injury. Clin. Kidney J. 2017, 10, 759–768. [Google Scholar] [CrossRef]
- Gonsalez, S.R.; Ferrão, F.M.; Souza, A.M.d.; Lowe, J.; Morcillo, L.d.S.L. Inappropriate activity of local renin-angiotensin-aldosterone system during high salt intake: Impact on the cardio-renal axis. Braz. J. Nephrol. 2018, 40, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Briet, M.; Schiffrin, E.L. Aldosterone: Effects on the kidney and cardiovascular system. Nat. Rev. Nephrol. 2010, 6, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Iglarz, M.; Touyz, R.M.; Viel, E.C.; Amiri, F.; Schiffrin, E.L. Involvement of oxidative stress in the profibrotic action of aldosterone: Interaction with the renin-angiotensin system. Am. J. Hypertens. 2004, 17, 597–603. [Google Scholar] [CrossRef]
- Kitiyakara, C.; Chabrashvili, T.; Chen, Y.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J. Am. Soc. Nephrol. 2003, 14, 2775–2782. [Google Scholar] [CrossRef]
- Nagase, M.; Fujita, T. Aldosterone and glomerular podocyte injury. Clin. Exp. Nephrol. 2008, 12, 233–242. [Google Scholar] [CrossRef]
- Washino, S.; Hosohata, K.; Jin, D.; Takai, S.; Miyagawa, T. Early urinary biomarkers of renal tubular damage by a high-salt intake independent of blood pressure in normotensive rats. Clin. Exp. Pharmacol. Physiol. 2018, 45, 261–268. [Google Scholar] [CrossRef]
- Farquhar, M.G.; Saito, A.; Kerjaschki, D.; Orlando, R.A. The Heymann nephritis antigenic complex: Megalin (gp330) and RAP. J. Am. Soc. Nephrol. 1995, 6, 35–47. [Google Scholar] [CrossRef]
- Leheste, J.-R.; Rolinski, B.; Vorum, H.; Hilpert, J.; Nykjaer, A.; Jacobsen, C.; Aucouturier, P.; Moskaug, J.Ø.; Otto, A.; Christensen, E.I. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol. 1999, 155, 1361–1370. [Google Scholar] [CrossRef]
- Schreiber, A.; Theilig, F.; Schweda, F.; Höcherl, K. Acute endotoxemia in mice induces downregulation of megalin and cubilin in the kidney. Kidney Int. 2012, 82, 53–59. [Google Scholar] [CrossRef]
- Marzolo, M.-P.; Farfán, P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol. Res. 2011, 44, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, M.; Zawada, E.T., Jr.; McClung, D.; TerWee, J. Preliminary report: Natriuretic effect of calcium supplementation in hypertensive women over forty. J. Am. Coll. Nutr. 1987, 6, 139–143. [Google Scholar] [CrossRef]
- Berthelot, A.; Esposito, J. Effects of dietary magnesium on the development of hypertension in the spontaneously hypertensive rat. J. Am. Coll. Nutr. 1983, 2, 343–353. [Google Scholar] [CrossRef]
- Chen, D.; Stegbauer, J.; Sparks, M.A.; Kohan, D.; Griffiths, R.; Herrera, M.; Gurley, S.B.; Coffman, T.M. Impact of angiotensin type 1A receptors in principal cells of the collecting duct on blood pressure and hypertension. Hypertension 2016, 67, 1291–1297. [Google Scholar] [CrossRef]
- Guyton, A.C. Blood pressure control—Special role of the kidneys and body fluids. Science 1991, 252, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Coffman, T.M.; Crowley, S.D. Kidney in hypertension: Guyton redux. Hypertension 2008, 51, 811–816. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiose, A.; Kuroda, J.; Tsuruya, K.; Hirai, M.; Hirakata, H.; Naito, S.; Hattori, M.; Sakaki, Y.; Sumimoto, H. A novel superoxide-producing NAD (P) H oxidase in kidney. J. Biol. Chem. 2001, 276, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Kopp, J.B.; Várnai, P.; Leto, T.L. Identification of renox, an NAD (P) H oxidase in kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 8010–8014. [Google Scholar] [CrossRef]
- Bussiere, F.I.; Gueux, E.; Rock, E.; Girardeau, J.-P.; Tridon, A.; Mazur, A.; Rayssiguier, Y. Increased phagocytosis and production of reactive oxygen species by neutrophils during magnesium deficiency in rats and inhibition by high magnesium concentration. Br. J. Nutr. 2002, 87, 107–113. [Google Scholar] [CrossRef]
- Gao, T.-C.; Cho, J.-Y.; Feng, L.-Y.; Chanmuang, S.; Park, S.-Y.; Ham, K.-S.; Auh, C.-K.; Pai, T.-K. Mineral-rich solar sea salt generates less oxidative stress in rats than mineral-deficient salt. Food Sci. Biotechnol. 2014, 23, 951–956. [Google Scholar] [CrossRef]
- Afanas’ev, I.B.; Suslova, T.B.; Cheremisina, Z.P.; Abramova, N.E.; Korkina, L.G. Study of antioxidant properties of metal aspartates. Analyst 1995, 120, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Matkovics, B.; Kiss, I.; Kiss, S. The activation by magnesium treatment of anti-oxidants eliminating the oxygen free radicals in Drosophila melanogaster in vivo. Magnes. Res. 1997, 10, 33–38. [Google Scholar] [PubMed]
- Zhang, Y.; Davies, L.R.; Martin, S.M.; Bawaney, I.M.; Buettner, G.R.; Kerber, R.E. Magnesium reduces free radical concentration and preserves left ventricular function after direct current shocks. Resuscitation 2003, 56, 199–206. [Google Scholar] [CrossRef]
- Das, S.; Choudhuri, D. Dietary calcium regulates the risk renal injury in high fat diet induced obese rats by regulating renal lipid metabolism, oxidative stress and inflammation. Arch. Physiol. Biochem. 2020, 1–11. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.M.; Boddu, R.; George, J.F.; Agarwal, A. Heme oxygenase-1 in kidney health and disease. Antioxid. Redox Signal. 2016, 25, 165–183. [Google Scholar] [CrossRef]
- Zhang, P.L.; Lun, M.; Schworer, C.M.; Blasick, T.M.; Masker, K.K.; Jones, J.B.; Carey, D.J. Heat shock protein expression is highly sensitive to ischemia-reperfusion injury in rat kidneys. Ann. Clin. Lab. Sci. 2008, 38, 57–64. [Google Scholar] [PubMed]
- Boute, N.; Gribouval, O.; Roselli, S.; Benessy, F.; Lee, H.; Fuchshuber, A.; Dahan, K.; Gubler, M.-C.; Niaudet, P.; Antignac, C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 2000, 24, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Roselli, S.; Gribouval, O.; Boute, N.; Sich, M.; Benessy, F.; Attié, T.; Gubler, M.-C.; Antignac, C. Podocin localizes in the kidney to the slit diaphragm area. Am. J. Pathol. 2002, 160, 131–139. [Google Scholar] [CrossRef]
- Liu, W.; Baker, S.S.; Baker, R.D.; Nowak, N.J.; Zhu, L. Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis. PLoS ONE 2011, 6, e24363. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Shu, X.-O.; Deng, X.; Xiang, Y.-B.; Li, H.; Yang, G.; Shrubsole, M.J.; Ji, B.; Cai, H.; Chow, W.-H. Modifying effect of calcium/magnesium intake ratio and mortality: A population-based cohort study. BMJ Open 2013, 3, e002111. [Google Scholar] [CrossRef]
- Hyun, Y.J.; Kim, J.G.; Kim, M.J.; Jung, S.K.; Kim, J.Y. Mineral-rich Jeju lava sea water suppresses lipid accumulation in 3T3-L1 adipocytes and ameliorates high-fat diet-induced obesity in C57BL/6 J mice. Food Sci. Biotechnol. 2021, 30, 299–304. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]




| Group | ||||
|---|---|---|---|---|
| CON (n = 8) | NaCl (n = 8) | HMLS (n = 8) | HMHC (n = 8) | |
| Body Weight (g) | ||||
| Initial Weight | 267.3 ± 5.9 | 268.4 ± 11.0 | 268.8 ± 10.6 | 267.6 ± 11.9 |
| Final Weight | 406.9 ± 15.1 | 393.0 ± 13.2 | 393.5 ± 13.2 | 395.5 ± 18.2 |
| Food Intake (g/day) | 25.6 ± 0.8 | 26.1 ± 0.6 | 26.1 ± 0.1 | 26.2 ± 0.5 |
| Water Intake (mL/day) | 49.0 ± 2.1 c | 113.5 ± 2.1 b | 149.0 ± 8.6 a | 139.6 ± 23.9 a |
| Kidney Weight (2ea) (g) | 2.62 ± 0.26 b | 3.07 ± 0.31 a | 3.09 ± 0.16 a | 3.03 ± 0.16 a |
| Blood Pressure (mmHg) | 113.1 ± 4.6 b | 161.8 ± 6.0 a | 161.1 ± 6.3 a | 160.7 ± 3.7 a |
| Gene ID | Gene Name | Fold Change |
|---|---|---|
| Down-regulated | ||
| Hbb | Hemoglobin Subunit beta | 0.238 |
| Nphs2 | nephrosis 2, idiopathic, steroid-resistant | 0.240 |
| Tbx18 | T-box18 | 0.282 |
| Col4a3 | collagen type IV alpha 3 chain, transcript variant X1 | 0.292 |
| Myocd | myocardin, transcript variant X2 | 0.340 |
| Apc | APC, WNT signaling pathway regulator, transcript variant X1 | 0.377 |
| Col4a4 | collagen type IV alpha 4 chain | 0.386 |
| Adamts1 | ADAM metallopeptidase with thrombospondin type 1 motif, 1 | 0.392 |
| Tsc1 | tuberous sclerosis 1, transcript variant X1 | 0.401 |
| Fat4 | FAT atypical cadherin 4, transcript variant X1 | 0.457 |
| Ctnnd1 | catenin delta 1, transcript variant X6 | 0.477 |
| Ahi1 | Abelson helper integration site 1 | 0.480 |
| Lrrk2 | leucine-rich repeat kinase 2, transcript variant X1 | 0.484 |
| Hoxc11 | homeobox C11 | 0.487 |
| Vegfa | vascular endothelial growth factor A, transcript variant 1 | 0.498 |
| Gene ID | Gene Name | Fold Change |
|---|---|---|
| Down-regulated | ||
| Hmox1 | Heme Oxygenase 1 | 0.062 |
| Hspb1 | Heat Shock Protein Family B (Small) Member 1 | 0.092 |
| Nr4a3 | Nuclear Receptor Subfamily 4 Group A Member 3 | 0.099 |
| Jun | Jun Proto-Oncogene, AP-1 Transcription Factor Subunit | 0.142 |
| Hbb | Hemoglobin Subunit beta | 0.238 |
| Klf6 | Kruppel Like Factor 6 | 0.323 |
| Arntl | Aryl Hydrocarbon Receptor Nuclear Translocator-like, transcript variant X4 | 0.375 |
| Uaca | Uveal Autoantigen with Coiled-coil domains and Ankyrin repeats, transcript variant X1 | 0.400 |
| ll1r1 | Interleukin 1 Receptor type 1, transcript variant X8 | 0.407 |
| Dusp1 | Dual Specificity Phosphatase 1 | 0.438 |
| Egfr | Epidermal Growth Factor Receptor | 0.440 |
| Abcc2 | ATP binding cassette subfamily C member 2 | 0.467 |
| Lrrk2 | Leucine-rich repeat kinase 2, transcript variant X1 | 0.484 |
| Category | Term | ID | p Value | Benjamini |
|---|---|---|---|---|
| GO, cellular component | Hemoglobin complex | GO:0005833 | 4.4 × 10−5 | 3.4 × 10−3 |
| Perinuclear region of cytoplasm | GO:0048471 | 4.5 × 10−5 | 3.4 × 10−3 | |
| Extracellular exosome | GO:0070062 | 3.6 × 10−4 | 1.8 × 10−2 | |
| GO, biological process | Negative regulation of inclusion body assembly | GO:0090084 | 1.1 × 10−5 | 8.0 × 10−3 |
| Oxygen transport | GO:0015671 | 7.4 × 10−5 | 2.2 × 10−2 | |
| Negative regulation of neuron apoptotic process | GO:0043524 | 9.0 × 10−5 | 2.2 × 10−2 | |
| GO, molecular function | Unfolded protein binding | GO:0051082 | 2.0 × 10−6 | 4.8 × 10−4 |
| Chaperone binding | GO:0051087 | 3.8 × 10−5 | 4.7 × 10−3 | |
| Oxygen transporter activity | GO:0005344 | 8.9 × 10−5 | 7.2 × 10−3 | |
| KEGG pathways | MAPK signaling pathway | − | 4.1 × 10−4 | 4.6 × 10−2 |
| African trypanosomiasis | − | 1.1 × 10−3 | 5.7 × 10−2 | |
| Estrogen signaling pathway | − | 1.7 × 10−3 | 5.7 × 10−2 |
| Sample | Mineral Contents (ppm) | Na/Mg | Mg/Ca | |||
|---|---|---|---|---|---|---|
| Na+ | Mg2+ | K+ | Ca2+ | |||
| DSW | 10700 | 1380 | 480 | 390 | 7.75 | 3.5 |
| HMLS | 36 | 179 | 1.7 | 41.5 | 0.2 | 4.3 |
| HMHC | 133 | 211 | 48 | 200 | 0.63 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.M.; Nam, J.; Park, S.-y.; Park, G.; Kim, B.G.; Jeong, G.-H.; Hurh, B.S.; Kim, J.Y. Effect of Mineral-Balanced Deep-Sea Water on Kidney Function and Renal Oxidative Stress Markers in Rats Fed a High-Salt Diet. Int. J. Mol. Sci. 2021, 22, 13415. https://doi.org/10.3390/ijms222413415
Jo SM, Nam J, Park S-y, Park G, Kim BG, Jeong G-H, Hurh BS, Kim JY. Effect of Mineral-Balanced Deep-Sea Water on Kidney Function and Renal Oxidative Stress Markers in Rats Fed a High-Salt Diet. International Journal of Molecular Sciences. 2021; 22(24):13415. https://doi.org/10.3390/ijms222413415
Chicago/Turabian StyleJo, So Min, Jain Nam, Soo-yeon Park, Geonhee Park, Byeong Goo Kim, Gwi-Hwa Jeong, Byung Serk Hurh, and Ji Yeon Kim. 2021. "Effect of Mineral-Balanced Deep-Sea Water on Kidney Function and Renal Oxidative Stress Markers in Rats Fed a High-Salt Diet" International Journal of Molecular Sciences 22, no. 24: 13415. https://doi.org/10.3390/ijms222413415
APA StyleJo, S. M., Nam, J., Park, S.-y., Park, G., Kim, B. G., Jeong, G.-H., Hurh, B. S., & Kim, J. Y. (2021). Effect of Mineral-Balanced Deep-Sea Water on Kidney Function and Renal Oxidative Stress Markers in Rats Fed a High-Salt Diet. International Journal of Molecular Sciences, 22(24), 13415. https://doi.org/10.3390/ijms222413415






